Abstract
Cognitive conflict is often experienced as a difficult, frustrating, and aversive state. Recent studies have indicated that conflict acts as an implicit cost during learning, valuation, and the instantiation of cognitive control. Here we investigated if an implicit manipulation of conflict also influences explicit decision making to risk. Participants were required to perform a Balloon Analogue Risk Task wherein the virtual balloon was inflated by performing a flankers task. By varying the percent of incongruent flanker trials between balloons, we hypothesized that participants would pump the balloon fewer times in conditions of higher conflict and that frontal midline theta would account for significant variance in this relationship. Across two studies, we demonstrate that conflict did not elicit reliable behavioral changes in this task across participants. However, individual differences in frontal theta power accounted for significant variance by predicting diminished balloon pumps. Thus, while conflict costs may act as investments to some individuals (invigorating behavior), it is aversive to others (diminishing behavior), and frontal midline theta power accounts for these varying behavioral tendencies between individuals. These findings demonstrate how frontal midline theta is not only a candidate mechanism for implementing cognitive control, but it is sensitive to the inherent costs therein.
Introduction
An emerging literature has catalogued the mechanisms by which effortful control tallies a cognitive cost. Humans implicitly seek cognitive efficiencies (Zipf, 1949), and effort is associated with more routine and habitual performance (Kool, McGuire, Rosen, & Botvinick, 2010), heightened perceived costs, diminished reward value, and a bias toward less demanding decisions (Botvinick, 2007; Kool et al., 2010; Westbrook & Braver, 2015). While there is considerable convergence in the understanding of effort costs on action selection and learning, there has not yet been a detailed study of similar costs on risky decision making. This topic is particularly appealing, since it involves explicitly managing known uncertainty instead of reducing unknown uncertainty (as in learning). In this report we examine whether the experience of cognitive conflict alters decision making under risk, and we seek to explain this phenomenon via a candidate mechanism of effort-related costs: frontal midline theta band EEG power.
Frontal midline theta band activity in the EEG offers a candidate measure of dorsomedial cortical operations, particularly to conflict, stopping, punishment and other events that elicit the need for cognitive control (Cavanagh & Frank, 2014; Van Noordt, Campopiano, & Segalowitz, 2016; Wessel, Danielmeier, Morton, & Ullsperger, 2012). The dorsal cingulate cortex appears to compute the combined reward value and effort cost for decisions (Croxson, Walton, O’Reilly, Behrens, & Rushworth, 2009; Klein-Flugge, Kennerley, Friston, & Bestmann, 2016), suggesting that it calculates the expected value of exerting control (Shenhav, Botvinick, & Cohen, 2013). Frontal midline theta also emerges with effort (Pellouchoud, Smith, McEvoy, & Gevins, 1999; Smit, Eling, & Coenen, 2004; Smit, Eling, Hopman, & Coenen, 2005; Wascher et al., 2014), effortful working memory maintenance (Gevins & Smith, 2000; Itthipuripat, Wessel, & Aron, 2013), and a bias towards response slowing and avoidance (Cavanagh & Shackman, 2014), suggesting it is a succinct indicator of the mid-frontal calculation of the need for control as well as its associated costs. In summary, a broad class of phenomena closely related to effort and difficulty are also associated with aversive biases, likely via common dorsomedial calculations.
Instead of pure effort, here we focus on a convergent construct of cognitive conflict. Cognitive conflict occurs when two competing response options compete for control of behavior (Botvinick, Braver, Barch, Carter, & Cohen, 2001), and it is reliably associated with dorsomedial activation (Shackman et al., 2011) and frontal midline theta (Cohen & Donner, 2013; Cohen, Ridderinkhof, Haupt, Elger, & Fell, 2008). Conflict can cause avoidance biases and stimulus devaluations (Cavanagh, Masters, Bath, & Frank, 2014; Dreisbach & Fischer, 2012; Fritz & Dreisbach, 2013; Schouppe, De Houwer, Richard Ridderinkhof, & Notebaert, 2012), and so can the similar phenomenon of stopping a pre-potent response (Wessel, O’Doherty, Berkebile, Linderman, & Aron, 2014; Wessel, Tonnesen, & Aron, 2015). Conflict can be reliably elicited and parametrically manipulated, suggesting that conflict can act as a succinct operationalization of dorsomedial computations affected by the implicit costs associated with effort.
In the present work we tested whether implicit conflict diminished explicit decision making, and if frontal midline theta could account for variance in this effect. To test this idea, we combined well-established tasks that separately elicit decision making for risky investments and response conflict. Risky decision making was assessed via the Balloon Analogue Risk Task (BART) (Lejuez et al., 2002). In the standard BART, a simulated “balloon” is inflated with each press of a button. Rewards accumulate with each inflation, and participants choose on each trial whether to press the button yet again or else to “cash out.” The risk on each trial therefore is that the balloon will be overinflated with the next button press and will burst, resulting in the loss of the rewards accumulated so far. In our novel modification of BART, conflict was introduced as a variable by incorporating the classic Eriksen flankers task (Eriksen & Eriksen, 1974). Specifically, congruent or incongruent flankers stimuli were presented on each BART trial requiring a button press to inflate the balloon, and it was the left/right key-press response to the flankers stimuli that would serve to inflate the BART balloon (Fig 1). Therefore, each decision in BART was accompanied by varying levels of conflict in flankers. To test the a priori hypotheses that increased conflict should result in more frontal midline theta as well as diminished decision making for risky investment, the proportion of incongruent flankers was manipulated across balloons. Two studies verified that individual differences in frontal midline theta predicted diminished investment in risky decision making in the face of conflict.
Figure 1.
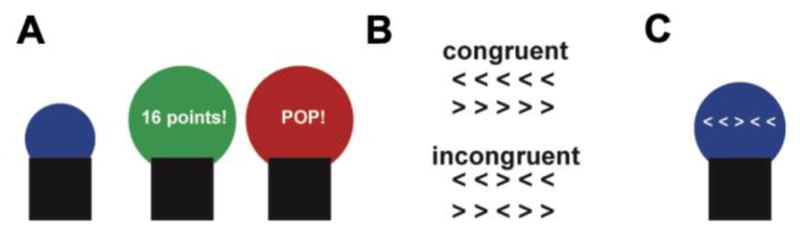
Modified BART task: A. The BART task. Participants inflate a virtual balloon with each key-press earning them one point. They can press a separate button to cash out on the points earned in each trial, unless they inflate the balloon too much and it “pops”, erasing that balloon’s accumulated points. B. The flankers task. Participants respond to the direction that the center arrow is pointing, ignoring the flanking arrows on both sides of the center arrow. When the center arrow points in the opposite direction than the other arrows, this proportion-incongruent which elicits conflict. C. Modified BART task. We combined the BART and flankers tasks by requiring participants to inflate the balloon with each correct response in the flankers task. Errors deflated the balloon by one pump. The influence of conflict on risky decision making was manipulated by varying the proportion of incongruent flanker trials within each balloon. Participants completed 15 balloons of each of the 5 levels of congruency (10%, 30%, 50%, 70%, 90% incongruent) for a total of 75 balloons.
Results
Experiment I
Performance
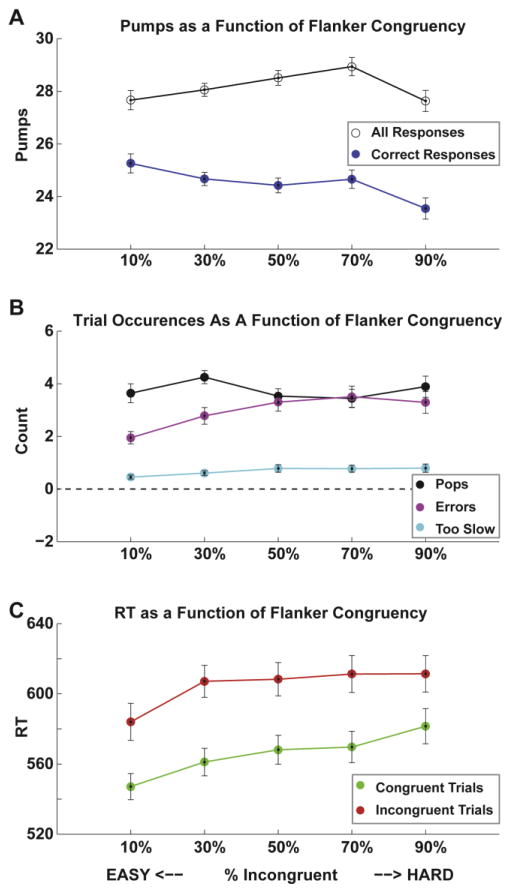
Contrary to our prediction that risky decision making behavior (pumps) would decrease with increasing levels of conflict (flankers proportion-incongruent), we found a non-significant increase in pumps with increasing levels of proportion-incongruent (t(35)= 1.14, p= .26), however there was no trend when considering the correct trials alone (t(35)= −.15, p= .88), see Fig 2A. This was not due to a failure of the congruency manipulation to elicit conflict (Fig 2B–C): there was a significant effect of proportion-incongruent on errors committed (t(35)= 9.95, p<.0001), trials that were too slow (t(35)= 4.59, p<.0001), as well as congruent RT(t(35)= 7.46, p<.0001) and incongruent RT (t(35)= 4.02, p<.0001). As expected, there was no relationship between flankers congruency and pops (t(35)= −.71, p=.48). In summary, while we did not find the expected decrease in risky behavior with increasing levels of proportion-incongruent, we are confident the participants experienced increased conflict as evidenced by increasing errors and reaction time. Thus the results do not support our hypothesis at the behavioral level, that increasing conflict would diminish risky decision making. Next, we investigated EEG markers of conflict and their relationship with behavior as a function of the congruency manipulation.
Figure 2.
Experiment I performance A. Average number of pumps as a function of the percentage of incongruent flankers. There was a slight (non-significant) positive relationship between the number of pumps and the percent of incongruent flankers; however this relationship was reversed when removing error trials. B. Errors and too-slow trials both increased with the proportion-incongruent manipulation, but the number of pops experienced was unrelated to this manipulation. C. Congruent and incongruent trial reaction times both increased with the proportion-incongruent manipulation. Bars are mean +/− SEM.
EEG to Flanker Cues
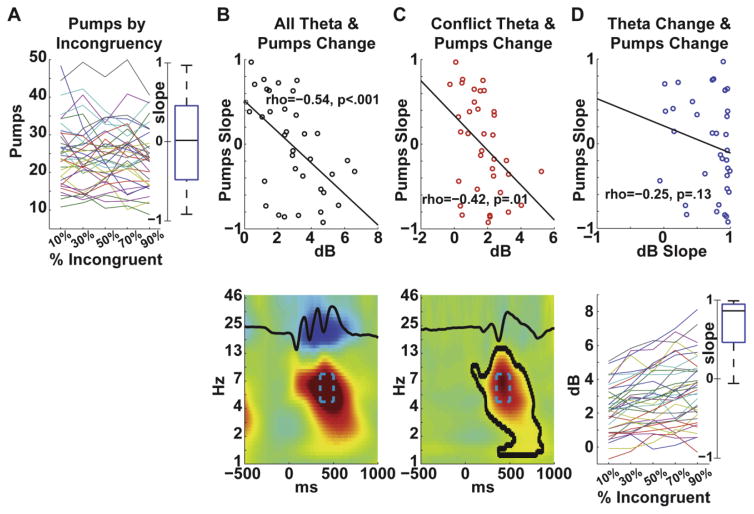
To investigate the relationship between theta band activity during cues and pumps across levels of congruency, we correlated the linear change in pumps across proportion-incongruent levels (shown in Fig 2A with individual slopes in Fig 3A; t(35)= 1.14, p= .26) with candidate theta band metrics. The first theta metric tested was total theta (congruent & incongruent trials) collapsed across levels of the proportion-incongruent manipulation, as a predictor of changes in risky decision making with increasing conflict. Figure 3B shows a significant negative correlation (rho(36)=−.54, p<.001) between these measures, indicating that individual differences in theta power predicted a decline in pumps as trials became more proportion-incongruent (similar relationships were observed with incongruent theta (rho(36)=−.56, p<.001) and congruent theta (rho(36)=−.53, p<.001)). Next we tested the conflict difference (incongruent minus congruent) in theta power collapsed across levels of the proportion-incongruent manipulation. Figure 3C shows that there was a significant negative correlation (rho(36)=−.42, p=.01) between these measures, indicating that conflict-specific theta also predicted a decline in pumps. Finally, we tested the slope in total theta power (congruent and incongruent) across levels of the proportion-incongruent manipulation with the slope of behavioral change. There was a significant effect of the congruency manipulation on theta power (t(35)=11.91, p<.0001), where increasing proportion-incongruent led to larger theta power. Yet there was a non-significant negative correlation (rho(36)=−.25, p=.13) between these two slopes (Fig 3D), suggesting that individual variation in theta power due to the manipulation was not as strong a predictor as obligatory or conflict-specific theta power during the flankers task. The scatterplot in Figure 3D suggests that this diminished relationship may be due to restricted range.
Figure 3.
Experiment I brain-behavior relationships. All scatterplot y-axis data are from (A), all x-axis data are shown below each scatterplot. A) The outcome variable was the slope of each participant’s behavioral change due to increasing percentages of incongruent stimuli. Each participant’s slope is shown here; the boxplot shows that the average slope did not differ from zero in the context of tremendous inter-individual variation. B) Total theta power (congruent & incongruent) inversely related to the behavioral slope. The theta band tf-ROI is highlighted in cyan, the average ERP is overlaid in black. C) The difference in theta power (incongruent minus congruent) also inversely related to the behavioral slope. The same tf-ROI is shown here, as are the ERP difference wave and the contour of t-test differences at a p<.001 threshold. D) The boxplot shows that nearly all participants had a positive slope of total theta power (congruent & incongruent) due to increasing percentages of incongruent stimuli. The slope in total theta power was non-significantly inversely related to the behavioral slope.
All three theta metrics correlated with each other (rhos > .41, ps < .01). A stepwise regression was used to predict the behavioral slope using the three theta band metrics depicted in Fig 3: only the total theta metric was retained (F(1,34)=14.44, p<.001, R2=.30), suggesting these three theta measures account for similar variance and the strongest reflection of this variance is the total amount of theta power during the flankers task. None of the measures correlated with average pumps collapsed across all conditions; behavioral slope (rho(36)=.004, p=.98); total theta power (rho(36)=.05, p=.79); conflict-specific theta power (rho(36)=.05, p=.77) with the exception of the theta slope (rho(36)=.38, p=.02), which was unexpected. If all trials (including errors and too-slow responses) are used for theta power and behavioral slope quantification then the correlations reported in Figure 3B–C remain significant and the correlation in Figure 3D becomes significant, suggesting these findings are robust.
Discussion
The behavioral data did not support our hypothesis that increasing conflict would be associated with decreased risky decision making. In fact, a non-significant trend occurred in the opposite direction as predicted if error trials were included. However, RT, error rate, and theta power all increased with increasing levels of proportion-incongruent flankers trials, suggesting the manipulation was effective in eliciting conflict. While some participants were fit by a negative slope over levels of increasing conflict (suggesting conflict can cause a reduction in risky behavior in some people), an equal number of participants showed the opposite trend (Fig 3a).
Even in the absence of an overall behavioral effect, individual differences in frontal theta predicted a decreasing slope of the numbers of pumps (i.e. correct flankers responses) with increasing flankers conflict. This suggests that theta power, as a signal of the need for control that is sensitive to the inherent costs therein, predicts the degree to which the manipulation of conflict acts as a cost on behavior. Notably, the strongest predictor was the most generic assessment of individual differences in theta power (total theta, not conflict modulated theta). While these findings were robust and nearly all measures were unrelated to individual differences in average BART pumps across conditions, there was an unanticipated relationship between the theta power slope and average BART pumps.
In Experiment II, we aimed to address three novel findings reported here: 1) In order to evoke a more robust behavioral trend, we increased the riskiness of the task to try to provoke larger effects (see Fig 4), 2) we expect the individual difference relationship between frontal theta power and the degree of change in pumps under conflict will replicate, 3) we will investigate if the unexpected finding between theta power and average BART pumps is replicable. Thus, Experiment II shared all the parameters of Experiment I with the exception of an increased risky environment.
Figure 4.
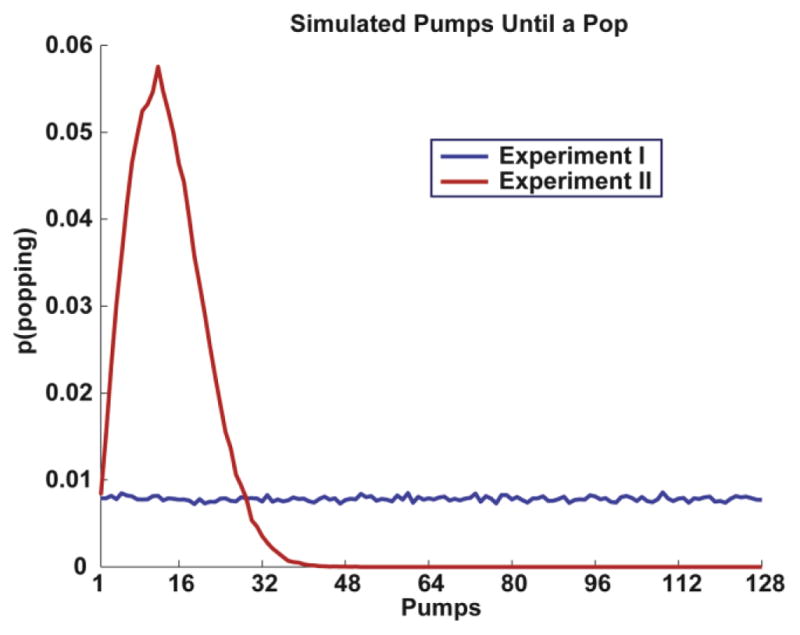
Simulated number of pumps until a pop for Experiments I and II. For Study I, each pump led to an increase in the risk of a pop due to a decreasing population of remaining ‘safe’ samples until the random (uniformly distributed) occurrence of the pop. However, across multiple balloons the median number of pumps one can take until a pop is also uniform. For Study II, the probability of a pop was re-set after each pump, amplifying the risk. This caused a much higher probability of a pop, with the median number of pumps until a pop peaking around 14 or 15.
Experiment II
Performance
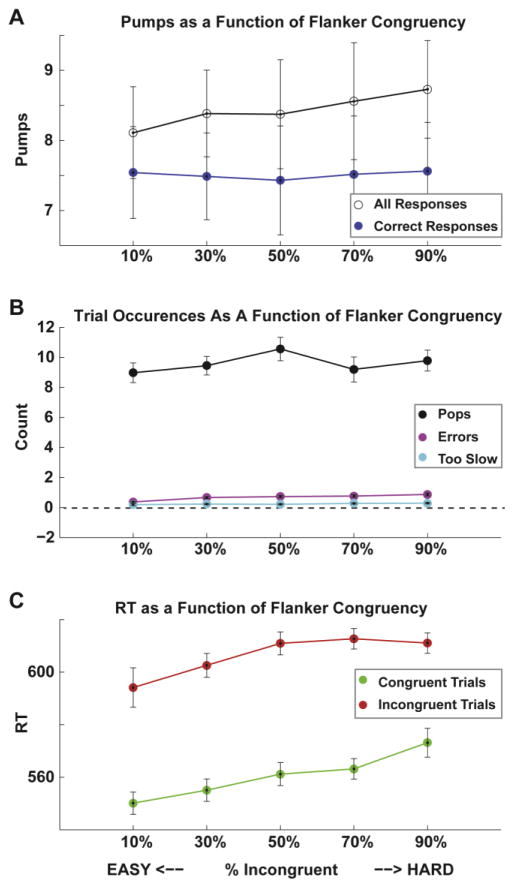
As shown in Figure 5A, we found an increase in pumps with increasing levels of proportion-incongruent flankers (t(35)= 3.98, p= .0003), however this effect was abolished when considering only correct responses (t(35)= .81, p= .42). As in Experiment I, the proportion-incongruent manipulation elicited conflict (Fig 5B–C) as evidenced by significant increases in errors committed (t(35)= 10.01, p<.0001) and too slow trials (t(35)= 5.34, p<.0001), as well as congruent RT (t(35)= 6.09, p<.0001) and incongruent RT (t(35)= 2.56, p=.02). As expected, there was no relationship between flankers congruency and pops (t(35)= 1.12, p= .27).
Figure 5.
Experiment II performance A. Average number of pumps as a function of the percentage of incongruent flankers. There was a (significant) positive relationship between the number of pumps and the percent of incongruent flankers. However this disappeared when error trials were removed. Again, B. Errors and too-slow trials both increased with the proportion-incongruent manipulation, but the number of pops experienced was unrelated to this manipulation. C. Congruent and incongruent trial reaction times both increased with the proportion-incongruent manipulation.
EEG to Flanker Cues
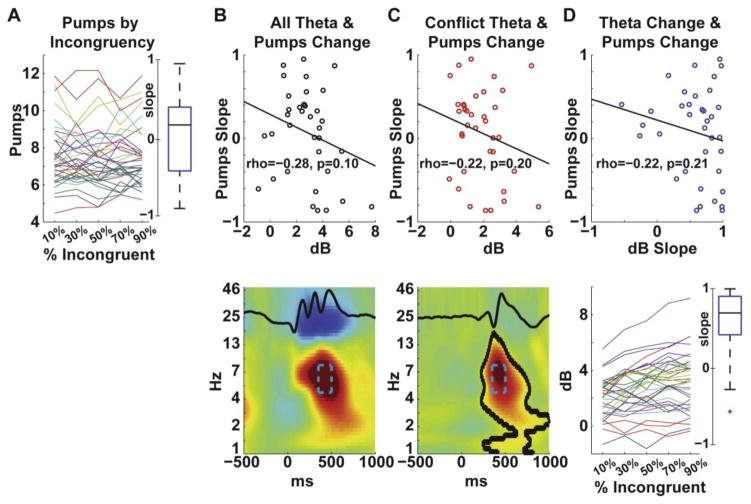
Figure 6A shows the slopes for each participant, of number of pumps per level of the flankers manipulation of proportion-incongruent trials. Figure 6B shows a scatterplot of the negative correlation (rho(36)=−.28, p=.10) between total theta power and behavioral slope. While this t-test was performed as a two-tailed test as in Experiment 1, if this directional hypothesis were tested as a one-tailed replication then the two-tailed p=.10 meets traditional levels of statistical significance as a one-tailed p=.05 (again, these were highly similar for incongruent trials and congruent trials: rho’s (36)=−.28, p’s=.10). Figure 6C shows a scatterplot of the non-significant negative correlation (rho(36)=−.22, p=.20) between the conflict difference (incongruent minus congruent) theta power and behavioral slope. Finally, Figure 6D shows a significant effect of the proportion-incongruent manipulation on theta power (t(35)=11.91, p<.0001), but a non-significant negative correlation (rho(36)=−.22, p=.21) between these measures.
Figure 6.
Experiment II brain-behavior relationships. A) Each participant’s slope is shown here; the boxplot shows that the average slope did not differ from zero yet there was tremendous inter-individual variation. B) Total theta power (congruent & incongruent) was inversely related to the behavioral slope. C) The difference in theta power (incongruent minus congruent) was non-significantly inversely related to the behavioral slope. D) The boxplot shows that nearly all participants had a positive slope of theta power (congruent & incongruent) due to increasing percentages of incongruent stimuli. Yet this slope in theta power was also non-significantly inversely related to the behavioral slope.
While behavioral slope nearly significantly correlated with individual differences in average pumps (rho(36)=−.31, p=.06); none of the EEG measures from Figure 6 did: total theta power (rho(36)=.03, p=.86), conflict-specific theta power (rho(36)=.08, p=.66); theta slope (rho(36)=.15, p=.38). Again all three of these measures were significantly correlated with each other (rhos>.70, ps<.001); however since none of them significantly predicted the behavioral slope we did not run a stepwise regression as in Experiment I.
Discussion
Experiment II replicated the basic patterns observed from Experiment I, albeit with a diminution in the strength of the brain-behavior relationships. It appears our manipulation of increased riskiness had an unintended consequence of restricted range. This assertion is supported by the finding that the behavioral slope and the mean number of pumps was negatively correlated in this experiment only, suggesting that those who pumped the balloon more often had a stronger effect of the proportion-incongruent manipulation due to increased sampling of the underlying probabilities. The error and RT findings were the same as in Experiment I, and the correlation between total theta and behavioral slope had a p-value of .10, which could be interpreted as a significant one-tailed test given the clear a priori predictions. Thus, Experiment II was a weaker replication of the set of patterns found in Experiment I: absent a group-wise behavioral trend, individual differences in frontal theta predicted reduced risky decision making under conflict.
Conclusion
In both studies, we found a negative association between frontal midline theta and risky decision-making during the experience of conflict. To our knowledge, no one has investigated how the experience of conflict affects risky decision-making. These findings are in line with studies of the cost of effort, and they further support the idea that conflict acts as an implicit cost (Cavanagh et al., 2014).
Contrary to our hypothesis, we did not find a reliable decrease in risky behavior with increasing levels of proportion-incongruent (Fig 2A & 5A). Participants experienced increasing conflict with increasing percentages of incongruent stimuli, as evidenced by significant correlations between errors, reaction time, and frontal midline theta. Multiple studies have implicated increased error rates and reaction times with the experience of conflict (Botvinick, 2007; Botvinick et al., 2001; Dreisbach & Fischer, 2012), as well as frontal midline theta and the related N2 ERP component (Cavanagh & Frank, 2014; Yeung, Botvinick, & Cohen, 2004). While the group average of correct responses were relatively constant across levels of proportion-incongruent, errors were generally additive in both studies, accounting for increased pumps as conditions became more difficult (significantly so in Experiment II). When considering the random effects of individual differences, participants had varied responses to the conflict manipulation: while many participants had decreased pumps as expected, an equal number had more pumps.
While there was no consistent group-level behavioral finding, a compelling amount of individual variability in the behavioral change due to conflict could be accounted for by frontal midline theta (Experiment I: R2=30%, Experiment II: R2=8%). In both studies, total theta, not conflict-modulated theta was the strongest predictor of this effect, and theta slope was the weakest (likely due to restricted range). All three of these theta measures were strongly correlated with each other (Experiment I: rhos>.41; Experiment II: rhos >.70), although there are no mathematical dependencies between them that require this to be true. Theta activities collapsed across high and low conflict conditions are rarely investigated as a candidate individual difference measure, but these studies suggest that this simple signal can be more predictive than manipulation-specific effects. While theta is expected to be isomorphic with the experience of conflict/effort, none of these phenomena are ubiquitous in being aversive (Goldfarb & Henik, 2014; Westbrook & Braver, 2015). For example, individuals may interpret sunken costs as an investment (invigorating behavior) or as a waste (diminishing behavior). Thus, reliable predictors of individual differences in effort costs may be extremely interesting, particularly in clinical groups with higher (e.g. anxiety) or lower (e.g. schizophrenia) frontal theta (Cavanagh & Shackman, 2014; Mathews, Perez, Delucchi, & Mathalon, 2012; Weinberg, Olvet, & Hajcak, 2010).
While we aimed to leverage a standardized measure of motivated decision making, the current findings likely reflect how conflict reduces effort-based decision making and not risky decisions per se. While risk tolerance is the primary interpretation of BART performance (White, Lejuez, & de Wit, 2008), and BART pumps correlate with many real-world risk taking behaviors (Hunt, 2005; Lejuez et al., 2003; Wallsten, Pleskac, & Lejuez, 2005), this task mixes aspects of learning, reward sensitivity, and performance consistency (Wallsten et al., 2005) as well as an under-appreciated amount of motor effort that combine towards manifest actions. The conflict manipulation likely affected decision making by reducing motor effort during this task, however this is still important given that the conflict was implicitly experienced and participants were motivated by the risky goal to achieve high value outcomes.
We predict that if a similar implicit conflict manipulation was used in other risky decision making tasks, theta power would account for intra- and inter-individual variability in a similar cost-of-conflict relationship on measures of reduced valuation (c.f. Wessel et al., 2014; Westbrook, Kester, & Braver, 2013) or diminished risk taking (c.f. Christopoulos, Tobler, Bossaerts, Dolan, & Schultz, 2009; Hewig et al., 2009). However, the predictive power of frontal midline theta to conflict/effort costs may be dissociated from delay-based costs that are calculated in other neural areas like orbitofrontal cortex (Rudebeck, Walton, Smyth, Bannerman, & Rushworth, 2006), and unpublished data from our lab suggests that decision making may need to be contingent on conflict/effort (e.g. not simply following it) in order to be affected by the manipulation.
It is unknown if cortical EEG is affected by the strong influences of striatal systems on motivating cost/reward decisions (Christopoulos et al., 2009; Croxson et al., 2009; Schmidt, Lebreton, Cléry-Melin, Daunizeau, & Pessiglione, 2012; Schouppe, Demanet, Boehler, Ridderinkhof, & Notebaert, 2014) and known dopaminergic moderation of these effects (Doya, 2008; Salamone, Correa, Farrar, Nunes, & Pardo, 2009; St Onge, Abhari, & Floresco, 2011; Treadway et al., 2012; Wardle, Treadway, Mayo, Zald, & de Wit, 2011). However, the findings discussed here provide clear predictions for utilizing frontal midline theta as a marker of cognitive effort costs in future pharmacological, genetic, and imaging studies.
In summary, the two studies reported here provide further evidence that conflict is associated with similar decision costs as effort. By extending these findings to explicit decision making in a risk-motivated environment, we extend the class of cognitive phenomena associated with effort costs. By accounting for individual differences in conflict costs, we demonstrate how frontal midline theta is not only a compelling mechanism for implementing cognitive control, but it is sensitive to the inherent costs therein.
Methods and Materials
Experiment I
Participants
The University of New Mexico Institutional Review Board approved this experiment, and all participants provided written informed consent. All participants were right handed, had normal or corrected-to-normal vision, no history of neurological psychiatric or any other relevant medical problem, and were free from current psychoactive medication use. Participants were recruited via the UNM Department of Psychology subject pool and received course credit for participation. Participants were informed that the top performer would earn $30 cash. There were 36 participants (26 female; mean age = 21.28, SD = 4.54; range 18 to 39 years). The task took an average of 59 minutes to complete.
Modified BART Task
As described earlier, we created a novel task to assess risk-taking behavior during the experience of conflict by amalgamating two well-established tasks: the Eriksen Flanker Task used to elicit conflict (Eriksen & Eriksen, 1974) and the Balloon Analogue Risk Task (BART) used to assess risk-taking behaviors (Lejuez et al., 2002), see Figure 1. We administered detailed instructions and a training session to familiarize the participants with each task separately, followed by the combined task.
In the BART component, riskiness is manipulated with a balloon-wise probability of pumps required to pop the balloon (uniform distribution between 1 and 128 pumps). In the Flankers task component, participants responded with a left or right key-press depending on the direction of a central target arrow, which was surrounded by flanking arrows pointing either in the same (congruent) or opposite (incongruent) direction as the target, i.e., (‘ < < < < <’ or ‘ > > > > >’) versus (‘ < < > < <’ or ‘ > > < > >’), respectively. The combined task used flanker performance to inflate the balloon (one pump per correct button left or right trigger button push). Errors deflated the balloon by one pump. If participants did not react to stimuli within 800 ms, they were informed their decision was ‘too slow’, and were awarded zero points. Participants cashed out by pressing a button with their thumb.
To manipulate conflict during BART performance, we altered the probability of conflicting flankers into 5 levels: 10%, 30%, 50%, 70%, and 90% incongruent. Each participant completed 75 balloons, with 15 balloons per congruency level. Trials were presented in random order. Total points gained were presented at the bottom of the screen throughout the task.
EEG Recording and Preprocessing
EEG was recorded continuously across 0.1–100Hz with a sampling rate of 500 Hz and an online CPz reference on a 64-channel Brain Vision system. EEG data were re-referenced to an average reference and CPz was re-created. Very ventral temporal sites were removed, as they tend to be unreliable, leaving 60 electrodes. Data were then epoched around the cues (−1,500 to 2,000 ms). Bad channels and bad epochs were identified using the FASTER algorithm (Nolan, Whelan, & Reilly, 2010) and were subsequently interpolated and rejected respectively. FASTER identifies artifacts based on 3 standard deviations of the absolute z-score of a variety of correlation and variance measures (see: Nolan, Whelan, & Reilly, 2010). Eye blinks and horizontal eye movements were removed following ICA (Delorme & Makeig, 2004). ICA components were algorithmically identified as potentially ocular if they were strongly correlated with vertical or horizontal electroencephalogram (greater than 3 standard deviations from all absolute z-scored ICA component correlations). All algorithmic suggestions were verified by visual inspection of the ICA topography prior to removal.
All analyses were performed on the FCz electrode. Time-Frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets (defined as a Gaussian-windowed complex sine wave: ei2πtfe−t^2/(2xσ^2), where t is time, f is frequency (which increase from 1–50Hz in 50 logarithmically spaced steps), and defines the width (or ‘cycles’) of each frequency band, set according to 4/(2πf)), and taking the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in estimates of instantaneous power (the magnitude of the analytic signal), defined as Z[t] (power time series: p(t) = real[z(t)]2 + imag[z(t)]2). Each epoch was then cut in length (−500 to 1,000ms). Power was normalized by conversion to a decibel scale (10 × log10[power(t)/power(baseline)]), controlling for spurious individual differences and allowing a direct comparison of effects across frequency bands. Event Related Potentials (ERPs) were low-pass filtered at 20 Hz. The baseline for time-frequency and ERP data consisted of the average power from −300 to −200 ms prior to the onset of the cues. Note that following wavelet convolution, each time point reflects a broad range of frequency-specific temporally common activity (calculated as 2*(cycles/(2pi*frequency))*1000), thus a 100 ms window captures multiple cycles of activity.
Statistical Analysis
The a priori hypothesis was that increasing conflict due to the flankers proportion-incongruent manipulation would diminish the investment in risky decisions, operationally defined as the number of BART pumps (Lejuez et al., 2002; Lejuez, Aklin, Zvolensky, & Pedulla, 2003). The statistical analysis across the five parametric manipulation conditions required an assessment of change over time, so the linear change in measures across proportion-incongruent levels (pumps, RT, theta power, etc.) were assessed via individual regression slopes. The mean number of pumps and theta power collapsed across all conditions were also important to understand, so these measures were included in some analyses. These two approaches can be considered assessments of the slope vs. the intercept of each phenomenon. The outcome variable of interest was the effect of the conflict manipulation on BART behavior (behavioral slope), but all theta band measures were possible predictors (total theta, conflict-specific theta, and theta slope across trials).
A theta band time-frequency Region-of-Interest (tf-ROI) of 4–8 Hz and 300–500 ms was selected from prior work and the visualization of the difference between incongruent and congruent flanker conditions (see Fig 3C). Only correct trials (no error trials or too-slow trials) were used for theta band analyses, unless otherwise reported. To control for differing numbers of incongruent stimuli per manipulation condition, three separate contrasts were used. First, total theta power (congruent + incongruent) collapsed across manipulation condition was used as a predictor of behavioral slope (akin to theta intercept). Then the difference in theta power (incongruent − congruent) collapsed across manipulation condition was tested. Finally, theta band slope across the proportion-incongruent conditions, collapsing across flankers trial type (congruent and incongruent) was tested as predictor of behavioral slope.
Statistical effects of performance differences were assessed via one-sample t-tests of each participant’s regression slope in order to test whether the average slope was significantly different than zero. Relationships between slopes were tested using Spearman’s correlations. A stepwise regression (F-to-enter <=5%, F-to-remove >= 10%) was used to predict the behavioral slope using the three theta band metrics in order to examine for independent vs. shared variance.
Experiment II
In Experiment II, we altered the probability of balloon pops. We changed it from a balloon-wise probability (for any balloon, the probability of popping remains fixed) to a pump-wise probability (for each pump, the probability of popping re-sets). All other methods were the same as described in the Experiment I. To demonstrate this manipulation of risk, we simulated each variant of the task for 100,000 iterations to determine the average number of pumps that one can execute in each Experiment until the balloon pops (Fig 4). In the standard BART task (Experiment I), the pop probability is reset after each balloon, producing a uniform distribution of pumps that can be expected to lead to a pop (i.e. if (number of pumps/128) > this balloon’s probability, then it will pop if pumped). Thus, while each pump does lead to increased riskiness of a pop, this is due to a decreasing population of remaining ‘safe’ samples until the random occurrence of the pop. Conversely, for Experiment II we reset the probability of a pop after each pump (i.e. if (number of pumps/128) > this trial’s probability, then it will pop if pumped), amplifying the risk. This yielded a median number of 14 to 15 potential pumps until a pop, and a near impossibility that a balloon could be pumped over 40 times as demonstrated by the simulation. Since this forced shorter trials, Experiment II consisted of 150 trials with 30 balloons per congruency level. All other methods were the same as described in the Experiment I, including sample size (also 36 participants, 20 female; mean age = 19.33, SD = 2.54; range 18 to 29 years), and motivation to earn $30 for top performance. The task took an average of 44 minutes to complete.
Acknowledgments
JFC is supported by NIGMS 1P20GM109089-01A1, NIMH 1UH2MH109168-01, and NIAAA R21AA0023947-01A1. The authors thank Jim Broadway for helpful comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7(4):356–366. doi: 10.3758/cabn.7.4.356. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18189009. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11488380. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014:1–8. doi: 10.1016/j.tics.2014.04.012. http://doi.org/10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed]
- Cavanagh JF, Masters SE, Bath K, Frank MJ. Conflict acts as an implicit cost in reinforcement learning. Nature Communications. 2014;5:5394. doi: 10.1038/ncomms6394. http://doi.org/10.1038/ncomms6394. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal Midline Theta Reflects Anxiety and Cognitive Control: Meta-Analytic Evidence. Journal of Physiology, Paris. 2014;109:3–15. doi: 10.1016/j.jphysparis.2014.04.003. http://doi.org/10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural Correlates of Value, Risk, and Risk Aversion Contributing to Decision Making under Risk. Journal of Neuroscience. 2009;29(40):12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. http://doi.org/10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Donner TH. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. Journal of Neurophysiology. 2013;110(12):2752–63. doi: 10.1152/jn.00479.2013. http://doi.org/10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. http://doi.org/S0006-8993(08)01872-6 [pii] [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. Effort-based cost-benefit valuation and the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(14):4531–41. doi: 10.1523/JNEUROSCI.4515-08.2009. http://doi.org/10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. http://doi.org/10.1016/j.jneumeth.2003.10.009S0165027003003479 [pii] [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7895011. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11(4):410–416. doi: 10.1038/nn2077. http://doi.org/nn2077 [pii]\r. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R. Conflicts as aversive signals. Brain and Cognition. 2012;78(2):94–8. doi: 10.1016/j.bandc.2011.12.003. http://doi.org/10.1016/j.bandc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Fritz J, Dreisbach G. Conflicts as aversive signals: conflict priming increases negative judgments for neutral stimuli. Cognitive, Affective & Behavioral Neuroscience. 2013;13(2):311–7. doi: 10.3758/s13415-012-0147-1. http://doi.org/10.3758/s13415-012-0147-1. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10(9):829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Goldfarb L, Henik A. Is the brain a resource-cheapskate? Frontiers in Human Neuroscience. 2014;8(October):1–3. doi: 10.3389/fnhum.2014.00857. http://doi.org/10.3389/fnhum.2014.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewig J, Straube T, Trippe RH, Kretschmer N, Hecht H, Coles MG, Miltner WH. Decision-making under risk: an fMRI study. J Cogn Neurosci. 2009;21(8):1642–1652. doi: 10.1162/jocn.2009.21112. http://doi.org/10.1162/jocn.2009.21112. [DOI] [PubMed] [Google Scholar]
- Hunt MK. Construct Validity of the Balloon Analog Risk Task (BART): Associations With Psychopathy and Impulsivity. Assessment. 2005;12(4):416–428. doi: 10.1177/1073191105278740. http://doi.org/10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Itthipuripat S, Wessel JR, Aron AR. Frontal theta is a signature of successful working memory manipulation. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 2013;224(2):255–62. doi: 10.1007/s00221-012-3305-3. http://doi.org/10.1007/s00221-012-3305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flugge MC, Kennerley SW, Friston K, Bestmann S. Neural signatures of value comparison in human cingulate cortex during decisions requiring an effort-reward trade-off. 2016;36(39):64105. doi: 10.1523/JNEUROSCI.0292-16.2016. bioRxiv. http://doi.org/10.1101/064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology. General. 2010;139:665–682. doi: 10.1037/a0020198. http://doi.org/10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. http://doi.org/10.1016/S0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, … Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. http://doi.org/10.1037/1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- Mathews Ca, Perez VB, Delucchi KL, Mathalon DH. Error-related negativity in individuals with obsessive-compulsive symptoms: toward an understanding of hoarding behaviors. Biological Psychology. 2012;89(2):487–94. doi: 10.1016/j.biopsycho.2011.12.018. http://doi.org/10.1016/j.biopsycho.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. Journal of Neuroscience Methods. 2010;192(1):152–62. doi: 10.1016/j.jneumeth.2010.07.015. http://doi.org/10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Pellouchoud E, Smith ME, McEvoy L, Gevins a. Mental effort-related EEG modulation during video-game play: comparison between juvenile subjects with epilepsy and normal control subjects. Epilepsia. 1999;40(Suppl 4):38–43. doi: 10.1111/j.1528-1157.1999.tb00905.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10487172. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9(9):1161–8. doi: 10.1038/nn1756. http://doi.org/10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:13. doi: 10.3389/neuro.08.013.2009. http://doi.org/10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Cléry-Melin ML, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology. 2012;10(2):e1001266. doi: 10.1371/journal.pbio.1001266. http://doi.org/10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouppe N, De Houwer J, Richard Ridderinkhof K, Notebaert W. Conflict: Run! Reduced Stroop interference with avoidance responses. The Quarterly Journal of Experimental Psychology. 2012;65(6):1052–1058. doi: 10.1080/17470218.2012.685080. http://doi.org/10.1080/17470218.2012.685080. [DOI] [PubMed] [Google Scholar]
- Schouppe N, Demanet J, Boehler CN, Ridderinkhof KR, Notebaert W. The role of the striatum in effort-based decision-making in the absence of reward. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(6):2148–54. doi: 10.1523/JNEUROSCI.1214-13.2014. http://doi.org/10.1523/JNEUROSCI.1214-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter Ha, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience. 2011;12(3):154–67. doi: 10.1038/nrn2994. http://doi.org/10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40. doi: 10.1016/j.neuron.2013.07.007. http://doi.org/10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AS, Eling PaTM, Coenen AML. Mental effort affects vigilance enduringly: after-effects in EEG and behavior. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2004;53(3):239–43. doi: 10.1016/j.ijpsycho.2004.04.005. http://doi.org/10.1016/j.ijpsycho.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PaTM, Hopman MT, Coenen AML. Mental and physical effort affect vigilance differently. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2005;57(3):211–7. doi: 10.1016/j.ijpsycho.2005.02.001. http://doi.org/10.1016/j.ijpsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(23):8625–33. doi: 10.1523/JNEUROSCI.1020-11.2011. http://doi.org/10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, … Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(18):6170–6. doi: 10.1523/JNEUROSCI.6459-11.2012. http://doi.org/10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noordt SJR, Campopiano A, Segalowitz SJ. A functional classification of medial frontal negativity ERPs: Theta oscillations and single subject effects. Psychophysiology. 2016;53(9):1317–1334. doi: 10.1111/psyp.12689. http://doi.org/10.1111/psyp.12689. [DOI] [PubMed] [Google Scholar]
- Wallsten TS, Pleskac TJ, Lejuez CW. Modeling behavior in a clinically diagnostic sequential risk-taking task. Psychological Review. 2005;112(4):862–80. doi: 10.1037/0033-295X.112.4.862. http://doi.org/10.1037/0033-295X.112.4.862. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MFS. The role of rat medial frontal cortex in effort-based decision making. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(24):10996–1003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12486195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Networks: The Official Journal of the International Neural Network Society. 2006;19(8):1302–14. doi: 10.1016/j.neunet.2006.03.005. http://doi.org/10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(46):16597–602. doi: 10.1523/JNEUROSCI.4387-11.2011. http://doi.org/10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E, Rasch B, Sänger J, Hoffmann S, Schneider D, Rinkenauer G, … Gutberlet I. Frontal theta activity reflects distinct aspects of mental fatigue. Biological Psychology. 2014;96:57–65. doi: 10.1016/j.biopsycho.2013.11.010. http://doi.org/10.1016/j.biopsycho.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. http://doi.org/10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(22):7528–37. doi: 10.1523/JNEUROSCI.6352-11.2012. http://doi.org/10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, O’Doherty JP, Berkebile MM, Linderman D, Aron AR. Stimulus devaluation induced by stopping action. Journal of Experimental Psychology: General. 2014;143(6):2316–2329. doi: 10.1037/xge0000022. http://doi.org/10.1037/xge0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Tonnesen AL, Aron AR. Stimulus devaluation induced by action stopping is greater for explicit value representations. Frontiers in Psychology. 2015 Oct;6 doi: 10.3389/fpsyg.2015.01640. http://doi.org/10.3389/fpsyg.2015.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Braver T. Cognitive effort: A neuroeconomic approach. Cognitive, Affective, & Behavioral Neuroscience. 2015;15:395–415. doi: 10.3758/s13415-015-0334-y. http://doi.org/10.3758/s13415-015-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Kester D, Braver TS. What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PloS One. 2013;8(7):e68210. doi: 10.1371/journal.pone.0068210. http://doi.org/10.1371/journal.pone.0068210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task (BART) Experimental and Clinical Psychopharmacology. 2008;16(6):565–570. doi: 10.1037/a0014083. http://doi.org/10.1037/a0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931–959. doi: 10.1037/0033-295X.111.4.939. http://doi.org/2004-19012-005 [pii] [DOI] [PubMed] [Google Scholar]
- Zipf GK. Human behavior and the principle of least effort. Cambridge, MA: Addison_Wesley; 1949. [Google Scholar]