Abstract
Background
Prenatal psychosocial stressors may increase the risk of wheeze in young offspring; yet little attention has been given to the effects that maternal ethnicity may have on this relationship.
Methods
From a population-based cohort of 1,193 children, we assessed the effect of maternal prenatal stressors on the risk of lifetime wheeze in young offspring. We further studied whether maternal Latina ethnicity modified these associations.
Results
The risk of wheeze in the offspring was increased from high levels of pregnancy anxiety (aRR 1.40, 95% CI 1.07, 1.83), negative life events (aRR 1.36, 95% CI 1.06, 1.75), or low paternal support (aRR 1.41, 95% CI 1.02, 1.96). The risk of lifetime wheeze was stronger in the offspring of Latina mothers than of White mothers for these same stressors.
Discussion
Multiple maternal prenatal stressors are associated with increased risk of lifetime wheeze in young offspring, with slight effect modification by Latina ethnicity.
Keywords: prenatal stress, childhood wheeze, Latina ethnicity, fetal programming
Background
Recently, several studies have investigated the contributions of prenatal psychosocial stressors to the occurrence of respiratory illness in the offspring (1–7). This hypothesis is part of the phenomenon known as “fetal programming,” which postulates that during critical periods of fetal development, certain stimuli or insults can have lifelong and even trans-generational effects (8). Maternal prenatal stress may alter the maturation of the fetal immune system, potentially shifting the balance towards a Th2-response that has been related to atopic disease, such as asthma. Additionally, the developing hypothalamus-pituitary-adrenal (HPA) axis may be affected by maternal stress mediated through excess placental secretion of corticotrophin releasing hormone (CRH), potentially influencing the developing fetal immune system (9,10).
Theoretical/Conceptual Framework
Different stressors (e.g.- chronic, acute, transient) may elicit different physical and emotional responses. Stress exposures, perceptions, and responses may also vary culturally. Ethnic minorities and low-income women tend to report more chronic stress and discrimination, and less practical or emotional support from family and friends, which may influence coping mechanisms (11–13). Previous reports have typically considered only one stressor or affective state per study (e.g.- maternal bereavement, negative life events, maternal demoralization) (3,4,6,7) and/or simply adjusted for race/ethnicity (1,2,5) rather than evaluating possible modifying effects of race/ethnicity which may be more elucidating when trying to understand the complex construct of perceived stress.
In the present analysis, we use population-based data from Los Angeles, CA to study three pregnancy stressors (pregnancy anxiety, acute stress from negative life events, chronic stress) and paternal support on subsequent risks for wheezing in the young (~3.5 year old) offspring. We also examine the influence of maternal ethnicity and nativity on these associations.
Methods
Participants
The UCLA Environment and Pregnancy Outcomes Study (EPOS) was originally designed to assess effects of air pollution on birth outcomes as previously described (14). The source population was identified from all live births between January 1–December 31, 2003 to mothers who resided in one of 111 Los Angeles County ZIP codes (41% of all LA County births). The final cohort consisted of 58,316 eligible births (87% of the original total). Cases of low birth weight (<2,500 g) or preterm birth (<37 completed weeks gestation) were selected, and an equal number of randomly sampled controls (≥2,500-g weight and full term). Of the 6,347 women sampled, 2,543 were interviewed in English or Spanish (40% response rate) at 3–6 months post-partum and provided detailed information on pregnancy exposures and behaviors.
In 2006–2007, the UCLA Environment and Child Health Outcomes Study (ECHOS) re-contacted EPOS participants to study the same offspring’s respiratory health at the average age of 3.5 years. 1,201 women participated in the survey by phone or mail (49.3% of those who agreed to be re-contacted); the majority of attrition resulted from the inability to locate women in Los Angeles from mail, telephone, or family/friend contacts initially provided three years prior (15).
Measures
In follow-up 2006–2007 interviews (ECHOS), we assessed respiratory health via maternal report according to the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire for 6–7-year-olds (16). For the primary diagnosis of interest ‘lifetime wheeze,’ we asked “has your child ever had wheezing or whistling in the chest at any time in the past?” In sensitivity analyses, we defined ‘current wheeze’ as “wheeze or whistling in the chest in the past 12 months,” ‘asthmatic symptoms’ as positive response to the question has a “doctor diagnosed asthma with dry cough at night and/or wheeze.” In exploratory analysis, ‘otitis media’ was defined as three or more “doctor diagnosed ear infections over the child’s life.” 1,193 children in the sample had information on respiratory outcomes.
We previously described our stress measures and performed all summations and categorizations for this analysis in the same manner (17). In the baseline survey (EPOS), we asked women to recall three types of stressors pertaining to the pregnancy period: pregnancy anxiety, chronic stress, acute stress due to negative life events, as well as paternal support. These measures are subsets of validated stress measures selected for their brevity and their ability to predict adverse birth outcomes (18–20). Pregnancy anxiety was operationalized by: “I was fearful about the health of my baby or about losing my baby during pregnancy.” Responses were provided via four-point Likert scales (not at all, moderately, somewhat, very much). Chronic stress was estimated using four questions from the Perceived Stress Scale (21), a validated instrument measuring perception of stress: 1) ability to control things, 2) difficulties piling up, 3) confidence in ability to handle problems, and 4) how often things were going the respondent’s way. Responses were given on a five-point Likert scale (never, almost never, sometimes, fairly often, very often). We calculated cumulative totals from the four questions and categorized the scores as low (4–8), moderate (9–12), and high (13+) chronic stress. Acute stress was assessed in terms of six major negative life events that occurred during pregnancy (1) loss of car/job/home, 2) serious arguments with partner, 3) close acquaintance with health, drug or legal problems, 4) anyone close having died, 5) having been threatened with physical harm, or 6) having been exposed to discrimination due to race/ethnicity. We summed the negative life events as 0 events, 1 event, and 2+ events.
Paternal support was operationalized in three questions: “does your partner show you respect/care,” “criticize you,” and “support you financially,” answered on a five-point Likert scale (never, almost never, sometimes, often, almost always). We summed the answer to the three questions and defined low support (3–8), moderate support (9–11) and high support (12+).
Covariates derived from the 2003 birth certificates and assessed in this analysis include maternal age at delivery, education and race/ethnicity (non-Latina White (hereafter referred to as White)), Latina, Black/African American, and Asian/other), gestational age at birth, preeclampsia, pregnancy complications, gestational diabetes and sex of the child. The 2003 baseline survey provided information on maternal pregnancy smoking and annual income. In 2006–2007 ECHOS interviews, we collected data on current smoking in the residence, maternal history of atopy, maternal nativity (US versus foreign born), and months of exclusive breastfeeding as defined in Table I.
Table I.
Demographic and pregnancy characteristics by child wheeze status among respondents to the Environment and Child Health Outcomes Study in Los Angeles County, California, 2006 (n=1193).
| Lifetime Wheeze | ||
|---|---|---|
| Yes n=304 (25.5) |
No n=889 (74.5) |
|
|
|
||
| Maternal age at child’s birth | ||
| < 20 | 18 (5.9) | 57 (6.4) |
| 20–24 | 54 (17.8) | 155 (17.4) |
| 25–34 | 174 (57.2) | 476 (53.5) |
| > 34 | 58 (19.1) | 201 (22.6) |
| Maternal education | ||
| < 11 years | 62 (20.4) | 256 (28.8) |
| 12 years | 65 (21.4) | 209 (23.5) |
| >13 years | 172 (56.6) | 404 (45.4) |
| missing | 5 (1.6) | 20 (2.5) |
| Maternal race/ethnicity | ||
| White | 79 (26.0) | 219 (24.6) |
| Latina | 168 (55.3) | 543 (61.4) |
| Foreign-born | 101 (60.1) | 383 (70.5) |
| Black | 31 (10.2) | 39 (4.4) |
| Asian/Other | 23 (7.6) | 82 (9.2) |
| missing | 3 (1.0) | 6 (0.7) |
| Preterm birth | ||
| Preterm (<37 weeks) | 137 (45.1) | 312 (35.1) |
| Preeclampsia | ||
| Yes | 3 (1.0) | 13 (1.5) |
| Pregnancy complications | ||
| Yes | 31 (10.2) | 83 (9.3) |
| Gestational diabetes | ||
| Yes | 3 (1.0) | 23 (2.5) |
| Sex of child | ||
| Male | 184 (60.5) | 421 (47.4) |
| Maternal pregnancy smoking | ||
| Nonsmoker | 193 (63.5) | 600 (67.5) |
| Former smoker | 93 (30.6) | 254 (28.6) |
| Pregnancy smoker | 18 (5.9) | 35 (3.9) |
| Current smoker in house | ||
| Yes | 6 (2.0) | 19 (2.1) |
| missing | 1 (0.3) | 0 (0.0) |
| History of maternal asthma, eczema, hayfever | ||
| Yes | 107 (35.2) | 162 (18.2) |
| Annual income | ||
| <$10,000 | 48 (15.8) | 137 (15.4) |
| $10–30,000 | 51 (16.8) | 161 (18.1) |
| $30–50,000 | 64 (21.1) | 170 (19.1) |
| <$50,000 | 105 (34.5) | 318 (35.8) |
| missing | 36 (11.8) | 103 (11.6) |
| Fearful about health of baby | ||
| Not at all | 85 (28.0) | 293 (33.0) |
| Moderately | 71 (23.4) | 210 (23.6) |
| Somewhat | 80 (26.3) | 236 (26.5) |
| Very much | 67 (22.0) | 142 (16.0) |
| missing | 1 (0.3) | 8 (0.9) |
| Chronic stress composite score | ||
| Low (4–8) | 151 (49.7) | 481 (54.1) |
| Medium (9–12) | 107 (35.2) | 293 (33.0) |
| High (13+) | 41 (13.5) | 94 (10.6) |
| missing | 5 (1.6) | 21 (2.3) |
| Negative life events | ||
| None (0) | 125 (41.1) | 463 (52.1) |
| Moderate (1) | 95 (31.3) | 244 (27.4) |
| High (2+) | 79 (26.0) | 169 (19.0) |
| missing | 5 (1.6) | 13 (1.5) |
| Paternal support composite score | ||
| Low (3–8) | 27 (8.8) | 45 (5.1) |
| Medium (9–11) | 48 (15.8) | 108 (12.1) |
| High (12+) | 226 (74.3) | 714 (80.3) |
| missing | 3 (1.0) | 22 (2.5) |
| Months exclusive breastfeeding (mean, sd) | 3.1 (3.8) | 3.6 (3.8) |
Analysis
We estimated risk ratios (RR) for the three types of stress and paternal support on wheeze, asthmatic symptoms and otitis media at age 3–4 years using Poisson regression models with robust error variance and a log link function (22). We analyzed the stressors individually in separate models due to the correlation between stressors. Multivariate analysis was conducted including potential confounders previously described, selected based upon literature or a >10% change in risk estimate for the pregnancy stressors. Pregnancy complications, preeclampsia and gestational diabetes showed no evidence of confounding the associations of interest and were thus removed from models. There were too few respondents (n=6) among those with wheeze who affirmed current smokers in the house to include in adjusted models, however, exclusion of those who currently smoked from adjusted models did not change estimates by >10% (23). Similarly, we were missing 11.6% of responses about household income, however, its inclusion in adjusted models did not change the effects by >10%. In our data, income had a correlation of 0.63 with maternal education, thus only maternal education was used in adjusted models. We adjusted models stratified by ethnicity using propensity scores to account for the limitations of small strata. Propensity scores were created by regressing covariates from the fully adjusted models on each individual stressor in a multinomial logistic regression, with inclusion of the predicted probabilities of exposure in the final model. Stratification by race/ethnicity was limited to White mothers and Latina mothers; strata for Black/African American or Asian/Other individuals were too small for analysis (n=70 and 105 respectively). Within the Latina stratum, we further stratified by maternal nativity (US-born vs. foreign-born).
In sensitivity analyses, inverse probability censoring weights (IPCW) were applied to assess the impact of attrition in the cohort. Within the EPOS (baseline) study, maternal age, race/ethnicity and education were all associated with the probability of being censored, and were regressed on a censoring variable to create censoring weights. In a separate sensitivity analysis examining each stressor and lifetime wheeze, the sample was re-weighted to adjust the prevalence of preterm births in the sample (39.7%) to equal 10% as observed in the general population. Finally, risk ratios for current wheeze, asthmatic symptoms and otitis media were calculated for each individual factor adjusting for the variables listed above.
The objective of UCLA Environment and Pregnancy Outcomes Study was to assess prenatal air pollution exposure on birth outcomes, and we did find an association between air pollution in the sample and preterm birth (14). However, we did not find an association between our measured air pollutants and wheeze in the offspring of our sample, and thus air pollution was not included in our adjusted models. Given the association between air pollution and preterm birth, we did adjust for preterm birth in addition to re-weighting of our preterm births in sensitivity analyses.
The UCLA Office for Protection of Research Subjects and the California Committee for the Protection of Human Subjects approved this research; informed consent was obtained from all participants.
Results
Of the 1,193 women in the ECHOS sample, a majority were Latina (59.6%) or non-Latina White (25%). Of the Latina mothers, 68.1% were foreign-born, predominantly in Mexico. Ages of the offspring ranged from 2.3–5.8 years, with a median age of 3.5 years. Of all women in the sample, 25.5% of the offspring (n=304) had a lifetime history of wheeze, and 14.1% (n=168) had wheezed within the last 12 months. Children with a lifetime history of wheeze were more likely to be born to Black/African American or US-born Latina women, to have been born preterm, and more frequently had a mother with a history of atopy. Frequencies of maternal pregnancy stress variables also differed as displayed in Table I.
In multivariate adjusted models (Table II), the risk of lifetime wheeze in the offspring was increased when the mother reported high pregnancy anxiety, high numbers of negative life events, or low paternal support in separate models. Level of chronic stress also increased the risk of lifetime wheeze, but confidence intervals crossed the null. IPCW analysis did not appreciably change the results.
Table II.
Crude, adjusted and inverse probability of censor weighted risk ratios for lifetime wheeze by stressor among respondents to the Environment and Child Health Outcomes Study in Los Angeles County, California, 2006 (n=1193).
| n (case)/n (non-case) | Crude risk ratio (95% CI) | Adjusted risk ratio (95% CI)1 | IPCW adjusted risk ratio (95% CI)2,3 | |
|---|---|---|---|---|
| Pregnancy anxiety | ||||
| Not at all | 85/293 | Reference | Reference | Reference |
| Moderately | 71/210 | 1.12 (0.85, 1.48) | 1.21 (0.92, 1.60) | 1.21 (0.91, 1.62) |
| Somewhat | 80/236 | 1.13 (0.86, 1.47) | 1.04 (0.80, 1.36) | 0.98 (0.74, 1.29) |
| Very much | 67/142 | 1.43 (1.09, 1.87) | 1.40 (1.07, 1.83) | 1.47 (1.11, 1.94) |
| Chronic stress (PSS) | ||||
| Low | 151/481 | Reference | Reference | Reference |
| Moderate | 107/293 | 1.12 (0.90, 1.39) | 1.15 (0.93, 1.43) | 1.11 (0.89, 1.40) |
| High | 41/94 | 1.27 (0.95, 1.70) | 1.22 (0.91, 1.63) | 1.18 (0.86, 1.63) |
| Negative life events | ||||
| None | 125/463 | Reference | Reference | Reference |
| 1 NLE | 95/244 | 1.32 (1.05, 1.66) | 1.30 (1.04, 1.63) | 1.28 (1.00, 1.63) |
| 2+ NLEs | 79/169 | 1.50 (1.18, 1.90) | 1.36 (1.06, 1.75) | 1.33 (1.02, 1.74) |
| Paternal support | ||||
| High | 226/714 | Reference | Reference | Reference |
| Moderate | 48/108 | 1.28 (0.99, 1.66) | 1.24 (0.95, 1.62) | 1.20 (0.90, 1.59) |
| Low | 27/45 | 1.56 (1.13, 2.15) | 1.41 (1.02, 1.96) | 1.34 (0.94, 1.59) |
Adjusted for maternal race, maternal age, maternal education, preterm birth, months exclusive breastfeeding, maternal atopy, child sex, pregnancy smoking and individually adjusted for each stressor.
Inverse probability censor weights (maternal age, race and education). Additionally, model is adjusted for all covariates in previous model.
Weighted sample n=2434.
When stressors were individually analyzed in models stratified by ethnicity (Figure 1), the risk of lifetime wheeze was higher in the offspring of Latina mothers than the offspring of White mothers when having reported high pregnancy anxiety, 2+ negative life events, or low paternal support in pregnancy, although the confidence intervals of the estimates largely overlapped. The risk of lifetime wheeze from high levels of prenatal chronic stress was higher in the offspring of White mothers than in offspring of Latinas. Further stratification among Latinas by nativity (data not shown) resulted in no appreciable differences in the risk of wheezing in the offspring between foreign-born and US-born Latinas resulting from the stressors, with the exception of low paternal support, where offspring of US-born Latinas had higher risk for wheeze (aRR 1.87, 95% CI 1.04, 3.36) than those of foreign-born Latinas (aRR 1.42, 95% CI 0.79, 2.54).
Figure 1. Risk of lifetime wheeze by race/ethnicity.
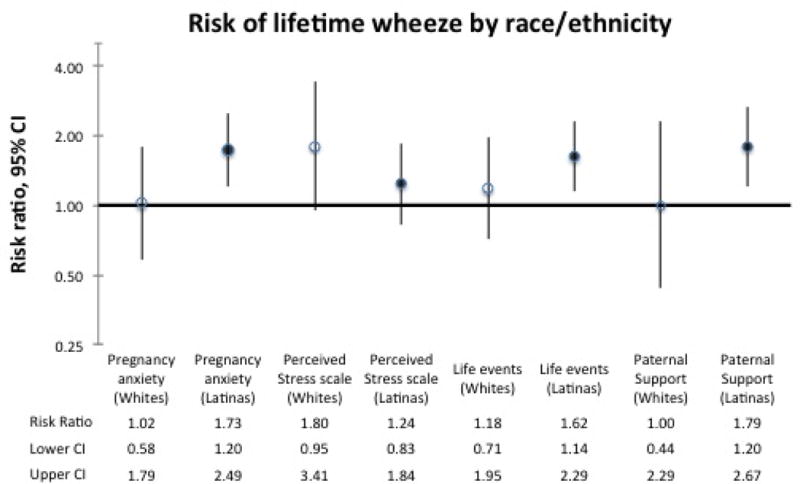
Adjusted risk ratios for lifetime wheeze stratified by race among respondents to the Environment and Child Health Outcomes Study in Los Angeles County, California, 2006 (n=1193). Risk ratios are adjusted with propensity scores for maternal age, maternal education, preterm birth, months of exclusive breastfeeding, maternal atopy, child sex, and pregnancy smoking, and individually adjusted for each stressor. Results are presented on a logarithmic scale. Estimates displayed as follows: pregnancy anxiety: very much vs. none at all; PSS: high vs. low; life events: 2+ vs. none; paternal support: low vs. high.
In sensitivity analyses (Table III) for current wheeze (within the last 12 months) we estimated similar sized effects as for lifetime wheeze for all factors except for paternal support, where low paternal support had a stronger association with current wheeze in the offspring. Point estimates for associations between the three stressors and paternal support and asthmatic symptoms in the offspring indicated increased risk from each of the stressors, but all confidence intervals crossed the null. Finally, 2+ negative life events and low paternal support increased the risk of three or more infections with otitis media, but confidence intervals crossed the null.
Table III.
Crude and adjusted risk ratios for current wheeze, asthmatic symptoms and otitis media by stressor among respondents to the Environment and Child Health Outcomes Study in Los Angeles County, California, 2006 (n=1193).
| Current wheeze (yes=168) | Asthmatic symptoms (yes=106) | Otitis media (yes=129) | ||||
|---|---|---|---|---|---|---|
| Crude risk ratio (95% CI) | Adjusted risk ratio (95% CI)1 | Crude risk ratio (95% CI) | Adjusted risk ratio (95% CI)1 | Crude risk ratio (95% CI) | Adjusted risk ratio (95% CI)1 | |
| Fearful about health of baby | ||||||
| Not at all | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderately | 0.95 (0.63, 1.43) | 1.03 (0.68, 1.55) | 1.12 (0.70, 1.89) | 1.22 (0.73, 2.05) | 1.15 (0.84, 1.59) | 1.18 (0.85, 1.62) |
| Somewhat | 1.17 (0.81, 1.70) | 1.04 (0.72, 1.50) | 1.23 (0.76, 2.01) | 1.07 (0.67, 1.71) | 1.27 (0.94, 1.72) | 1.16 (0.85, 1.57) |
| Very much | 1.45 (0.99, 2.14) | 1.45 (1.00, 2.14) | 1.43 (0.85, 2.41) | 1.39 (0.83, 2.35) | 0.99 (0.68, 1.43) | 0.96 (0.66, 1.39) |
| Chronic stress (PSS) | ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 1.02 (0.75, 1.39) | 1.03 (0.76, 1.41) | 0.75 (0.49, 1.15) | 0.75 (0.49, 1.14) | 1.17 (0.91, 1.49) | 1.42 (1.11, 1.82) |
| High | 1.23 (0.81, 1.87) | 1.14 (0.76, 1.72) | 1.33 (0.80, 2.21) | 1.11 (0.67, 1.86) | 0.95 (0.64, 1.42) | 1.18 (0.78, 1.77) |
| Negative life events | ||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate (1) | 1.28 (0.92, 1.79) | 1.23 (0.89, 1.70) | 1.28 (0.84, 1.95) | 1.21 (0.80, 1.83) | 0.89 (0.67, 1.18) | 0.92 (0.69, 1.22) |
| High (2+) | 1.44 (1.01, 2.05) | 1.22 (0.85, 1.76) | 1.24 (0.77, 1.98) | 0.96 (0.58, 1.57) | 1.16 (0.87, 1.54) | 1.22 (0.91, 1.64) |
| Paternal support | ||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 1.37 (0.93, 2.00) | 1.35 (0.92, 1.96) | 1.56 (0.97, 2.50) | 1.54 (0.95, 2.45) | 0.92 (0.64, 1.32) | 0.98 (0.68, 1.41) |
| Low | 2.06 (1.35, 3.13) | 1.82 (1.15, 2.87) | 1.96 (1.09, 3.51) | 1.54 (0.84, 2.81) | 1.19 (0.77, 1.84) | 1.45 (0.93, 2.27) |
Adjusted for maternal race, maternal age, maternal education, preterm birth, months exclusive breastfeeding, maternal atopy, child sex, pregnancy smoking and individually adjusted for each stressor.
When preterm birth was down-weighted to represent 10% of the sample, point estimates did not change by greater than 10%; confidence intervals became slightly wider (data not shown).
Discussion
Using measures on three types of stress and paternal support during pregnancy, we found that a woman’s anxiety about the health of the baby during pregnancy, negative life events, and low paternal support in pregnancy increased the risk of lifetime wheeze in the offspring. The strength of the association with lifetime wheeze in the offspring did not differ by stressor. However, upon examination by ethnicity, Latina mothers tended to have greater magnitude risk estimates for wheeze in their offspring resulting from pregnancy stressors.
Our interest in examining ethnicity as an effect modifier stemmed from a recent analysis suggesting that the perception and vulnerability to stress may vary by race/ethnicity. In the analysis, levels of maternal perceived stress, maternal negative life events and pregnancy stress differed by race/ethnicity within income categories (24) suggesting that racial/ethnic differences in stress burden persist independent of income. While our sample sizes within strata were small, we also found evidence for differences in risk of wheeze to the offspring of White and Latina mothers, with greater risk of wheeze incurred in the offspring of Latinas from pregnancy anxiety, 2+ negative life events, and low paternal support. This modification by ethnicity may be a result of Latinas having less access to resources that influence coping mechanism, such as financial means, adequate nutrition and exercise, increasing the vulnerability to wheeze in their young offspring. Pregnancy support programs designed to offer additional social support to low-income or minority women through prenatal care, social interaction and meditation have shown positive effects on preterm birth and birth weight (25). While our paper was not designed to assess interventions such as these, investigation into whether these programs may also impact the respiratory health in the offspring through mitigation of pregnancy stressors may be warranted.
We were interested to find that low paternal support was modified by nativity, with stronger risk of wheeze in the offspring of US-born Latinas. One explanation for our findings with respect to paternal support is that compared with both non-Latina White and foreign-born Latinas, US-born Latinas were less likely to be married or living with a partner, more likely to be teenage mothers, and less likely to breastfeed. Although we adjusted for these factors in our models, these patterns suggest there may be other unmeasured and less healthy behaviors during pregnancy, and the additional stress of low paternal support may then be even more detrimental to health in pregnancy and of young offspring among the US-born Latinas.
The few studies that have examined the risk of asthma from prenatal stressors found stronger evidence of an increased risk of asthma in older children (age 7+) (1) and males (6). Our attenuated findings of prenatal stressors and asthmatic symptoms may reflect the difficulty of diagnosing asthma at the young age of our children. In addition, 39.6% of those with asthmatic symptoms in our sample had mothers with atopy, and our wide confidence intervals may reflect heterogeneity in those with asthmatic symptoms and potentially different fetal programming mechanisms that result from prenatal stress in atopic and non-atopic respiratory disease (26). Finally, we saw suggestions of an increased risk of otitis media from 2+ negative life events and low paternal support. Otitis media results from infection with the hypothesized pathway of HPA alteration in the developing fetus resulting in compromised immune function (27). Little has been published on prenatal stress and infections in human offspring, however, researchers did find an increased risk of hospitalization from any type of infection prior to age 15 in the offspring of mothers who suffered prenatal bereavement (RR 1.31, 95% CI 1.27, 1.35) (28). While our observations for otitis media are only suggestive, more research targeting this outcome may be warranted.
It was not possible in our sample to consider the effect of postnatal stressors as we did not ask about postnatal maternal stress in follow up interviews. A recent study found prenatal and postnatal stressors to be correlated (ρ=0.56–0.6 in first 3 years of life) (4), and one could hypothesize that alteration to the HPA axis from prenatal stress could prime the offspring for vulnerability to postnatal stress, further increasing the risk of childhood wheeze. However, given the biologic plausibility of the fetal programming hypothesis and work that has been done to elucidate the role of prenatal stress on immune function in animal models (29,30) and in human cord blood mononuclear cells (31), it is convincing that the mental health and wellbeing of the mother during the prenatal time frame independently contributes to the respiratory health in her offspring.
Strengths of this study include the large population of immigrant Latinas, allowing us to investigate this often-understudied group to examine effect measure modification by ethnicity and nativity, which, to our knowledge, has not been explored previously. Additionally, using three types of pregnancy stressors and paternal support allowed us to test whether different aspects or types of stress had differential effects on the risk of wheeze, which our data did ultimately not support.
Limitations in our study include that our stressors were recalled 3–6 months postpartum. This allows for the possibility of recall bias if wheezing occurred in the first 3–6 months; however, the similar magnitude in risk ratios observed with current wheeze in sensitivity analyses increased our confidence in the time sequence of events. Another limitation is our single-time point collection of respiratory outcomes, prohibiting the ability to distinguish between transient, persistent, or early or late-onset wheeze. Wheezing phenotypes are not reliably established until the age of six, and are quite different in their risk for subsequent development of asthma (32,33). There was also the potential of selection bias, as we were unable to locate 51% of our sample between 2003 and 2006. However, our analyses utilizing IPCW did not meaningfully change our estimates of interest. Finally, our cohort was at higher risk than the general population as preterm birth may be on the causal path to childhood wheeze. In sensitivity analysis re-weighting the cohort to mirror the population prevalence of preterm birth, estimates remained stable with slightly wider confidence intervals, suggesting that the increased risk of wheeze from prenatal stressors still persists in our sample without the over-contribution from those with higher risk.
New Contribution to the Literature
This work adds to the literature by examining different maternal prenatal stressors and the risk of lifetime wheeze in their offspring, and further exploring differences in the magnitude of risk between Whites and Latinas. As both asthma and wheeze have higher prevalence in minorities and disproportionately affect children (34), the identification of potentially modifiable risk factors such as maternal prenatal stress is of great public health importance. Further, the identification of stressors that may be more detrimental to select races/ethnicities could aid in targeted approaches to reducing maternal stress during this crucial development period.
Acknowledgments
We would like to thank Susan Cochran, Marjan Javanbakht and May Wang for their insightful comments and suggestions to the project. This work was supported by funding from the National Institutes of Environmental Health Sciences [R01ES010960-01].
References
- 1.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers’ anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009 Apr;123(4):847–53.e11. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guxens M, Sonnenschein-van der Voort AMM, Tiemeier H, Hofman A, Sunyer J, de Jongste JC, et al. Parental psychological distress during pregnancy and wheezing in preschool children: the Generation R Study. J Allergy Clin Immunol. 2014 Jan;133(1):59–67.e1–12. doi: 10.1016/j.jaci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Mathilda Chiu Y-H, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012 Jul 15;186(2):147–54. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM, et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol. 2011 Jul;107(1):42–9.e1. doi: 10.1016/j.anai.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood RA, Bloomberg GR, Kattan M, Conroy K, Sandel MT, Dresen A, et al. J Allergy Clin Immunol. 4. Vol. 127. Elsevier Ltd; 2011. Apr, Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) pp. 913–9.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang F, Höglund CO, Arck P, Lundholm C, Långström N, Lichtenstein P, et al. Maternal bereavement and childhood asthma-analyses in two large samples of Swedish children. PLoS One. 2011 Jan;6(11):e27202. doi: 10.1371/journal.pone.0027202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khashan AS, Wicks S, Dalman C, Henriksen TB, Li J, Mortensen PB, et al. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosom Med. 2012;74(6):635–41. doi: 10.1097/PSY.0b013e31825ac5e7. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2007 Sep 27;4(2b) doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 9.Wright RJ. Biol Psychol. 1. Vol. 84. Elsevier B.V.; 2010. Apr, Perinatal stress and early life programming of lung structure and function; pp. 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provençal N, Binder E. Exp Neurol. Elsevier B.V.; 2014. Sep 9, The effects of early life stress on the epigenome: From the womb to adulthood and even before; pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Braveman P, Marchi K, Egerter S, Kim S, Metzler M, Stancil T, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Matern Child Health J. 2010 Jan;14(1):20–35. doi: 10.1007/s10995-008-0427-0. [DOI] [PubMed] [Google Scholar]
- 12.Lauderdale DS, Wen M, Jacobs EA, Kandula NR. Immigrant perceptions of discrimination in health care: the California Health Interview Survey 2003. Med Care. 2006 Oct;44(10):914–20. doi: 10.1097/01.mlr.0000220829.87073.f7. [DOI] [PubMed] [Google Scholar]
- 13.Wright RJ, Rodriguez M, Cohen S. Occasional reviews Review of psychosocial stress and asthma : an integrated biopsychosocial approach. Thorax. 1998;53:1066–74. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JKC. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007 Nov 1;166(9):1045–52. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 15.Hoggatt KJ, Flores M, Solorio R, Wilhelm M, Ritz B. The “Latina epidemiologic paradox” revisited: the role of birthplace and acculturation in predicting infant low birth weight for Latinas in Los Angeles, CA. J Immigr Minor Health. 2012 Oct;14(5):875–84. doi: 10.1007/s10903-011-9556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995 Mar 1;8(3):483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh JKC, Wilhelm MH, Dunkel-Schetter C, Lombardi CA, Ritz BR. Paternal support and preterm birth, and the moderation of effects of chronic stress: a study in Los Angeles county mothers. Arch Womens Ment Health. 2010 Aug;13(4):327–38. doi: 10.1007/s00737-009-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunkel-Schetter C, Glynn L. Stress in Pregnancy: Empirical Evidence and the Theoretical Issues to Guide Interdisciplinary Research. The Handbook of Stress Science. 2010 p. Ch 24. [Google Scholar]
- 19.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011 Jan;62:531–58. doi: 10.1146/annurev.psych.031809.130727. November 2010. [DOI] [PubMed] [Google Scholar]
- 20.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999 Jul;18(4):333–45. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck TMR. A global measure of perceived stress. J Heal Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 22.Fang J. Using SAS ® Procedures FREQ, GENMOD, LOGISTIC, and PHREG to Estimate Adjusted Relative Risks – A Case Study. SAS Global Forum. 2011:345. 2011. [Google Scholar]
- 23.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989 Jan;129(1):125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 24.Dunkel Schetter C, Schafer P, Lanzi RG, Clark-Kauffman E, Raju TNK, Hillemeier MM. Shedding Light on the Mechanisms Underlying Health Disparities Through Community Participatory Methods: The Stress Pathway. Perspect Psychol Sci. 2013 Nov 4;8(6):613–33. doi: 10.1177/1745691613506016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ickovics JR, Kershaw TS, Westdahl C, Rising SS, Klima C, Reynolds H, et al. Group prenatal care and preterm birth weight: Results from a matched cohort study at public clinics. Obstet Gynecol. 2003;102(5):1051–7. doi: 10.1016/s0029-7844(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 26.Wright RJ. Epidemiology of stress and asthma: from constricting communities and fragile families to epigenetics. Immunol Allergy Clin North Am. 2011 Mar;31(1):19–39. doi: 10.1016/j.iac.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AH. Reviews and Overviews When Not Enough Is Too Much : The Role of Insufficient Glucocorticoid Signaling in the Pathophysiology of Stress-Related Disorders. Am J Psychiatry. 2003 Sep;160:1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen NM, Hansen AV, Simonsen J, Hviid A. Prenatal stress and risk of infectious diseases in offspring. Am J Epidemiol. 2011 May 1;173(9):990–7. doi: 10.1093/aje/kwq492. [DOI] [PubMed] [Google Scholar]
- 29.Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008 Jan;22(1):42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Veru F, Laplante DP, Luheshi G, King S. Prenatal maternal stress exposure and immune function in the offspring. Stress. 2014;17(2):133–48. doi: 10.3109/10253890.2013.876404. [DOI] [PubMed] [Google Scholar]
- 31.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010 Jul 1;182(1):25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008 Nov;63(11):974–80. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005 Nov 15;172(10):1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelfand EW. THE IMPACT OF ASTHMA ON THE PATIENT. Johns Hopkins Adv Stud Med. 2008;8(3):57–63. [Google Scholar]


