Abstract
Elevated homocysteine levels are a well-known independent risk factor for cardiovascular disease. To date, relatively few selective fluorescent probes for homocysteine detection have been reported. The lack of sensing reagents and remaining challenges largely derive from issues of sensitivity and/or selectivity. For example, homocysteine is a structural homologue of the more abundant (ca, 20–25 fold) aminothiol cysteine, differing only by an additional methylene group side chain. Fluorescein tri-aldehyde, described herein, has been designed and synthesized as a sensitive and selective fluorophore for the detection of homocysteine in human plasma samples. It responds to analytes selectively via a photoinduced electron transfer (PET) inhibition process that is modulated by predictable analyte-dye product hybridization and ionization states. Mulliken population analysis of fluorescein tri-aldehyde and its reaction products reveals that the characteristic formation of multiple cationic of homocysteine-derived heterocycles leads to enhanced relative negative charge build up on the proximal phenolate oxygen of the fluorophore as a contributing factor to selective emission enhancement.
Keywords: Homocysteine, Thiazinane, Thiazolidine, Fluorescein aldehydes, Fluorescence
Introduction
Homocysteine (Hcy) is a naturally-occurring amino thiol. It is an intermediate in the metabolism of methionine (Met) [1] and a precursor to cysteine (Cys) [2]. The conversion of Hcy to Met by remethylation and to Cys by transsulfuration steps modulates Hcy levels in human plasma [3]. Metabolic irregularities can lead to Hcy concentrations above 15 μM, a condition known as hyperhomocysteinemia (HHcy) [4]. HHcy can be caused by several factors including chronic renal failure, enzymatic defects, vitamin deficiency [1], overburdening of Met, and accumulation of reactive oxygen species [4]. HHcy is a well-known independent risk factor for cardiovascular disease [5] and is additionally associated with other major disorders including Alzheimer's [6], osteoporosis [7], renal failure [8], cancer [9] and neural tube defects [10].
The association of elevated Hcy levels with major disease risk has led to broad interest in its detection. Clinical monitoring is currently performed via chromatography, immunoassays or enzyme cycling [11–13]. Direct optical detection without separations or biological materials has been of great interest for determining Hcy and/or Cys, due to their relative ease of operation and low cost [14–19]. Hcy and Cys have very similar structures; additionally, circulating Cys levels are typically 15–20 times higher than those of Hcy. Therefore the selective detection of Hcy by abiotic chemosensors or dosimeters has been challenging. For example, relatively few optical sensors have been reported for the detection of Hcy over Cys and other amino acids [20–27].
Recently, we described a photochemical method for the detection of Hcy in human plasma via a redox mechanism [22] as well as an aldehyde-functionalized fluorescein method based on pH modulation [27]. Another recent example of a redox probe for Hcy is the dansyl derivative reported by Wang [23]. The well-known reaction of aldehyde fluorophores [14–16, 20, 28] with Hcy and Cys has promoted the design of three other Hcy probes. These include an iridium complex [24], BODIPY [25] and pyrene [20] fluorophores. Each of the reported probes to date has inherent challenges including either low sensitivity at physiological levels, poor selectivity over more abundant thiols, fluorescence via only short wavelength excitation, and/ or lack of function in aqueous solvents. To address the current issues and increase the pool of useful optical indicators available for Hcy detection, herein the synthesis of fluorescein tri-aldehyde and its selectivity for Hcy under physiological conditions is reported.
Experimental
Materials
All chemicals were purchased from Sigma-Aldrich and used as received. Ultrapure water obtained from a Milli-Q™ direct water purification system was utilized to prepare all aqueous solutions. All spectroscopic measurements were conducted in DMSO:buffer (1:99) solutions.
Methods
1H NMR and 13C NMR were recorded on a ARX-400 Advance Bruker spectrometer performing at 400.13 MHz (1 H) and 100.61 MHz (13C). All chemical shifts (δ) were reported in ppm using DSS (sodium 2,2-dimetyl-2-silapentane-5-sulfonate) as an internal standard (δ = 0.000) for 1H NMR studies. CF3COOD was used as an NMR solvent to record 1H NMR spectra. The 13C NMR of 1 was obtained in a 1:4 CDCl3:CF3COOD solvent system. ESI-HRMS (high resolution mass spectrometry) spectra were recorded at the PSU Bioanalytical Mass Spectrom-etry Facility on a ThermoElectron LTQ-Orbitrap high resolution mass spectrometer. FT-IR spectra were aquired on a Thermo Scientific Nicolet iS10 spectrophotometer equipped with a single-bounce diamond ATR attachment. Deproteinization of plasma was carried out by standard procedures [27].
Spectrophotometric Measurements
UV-visible spectra were recorded on a Cary 50 UV-Vis spectrophotometer (Agilent Technologies). Fluorescence spectra were recorded on a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies) with slit widths set at 5 nm for both excitation and emission, respectively. Fluorescence spectra were corrected for the wavelength dependent response of the R928 photomultiplier tube with the help of a manufacturer generated correction file. All spectrophotometric measurements were conducted at rt.
Mulliken Charge Calculations
Geometry optimization and Mulliken population analysis were performed using the PM7 hamiltonian in MOPAC2012 [29, 30] for compounds 1, 1a and 1b.
Synthesis of 3
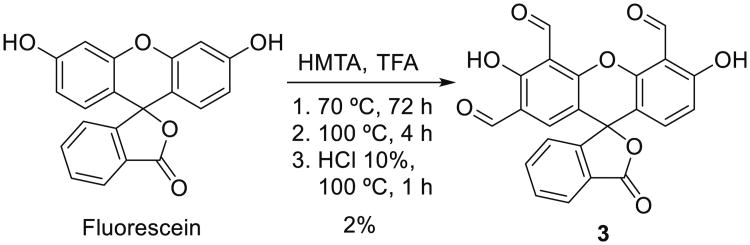
Fluorescein (1 equiv., 7.52 mmol) is dissolved in a minimum amount of TFA. Hexamethylene tetraamine (HMTA, 10 equiv., 75.23 mmol) is added slowly to the fluorescein solution under Ar at rt. The reaction mixture is stirred at 70 °C for 72 h and at 100 °C for 4 h. A 30 mL solution of 1 N HCl is added and the mixture stirred at 100 °C for 1 h. Formation of an orange precipitate is observed. The reaction mixture is left at rt. for 15 h, the precipitates filtered, washed with H2O and dried in vacuo. Probe 3 is purified by column chromatography (CHCl3:MeOH, 98:2 and DCM:EtOAc 97:3) and obtained as a colorless solid (yield: 62 mg, 2.0 %). Rf = 0.3 (3 % EtOAc in DCM, silica gel); 1H NMR (400 MHz, CDCl3) δ 10.74 (s, 1 H), 10.62 (s, 1 H), 9.93 (s, 1 H), 8.17 (d, J = 7.7 Hz, 1 H), 7.84 (s, 1 H), 7.74 (m, 2 H), 7.25 (m, 2 H), 6.94 (m, 1 H); 13C NMR (101 MHz, CDCl3) δ 189.69 (CHO), 187.58 (overlapping, CHO), 166.3 (COOH), 153.6, 152.8, 137.8, 133.5, 131.8, 127.8, 125.0, 122.4, 122.04, 120.7, 117.9, 115.4, 111.8, 110.8, 109.6 108.0 (overlapping), 104.36; FTIR, νmax (film), cm−11738 (C = O);. Electrospray ionization (ESI) mass spectrometry (negative electron ionization) m/z: 415.04610 [M-H] −, calculated for C23H12O8 m/z: 416.05322.
Results
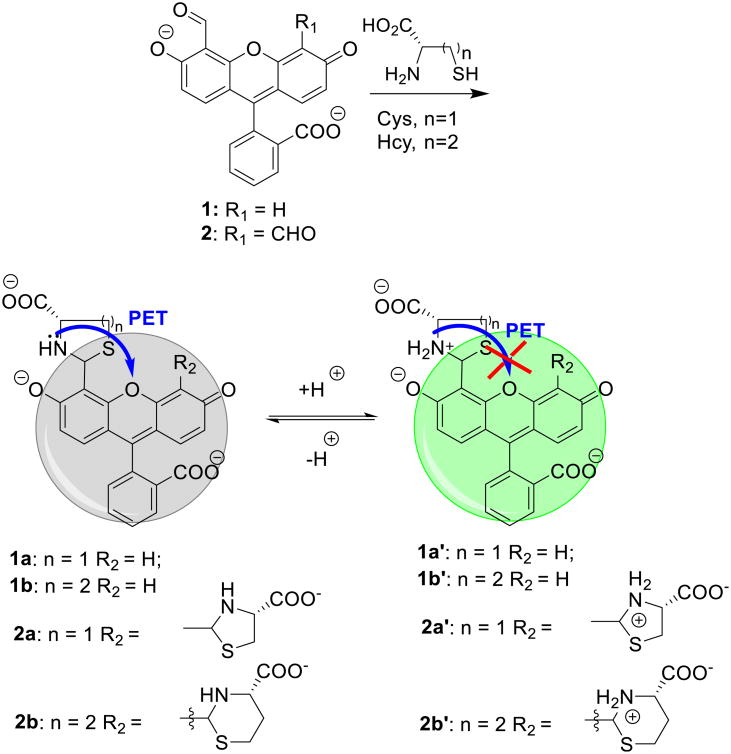
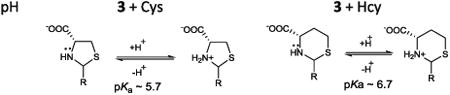
Recently, we showed that fluorescein aldehyde probes could respond to the presence of Cys/Hcy in a controllable manner, affording either quenching or enhancement. The presence of Hcy could be selectively distinguished from that of Cys at pH 6, whereby Hcy addition promoted enhanced emission, while the Cys-induced emission response was relatively very weak [27]. Under more basic conditions (pH 9.5), both Hcy and Cys quenched the fluorescence. The pH-dependent modulation of the emission by Cys/Hcy is due to a PET mechanism. For example, it is known that thiazinane and thiazolidine lone pairs can quench proximal fluorophore emission. Since the pKa of the amine of the thiazolidine (∼ 5.7) is lower than that of the analogous thiazinane (∼ 6.7) due to hybridization effects, the protonation of the thiazinane, affording concomitant PET inhibiton, can be modulated selectively as a function of careful solution pH selection (Scheme 1) [27].
Scheme 1. Mechanism of the signal transduction in fluorescein aldehydes upon reaction with Hcy and Cys.
The successful discrimination of Hcy from Cys using relatively high (≥ 50 μM) Hcy levels motivated the design of a more sensitive fluorophore (3). The synthesis of 3 was carried out via a one-pot Duff formylation of fluorescein (Scheme 2).
Scheme 2. One pot synthesis of 3 (yield not optimized).
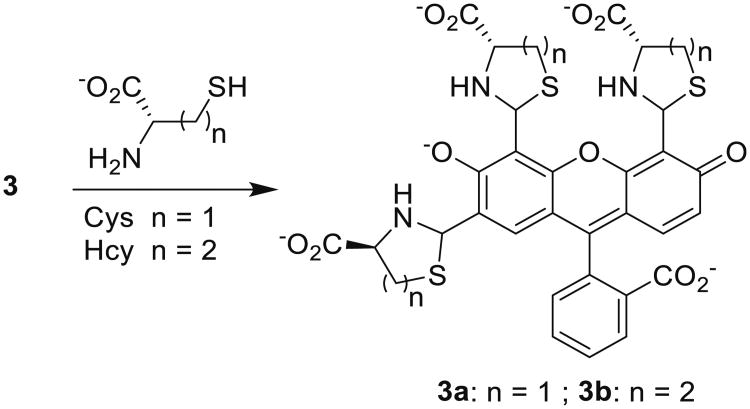
Similar to 1 and 2, compound 3 (fluorescein tri-aldehyde) exhibits fluorescence enhancement in the presence of Hcy and no response to Cys. The fluorescence sensing behavior of 3 towards Hcy and Cys was examined at pH 6.0 at their physiological concentrations (Fig. 1). Upon reaction of Hcy and Cys with 3, formation of thiazolidine and thiazinane takes place respectively (Scheme 3). We noticed that upon adding Hcy in the presence of Cys to a solution of 1, an enhancement in the fluorescence was obtained whereas Cys by itself does not produce any appreciable change in the optical response of 2 (Fig. 1). Thus, the presence of excess of Cys does not cause interference in the detection of Hcy (Fig. 1).
Fig. 1.
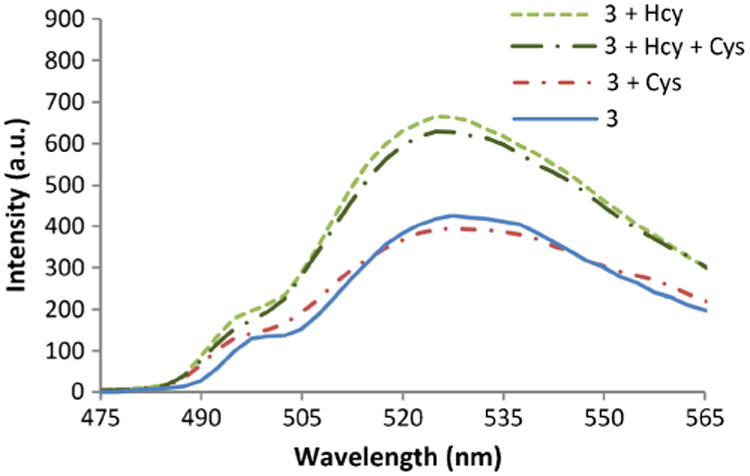
The fluorescence spectra of solutions of 3 and thiols after 2 h. Physiological concentrations of the thiols are used: 3 (4 μM), Cys (250 μM) and Hcy (15 μM) in phosphate buffer (100 mM, pH 6.0): DMSO 99:1 at 20 °C, λex = 495 nm. Cys does not interfere with Hcy detection
Scheme 3. Reaction of 3 with Hcy and Cys.
Moreover, at physiologically-relevant levels of thiols, we studied the response of 3 towards Hcy and Cys in deproteinized plasma, where the addition of Hcy to a solution of 3 enhances the fluorescence. In contrast, no significant change in the emission intensity was observed upon reaction with Cys (Fig. 2).
Fig. 2.
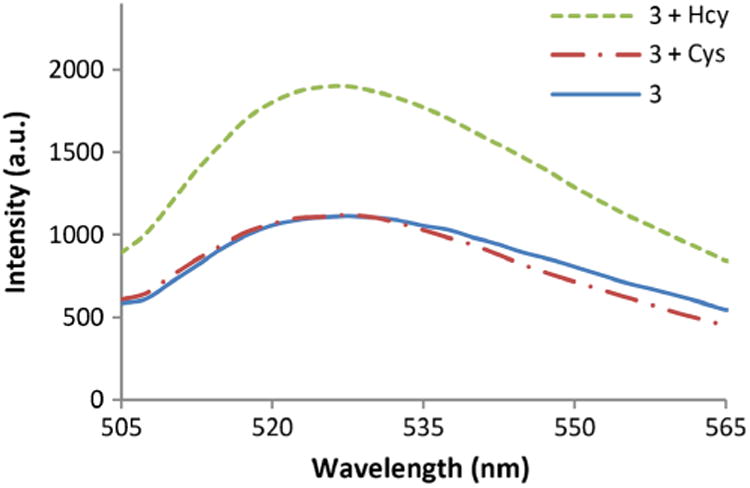
Optical sensing behavior of 3 towards Cys and Hcy in deproteinized plasma at pH 6.0 after 2 h. Solutions are composed of 3 (20 μM), Cys (250 μM), and Hcy (15 μM) in phosphate buffer (100 mM, pH 6.0):DMSO 99:1 at 20 °C, λex = 495 nm
The response to Hcy was also investigated in the presence of various amino acids (Gly, Ala, Met, Gln, Glu, and Asp) in addition to Cys. Figure 3 shows that only Hcy produces a distinct response towards 2 in the presence of these interfering analytes. The response to glutathione (GSH) towards 3 was also studied; however no significant change in emission intensity was observed (Fig. 3). Overall, these results further demonstrate that 3 exhibits a distinct selectivity for Hcy.
Fig. 3.
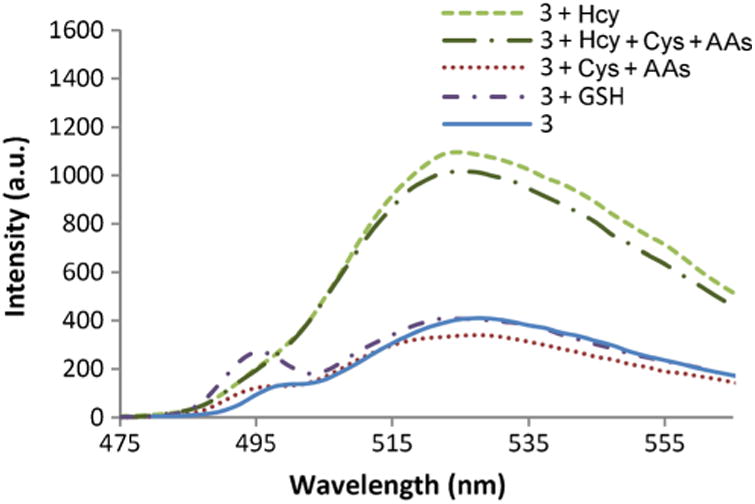
Fluorescence emission of 3 with Cys and Hcy in the presence and absence of amino acids (AAs: Gly, Ala, Met, Gln, Glu and Asp) and GSH after 2 h. Solutions are composed of 3 (4 μM) and each analyte (50 μM) in phosphate buffer (100 mM, pH 6.0):DMSO 99:1 at 20 °C, λex = 495 nm and λex 525 nm
A concentration dependent experiment was also performed, which indicates that upon increasing concentrations of Hcy, the fluorescence response of 3 increases linearly, and with a limit of detection (LOD) of 1.88 μM (Fig. 4) that is below physiological human plasma levels.
Fig. 4.
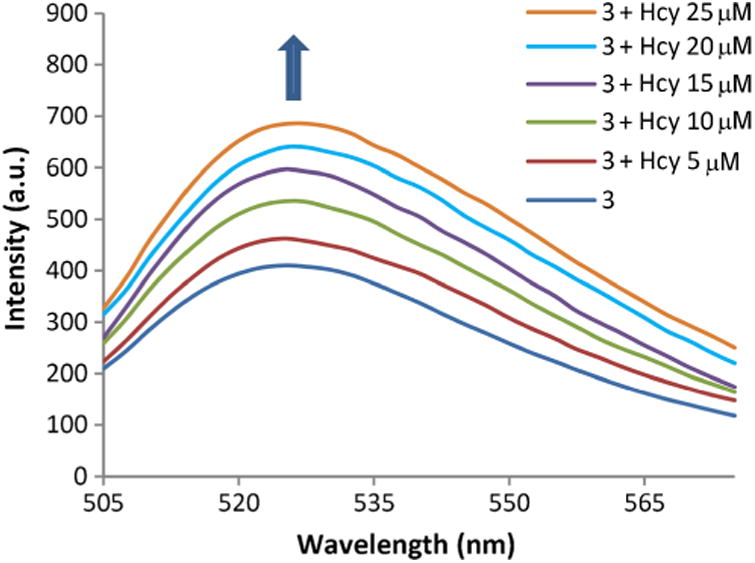
Spectral response of 3 towards increasing levels of Hcy in phosphate buffer (100 mM, pH 6.0). Solutions are composed of 3 (4 μM) with Hcy (0–25 μM) in phosphate buffer (100 mM, pH 6.0): DMSO 99:1 at 20 °C, λex = 495 nm
Discussion
As previously described, the fluorescence enhancement of solutions of 3 upon addition of Hcy is due to the formation of a thiazinane-4-carboxylic acid, and subsequent protonation of the heterocyclic amine affording PET inhibition. To confirm the mechanism and the origin of the Hcy selectivity, we studied the reaction of Hcy and Cys at pH 5.0, 6.0 and 9.5. Figure 5a shows that emission is enhanced at pH = 5 in the case of both Cys and Hcy additions to 3. This is due to the expected protonation of the amines of both the Hcy-derived thiazinane and the Cys- derived thiazolidine (Table 1).
Fig. 5.
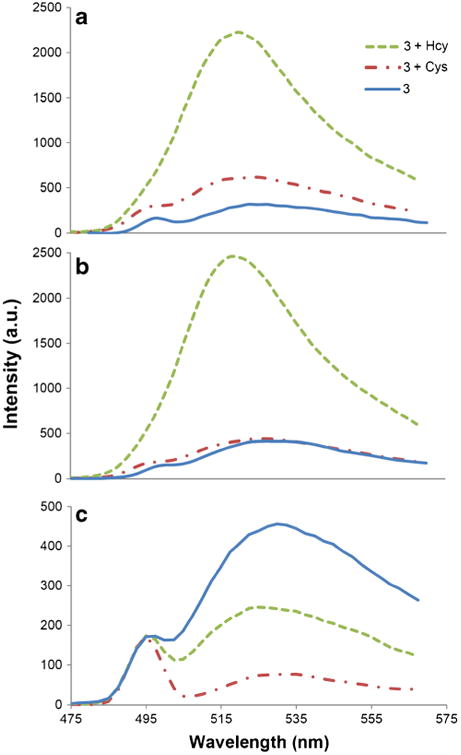
Fluorescence spectra of 3 due to Cys and Hcy in buffer after 2 h. (a) At pH 5.0 in phosphate buffer, (b) at pH 6.0 in phosphate buffer, (c) at pH 9.5 in carbonate buffer. Solutions are composed of 3 (4 μM) and analyte (1 mM) in 100 mM buffer solution: DMSO(99:1) at 20 °C, λex = 495 nm
Table 1. Signal transduction of 3 upon reaction with Cys and Hcy as a function of pH.
| ||||
|---|---|---|---|---|
| 5.0 | minor | major | minor | major |
| 6.0 | comparable amounts | minor | major | |
| 9.5 | major | minor | major | minor |
At pH = 6.0, the ammonium form of the Hcy-derived thiazinane is the predominant species (Fig. 5b, Table 1) and affords emission enhancement. In contrast, at pH = 6.0, in the case of the Cys-derived thiazolidine, the equilibrating states of the amine and ammonium species, are each present in relatively close amounts. This affords a very weak signal change upon Cys addition due to the respective competing PET -on and -off states. At higher pH (9.5), the free amine of both het-erocycles predominates, and thus fluorescence quenching is observed in solutions of either Cys or Hcy (Fig. 5c, Table 1).
Previously, Huang et al. calculated surface charges based on density functional theory (DFT) for an aldehyde-bearing iridium (III) complex. They found that the charges on the N and S atoms of the Cys-derived thiazolidine are more negatively polarized than the charges on the N and S of the Hcy- thiazinane. The higher negative charges on the thiazolidine were ascribed to an intramolecular electron-transfer process from the thiazolidine to the excited complex, causing a weak photoluminescence of the probe-Cys complex and larger signal from the probe-Hcy complex [24].
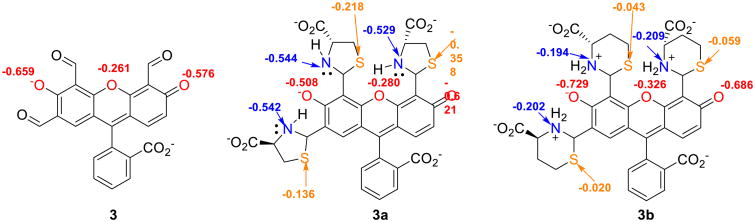
In compound 3 (Fig. 6), we also find that the Mulliken charges on the N and S atoms for Cys-derived thiazolidine are more negative (N, –0.542, –0.544, –0.529 and S,–0.136, –0.218,–0.358) as compared to the N and S atoms of Hcy-derived thiazinane (N, –0.202, –0.194, –0.209 and S, –0.020,–0.043, –0.059). This is in agreement with the results reported by Huang et al. for a PET mechanism intramolecular electron transfer process from the thiazolidine group resulting in fluorescence quenching or weak enhancement.
Fig. 6.
Predominant species of compounds 3, 3a and 3b at pH 6.0. Calculated Mulliken charges are shown for the oxygen atoms in the fused ring system
The enhanced sensitivity of probe 3 compared to previously reported 1 and 2 is attributed to the presence of three aldehyde groups rendering the fluorophore more electrophilic. Further, the selective signaling for Hcy is also consistent with an investigation of the ionic charges of the xanthene phenolate oxygens. From Mulliken charge calculations, it is observed that the phenolate anion (and the bridging ether oxygen) of the thiazinane (Hcy)-dye adduct bear higher negative charges (–0.729, – 0.686 and –0.326, respectively) compared to those of the Cys-derived thiazolidine (–0.508, –0.621 and –0.280, respectively) and 3 alone (–0.659, –0.576 and –0.261, respectively (Fig. 6). The development of enhanced negative charge density on the fluorophore phenolate oxygen upon formation of the Hcy-derived thiazinane results in an increase in fluorescence emission. Phenolate ion formation is well known to lead to emission enhancement in xanthene dyes.
Conclusion
In summary, a highly selective fluorescein tri-aldehyde for the detection of Hcy at its physiological levels in biological media has been developed. At pH = 6.0, protonation of the amine of the Hcy-derived thiazinane takes place in a selective manner due to its higher basicity compared to the amine of the Cys-derived thiazol-idine. The protonation results in inhibition of PET quenching and selective signaling for Hcy. The presence of three electron-withdrawing aldehyde groups makes the fluorophore highly reactive and enhances the Hcy detection limit. Mulliken charge calculations confirm the PET mechanism and also reveal that the Hcy-derived thiazinane renders the fluorophore phenoxide oxygen relatively electron rich as compared to the Cys-derived thiazolidine. This affords an enhanced emission signal upon addition of Hcy to 3.
Acknowledgments
This work was supported by the National Institutes of Health (grant R15EB016870).
References
- 1.Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med. 2005;5(2):77–86. doi: 10.1055/s-2005-872394. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157(Suppl 2):S40–S44. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- 3.Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1–2):554–561. doi: 10.1016/j.jchromb.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldyrev A. Molecular mechanisms of homocysteine toxicity and possible protection against hyperhomocysteinemia. Free Radic Biol Med. 2009;47:S137–S138. [Google Scholar]
- 5.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PWF, Wolf PA. Plasma homocyste-ine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann M, Widmann T, Herrmann W. Homocysteine–a newly recognised risk factor for osteoporosis. Clin Chem Lab Med. 2005;43(10):1111–1117. doi: 10.1515/CCLM.2005.194. [DOI] [PubMed] [Google Scholar]
- 8.Bernasconi AR, Liste A, Del Pino N, Rosa Diez GJ, Heguilen RM. Folic acid 5 or 15 mg/d similarly reduces plasma homocysteine in patients with moderate-advanced chronic renal failure. Nephrology (Carlton) 2006;11(2):137–141. doi: 10.1111/j.1440-1797.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Ozkan Y, Yardim-Akaydin S, Firat H, Caliskan-Can E, Ardic S, Simsek B. Usefulness of homocysteine as a cancer marker: total thiol compounds and folate levels in untreated lung cancer patients. Anticancer Res. 2007;27:1185–1189. [PubMed] [Google Scholar]
- 10.Mills JL, Scott JM, Kirke PN, McPartlin JM, Conley MR, Weir DG, Molloy AM, Lee YJ. Homocysteine and neural tube defects. J Nutr. 1996;126(3):S756–S760. doi: 10.1093/jn/126.suppl_3.756S. [DOI] [PubMed] [Google Scholar]
- 11.Ables GP, Ouattara A, Hampton TG, Cooke D, Perodin F, Augie I, Orentreich DS. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci Rep. 2015;5 doi: 10.1038/srep08886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Ham M, Jans JJ, Visser G, van Hasselt PM, Prinsen HC, Verhoeven-Duif NM. Suitability of methylmalonic acid and total homocysteine analysis in dried bloodspots. Anal Chim Acta. 2015;853:435–441. doi: 10.1016/j.aca.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Taysi S, Keles M, Gumustekin K, Akyuz M, Boyuk A, Cikman O, Bakan N. Plasma homocysteine and liver tissue S-adenosylmethionine, S-adenosylhomocysteine status in vitamin B6-deficient rats. Eur Rev Med Pharmacol Sci. 2015;19(1):154–160. [PubMed] [Google Scholar]
- 14.Rusin O, St Luce NN, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM. Visual detection of cysteine and homocysteine. J Am Chem Soc. 2004;126(2):438–439. doi: 10.1021/ja036297t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WH, Rusin O, Xu XY, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM. Detection of homocysteine and cysteine. J Am Chem Soc. 2005;127(45):15949–15958. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim S, Escobedo JO, Lowry M, Xu X, Strongin R. Selective fluorescence detection of cysteine and N-terminal cysteine peptide residues. Chem Commun. 2010;46(31):5707–5709. doi: 10.1039/c0cc01398f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobedo JO, Rusin O, Wang W, Alpturk O, Kim KK, Xu X, Strongin RM. Detection of biological thiols. In: Geddes CD, Lakowicz JR, editors. Reviews in fluorescence 2006. Vol. 3. Springer; US: 2006. pp. 139–162. [Google Scholar]
- 18.Jung HS, Chen X, Kim JS, Yoon J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem Soc Rev. 2013;42(14):6019–6031. doi: 10.1039/c3cs60024f. [DOI] [PubMed] [Google Scholar]
- 19.Peng H, Chen W, Cheng Y, Hakuna L, Strongin R, Wang B. Thiol reactive probes and chemosensors. Sensors. 2012;12(11):15907–15946. doi: 10.3390/s121115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Choi YP, Kim S, Yoon T, Guo Z, Lee S, Swamy KM, Kim G, Lee JY, Shin I, Yoon J. Selective homocysteine turn-on fluorescent probes and their bioimaging applications. Chem Commun. 2014;50(53):6967–6969. doi: 10.1039/c4cc00243a. [DOI] [PubMed] [Google Scholar]
- 21.Escobedo JO, Wang W, Strongin RM. Useof a commercially available reagent for the selective detection of homocysteine in plasma. Nat Protoc. 2006;1(6):2759–2762. doi: 10.1038/nprot.2006.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakuna L, Escobedo JO, Lowry M, Barve A, McCallum N, Strongin RM. A photochemical method for determining plasma homocysteine with limited sample processing. Chem Commun. 2014;50(23):3071–3073. doi: 10.1039/c4cc00432a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H, Wang K, Dai C, Williamson S, Wang B. Redox-based selective fluorometric detection of homocysteine. Chem Commun. 2014;50(89):13668–13671. doi: 10.1039/c4cc03677h. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Zhao Q, Wu Y, Li F, Yang H, Yi T, Huang C. Selective phosphorescence chemosensor for homocysteine based on an iridium (III) complex. Inorg Chem. 2007;46(26):11075–11081. doi: 10.1021/ic7010887. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Jiang XD, Shao X, Zhao J, Su Y, Xi D, Yu H, Yue S, L-j X, Zhao W. A turn-on NIR fluorescent probe for the detection of homocysteine over cysteine. RSC Adv. 2014;4(96):54080–54083. doi: 10.1039/c4ra08771b. [DOI] [Google Scholar]
- 26.Yu C, Zeng F, Luo M, Wu S. A silica nanoparticle-based sensor for selective fluorescent detection of homocysteine via interaction differences between thiols and particle-surface-bound polymers. Nanotechnology. 2012;23(30):305503. doi: 10.1088/0957-4484/23/30/305503. [DOI] [PubMed] [Google Scholar]
- 27.Barve A, Lowry M, Escobedo JO, Huynh KT, Hakuna L, Strongin RM. Differences in heterocycle basicity distinguish homo-cysteine from cysteine using aldehyde-bearing fluorophores. Chem Commun. 2014;50(60):8219–8222. doi: 10.1039/c4cc03527e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TK, Lee DN, Kim HJ. Highly selective fluorescent sensor for homocysteine and cysteine. Tetrahedron Lett. 2008;49(33):4879–4881. doi: 10.1016/j.tetlet.2008.06.003. [DOI] [Google Scholar]
- 29.Stewart JJP. Stewart Computational Chemistry, Version 14335 W MOPAC2012. [Google Scholar]
- 30.Maia JDC, et al. J Chem Theory Comput. 2012;8:3072–3081. doi: 10.1021/ct3004645. [DOI] [PubMed] [Google Scholar]