Abstract
RATIONALE
We hypothesized that Cluster of differentiation 74 (CD74) downregulation of placental macrophages, leading to altered macrophage-trophoblast interaction, is involved in preeclampsia.
OBJECTIVE
Preeclamptic pregnancies feature hypertension, proteinuria and placental anomalies. Feto-placental macrophages regulate villous trophoblast differentiation during placental development. Disturbance of this well-balanced regulation can lead to pathological pregnancies.
METHODS AND RESULTS
We performed whole genome expression analysis of placental tissue. CD74 was one of the most downregulated genes in placentas from preeclamptic women. By RT-PCR, we confirmed this finding in early onset (<34 gestational week, n=26) and late onset (≥34 gestational week, n=24) samples from preeclamptic women, compared to healthy pregnant controls (n=28). CD74 protein levels were analyzed by Western blot and flow cytometry. We identified placental macrophages to express CD74 by immunofluorescence, flow cytometry and RT-PCR. CD74-positive macrophages were significantly reduced in preeclamptic placentas compared to controls. CD74-silenced macrophages showed that the adhesion molecules ALCAM, ICAM4, and Syndecan-2, as well as macrophage adhesion to trophoblasts were diminished. Naïve and activated macrophages lacking CD74 showed a shift towards a pro-inflammatory signature with an increased secretion of TNFα, CCL5, and MCP-1, when co-cultured with trophoblasts compared to control macrophages. Trophoblasts stimulated by these factors express more CYP2J2, sFlt1, TNFα and IL-8. CD74-knockout mice showed disturbed placental morphology, reduced junctional zone, smaller placentas and impaired spiral artery remodeling with fetal growth restriction.
CONCLUSIONS
CD74 downregulation in placental macrophages is present in preeclampsia. CD74 downregulation leads to altered macrophage activation towards a pro-inflammatory signature and a disturbed crosstalk with trophoblasts.
Keywords: CD74, preeclampsia, macrophages, trophoblasts, immunology
INTRODUCTION
Preeclampsia (PE) is a pregnancy-related disorder characterized by new-onset hypertension (>140/90 mm Hg) after the 20th week of pregnancy and proteinuria (>300 mg/l per 24-hour) or in association with thrombocytopenia, impaired liver function, development of renal insufficiency, pulmonary edema, or new-onset cerebral or visual disturbances.1 Overall, 5–10% of all pregnancies worldwide develop PE, the leading cause of morbidity and mortality in mother and child.2
Immune cell and trophoblastic-cell interactions facilitate placental growth by a well-balanced regulation, including interactions between maternal immune cells and extravillous trophoblast as well as between feto-placental immune cells and villous trophoblast. Pregnancy initiates a profound adaptation of the maternal immune system to the semiallogeneic fetus, and a significant inflammatory response has been described in preeclamptic women.3, 4 Chemokines such as monocyte chemotactic protein-1 (MCP-1) and chemokine (C-C motif) ligand 5 (CCL5) are elevated in PE and enhance maternal monocytes/macrophage recruitment.5–7 Maternal macrophages have been shown to participate in intrauterine growth restriction (IUGR) and PE, perhaps related to cytokine release.8 In the decidua, activated maternal macrophages produce high levels of tumor necrosis factor α (TNFα). Extravillous trophoblasts express the receptors of TNFα, and interactions between TNFα and its receptor are described to induce trophoblast apoptosis in vitro.9, 10 Moreover, invasion, migration, and proliferation of extravillous trophoblasts appear to be regulated by local cytokine concentrations.11–14
Hofbauer cells are placental macrophages of fetal origin and play a direct role in early placental development. They differentiate from progenitor cells within the population of mesenchymal cells in the villous stroma or from penetration of embryonic/fetal bone marrow-derived monocytes into the villous stroma.15 Hofbauer cells are associated with several pregnancy complications such as chorioamnionitis, spontaneous abortion, and fetal metabolic storage disease.16 Hofbauer cells contribute to the placental expression of anti-angiogenic factors and their dysregulation in preeclamptic placenta.17
The HLA class II histocompatibility antigen gamma chain, also known as cluster of differentiation 74 (CD74) when expressed on cell surfaces, is the MHC II invariant chain (Ii) protein that is involved in antigen presentation and crucial for biogenesis.18 CD74 is also a high-affinity binding protein for the pleiotropic inflammatory cytokine macrophage migration inhibitory factor (MIF). Following its activation on the cell membrane, CD74 transduces signaling and triggers various downstream responses including inflammatory protein expression and modulation of cell survival.19 MIF also acts as a major regulator of inflammatory cell recruitment and atherogenesis by activating CXCR2/4.20 In the first-trimester placenta, trophoblasts express and release MIF that modulates monocyte activity.21–23 Furthermore, CD74 has been described in a soluble form (sCD74) that neutralizes MIF signal transduction in patients with autoimmune liver disease.24 Soluble CD74 plasma levels correlate with the concentration of a complex that is formed between sCD74 and soluble HLA-DR (sCD74/sHLA-DR).25 Interestingly, sHLA-DR levels are significantly lower in the circulation of preeclamptic women when compared to women with normally progressing pregnancies.26 Based on microarray gene expression data from clinical specimens, we tested the hypothesis that preeclampsia is associated with a CD74-induced disturbed placentation. We used in vitro models and a knockout mouse model in vivo to study the pathophysiological significance of CD74.
METHODS
Patients
Human placenta, decidua and blood sampling was approved by the Regional Committee of Medical Research Ethics in Eastern Norway and the Medical Faculty of Charité Berlin. Placental biopsies and decidual tissue were obtained from 50 preeclamptic women and 28 women with normotensive and uncomplicated pregnancies. The PE group was divided into early onset PE (<34 gestational week, n=26) and late onset PE (≥34 gestational week, n=24). Patient characteristics are shown in Supplemental Table S1.
Animals
Local authorities (LaGeSo, Berlin, Germany) approved all experiments. Primary cultures of macrophages were generated from the bone marrow of male 10–12 week old wild-type (C57Bl/6JOlaHsd) mice; Harlan Laboratories, Rossdorf, Germany) or CD74 knockout (B6-(Cd74)tm) mice. To evaluate the preeclamptic phenotype, wild-type (C57Bl/6JOlaHsd) and CD74 knockout (B6-(Cd74)tm) mice were bred. Doppler studies were performed on day 15/17 on anesthetized (1.5% isoflurane) mice. The hair was removed from the abdomen, and pre-warmed gel was used as an ultrasound-coupling medium. The pregnant mice were imaged with an ultrasound biomicroscope and a 30-MHz or 40-MHz transducer at 30 frames per second (Model Vevo 660, VisualSonics Inc). Peak systolic velocity (PSV) and end-diastolic velocity (EDV) were measured and the resistance index (RI=(PSV EDV)/PSV) was calculated.
Isolation of primary cells from human placenta
All placentas were removed from fetal membranes, basal plate, umbilical cord and fibrotic tissue. Villous tissue was minced and washed several times. Various trypsin (Sigma) and DNase I (Roche) digestion steps were used and supernatants were filtered and collected in New Born Calf Serum (Biochrome). For Hofbauer cell isolation supernatant was discarded and undigested tissue was digested further with collagenase A (Roche), DNase I and cell suspensions were loaded on Percoll (GE Healthcare) gradual gradients. For primary trophoblasts, cells were negatively immunopurified using HLA-ABC (DakoCytomation) and magnetic dynabeads (Dynal Biotech).
Macrophage preparation and cell culture
Macrophages were generated from two different origins: Human peripheral blood mononuclear cells (PBMCs) and mouse bone marrow-derived (BMD). PBMCs derived macrophages: 80 ml of blood were drawn from human healthy donors. The study was approved by the Regional Committee of Medical Research Ethics (Charité). PBMCs were purified by Ficoll gradient centrifugation and an adhesion step.
BMD macrophages: Cells were isolated from the femur and tibia of freshly euthanized mice. For macrophage differentiation, 10 × 106 bone-marrow derived cells were cultivated in 50 ml of differentiation media for 7 d in sealed, hydrophobic Teflon® bags (FT FEP 100 C (DuPont), American Durafilm).
SGHPL-4 cells derived from primary human first trimester extravillous trophoblasts (transfected with the early region of SV40) were a kind gift from Judith E. Cartwright (St George’s University of London, London, United Kingdom).
Microarray analysis
Microarray analysis of M(−) and M(IL-4) was done with the Illumina HumanHT-12_V3_0_R2 according the Minimum Information About a Microarray Experiment (MIAME) criteria. Data is provided at ArrayExpress under accession number E-MTAB-3309.
Expanded methods are described in the online supplement.
RESULTS
Downregulation of placental and circulating CD74 in PE
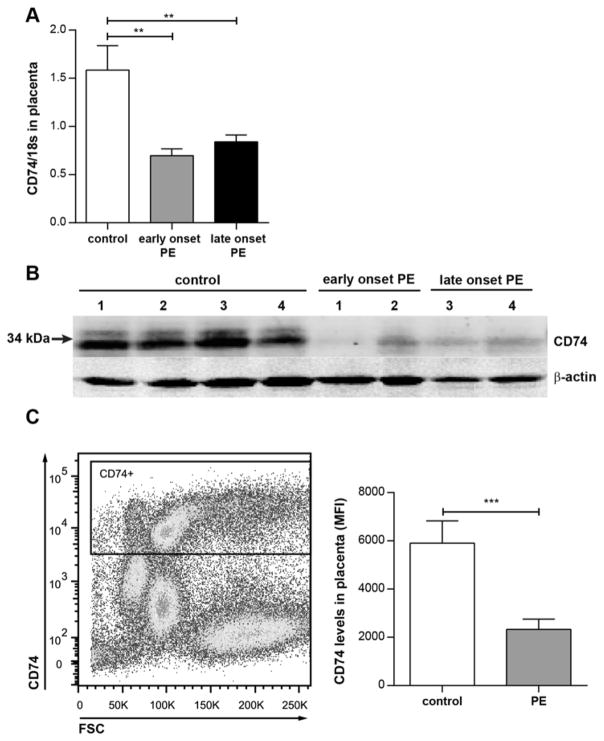
Microarray analysis performed on placental tissue showed downregulation of CD74 expression by 3 different nucleotide sequences (ILMN_1761464, ILMN_2379644, ILMN_1736567).27 This observation was confirmed in a different cohort of patients, dividing the preeclamptic group into early onset (<34 gestational week) and late onset (≥34 gestational week) of preeclampsia (Supplemental Table S1) by real-time RT-PCR. We found that CD74 was significantly downregulated in early onset PE by a factor of 2.3 (p<0.001) and late onset PE by a factor of 1.9 (p<0.01) (Figure 1A). Western blot analysis of 4 controls vs. 4 preeclamptic placentas confirmed this finding. CD74 protein level was clearly diminished in both early- and late onset preeclamptic placentas (Figure 1B). Moreover, flow cytometry analysis on the whole placental cell population of 6 healthy and 10 late onset preeclamptic placentas showed a significantly lower presentation of CD74 in cells of late onset preeclamptic placentas (Figure 1C). Decidua showed no differential CD74 expression between groups (Supplemental Figure S1A). In the PE group, placental CD74 expression negatively correlated to the serum ratio of the soluble Fms-like tyrosine kinase 1 (sFlt1) and the placental growth factor (PLGF), the sFlt1/PLGF ratio, but not in controls (Supplemental Figure S1D). CD74 not only serves as invariant chain for MHC class II proteins and as a MIF receptor on the cell surface, but also circulates as a soluble ectodomain, termed sCD74.24 We thus also analyzed serum sCD74 levels in our cohort and detected somewhat lower mean levels in both preeclamptic groups compared to controls, although these results were not statistically significant (Supplemental Figure S1B). Circulating levels of the binding partner of sCD74, macrophage migration inhibitory factor (MIF), were correspondingly enhanced during preeclamptic disease, but without reaching statistical significance (Supplemental Figure S1C).
Figure 1. CD74 is downregulated in preeclamptic placenta.
A) CD74 expression is downregulated in placentas of preeclamptic women (PE) compared to healthy women (control; n=28). PE is subdivided in early onset PE (delivery <34 week of gestation; grey bar; n=26) and late onset PE (delivery ≥34 week of gestation; black bar; n=24) (**p<0.01; ***p<0.001; Bonferroni’s multiple comparisons test). B) CD74 protein (33 and 35 kDa) is lowered in placenta lysates of early and late onset PE (n=2 each). C) Confirmation by flow cytometry; representative CD74-positive gating is shown for whole placenta cell population (left panel). Mean fluorescent intensity (MFI) of CD74 is lowered in general placenta cell population of late PE vs. control (right panel) (n=10 (PE) and 6 (control); ***p=0.001; Mann Whitney test).
Localization of CD74 in placental macrophages (Hofbauer cells)
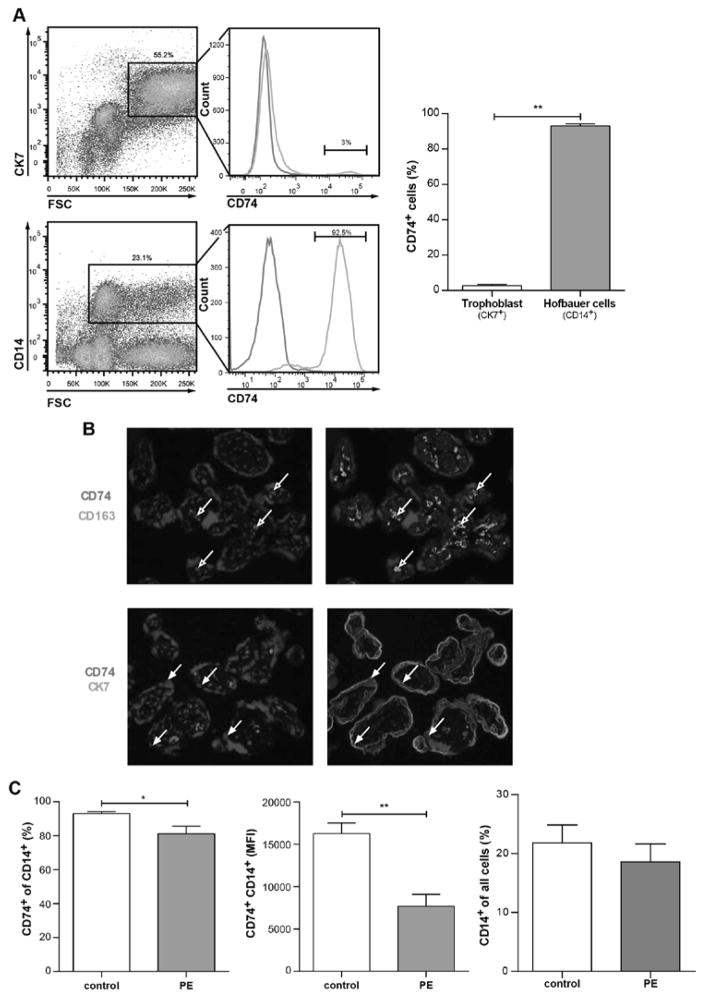
Following differential expression of CD74 in preeclamptic placentas, we identified the specific cell type that expresses CD74. Given the low amounts of lymphocytes in placenta, we focused on CD74 levels in two main placental populations – trophoblasts (CK7+) and macrophages (CD14+). It appeared that in healthy control placentas only 2.6±0.8% of trophoblasts were positive for CD74 (CK7+CD74+), in contrast to 93.1±1.1% of macrophages (CD14+CD74+) (Figure 2A). Furthermore, immunofluorescence co-staining was performed on placental villous tissue for trophoblasts (CK7) and Hofbauer cells (CD163) with CD74. We could confirm that trophoblasts were negative for CD74 (CK7+CD74−) while Hofbauer cells were positive (CD163+CD74+) (Figure 2B).
Figure 2. CD74 is highly expressed in placental macrophages (Hofbauer cells) and downregulated in preeclamptic Hofbauer cells.
A) By flow cytometry; representative CK7-positive gating (trophoblast marker) and CD14-positve gating (macrophage marker) is shown for whole placenta cell population (left panel) of healthy controls. Solely 2.6±0.8 % of trophoblasts (CK7-positive) vs. 93.1±1.1 % of Hofbauer cells (CD14-positive) were positive for CD74 staining (right panel) (n=6; p<0.01; Mann Whitney test). B) Immunostaining showed that CD74 (red) is co-localized with CD163 (green) (Hofbauer cells; open arrows; upper panel) but not with CK7 (green). C) Flow cytometry on whole placenta cell population revealed that CD74 was less present in CD14-positive cells of placentas from late preeclamptic women (PE) compared to healthy women (control) (left panel). The mean fluorescent intensity (MFI) of CD74-CD14-positive cells was lower in PE vs. control (middle panel). Percentage of CD14-positive cells in all placental cells was not changed in PE (n=10) vs. control (n=6) (right panel) (*p<0.05, **p<0.01; Mann Whitney test).
To investigate CD74 mRNA expression levels we isolated primary trophoblasts and primary Hofbauer cells from healthy human term placentas and compared them with placental tissue, early trophoblast cell line (SGHPL-4) and blood-derived macrophages (human M(IL4)). We confirmed that isolated Hofbauer cells showed high levels of CD74 expression, similar to human M(IL4) and M(−), while primary trophoblasts and SGHPL-4 cells showed low levels (Supplemental Figure S2). Accordingly, in Hofbauer cells and blood derived macrophages, but not in SGHPL-4 cells the phosphorylation of ERK (p44/42) could be activated by stimulation with the CD74 ligand MIF (Supplemental Figure S3).
To combine both findings, the observed placental downregulation of CD74 in preeclamptic placentas and the expression of CD74 on placental macrophages, we then compared CD74 levels expressed on CD14+ Hofbauer cells from healthy and preeclamptic placentas. Significantly fewer placental CD14+ macrophages co-expressed CD74 in late preeclampsia (81.2±4.4%), compared with healthy controls (93.1±1.1%) (p<0.05) (Figure 2C, left panel). Additionally, CD74+ cells of preeclamptic placentas showed lower intensity of CD74 staining as compared to controls, shown by mean fluorescence intensity of CD74 (16283±1244 vs 7693±1392 a.u., p<0.01) (Figure 2C, middle panel). The frequency of CD14+ macrophages did not differ between control and preeclamptic placentas (Figure 2C, right panel).
Impaired function of macrophages lacking CD74
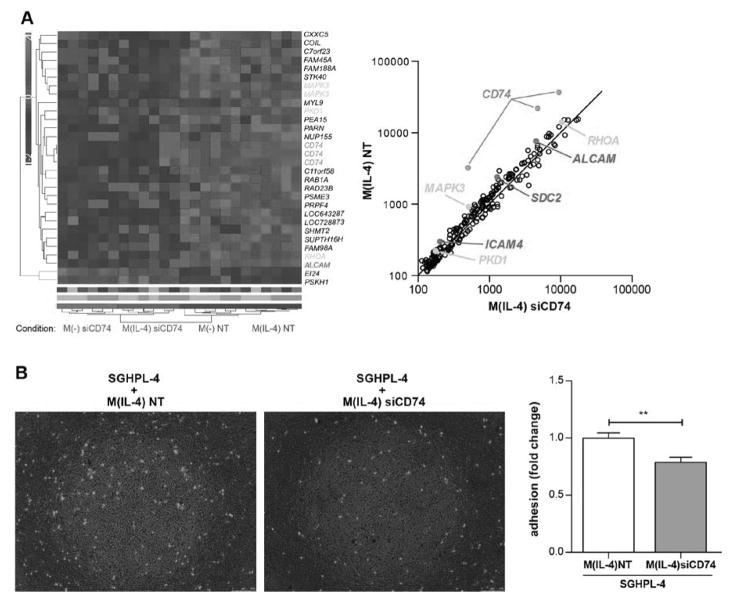
Since Hofbauer cells are considered to be M2 (also named alternatively activated) macrophages28, we aimed to establish an in vitro preeclamptic-model of CD74 downregulated Hofbauer cells by transfecting M(IL-4) with small interfering RNA (siRNA) against CD74 (siCD74) and an appropriate non-targeting control (NT). However, the current characterization of placental macrophages might be not sufficient regarding activation status, we therefore used M(−) for silencing protocols additionally. Downregulation of CD74 was confirmed by gene expression via real-time RT-PCR (Supplemental Figure S4A) and at the protein level by Western blot (Supplemental Figure S4B) (data shown for M(IL-4)). mRNA was processed and differential expression (NT vs. siCD74) (n=6 each) was revealed by whole transcriptome analysis. Subdued parametric three way ANOVA resulting p-values were Benjamini Hochberg FDR corrected, furthermore, probes undergoing 5% FDR in “silencing” were average linkage-clustered using standardized signal values and the Euclidean distance function. 30 probes, representing 27 known genes, were significantly differentially expressed by CD74 silencing (5% FDR), 25 of them were downregulated in CD74-silenced samples and 2 were up-regulated and represented on the heat map (Figure 4A, left panel). These 27 genes were matched to the literature in respect to placenta. We found that Ras homologue gene family, member A (RHOA), mitogen-associated protein kinase 3 (MAPK3) and polycystic kidney disease gene 1 (PKD1) were linked to pregnancy and are highlighted in orange. For further analysis, genes that followed FDR <65% were plotted and are shown in Figure 4A (right panel). To determine possible interactions of differently regulated genes, datasets of these 226 genes with altered expression were analyzed using Ingenuity Pathway Analysis Tool (IPA). The top ten list of canonical pathways was identified by IPA. The pro-inflammatory IL-1 signaling pathway was the most significantly enriched. Among the identified biological functions we found cell morphology, connective tissue disorders and immunological diseases (Supplemental Figure S5). Strongly downregulated genes within the 226 dysregulated genes were adhesion molecules – activated leukocyte cell adhesion molecule (ALCAM), intracellular adhesion molecule 4 (ICAM4) and syndecan-2 (SDC2) – which are presented in red (Figure 4A, right panel). We confirmed ALCAM, ICAM4 and SDC2 downregulation in M(IL-4) lacking CD74 by real-time RT-PCR (n=27 each) (Supplemental Figure S6).
Figure 4. Dysregulated expression and function of CD74 silenced M(−) and M(IL-4) macrophages.
A) Heatmap represents microarray analysis for indicated conditions (left panel). Genes were pre-selected by 5% FDR (Subdued parametric three way ANOVA, Benjamini Hochberg FDR corrected) for CD74 silenced (siCD74) vs. non-targeting control (NT). Genes undergoing 65% FDR were plotted for siCD74 vs NT in M(IL-4) macrophages (right panel). Genes in orange correlate to “placenta” (by literature), genes in red encode adhesion molecules and CD74 is indicated in green (both panels). B) Adhesion of CD74 silenced macrophages (M(IL-4) siCD74) to first trimester trophoblast derived cell line (SGHPL-4) was lowered compared to control macrophages (M(IL-4) NT) as shown in representative pictures (left panel) and in summary of 3 independent experiments (right panel) (**p<0.01; unpaired t test).
As gene expression of adhesion molecules was downregulated in macrophages lacking CD74, adhesion assay with M(IL-4) macrophages and first trimester trophoblast cell line SGHPL-4 was performed. There was significantly less M(IL-4) adherence to the SGHPL-4 layer in the siCD74-treated group when compared to NT, as documented by microscopy and measured by fluorescence intensity (Figure 4B).
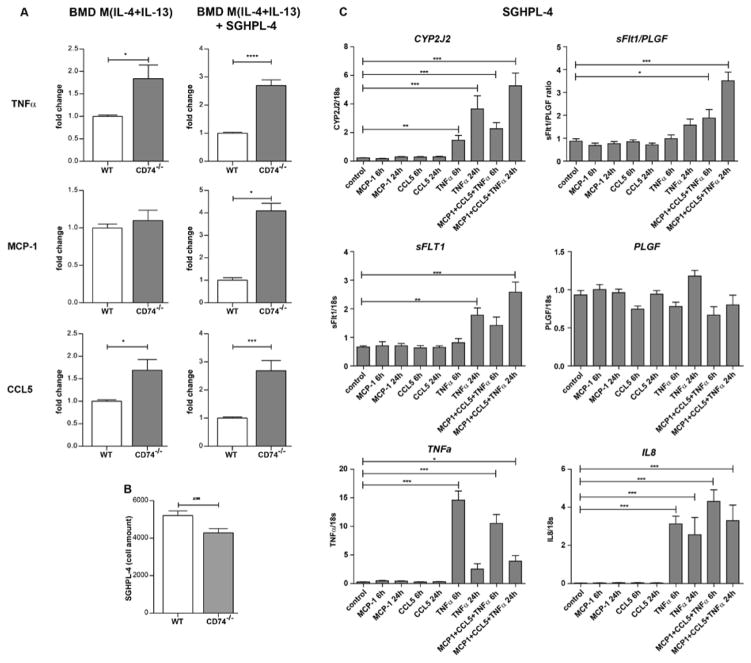
To reveal the impact of CD74 deficiency on M2 macrophages, we also generated BMD macrophages from CD74 knockout (CD74−/−) mice. BMD M(−) and M(IL-4+IL-13) showed high CD74 expression when derived from WT, which was absent in CD74−/− macrophages (Supplemental Figure S7). First, we activated CD74−/− and WT macrophages with IL-4+IL-13 to promote a M2 phenotype and analyzed the secretion of different cytokines (Figure 5A). Surprisingly, M2 macrophages from CD74−/− mice secreted significantly more TNFα (1.8-fold) and CCL5 (1.7-fold) compared to WT-derived macrophages indicating a shift towards a pro-inflammatory phenotype. Co-culturing M2 CD74−/− macrophages with trophoblast cell line SGHPL-4 further increased the secretion of these cytokines TNFα (2.7-fold) and CCL5 (2.7-fold)). Interestingly, the secretion of chemokine MCP-1 was 4.1-fold only induced in BMD M(IL-4+IL-13) from CD74−/− (compared to WT) when co-cultured with SGHPL-4. SGHPL-4 cell count was significantly lowered when co-cultured with BMD M(IL-4+IL-13) from CD74−/− compared to BMD M(IL-4+IL-13) from WT (4284±225 vs. 5200±252 cells) (Figure 5B). SGHPL-4 cells stimulated with the combination of MCP-1, CCL5 and TNFα for 6h and 24h expressed more Cytochrome P450 Subfamily 2J Polypeptide 2 (CYP2J2), sFlt1, TNFα and Interleukin 8 (IL-8). The sFlt1/PLGF ratio was also enhanced (Figure 5C).
Figure 5. Cytokine/chemokine-upregulation of macrophages derived from CD74 knockout mice (CD74−/−) and expression profile in a trophoblast cell line.
A) In vitro activated bone marrow derived (BMD) macrophages (M(IL-4+IL-13)) from CD74−/− mice produced more tumor necrosis factor α (TNFα) and chemokine (C-C motif) ligand 5 (CCL5) compared to BMD M(IL-4+IL-13) from wild type (WT) mice (left panel) (n=6 each; *p<0.05, unpaired t test). Co-culture with human first trimester trophoblast derived cell line (SGHPL-4) enhanced the effect (right panel) (n=6 each, ***p<0.001, ****p<0.0001; unpaired t test). Secretion of monocyte chemoattractant protein-1 (MCP-1) was solely enhanced under co-culture conditions (n=4 each, *p<0.05, Mann Whitney test). All factors were secreted by macrophages, as Luminex assay was specific for mice. B) Co-culturing of BMD M(IL-4+IL-13) from CD74−/− with SGHPL-4 lowered cell amount of SGHPL-4 when compared to BMD M(IL-4+IL-13) from WT (n=30 each; **p<0.01, unpaired t test). C) SGHPL-4 stimulated by the combination of MCP-1, CCL5 and TNFα for 6h and 24h express more Cytochrome P450 Subfamily 2J Polypeptide 2 (CYP2J2), soluble Fms-like tyrosine kinase 1 (sFlt1), TNFα and Interleukin 8 (IL-8). The ratio of the expression of sFlt1 and the placental growth factor (PLGF), the sFlt1/PLGF ratio was also enhanced (n=9, *p<0.05, **p<0.01, ***p<0.001; ANOVA followed by Dunnett post hoc testing).
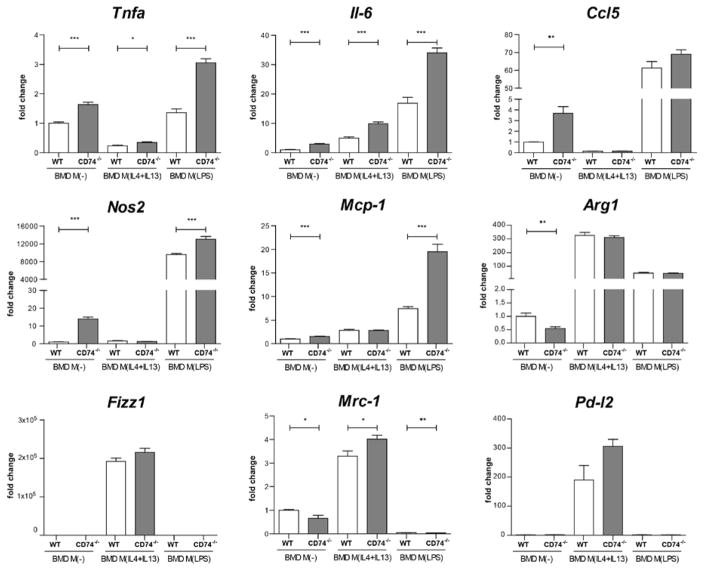
To reveal the impact of CD74−/− on M1 and M2 macrophage activation, we analyzed for M1 activation the pro-inflammatory marker genes Tnfα, Il-6, Ccl5, nitric oxide synthase 2 (Nos2), Mcp-1 and for M2 activation Resistin-like molecule alpha1 (Fizz1), mannose receptor 1 (Mrc-1) and programmed death ligand 2 (Pd-l2) in BMD M(−) and M(IL-4+IL-13) from CD74−/− compared to WT (Figure 6). Arginase 1 (Arg1) is known to be activated after M1 and M2 stimuli. LPS used to induce M1 activation (M(LPS)). The activation by IL-4+IL-13 and LPS led to a typical marker gene signature in BMD macrophages from both origins, CD74−/− and WT. Non-activated M(−) macrophages from CD74−/− origin showed an enhanced expression of Tnfα (1.6-fold), Il-6 (2.9-fold), Ccl5 (3.7-fold), Nos2 (14-fold) and Mcp-1 (1.5-fold) compared to M(−) WT macrophages, indicating a shift towards a pro-inflammatory signature. Under M2 conditions, Tnfα and Il-6 were also higher expressed in M(IL-4+IL-13) CD74−/− macrophages. LPS activation (M(LPS)) led to a higher expression of Tnfα, Il-6, Nos2 and Mcp-1 in CD74−/− macrophages indicating again a shift towards a pro-inflammatory macrophage phenotype.
Figure 6. Pro-inflammatory signature of macrophages derived from CD74 knockout mice (CD74−/−).
In vitro differentiated bone marrow derived (BMD) macrophages (M(−)) from CD74−/− mice express more tumor necrosis factor α (Tnfα), interleukin 6 (Il-6), chemokine (C-C motif) ligand 5 (Ccl5), nitric oxide synthase 2 (Nos2) and monocyte chemoattractant protein-1 (Mcp-1) compared to BMD M(−) from wild type (WT) mice. Tnfα and Il-6 were also higher expressed in BMD M(IL-4+IL-13) from CD74−/− mice compared to WT. Marker for LPS signature (Nos2, Ccl5, IL-6, Tnfα, Mcp-1) and IL-4+IL-13 signature (arginase 1 (Arg1), Resistin-like molecule alpha1 (Fizz1), mannose receptor 1 (Mrc-1), programmed death ligand 2 (Pd-l2)) were fully activated in M(LPS) and M(IL-4+IL-13) respectively and pro-inflammatory activation by LPS was further increased by CD74−/− in regard of Tnfα, Il-6, Nos2 and Mcp-1 (n=6 each;*p<0.05, **p<0.01, ***p<0.001; unpaired t test).
Disturbed placental/fetal phenotype of CD74 knockout (CD74−/−) mice
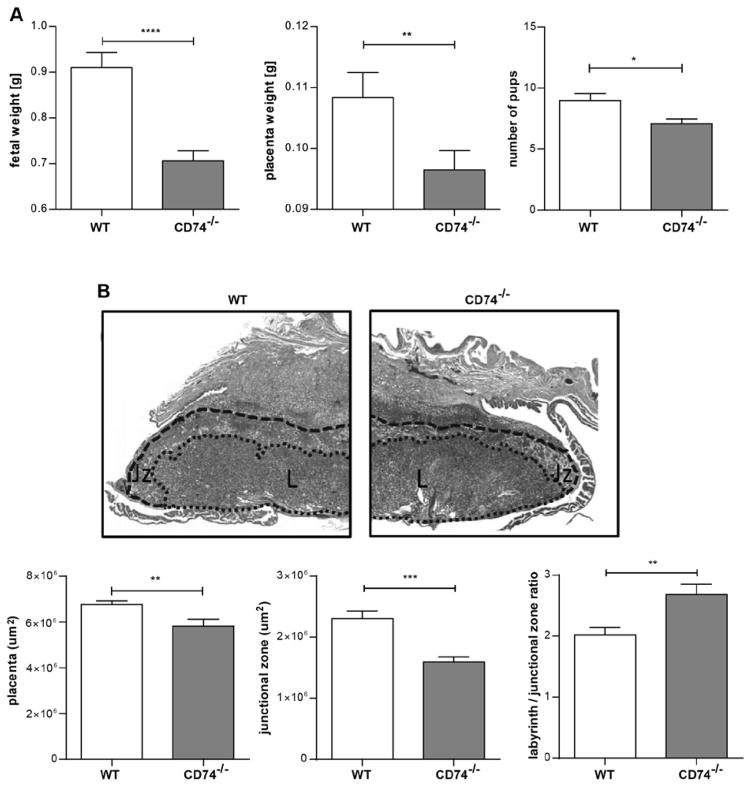
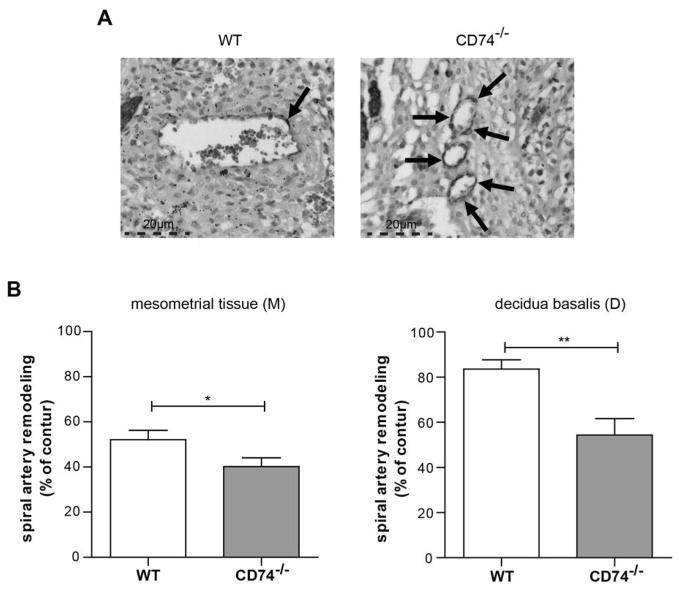
To test the hypothesis that CD74 is important for pregnancy and fetal development, we analyzed the pregnancy and the uteroplacental unit of the CD74−/− mice. CD74 expression was absent on protein level in the placenta of CD74−/− and clearly detectable in wild-type (WT) mice (Supplemental Figure S8). Fetal weight on day 18 was significantly lower in CD74−/− mice (0.71±0.02 g) compared to WT (0.91±0.03 g) (Figure 7A). Placenta weight (0.1 g in CD74−/− vs. 0.11 g in WT) and litter size (7 in CD74−/− vs. 9 in WT pups) was also diminished. Histomorphological analysis revealed a significant overall reduction of the placental area in CD74−/− mice, compared to WT placentas (Figure 7B). Additionally, CD74−/− placentas presented significant structural changes, as the ratio between two functional placental zones, the labyrinth and the junctional zone, was increased in placentas from CD74−/− placentas compared to WT controls (Figure 7B, lower panel). This increase was due to a significant reduction of the junctional zone in CD74−/− placentas, whereas the labyrinth area remained unaltered between CD74−/− and WT mice (Supplemental Figure S9). The spiral artery remodeling was also impaired in arteries of the decidua basalis and mesometrial tissue (Figure 8). Areas are indicated in Supplemental Figure S10A. CD74−/− mice showed significantly more α-actin, representing an abnormal remodeling process (Figure 8). Arteries of CD74−/− mice were smaller, indicated by artery area (μm2) and perimeter (μm) when compared to WT mice (Supplemental Figure S10B). Doppler waveform analysis of the distal uterine artery showed a significant increase of the resistance index (RI) of the CD74−/− mice (0.51±0.02 RI) compared to WT (0.44±0.02 RI) (Supplemental Figure S11).
Figure 7. Fetal growth restriction and disturbed placenta morphology in CD74 knockout mice (CD74−/−).
A) Pup and placental weights were lower in CD74−/− (n=32) vs. WT (n=33). Litter size was also decreased in CD74−/− (n=17) vs. WT (n=28) (*p<0.05, **p<0.01 and ****p<0.0001; Mann Whitney test). B) Representative pictures of Masson stained mid-sagittal placental tissue sections used to carry out the histomorphological analysis (upper panel). Zones are marked and indicated by L (labyrinth zone) and Jz (junctional zone). The overall size of the placenta was decreased in CD74−/− tissues (lower panel) whereas the ratio between the labyrinth/junctional zones was enhanced in CD74−/− (n=13) in comparison to WT (n=17). The area of the junctional zone of CD74−/− placentas was smaller compared to those of WT. (**p<0.01, ***p<0.001; unpaired t test).
Figure 8. Impaired spiral artery remodeling in CD74 knockout mice (CD74−/−).
A) α-actin staining is shown in representative pictures of arteries. Arrows are indicating α-actin stained VSMC’s. B) Spiral arteries of CD74−/− showed more α-actin in the vessel wall when compared to wild type (WT), indicating an impaired remodeling. Bar graphs show % of remodeling of artery (total loss of α-actin = 100%). (M): n=85 (WT), n=95 (CD74−/−); (D): n=36 (WT), n=24 (CD74−/−); *p<0,05, **p<0,01; unpaired t test.
A putative maternal syndrome was characterized by blood pressure and albuminuria (Supplemental Figure S12). However, on day 15 of pregnancy, mean arterial pressure (MAP) and albuminuria were not significantly changed in CD74−/− mice. To analyze the effects of whole body knock out (CD74−/−) in the female mice on fetal growth restriction and lowered placental weights, we transferred fertilized oocytes from CD74−/− mice to WT foster mothers. Fetuses and placentas resulting from this model were also smaller compared to fetuses and placentas resulting from WT fertilized oocytes (Supplemental Figure S13).
DISCUSSION
We present novel data to support the notion that CD74 is significantly downregulated in third trimester preeclamptic placentas. We successfully localized the origin of CD74 expression to placental macrophages (Hofbauer cells) and inversely correlated the placental CD74 expression to the serum sFlt1/PLGF ratio that is enhanced in the preeclamptic circulation. In maternal blood, the shed soluble CD74 and its ligand MIF were not statistically significantly altered in the circulation of preeclamptic women. Importantly, macrophages that lack CD74 showed a dysregulated interaction with trophoblasts. The adhesion of such macrophages to trophoblasts was diminished, while CD74 deficient macrophages exhibited a pro-inflammatory phenotype when co-cultured with trophoblasts. Trophoblasts activated by those pro-inflammatory stimulus expressed more vasoactive and anti-angiogenic factors. M1 and M2 activation under LPS and IL-4+IL-13 led to the induction of the respective signature genes. Interestingly, M(IL-4+IL-13) CD74−/− M2 macrophages also expressed pro-inflammatory genes suggesting a shift towards an altered M2 phenotype. Importantly, CD74−/− mice showed altered placenta morphology, evidenced by a smaller junctional zone and an impaired spiral artery remodeling. This abnormal placentation was accompanied by fetal growth restriction and an increased resistance index of the distal uterine arteries. Our results suggest a novel link between preeclampsia and the regulatory interaction of placental macrophages and villous trophoblasts with a specific role for CD74.
The most studied function of CD74 is its chaperone activity, in which CD74 acts as an invariant chain for MHC class II proteins and is crucial for antigen presentation.18 Due to the importance of antigen presentation in the recognition of fetal cells by the mother, it is surprising that little is known about the expression of CD74 in the placenta. Peyman et al. showed that trophoblasts do not present MHC class II proteins and CD74 on their cell surface. Moreover, those genes seem to be inactive, as the mRNA expression was not inducible by interferon-γ, which has been shown to upregulate these proteins in macrophages.29 These observations are in line with our findings. We also found that CD74 is expressed by placental macrophages and not by trophoblast cells. In human placental explants infected by Toxoplasma gondii, a model for induction and suppression of infection, CD74 was detected in first but not third trimester trophoblasts, as well as in Hofbauer cells.30 However, colocalization of CD74 with a marker like CD163 for macrophages or CK7 for trophoblast was not performed. The expression of CD74 in general was higher in first trimester explants. Interestingly, in this study the production and secretion of the CD74 ligand MIF was enhanced in first-trimester placenta after activation of inflammatory processes by pathogens. MIF may play an essential role as an autocrine/paracrine mediator in placental infection.23, 30 In this context, our finding that expression of CD74 is downregulated in preeclampsia suggests a disturbance of the normally balanced immune response to antigens in the preeclamptic placenta. MIF expression and concentrations were described in pregnancy and preeclampsia by Cardaropoli et al.31 Only in serum of preeclamptic women accompanied by fetal growth restriction they found significantly higher MIF levels compared to controls. However, that study also failed to show a statistically significant up-regulation in the preeclamptic group without fetal growth restriction, which is in line with our findings.
Inflammatory dysfunction in preeclampsia is mostly described on the maternal side of the uteroplacental unit or in maternal circulation. Brewster et al. described that the monocyte secretory capacity for different cytokines, including MCP-1 and TNFα, is higher in preeclamptic women than in controls.32 MCP-1 secretion can be stimulated by TNFα and IL-1β in first trimester derived decidual explants.33 The chemokines MCP-1 and CCL5, as well as the adhesion molecules ICAM-1 and VCAM-1, are elevated in preeclampsia compared with healthy pregnancies, resulting in an overall pro-inflammatory systemic environment.5–7 Circulating levels of the inflammatory cytokines TNFα, IL-10, INF-γ are controversially discussed.34 Our results from the in vitro model also suggest an upregulated expression of macrophage signaling molecules such as MCP-1, TNFα and CCL5 as well as a diminished adhesion of macrophages to trophoblasts in preeclampsia that may be caused by downregulated CD74. The in-vitro findings show a dysregulation of the well-balanced interaction between villous trophoblasts and placental macrophages towards a pro-inflammatory phenotype. However, this does not illuminate the nature of the differences in circulatory in the maternal system. Fest et al. could show that the human THP-1 monocyte line expressed and secreted more cytokines and chemokines when co-cultured with trophoblasts compared to mono culture. Among these factors were MCP-1 and CCL5. 35 Our findings are in line with this observation. We also show an effect of trophoblasts on macrophages regarding secretion of TNFα, MCP-1 and CCL5. Furthermore, we showed that this effect is enhanced by CD74 downregulation. Further induction of an already existing robust inflammatory response during pregnancy by inflammatory cytokines and chemokines is already described in preeclampsia.3, 4 Here, we could show that the activation of a trophoblast cell line by TNFα, MCP-1 and CCL5 led to an upregulation of the vasoactive CYP2J2, the anti-angiogenic factor sFlt1 and the pro-inflammatory TNFa and IL-8. All these factors are described to be mechanistically involved in the preeclamptic syndrome.27, 34, 36, 37 Recently, we described the fact that the CYP2J2 expression is upregulated in preeclamptic trophoblasts.27 This uteroplacental upregulation resulted in enhanced CYP2J2 metabolite levels in the circulation of preeclamptic women. Furthermore, we tested the vasoactive function the metabolite and could show that it induced a chronotropic effect, an endothelial dysfunction and a down regulation of the KCa1.1 channel activity. Its function was synergistic to angiotensin II. In preeclampsia, angiotensin II sensitivity, endothelial dysfunction and alterations in endothelium-dependent vascular contractile properties, are part of the maternal syndrome. We now can conclude that the recently described CYP2J2 upregulation in preeclamptic trophoblasts could be a mechanism of the here described CD74 depended macrophage-trophoblast interaction. We also could show that CD74−/−-macrophages show a pro-inflammatory shift towards an M(LPS) (or classical macrophage (M1)) phenotype. This result is in line with recent findings. Similar shift toward pro-inflammatory M1 phenotype was also described in preeclamptic placentas were the increase of M1 macrophages and the decrease of M2 macrophages lead to an acute atherosis.38
Hofbauer cells may play a direct role in early placental development, as early placental vasculogenesis is influenced by close contact of Hofbauer cells with endothelial progenitor cells in primitive vessels in the first trimester placenta.39 With respect to the importance of Hofbauer cells in the early placenta, our study of early third trimester placentas show the relevance of CD74 and Hofbauer cells in the middle stage of pregnancy which may be of importance in the pathophysiology of preeclampsia and fetal growth restriction. We can only speculate that CD74 downregulation is already a feature of immunological dysregulation at the beginning of pregnancy or even before conception, the latter being viewed as relevant for the development of preeclampsia and/or fetal growth restriction.40 Little is known about the transcriptional regulation of CD74. CD74 expression is described to be increased in tissue injury disorders and decreased in brain tissue in schizophrenia and bipolar disorder.41, 42 However, none of these studies could reveal the regulatory mechanisms that underlie the regulation of CD74 expression. The regulation of CD74 in different cell types correlates with glucose concentrations and an immune activation.41, 43 The inaccessibility of tissue from histiotrophic placentas let us just speculate about the mechanisms that lead to the downregulation of CD74 in the preeclamptic placental macrophages. Glucose regulates the Heme oxygenase-1 that is a central player for immune regulation.44 Furthermore, glucose regulates the exosomal signaling by placental cells and the release of cytokines.45 It might be that a dysregulation in glucose metabolism at first trimester in pregnancies has adverse outcome on the CD74 expression and therefore associates with placental insufficiency. Currently, no single nucleotide polymorphism (SNP) is described for the CD74 gene or its promoter region. Analysis of SNPs and their association to the genesis of the preeclamptic syndrome are growing. Association studies of polymorphisms in inflammatory genes involved in preeclampsia are controversial.46 However, further studies to reveal the mechanisms that underlie the transcriptional regulation of CD74 are important.
The CD74−/− mouse model was established and first described in 1993 by Bikoff et al.47 Most publications described the role of CD74 in regulating MHC class II expression and function.48 Recently, CD74−/− mice were described to develop spontaneous emphysema in association with MIF.49 Mun et al. isolated bone-marrow cells from CD74−/− mice and described the mice as indistinguishable from WT littermates in their general health, growth rate as well as breeding performance.50 However, this does not reflect our observations. This result could be due to different murine backgrounds, as the prior authors crossed heterozygous CD74 knockout mice in order to generate homozygous CD74−/− littermates, or to an insufficient characterization of fetuses and placentas. Herein, we report that CD74 deficiency was associated with an altered placentation at late gestation, evidenced by smaller placentas and an impaired spiral artery remodeling. Placentas from CD74−/− mice also revealed an increased L/Jz ratio, which was mainly due to a decrease of the junctional zone area. The junctional zone is composed of spongiotrophoblast, glycogen cells, and a layer of giant cells reaching the decidual tissue.51 Although the function of the junctional zone in mice is not yet fully understood, it has been shown to support the growth and expansion of the labyrinth.51 Moreover, the junctional zone synthesizes and secretes a vast array of cytokines and hormones such as placental lactogens, hereby maintaining fetal growth.52–54 Hence, the decreased junctional zone observed in CD74−/− mice at late gestation and the impaired spiral artery remodeling could account for the increased resistance index of the distal uterine arteries and the decreased fetal weight. Moreover, we provide evidence that while the area of the labyrinth is unaffected in CD74−/− mice, the relative fraction of the placenta occupied by the labyrinth is increased. This relative increase in the size of the labyrinth has been reported to naturally occur in small placentas and can be interpreted as an adaptation attempt to meet the demands for fetal growth.55 The labyrinth comprises a complex vascular network of maternal and fetal blood vessels in close proximity, hereby promoting nutrient and gas transfer from mother to fetus. Interestingly, it was shown that the relative increase in the labyrinth from small placentas occurs at the expense of the junctional zone, which could provide an explanation for the decrease in the area of the junctional zone observed in the CD74−/− placentas. We here assessed placental tissue from CD74−/− mice and respective WT controls late in gestation and hence, can only speculate that the adaptive changes observed in the placentas of CD74−/− mice may have occurred earlier in gestation. A limitation of the mouse-model might be the knock-out effect on other cell types than the placental macrophages and the whole body knock-out effects in the maternal body. To exclude the CD74−/− effect in organs of the female mice, we also provided the CD74−/− two-cell stage embryo transfer in WT mice. The observed fetal and placental growths restrictions underline the significance of the CD74−/− on the uteroplacental unit. However, in this model, we just can speculate that the effects are macrophage driven and not co-stimulated by CD74−/− in other placental cell types. Further experiments with a placental macrophage specific knock-out model could complete rule out the interaction between macrophages and trophoblasts. The maternal syndrome was not developed in CD74−/− mice. One explanation for the lack of hypertension in CD74−/− mice could be related to the C57BL/6 genetic background of the knockout mice. European C57BL/6 are often resistant to hypertension. Therefore, we cannot completely rule out a role of CD74 on blood pressure. Our hypothesis is that the CD74 downregulation leads to an adverse outcome in placentation resulting in vulnerability for preeclampsia and IUGR. We hypothesize that additional triggers could promote a maternal syndrome and further studies will be necessary to investigate which second hit is necessary in addition to downregulation of CD74 to promote the full clinical picture of preeclampsia. Furthermore, our findings are in line with the clinical discussion of preeclampsia and IUGR. Both pregnancy complications share risk factors, molecular pathways and the risk for developing future cardiovascular.56 Moreover, as preeclampsia is a complex syndrome, the lack of CD74 in the mouse model we here used may have been compensated by other pathways. Such redundancy of single pathways in the context of maintaining successful reproduction has been described in a number of genetically engineered mice.57
CD74 is significantly downregulated in third trimester preeclamptic placentas. Therefore, we propose that CD74 might be involved in the development of preeclampsia. Our data suggests that CD74 is a potential candidate for controlling the well-balanced regulation between villous trophoblasts and placental macrophages. Thus, studies on CD74 and the macrophage-trophoblast interaction in pregnancy and preeclampsia are essential to enhance our knowledge of its modes of action and to evaluate its potential as a therapeutic agent in preeclampsia and other placenta dysfunctional complications of pregnancy.
Supplementary Material
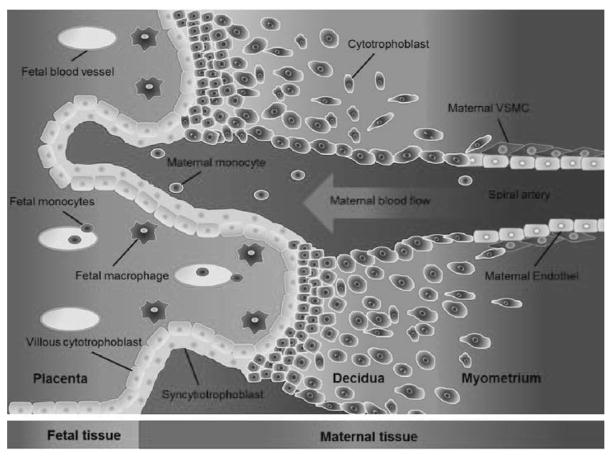
Figure 3. Illustration of the maternal-fetal-interface.
Tissue and cells from fetal origin are illustrated in blue. Maternal tissue and cells are illustrated in red. Fetal cytotrophoblasts invade into the maternal tissue and remodel the maternal spiral artery. Fetal macrophages (Hofbauer cells) interact with the fetal villous cytotrophoblast via adhesion molecules and cytokines.
Acknowledgments
We thank Juliane Anders, Jutta Meisel, Ilona Kamer, Gabriele N’Diaye, Jana Czychi, Dr. Hongqi Lue, May-Britt Köhler, Stefanie Schelenz, Martin Taube, Anika Wehner and Thomas Andreas for their excellent technical assistance. We also thank Lise Øhra Levy for valuable assistance in patient recruitment and biobank handling and Ralf Kuehn, Rainer Kabisch and Sabine Manz from animal facility of MDC/BIH.
SOURCES OF FUNDING
The Deutsche Forschungsgemeinschaft (DFG) supported Dr. Herse (HE 6249/1-2, HE 6249/4-1), Dr. Dechend (DE 631/9-1) and Dr. Müller. The German Centre for Cardiovascular Research (DZHK) supported Dr. Müller. Dr. Bernhagen acknowledges support by DFG grant SFB1123/A03 and RWTH Aachen-IZKF grant K7-1
Footnotes
DISCLOSURES
NONE
References
- 1.Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstetrics and gynecology. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Fayyad AM, Harrington KF. Prediction and prevention of preeclampsia and iugr. Early human development. 2005;81:865–876. doi: 10.1016/j.earlhumdev.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 4.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 5.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunology. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. Journal of reproductive immunology. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Mellembakken JR, Solum NO, Ueland T, Videm V, Aukrust P. Increased concentrations of soluble cd40 ligand, rantes and gro-alpha in preeclampsia--possible role of platelet activation. Thrombosis and haemostasis. 2001;86:1272–1276. [PubMed] [Google Scholar]
- 8.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biology of reproduction. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 9.Hunt JS. Macrophages in human uteroplacental tissues: A review. Am J Reprod Immunol. 1989;21:119–122. doi: 10.1111/j.1600-0897.1989.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 10.Yui J, Hemmings D, Garcia-Lloret M, Guilbert LJ. Expression of the human p55 and p75 tumor necrosis factor receptors in primary villous trophoblasts and their role in cytotoxic signal transduction. Biology of reproduction. 1996;55:400–409. doi: 10.1095/biolreprod55.2.400. [DOI] [PubMed] [Google Scholar]
- 11.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- 12.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. The Journal of clinical endocrinology and metabolism. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 14.Xu B, Nakhla S, Makris A, Hennessy A. Tnf-alpha inhibits trophoblast integration into endothelial cellular networks. Placenta. 32:241–246. doi: 10.1016/j.placenta.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Huppertz B. The anatomy of the normal placenta. Journal of clinical pathology. 2008;61:1296–1302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 16.Tang Z, Abrahams VM, Mor G, Guller S. Placental hofbauer cells and complications of pregnancy. Annals of the New York Academy of Sciences. 2011;1221:103–108. doi: 10.1111/j.1749-6632.2010.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripathi R, Rath G, Jain A, Salhan S. Soluble and membranous vascular endothelial growth factor receptor-1 in pregnancies complicated by pre-eclampsia. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2008;190:477–489. doi: 10.1016/j.aanat.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Long EO, Strubin M, Wake CT, Gross N, Carrel S, Goodfellow P, Accolla RS, Mach B. Isolation of cdna clones for the p33 invariant chain associated with hla-dr antigens. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:5714–5718. doi: 10.1073/pnas.80.18.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via cd44, cd74, and mapk signaling pathways. The Journal of clinical endocrinology and metabolism. 2010;95:E403–412. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 20.Noels H, Bernhagen J, Weber C. Macrophage migration inhibitory factor: A noncanonical chemokine important in atherosclerosis. Trends in cardiovascular medicine. 2009;19:76–86. doi: 10.1016/j.tcm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Arcuri F, Cintorino M, Vatti R, Carducci A, Liberatori S, Paulesu L. Expression of macrophage migration inhibitory factor transcript and protein by first-trimester human trophoblasts. Biology of reproduction. 1999;60:1299–1303. doi: 10.1095/biolreprod60.6.1299. [DOI] [PubMed] [Google Scholar]
- 22.Castro AS, Alves CM, Angeloni MB, Gomes AO, Barbosa BF, Franco PS, Silva DA, Martins-Filho OA, Mineo JR, Mineo TW, Ferro EA. Trophoblast cells are able to regulate monocyte activity to control toxoplasma gondii infection. Placenta. 2013 doi: 10.1016/j.placenta.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Ferro EA, Mineo JR, Ietta F, Bechi N, Romagnoli R, Silva DA, Sorda G, Bevilacqua E, Paulesu LR. Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. The American journal of pathology. 2008;172:50–58. doi: 10.2353/ajpath.2008.070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assis DN, Leng L, Du X, Zhang CK, Grieb G, Merk M, Garcia AB, McCrann C, Chapiro J, Meinhardt A, Mizue Y, Nikolic-Paterson DJ, Bernhagen J, Kaplan MM, Zhao H, Boyer JL, Bucala R. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2014;59:580–591. doi: 10.1002/hep.26664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebmann V, Dornmair K, Grosse-Wilde H. Biochemical analysis of plasma-soluble invariant chains and their complex formation with soluble hla-dr. Tissue antigens. 1997;49:438–442. doi: 10.1111/j.1399-0039.1997.tb02776.x. [DOI] [PubMed] [Google Scholar]
- 26.Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Soluble hla-dr levels in the maternal circulation of normal and pathologic pregnancy. American journal of obstetrics and gynecology. 2003;188:473–479. doi: 10.1067/mob.2003.55. [DOI] [PubMed] [Google Scholar]
- 27.Herse F, Lamarca B, Hubel CA, Kaartokallio T, Lokki AI, Ekholm E, Laivuori H, Gauster M, Huppertz B, Sugulle M, Ryan MJ, Novotny S, Brewer J, Park JK, Kacik M, Hoyer J, Verlohren S, Wallukat G, Rothe M, Luft FC, Muller DN, Schunck WH, Staff AC, Dechend R. Cytochrome p450 subfamily 2j polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation. 2012;126:2990–2999. doi: 10.1161/CIRCULATIONAHA.112.127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joerink M, Rindsjo E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta. 2011;32:380–385. doi: 10.1016/j.placenta.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Peyman JA, Hammond GL. Localization of ifn-gamma receptor in first trimester placenta to trophoblasts but lack of stimulation of hla-dra, -drb, or invariant chain mrna expression by ifn-gamma. J Immunol. 1992;149:2675–2680. [PubMed] [Google Scholar]
- 30.de Oliveira Gomes A, de Oliveira Silva DA, Silva NM, de Freitas Barbosa B, Franco PS, Angeloni MB, Fermino ML, Roque-Barreira MC, Bechi N, Paulesu LR, Dos Santos MC, Mineo JR, Ferro EA. Effect of macrophage migration inhibitory factor (mif) in human placental explants infected with toxoplasma gondii depends on gestational age. The American journal of pathology. 2011;178:2792–2801. doi: 10.1016/j.ajpath.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardaropoli S, Paulesu L, Romagnoli R, Ietta F, Marzioni D, Castellucci M, Rolfo A, Vasario E, Piccoli E, Todros T. Macrophage migration inhibitory factor in fetoplacental tissues from preeclamptic pregnancies with or without fetal growth restriction. Clinical & developmental immunology. 2012;2012:639342. doi: 10.1155/2012/639342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brewster JA, Orsi NM, Gopichandran N, Ekbote UV, Cadogan E, Walker JJ. Host inflammatory response profiling in preeclampsia using an in vitro whole blood stimulation model. Hypertension in pregnancy. 2008;27:1–16. doi: 10.1080/10641950701826067. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: Implications for preeclampsia. The American journal of pathology. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. Il-10, tnf-alpha & ifn-gamma: Potential early biomarkers for preeclampsia. Cellular immunology. 2013;283:70–74. doi: 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G. Trophoblast-macrophage interactions: A regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 36.Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma il-4, il-8, il-12, interferon-gamma and crp levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birth weight. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:1569–1573. doi: 10.3109/14767058.2011.648233. [DOI] [PubMed] [Google Scholar]
- 37.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Frontiers in immunology. 2014;5:298. doi: 10.3389/fimmu.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seval Y, Korgun ET, Demir R. Hofbauer cells in early human placenta: Possible implications in vasculogenesis and angiogenesis. Placenta. 2007;28:841–845. doi: 10.1016/j.placenta.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, Izquierdo MC, Selgas R, Egido J, Ortiz A. Mif, cd74 and other partners in kidney disease: Tales of a promiscuous couple. Cytokine & growth factor reviews. 2013;24:23–40. doi: 10.1016/j.cytogfr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Morgan LZ, Rollins B, Sequeira A, Byerley W, DeLisi LE, Schatzberg AF, Barchas JD, Myers RM, Watson SJ, Akil H, Bunney WE, Jr, Vawter MP. Quantitative trait locus and brain expression of hla-dpa1 offers evidence of shared immune alterations in psychiatric disorders. Microarrays (Basel) 2016:5. doi: 10.3390/microarrays5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghiglione Y, Rodriguez AM, De Candia C, Carobene M, Benaroch P, Schindler M, Salomon H, Turk G. Hiv-mediated up-regulation of invariant chain (cd74) correlates with generalized immune activation in hiv+ subjects. Virus research. 2012;163:380–384. doi: 10.1016/j.virusres.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Zenclussen ML, Linzke N, Schumacher A, Fest S, Meyer N, Casalis PA, Zenclussen AC. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Frontiers in pharmacology. 2014;5:291. doi: 10.3389/fphar.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, Mitchell MD, Salomon C. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. The Journal of clinical endocrinology and metabolism. 2015;100:E1280–1288. doi: 10.1210/jc.2015-2270. [DOI] [PubMed] [Google Scholar]
- 46.Harmon QE, Engel SM, Wu MC, Moran TM, Luo J, Stuebe AM, Avery CL, Olshan AF. Polymorphisms in inflammatory genes are associated with term small for gestational age and preeclampsia. Am J Reprod Immunol. 2014;71:472–484. doi: 10.1111/aji.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class ii assembly, transport, peptide acquisition, and cd4+ t cell selection in mice lacking invariant chain expression. The Journal of experimental medicine. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shachar I, Flavell RA. Requirement for invariant chain in b cell maturation and function. Science. 1996;274:106–108. doi: 10.1126/science.274.5284.106. [DOI] [PubMed] [Google Scholar]
- 49.Sauler M, Leng L, Trentalange M, Haslip M, Shan P, Piecychna M, Zhang Y, Andrews N, Mannam P, Allore H, Fried T, Bucala R, Lee PJ. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. American journal of physiology. Lung cellular and molecular physiology. 2014;306:L487–496. doi: 10.1152/ajplung.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mun SH, Won HY, Hernandez P, Aguila HL, Lee SK. Deletion of cd74, a putative mif receptor, in mice enhances osteoclastogenesis and decreases bone mass. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28:948–959. doi: 10.1002/jbmr.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nature reviews Genetics. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 52.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 53.Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine prolactin/placental lactogen-related genes is not associated with their position in the locus. BMC genomics. 2008;9:352. doi: 10.1186/1471-2164-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croy BA. Reproductive immunology issue one: Cellular and molecular biology. Cellular & molecular immunology. 2014;11:405–406. doi: 10.1038/cmi.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. The Journal of physiology. 2008;586:4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasmussen LG, Lykke JA, Staff AC. Angiogenic biomarkers in pregnancy: Defining maternal and fetal health. Acta obstetricia et gynecologica Scandinavica. 2015;94:820–832. doi: 10.1111/aogs.12629. [DOI] [PubMed] [Google Scholar]
- 57.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nature medicine. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.