Abstract
Ligand-conjugated siRNAs have potential to achieve targeted delivery and efficient silencing in neurons following local administration in the central nervous system (CNS). We recently described the activity and safety profile of a docosahexaenoic acid (DHA)-conjugated, hydrophobic siRNA (DHA-hsiRNA) targeting Huntingtin (Htt) mRNA in mouse brain. Here, we report the synthesis of an amide-modified, phosphocholine-containing DHA-hsiRNA conjugate (PC-DHA-hsiRNA), which closely resembles the endogenously esterified biological structure of DHA. We hypothesized that this modification may enhance neuronal delivery in vivo. We demonstrate that PC-DHA-hsiRNA silences Htt in mouse primary cortical neurons and astrocytes. After intrastriatal delivery, Htt-targeting PC-DHA-hsiRNA induces ~80% mRNA silencing and 71% protein silencing after one week. However, PC-DHA-hsiRNA did not substantially outperform DHA-hsiRNA under the conditions tested. Moreover, at the highest locally administered dose (4 nmol, 50 µg), we observe evidence of PC-DHA-hsiRNA-mediated reactive astrogliosis. Lipophilic ligand conjugation enables siRNA delivery to neural tissues, but rational design of functional, non-toxic siRNA conjugates for CNS delivery remains challenging.
TOC Graphic
Introduction
The clinical success of therapeutic siRNA depends on efficient delivery to relevant tissues and uptake by the target cell types. Therapeutic siRNAs suffer from poor serum stability, rapid renal clearance, inadequate tissue retention due to inefficient cellular uptake, and nonproductive intracellular localization.2–3 Many diverse strategies have been investigated to improve tissue delivery following systemic (e.g. intravenous or subcutaneous) administration of therapeutic siRNA, including nanoparticle encapsulation, formulation with cationic carrier molecules, and direct chemical conjugation.4–11 The most clinically advanced technology employs a trivalent N-acetylgalactosamine (GalNAc)-siRNA conjugate12–14, in which a single injection was shown to induce several months of gene silencing in the liver15. Productive delivery to organs beyond the liver and kidney, particularly the brain, remains a challenge16–19. Typical lipid-mediated siRNA transfection strategies show acute toxicity when administered locally in the brain18, 20–21. Moreover, unmodified (retaining 2´-OH) or partially modified siRNAs require continuous, high dose local infusions to maintain gene silencing in neighboring tissues19, 22.
There are many genetically defined neurological disorders that would benefit greatly from a technology permitting non-toxic and targeted delivery to the brain. One synthetic strategy that holds promise for non-formulated siRNA delivery to the CNS is direct conjugation of biologically occurring ligands, including hydrophobic modifications such as cholesterol or free fatty acids23–25. Efficient delivery to neuronal cells has been described for polyunsaturated fatty acid-siRNA conjugates both in vitro and in vivo1, 26–27. We recently demonstrated that a docosahexaenoic acid (DHA) conjugate permits enhanced intracranial distribution and Huntingtin mRNA (Htt) silencing compared to an unconjugated siRNA following direct intrastriatal administration in wild-type FVB/NJ mice (Scheme 1a)1. DHA was conjugated to a chemically stabilized, hydrophobic siRNA scaffold (hsiRNA), which is necessary for stability in vivo (Hassler et al., manuscript in review). Notably, this compound did not elicit an innate immune response or induce neuronal toxicity over a broad range of concentrations, as cholesterol-conjugated hsiRNAs of the same chemical composition are strongly retained around the site of injection and induce local neuronal toxicity in FVB/NJ mice28. These results strongly suggest that chemical tuning of hydrophobic conjugates is a promising strategy for enhancing CNS distribution and improving their safety profile.
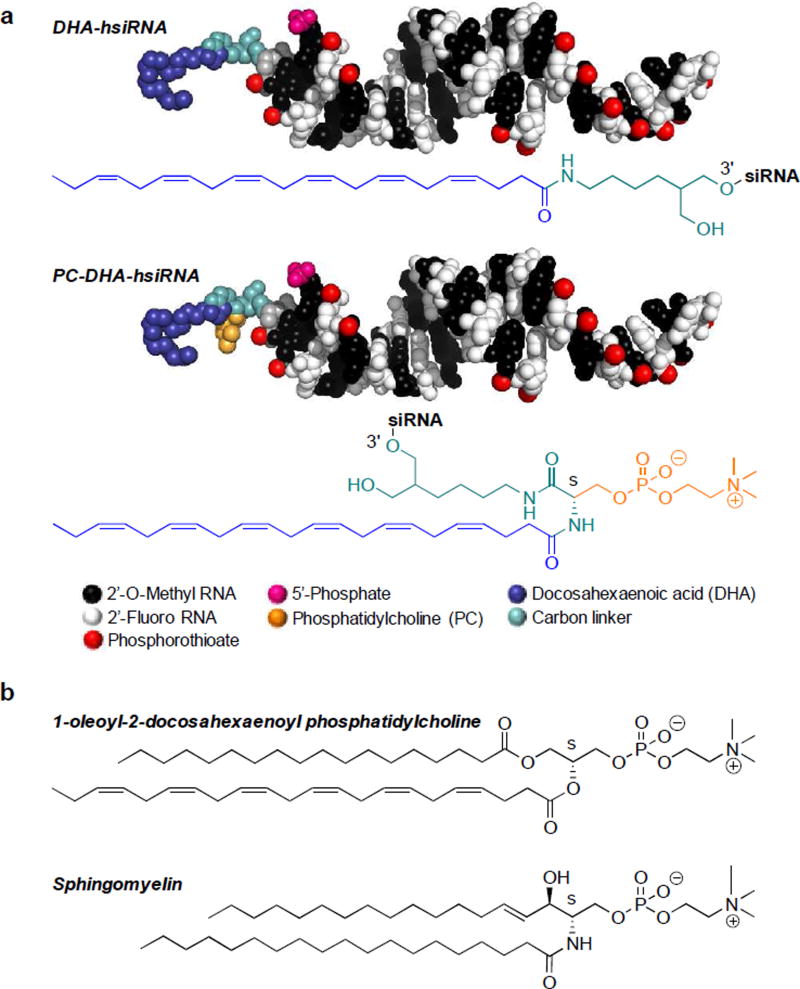
Scheme 1. Chemical structures of DHA-hsiRNA conjugates and derivatives.
(a) Fully chemically stabilized (alternating 2´-fluoro, 2´-O-methyl substituted), hydrophobically modified siRNA (hsiRNA) containing a 5´-phosphate on the antisense strand and a 3´-DHA or phosphocholine-DHA conjugate attached via a carbon linker on the sense strand. Molecular models of hsiRNA represented to scale using PyMOL. The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC. (b) Chemical structures of 1-oleoyl-2-docosahexaenoyl phosphatidylcholine (a naturally esterified form of DHA) and sphingomyelin (an important lipid component of nerve cell membranes).
DHA is highly enriched in the membrane phospholipids of brain and eye tissues, and is actively transported across the blood-brain barrier (BBB)29–30. The major endothelial receptor implicated in BBB trafficking is major facilitator superfamily domain containing 2a (MFSD2A)31. This receptor preferentially recognizes and transports DHA as a lysophosphatidylcholine (LPC) ester, which is synthesized from unesterified DHA in the liver (Scheme 1b)32. In human plasma, ~55% of DHA is present as the LPC ester32. Based on these considerations, we were interested in investigating whether the use of an esterified DHA bioconjugate (phosphocholine-DHA; PC-DHA) would improve siRNA retention and neuronal uptake throughout the brain following local or systemic administration33. Although lipid-based conjugates and nanoparticles are routinely used in siRNA formulation, direct phospholipid conjugation to oligonucleotides is rare and generally involves major alteration of the phospholipid head group34–36. In our original scheme for DHA-hsiRNA synthesis, we conjugated DHA through its terminal carboxyl group, rendering it inert to endogenous esterification. Here, we report the design, synthesis, in vitro, and in vivo activity of an O-phosphocholine-N-docosahexaenoyl-L-serine siRNA conjugate (PC-DHA-hsiRNA) that is structurally analogous to naturally occurring phosphocholines and sphingomyelins (Scheme 1a,b) and is orthogonally compatible with solid-phase RNA synthesis.
Results
Synthesis of PC-DHA-hsiRNA Bioconjugates
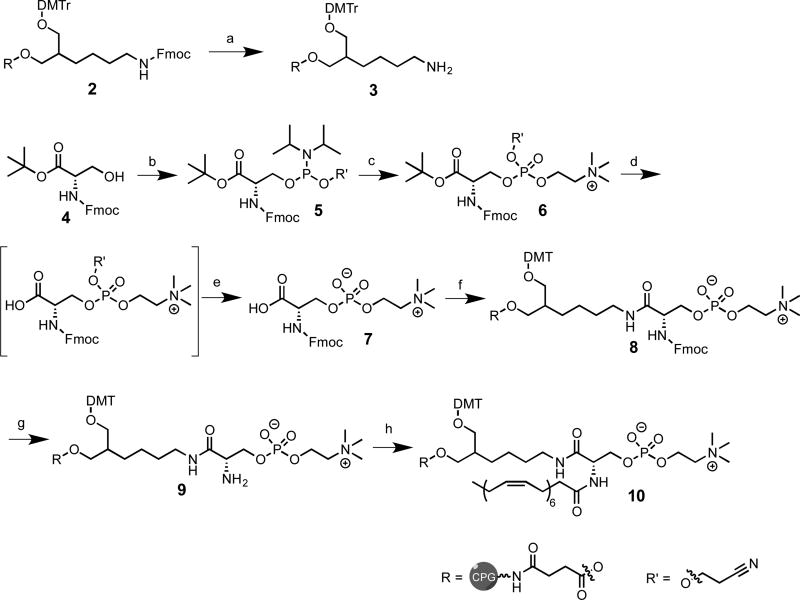
A synthetic route for the preparation of PC-DHA-hsiRNAs was developed utilizing mild conditions and simple precursors, including docosahexaenoic acid, a protected L-serine, a phosphocholine head group, and a commercially available bifunctional linker immobilized on CPG (see Supplementary Information, Fig. S1-S10, Scheme 2). This route involved reacting Nα-Fmoc-L-serine tert-butyl ester (4) with 2-cyanoethyl N,N-diisopropylchlorophosphoramidite to afford 5 as two diastereomers (S , Sp and S , Rp) in 95% yield. 5 was then reacted with choline tosylate37 in the presence of 5-ethylthio-1H-tetrazole (ETT) as activator and oxidized with meta-chloroperoxybenzoic acid (mCPBA) to afford 6 as a mixture of tetrazolium (~95%) and tosylate salt (~5%) in 69% yield. Following this, the ester group of 6 was removed by treatment with trifluoroacetic acid (TFA) in dichloromethane (DCM) and the cyanoethyl group was then eliminated by using 10% diisopropylamine in acetonitrile (ACN) to obtain 7 in 74% yield (over two steps). This last deprotection is advantageous from a synthetic perspective as it affords a phosphodiester (7), which is stable to base catalyzed β-elimination of phosphoserines38–39. In a parallel line, the Fmoc group on the 1-O-DMT-6-N-Fmoc-2-hydroxymethylhexane40 support (2) was removed using a solution of 20% piperidine in N,N-dimethylformamide (DMF) to give 3. Subsequently, 7 and 3 were coupled in the presence of (Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP), hydroxybenzotriazole (HOBt) and 2,4,6-collidine to yield 8. The Fmoc group on 8 was then removed using 20% piperidine in DMF. Lastly, the free amine on 9 was coupled to DHA in the presence of 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxidhexafluorophosphate (HATU) and diisopropylethylamine (DIPEA) to afford the final functionalized support 10. All sense strands were synthesized on this support following standard solid-phase synthesis protocols. Following synthesis, the oligonucleotides were cleaved and deprotected with 40% methylamine in water (45°C, 1 hour). Deprotected oligonucleotides were purified by high-performance liquid chromatography (HPLC) and characterized by liquid chromatography–mass spectrometry (LC-MS) (Figure S10).
Scheme 2. Synthetic route to PC-DHA-controlled pore glass (CPG).
Reagents and conditions: (a) 20% piperidine in DMF (2×15 min); (b) 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, DIEA, DCM, 2 h, rt, 95%; (c) choline tosylate, ETT, MeCN, 2 h, rt, followed by mCPBA, 10 min, rt, 69%; (d,e) TFA in dry DCM (1:1), triisopropylsilane, 2 h, rt, then 10% diisopropylamine in MeCN, 1.5h, rt 74%(f) 3, BOP, HOBt, DMF, 2,4,6-collidine, rt, 12 h; (g) 20% piperidine in DMF (2×15 min), rt; (h) DHA, HATU, DMF, rt, 12 h
PC-DHA-hsiRNA Exhibits Intracellular Uptake and Enhanced Huntingtin mRNA Silencing In Vitro
The hsiRNA duplexes used in this study (hsiRNAHtt) were designed to target both human and mouse huntingtin mRNA (Htt). Their sequences and chemical modification patterns are presented in Table S1. These oligonucleotides contain alternating 2´-fluoro and 2´-O-methyl ribose modifications to enhance stability41 and a partially phosphorothioated backbone to increase serum half-life and improve cellular uptake (Hassler et al., manuscript in revision).
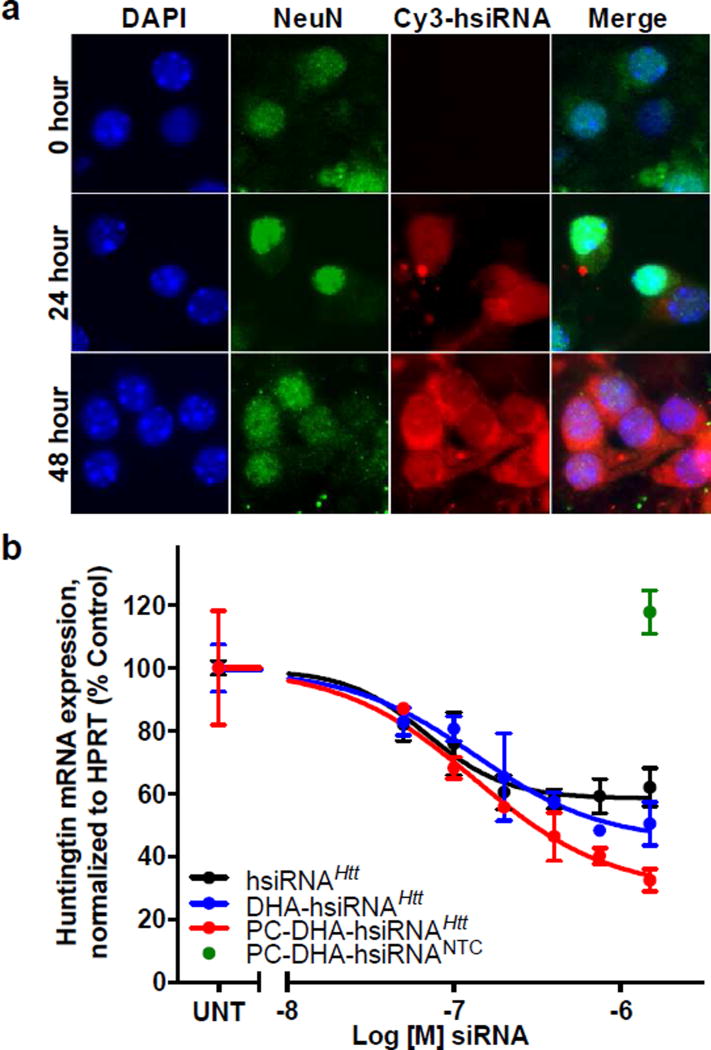
We first evaluated the ability of PC-DHA-hsiRNA to enter mouse primary cortical neurons (Figure 1a). Here, the duplex was labeled for imaging by addition of a Cy3 fluorophore to the 5´-end of the sense strand. Modifications on the 5´-end of the sense strand, unlike the antisense strand, do not interfere with RNA Induced Silencing Complex (RISC) loading42. Cy3-labeled PC-DHA-hsiRNAHtt (Figure 1a, red) was readily detectable in the cytoplasm of neuronal biomarker NeuN-positive neurons (Figure 1a, green) after 48 hours, demonstrating both cytoplasmic and membrane localization and few intracellular foci. Intracellular staining was most concentrated in the perinuclear space, where productive RISC loading and mRNA silencing is thought to occur43–44. We also observed a small fraction of nuclear-localized PC-DHA-hsiRNAs. Active nuclear RISC complexes have been reported previously45–46. The slow kinetics of delivery were analogous to the first generation of DHA-hsiRNA conjugates47. We directly compared the ability of PC-DHA-hsiRNAHtt and DHA-hsiRNAHtt to silence Huntingtin mRNA in purified primary mouse cortical neurons or astrocytes. Following passive delivery in neuronal culture (i.e. no transfection reagent), PC-DHA-hsiRNAHtt achieves a 68% reduction in Htt mRNA, while DHA-hsiRNAHtt silences by 55% (Figure 1b, 1.5 µM dose). The calculated IC50 values for both compounds are similar (135 nM and 133 nM, respectively). Furthermore, at the 1.5 µM dose, the PC-DHA-hsiRNA non-targeting control shows no effect on Htt expression (Figure 1b, green point). A non-conjugated hsiRNA control is capable of silencing Htt mRNA by ~40% in vitro (Figure 1b, black line); this residual activity has been reported from non-specific cellular internalization mediated by the single-stranded phosphorothioate tail, in a manner similar to that of conventional therapeutic antisense oligonucleotides28. Treatment of mouse primary cortical neurons with non-conjugated hsiRNA, DHA-hsiRNA, or PC-DHA-hsiRNA with doses from 0.05 – 3 µM has no significant effect on cell viability as measured by the Alamar Blue cytotoxicity assay (Figure S11).
Figure 1. Neuronal uptake and efficacy of PC-DHA-hsiRNA.
(a) Cellular internalization of Cy3-labeled PC-DHA-hsiRNA in mouse primary cortical neurons. Cells incubated for 0, 24, or 48 h with PC-DHA-hsiRNAHtt. Images acquired with Leica DMi8 microscope (63X). Nuclei (Hoechst), blue; PC-DHA-hsiRNA (Cy3), red; NeuN (AlexaFluor 488), green (b) Primary mouse cortical neurons were incubated with 0–1.5 µM Htt-targeting compounds at concentrations shown for one week. Htt mRNA levels were measured using QuantiGene® (Affymetrix), normalized to a housekeeping gene, Hprt (Hypoxanthine-guanine phosphoribosyl transferase), and presented as percent of untreated control (n=3, mean ± SD). UNT—untreated; NTC—non-targeting control.
PC-DHA-siRNA Shows Broad Intracranial Distribution and Parenchymal Retention Within the Injected Hemisphere
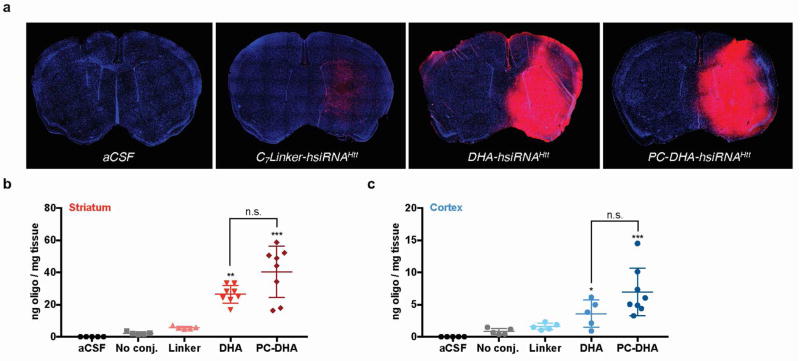
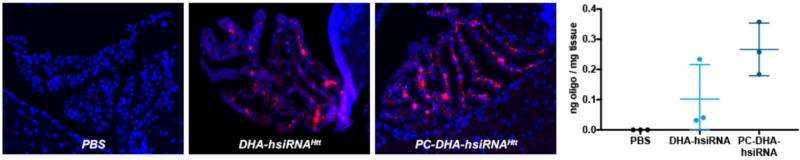
We first qualitatively examined the local intracranial distribution of Cy3-labeled PC-DHA-hsiRNA in wild-type (FVB/NJ) mouse brain by fluorescence microscopy. Animals (n = 2 per group) were administered 2 nmol (25 µg, 2 µL) of active Cy3-labeled DHA-hsiRNAHtt or PC-DHA-hsiRNAHtt, which were compared to an equimolar injection of a control construct lacking the phosphocholine and DHA moieties (C7linker-hsiRNAHtt). After 48 hours, animals were perfused with phosphate-buffered saline and fixed with formalin. Brains were processed into 4 µm coronal sections, stained with DAPI to visualize nuclei, and imaged on a Leica DMi8 inverted microscope (Figure 2a). At this dose, both DHA-hsiRNAHtt and PC-DHA-hsiRNAHtt exhibited diffuse staining throughout the ipsilateral (injected) striatum and cortex, while the C7linker-hsiRNA control was barely detectable. Next, we directly measured the striatal and cortical accumulation of each compound using a quantitative peptide-nucleic acid (PNA) hybridization assay (n = 5 per group) (Figure 2b,c). In these studies, hsiRNAs were not labeled with Cy3, to remove any contribution of the fluorophore to distribution and cellular uptake. Animals were injected with 2 nmol (25 µg, 2 µL) of non-Cy3 labeled unconjugated hsiRNA, C7linker-hsiRNA, DHA-hsiRNA, or PC-DHA-hsiRNA. After 48 hours, animals were perfused with phosphate-buffered saline and punch biopsies (2 mm) were lysed, hybridized to a fully complementary PNA probe, and analyzed by HPLC as described previously1. At the 2 nmol (25 µg) dose, we observe that PC-DHA-hsiRNA (40.4 ± 16.0 ng/mg tissue) and DHA-hsiRNA (26.5 ±5.4 ng/mg tissue) are both highly retained in the striatum, compared to the unconjugated and C7linker-conjugated hsiRNA controls (2.2 ±0.9 and 5.6 ±0.9 ng/mg tissue, respectively) (Figure 2b). At this dose and time point, we also observe significant cortical retention of both PC-DHA-hsiRNA (6.7 ±3.7 ng/mg tissue) and DHA-hsiRNA (3.6 ±2.1 ng/mg tissue), compared to the unconjugated and C7linker-conjugated hsiRNA controls (0.8 ±0.5 and 1.6 ±0.5 ng/mg tissue, respectively) (Figure 2c). Statistical significance was calculated using a Kruskal-Wallis one-way ANOVA with Dunn’s post hoc analysis.
Figure 2. PC-DHA-hsiRNA shows enhanced distribution and retention in mouse striatum and cortex.
(a) aCSF, Cy3-C7linker-hsiRNA, Cy3-DHA-hsiRNA, and Cy3-PC-DHA-hsiRNA were administered by unilateral intrastriatal injection (2 nmol, 25 µg). After 48 hours, fluorescent images were acquired with a Leica DMi8 tiling microscope using equivalent exposure and acquisition settings (10X). aCSF and C7linker-hsiRNAHtt images were publisher previously.1 (b) Tissue concentrations of animals injected with 2 nmol of non-Cy3-labeled unconjugated hsiRNA, C7linker-hsiRNA, DHA-hsiRNA, and PC-DHA-hsiRNA (n=8) measured by RNA hybridization from the ipsilateral (injected) striatum (b) or cortex (c). (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Kruskal-Wallis one-way ANOVA with Dunn's post hoc analysis). aCSF – artificial cerebrospinal fluid (vehicle); n.s. - non-significant.
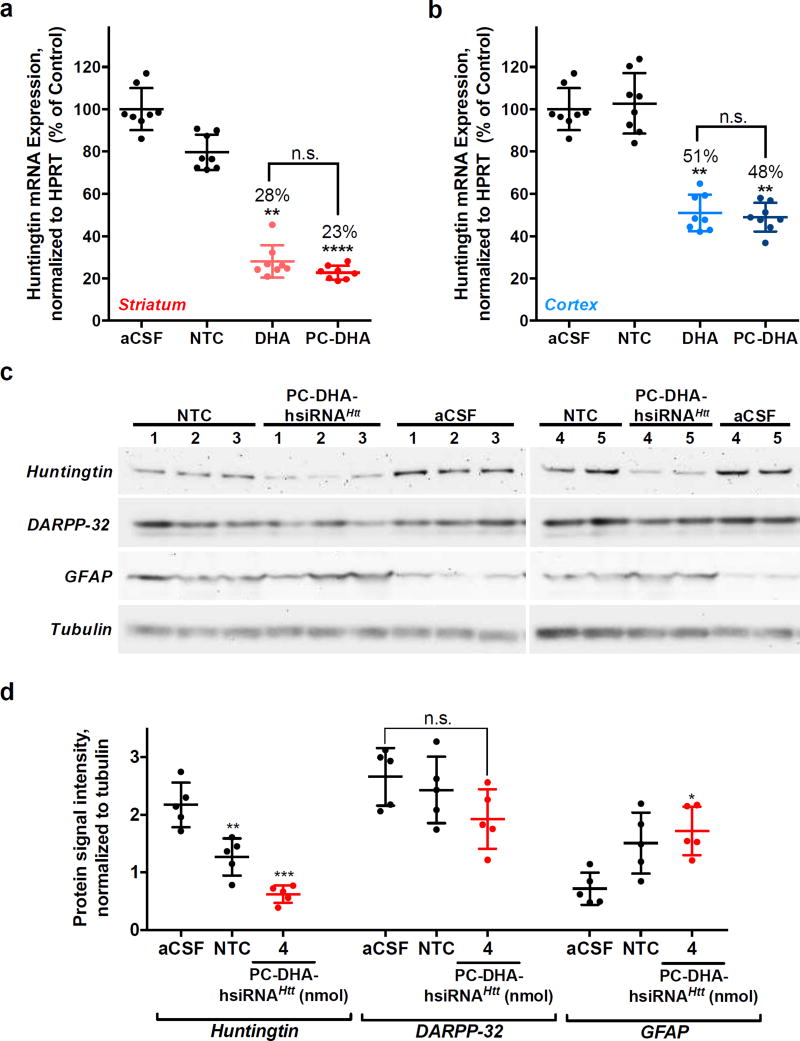
Striatal Huntingtin mRNA and Protein Silencing by PC-DHA-hsiRNAHtt
We investigated whether addition of the phosphocholine head group to the DHA-hsiRNA scaffold was capable of sustaining or increasing gene-silencing activity in vivo following local administration in mouse brain. Wild-type mice (FVB/NJ, n = 8 per group) were injected into the right striatum with aCSF, a non-targeting control hsiRNA (PC-DHA-hsiRNANTC, 4 nmol, 50 µg), DHA-hsiRNAHtt (2 nmol, 25 µg), and PC-DHA-hsiRNAHtt (2 nmol, 25 µg). After one week, the levels of Htt mRNA expression in the ipsilateral striatum and cortex were measured by the QuantiGene® assay, normalized to a housekeeping gene (Hprt1), and presented as percentage of aCSF-treated control48. Huntingtin mRNA levels are reduced by 72% and 77% in the striatum after administration of 2 nmol of DHA-hsiRNA or PC-DHA-hsiRNAHtt, respectively (Figure 3a). We assessed protein levels at the 4 nmol dose by Western blot and detected 71% Huntingtin protein silencing in the striatum (Figure 3c,d). In the cortex, DHA-hsiRNAHtt and PC-DHA-hsiRNAHtt silenced Htt mRNA by 49% and 52% after treatment with 2 nmol, respectively (Figure 3b). A statistical comparison of DHA-hsiRNA and PC-DHA-hsiRNA at this dose was performed using a Kruskal-Wallis one-way ANOVA with Dunn’s post-hoc analysis. Although significant levels of mRNA silencing were achieved in both striatum and cortex, there was no discernable difference between DHA-hsiRNA conjugates with or without the phosphocholine head group (Figure 3a,b).
Figure 3. Huntingtin mRNA and protein are efficiently silenced by PC-DHA-hsiRNAHtt.
aCSF, PC-DHA-hsiRNANTC (4 nmol, 50 µg), DHA-hsiRHAHtt, and PC-DHA-hsiRNAHtt (2 nmol, 25 µg) were unilaterally injected into the striatum of FVB/NJ mice (n = 8 per group). Punch biopsies (5 mg) of the striatum (a) and cortex (b) were collected after one week. Level of Htt mRNA was measured using QuantiGene® (Affymetrix), normalized to a housekeeping gene (Hprt), and presented as percentage of untreated control (mean ± SD). DHA-hsiRNAHtt efficacy was previously reported.1 (c,d) Protein expression levels of Huntingtin, Dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP32), and Glial fibrillary acidic protein (GFAP) levels in the ipsilateral striatum of wild-type (FVB/NJ) mice after a one-week treatment with PC-DHA-hsiRNANTC or PC-DHA-hsiRNAHtt (4 nmol, 25 µg). (n=5, mean ± SD). NTC—non-targeting control; aCSF—artificial cerebrospinal fluid; n.s. – nonsignificant (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s post hoc analysis).
We observed a 21% reduction of Htt mRNA in animals receiving a non-targeting control (PC-DHA-hsiRNANTC) around the site of injection (Figure 3a). This resulted in 42% nonspecific silencing of Huntingtin protein expression after normalization to tubulin (compared to an untreated control, Figure 3d, left). To ascertain the cause of this aberrant activity, we examined the expression of dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) levels in treated tissues. DARPP-32 expression is routinely used to monitor dopamine receptor integrity and cell viability in medium spiny neurons49. We noted that DARPP-32 staining was not significantly reduced in animals treated with the highest dose (4 nmol) of PC-DHA-hsiRNAHtt (Figure 3d, middle panel). This result suggested that PC-DHA-hsiRNA is not inducing acute neuronal toxicity after local administration. We also monitored changes in glial fibrillary acidic protein (GFAP) expression. GFAP upregulation is a hallmark of reactive astrogliosis, an inflammatory response to mechanical or chemical brain trauma50. We observed a ~two-fold increase in GFAP expression in animals receiving PC-DHA-hsiRNANTC and PC-DHA-hsiRNAHtt compared to an aCSF-injected control (Figure 3d, right). These data suggested that astrocyte activation was sequence-independent and therefore mediated by the PC-DHA-hsiRNA bioconjugate or oligonucleotide chemical modifications.
Transvascular brain delivery of DHA-hsiRNAHtt and PC-DHA-hsiRNAHtt
The original motivation behind the design of the PC-DHA bioconjugate was to enable efficient BBB transfer. To determine if DHA-hsiRNA or PC-DHA-hsiRNA could penetrate the blood-brain or blood-cerebrospinal fluid (CSF) barriers after intravenous delivery, we injected wild-type animals (FVB/NJ, n = 2) with a two-day regimen of PBS, DHA-hsiRNAHtt or PCDHA- hsiRNAHtt (20 mg/kg per injection). Animals were perfused and brains were sectioned and DAPI-stained as described previously. After systemic injection, we observed low, but detectable levels of Cy3 fluorescence in the brains of both DHA-hsiRNA- and PC-DHA-hsiRNA-treated animals. This fluorescence was limited to the epithelial cells of the choroid plexus, the principal source of CSF production (Figure 4). Oligonucleotide accumulation in the choroid plexus is unsurprising, as its fenestrated endothelium is highly permeable to ions, small molecules, and immune cells51. We did not observe significant accumulation in other brain regions, minimizing the applicability of this delivery method to the treatment of Huntington’s disease, which primarily affects the striatum and cortex28. We quantified oligonucleotide accumulation in wild-type animals at the same dose (FVB/NJ, n = 3) by PNA hybridization, and noted that both DHA-hsiRNA and PC-DHA-hsiRNA are retained in the choroid plexus (DHA-hsiRNA: 0.1 ng/mg; PC-DHA-hsiRNA: 0.3 ng/mg). Under these conditions, the levels of DHA-hsiRNA and PC-DHA-hsiRNA accumulation are not high enough to support gene silencing (data not shown). However, a higher bolus intravenous dosing regimen may improve BBB penetration.
Figure 4. DHA-hsiRNA and PC-DHA-hsiRNAHtt accumulation in mouse brain choroid plexus after systemic administration.
(left panel) PBS (vehicle), Cy3-DHA-hsiRNAHtt, and Cy3-PC-DHA-hsiRNAHtt (two daily 20 mg/kg) were administered by intravenous injection in FVB/NJ mice (n=3 per group). After 48 hours, fluorescent images were acquired with a Leica DMi8 tiling microscope (63X). (right panel) Whole brain lysate measurement of Htt antisense strand accumulation following intravenous administration PBS, DHA-hsiRNAHtt, and PC-DHA-hsiRNAHtt (two daily 20 mg/kg) via PNA hybridization. PBS—phosphate-buffered saline.
Conclusion
Gene silencing by RNA interference (RNAi) is a highly promising approach for the pharmaceutical treatment of genetically defined neurological disorders, including Huntington’s disease. Many non-viral strategies have been attempted to efficiently deliver siRNAs into the CNS, including liposome formulation and conjugation of cell-penetrating peptides, with limited success16, 52. Previously, we validated the efficacy and safety of Htt-targeting DHA-hsiRNA for CNS delivery. We reported efficient Htt silencing in the ipsilateral striatum and cortex with no neural cell death or measureable innate immune activation at concentrations over 20-fold above the efficacious dose1. Here, we have investigated the utility of further modifying DHA-hsiRNA to mimic the structure of lysophosphatidylcholine DHA ester, a molecule which is actively transported into neurons. We report that PC-DHA-hsiRNA shows similar intracranial distribution and tissue retention compared to the previous generation of DHA-hsiRNA conjugates47. Both PC-DHA-hsiRNA and DHA-hsiRNA are significantly more hydrophobic than an unconjugated hsiRNA, as evidenced from their higher retention time on a reverse-phase column (Figure S12). This property may enable the dramatic increase in retention of these compounds compared to the unconjugated control1.
PC-DHA-hsiRNA induced Htt mRNA and protein silencing in the striatum (~80% mRNA and ~71% protein silencing following a single, 50 µg intrastriatal injection). This degree of Htt reduction is on par with current antisense oligonucleotides under clinical investigation53 (ClinicalTrials.gov, NCT02519036). However, this silencing activity occurs with a disturbing increase in neuronal toxicity and astrogliosis. The precise mechanism behind these adverse effects is unknown. There is a precedent for phosphorothioate and 2´-fluoro-mediated toxicity in heavily modified oligonucleotides54–56. We do not exclude the possibility that the phosphocholine itself is stimulating an immunogenic response57.
We observed that PC-DHA-hsiRNA is capable of low, but measurable transfer across the blood-CSF barrier into the choroid plexus. Although oligonucleotide accumulation in this tissue is not optimal for the treatment of Huntington’s disease, there are several potential disorders which may benefit from an RNAi-based therapeutic which enters the choroid epithelium, including leptomeningeal amyloidosis. An antisense oligonucleotide that suppresses choroid transthyretin (TTR) production in a leptomeningeal amyloidosis mouse model has been previously described58. For these applications, delivery of PC-DHA-hsiRNA to the choroid plexus following intravenous injection could be further improved by the use of a large, bolus dose, and supported by a multi-day dosing regimen.
After a direct intrastriatal injection, we observed no diffusion or Htt silencing within the contralateral (non-injected) striatum or cortex (Figures 2a, S13). Different routes of administration can be explored to promote more diffuse distribution throughout the CSF, including intrathecal (lumbar spinal cord) and intracerebroventricular (lateral ventricle) injections. These different routes of injection may help to reduce the local toxicity observed following a direct parenchymal injection.
The synthetic methodology described herein can be used for the preparation of a broad range of biologically active siRNA conjugates containing at least one saturated or unsaturated fatty acid and a polar or neutral head group. Current efforts are focused on expanding this chemistry to other classes of fatty acids and on studying their distribution and efficacy following both CNS and systemic administration.
Materials and Methods
Oligonucleotide synthesis and purification
All oligonucleotide synthetic schemes and corresponding spectra are presented in the Supporting Information.
Preparation and treatment of primary cortical neurons and astrocytes
Primary cortical neurons were isolated and cultured from FVN/NJ embryos (E15.5) as described previously1. Briefly, the cortices from both embryonic brain hemispheres were dissected, and a solution of papain and DNAse I (Worthington, Lakewood, NJ) in Hibernate E medium (Brainbits, Springfield, IL) was used to disrupt the extracellular matrix prior to trituration to a single-cell suspension using a fire-polished glass pipette. For mRNA silencing experiments, cells were plated at 1×105 cells/well in a poly-L-lysine coated 96-well plate and treated with oligonucleotides on day 3 after plating. For cellular uptake experiments, neurons were plated at 2×105 cells per poly-L-lysine coated, 35 mm glass-bottom dish. For imaging, neurons were maintained in phenol red-free NbActiv4 medium (BrainBits). Prior to imaging, cells were stained according to the manufacturer’s protocol with NucBlue (Life Technologies), washed three times with PBS, and fixed with 4% paraformaldehyde.
Fluorescence imaging
All fluorescent images were acquired with a Leica DMi8 inverted tiling microscope (Leica Microsystems). Images were processed using the Leica software suite (LAS X and LAS X Lite).
mRNA quantification in cells and tissue punches
mRNA was quantified from both cells and tissue punches using the QuantiGene® 2.0 DNA Assay (Affymetrix) exactly as described previously1, 48. Probe sets (Mouse Htt or Hprt) were diluted and used according to the manufacturer’s recommended protocol. Briefly, tissue punches (5 mg) were homogenized in 300 µl of Homogenizing Buffer (Affymetrix) containing 2 µg/µl proteinase K in 96-well plate format on a QIAGEN TissueLyser II, and 40 µl of each lysate was added to a bDNA capture plate. Htt or Hprt probe sets (60 µL) were added to each well of the capture plate for a final volume of 100 µL. Signal was amplified according to the Affymetrix protocol. Luminescence was detected on a Tecan M1000 luminometer.
PNA hybridization assay
Levels of hsiRNA guide (antisense) strand accumulation in mouse brain were quantified using a PNA hybridization assay, as described previously1. Briefly, tissue punches were homogenized in MasterPure Tissue Lysis Solution (EpiCentre) with added proteinase K (2 mg/mL, Invitrogen) and homogenized using a TissueLyser II (Qiagen), using 100 µL of lysis solution per 10 mg tissue. Following lysis, sodium dodecyl sulfate was precipitated with KCl (3 mol/l) and cleared supernatant was hybridized to a Cy3-labeled PNA oligonucleotide fully complementary to the guide strand (PNABio). This mixture was analyzed by high-performance liquid chromatography. Cy3-labeled peaks were integrated and plotted on an internal calibration curve. Mobile phase for HPLC was 50% water, 50% acetonitrile, 25 mmol/l Tris-HCl (pH 8.5) and 1 mmol/l ethylenediamine-tetraacetate. The salt gradient was 0–800 mmol/l NaClO4.
Stereotaxic injections and tissue collection
Wild-type (FVB/NJ) mice (6–8 weeks old) were deeply anesthetized with 1.2% Avertin (Sigma) and microinjected by stereotactic placement into the right striatum (coordinates relative to bregma: 1.0 mm anterior, 2.0 mm lateral, and 3.0 mm ventral). For efficacy studies, animals were euthanized after seven days, 300 µm coronal sections were collected using a cryotome, and a 2 mm punch biopsy was taken from both injected and non-injected hemispheres. Each punch was placed in RNA later (Ambion) for 24 hours at 4°C. Punches were processed for the QuantiGene® 2.0 DNA Assay as described previously1. For biodistribution studies, animals were injected as described, euthanized after 48 hours, perfused with PBS and 10% formalin. Brains were postfixed for 48 hours, paraffin-embedded, and sliced into 4 µm sections. Each brain section was mounted on a glass slide and stained with DAPI to visualize nuclei prior to image collection on a Leica DMi8 inverted microscope. All animal procedures were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee (IACUC, protocol number A-2411).
Western blot
Protein levels were quantified from tissue punches collected and snap-frozen seven days after intrastriatal injection, as described previously28. Briefly, cell lysates (25 µg) were separated by SDS-PAGE using 3–8% Tris-acetate gels (Life Technologies) and transferred to nitrocellulose using a TransBlot Turbo apparatus (BioRad). Blots were blocked in 5% nonfat dry milk (BioRad) diluted in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 hour at room temperature and then incubated with primary anti-huntingtin Ab1, anti-DARPP-32, or anti-GFAP antibodies overnight at 4°C with agitation. Blots were washed with TBST and incubated with peroxidase-labeled antirabbit IgG (Jackson ImmunoResearch) for 1 hour at room temperature. Protein signal was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and Hyperfilm ECL (GE Healthcare). Blots were reprobed with anti-β tubulin antibody (Sigma) as a loading control. After films were scanned (Epson Perfection V750), bands were measured and densitometry was performed using ImageJ software (v. 1.47). Huntingtin, DARPP-32, and GFAP signals were normalized to the tubulin signal for each set of samples (n = 5).
Statistical analysis
Data were analyzed using GraphPad Prism 6 software. Concentration-dependent IC50 curves for primary neuron experiments were fitted using a log(inhibitor) versus response—variable slope (four parameter). In vivo data were analyzed using a Kruskal-Wallis one-way ANOVA with Dunn’s post-hoc analysis
Supplementary Material
Acknowledgments
This project was funded by the CHDI Foundation (research agreement A-6119 and JSC A6367), RO1GM10880302, RO1NS038194, UH3TR00088803, and F32NS095508 (to M.F.O). We thank Michael Moazami for technical assistance and proofreading. We thank the members of the Khvorova Laboratory for helpful discussions and editorial feedback.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website. Oligonucleotide synthesis experimental details and spectra, supporting figures and methods.
Author contributions
M.N. conceived of the PC-modified DHA concept, designed and executed the multistep synthesis, and assisted in manuscript preparation, A.B. optimized the protection group synthetic strategy, characterized all intermediates and final products, and prepared the Supporting Information, A.H.C. and B.G. performed all in vivo efficacy and distribution experiments, R.A.H. quantified the tissue accumulation of each compound, E.S. evaluated protein silencing, M.N., D.E. and A.B. synthesized oligonucleotide variants, M.D., N.A., and A.K. supervised experimental design and assisted with manuscript editing, and M.F.O. performed all in vitro experiments, assisted with the in vivo distribution experiments, critically analyzed the data, prepared figures, and wrote the final manuscript.
A.K. owns stock at RXi Pharmaceuticals and Advirna LLC, which holds a patent and license on asymmetric, hydrophobically modified siRNAs. Other authors do not have any competing financial interest to disclose.
References
- 1.Nikan M, Osborn MF, Coles AH, Godinho BM, Hall LM, Haraszti RA, Hassler MR, Echeverria D, Aronin N, Khvorova A. Docosahexaenoic Acid Conjugation Enhances Distribution and Safety of siRNA upon Local Administration in Mouse Brain. Mol Ther Nucleic Acids. 2016;5:344. doi: 10.1038/mtna.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet. 2015;16:543–52. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deleavey GF, Damha MJ. Designing chemically modified oligonucleotides for targeted gene silencing. Chem Biol. 2012;19:937–54. doi: 10.1016/j.chembiol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 5.Juliano RL, Ming X, Carver K, Laing B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic Acid Ther. 2014;24:101–13. doi: 10.1089/nat.2013.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ming X, Laing B. Bioconjugates for targeted delivery of therapeutic oligonucleotides. Adv Drug Deliv Rev. 2015;87:81–9. doi: 10.1016/j.addr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JH, Mok H, Oh YK, Park TG. siRNA conjugate delivery systems. Bioconjug Chem. 2009;20:5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Shum KT, Burnett JC, Rossi JJ. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals (Basel) 2013;6:85–107. doi: 10.3390/ph6010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stoter M, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–46. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 10.Gooding M, Browne LP, Quinteiro FM, Selwood DL. siRNA delivery: from lipids to cell-penetrating peptides and their mimics. Chem Biol Drug Des. 2012;80:787–809. doi: 10.1111/cbdd.12052. [DOI] [PubMed] [Google Scholar]
- 11.Stanton MG, Colletti SL. Medicinal chemistry of siRNA delivery. J Med Chem. 2010;53:7887–901. doi: 10.1021/jm1003914. [DOI] [PubMed] [Google Scholar]
- 12.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel'in AV, Milstein S, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–61. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 13.Rajeev KG, Nair JK, Jayaraman M, Charisse K, Taneja N, O'Shea J, Willoughby JL, Yucius K, Nguyen T, Shulga-Morskaya S, et al. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. Chembiochem. 2015;16:903–8. doi: 10.1002/cbic.201500023. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda S, Keiser K, Nair JK, Charisse K, Manoharan RM, Kretschmer P, Peng CG, A VKI, Kandasamy P, Willoughby JL, Liebow A, et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem Biol. 2015;10:1181–7. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med. 2016 doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathupala SP. Delivery of small-interfering RNA (siRNA) to the brain. Expert Opin Ther Pat. 2009;19:137–40. doi: 10.1517/13543770802680195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zukiel R, Nowak S, Wyszko E, Rolle K, Gawronska I, Barciszewska MZ, Barciszewski J. Suppression of human brain tumor with interference RNA specific for tenascin-C. Cancer Biol Ther. 2006;5:1002–7. doi: 10.4161/cbt.5.8.2886. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–77. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 19.Grondin R, Ge P, Chen Q, Sutherland JE, Zhang Z, Gash DM, Stiles DK, Stewart GR, Sah DW, Kaemmerer WF. Onset Time and Durability of Huntingtin Suppression in Rhesus Putamen After Direct Infusion of Antihuntingtin siRNA. Mol Ther Nucleic Acids. 2015;4:e245. doi: 10.1038/mtna.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm D. The dose can make the poison: lessons learned from adverse in vivo toxicities caused by RNAi overexpression. Silence. 2011;2:8. doi: 10.1186/1758-907X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krichevsky AM, Kosik KS. RNAi functions in cultured mammalian neurons. Proc Natl Acad Sci U S A. 2002;99:11926–9. doi: 10.1073/pnas.182272699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A. 2007;104:17204–9. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Kwong B, Irvine DJ. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew Chem Int Ed Engl. 2011;50:7052–5. doi: 10.1002/anie.201101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo T, Yanagihara K, Takei Y, Mihara K, Morita Y, Seyama T. Palmitic acid-conjugated 21-nucleotide siRNA enhances gene-silencing activity. Mol Pharm. 2011;8:2193–203. doi: 10.1021/mp200250f. [DOI] [PubMed] [Google Scholar]
- 26.Willibald J, Harder J, Sparrer K, Conzelmann KK, Carell T. Click-modified anandamide siRNA enables delivery and gene silencing in neuronal and immune cells. J Am Chem Soc. 2012;134:12330–3. doi: 10.1021/ja303251f. [DOI] [PubMed] [Google Scholar]
- 27.Felber AE, Bayo-Puxan N, Deleavey GF, Castagner B, Damha MJ, Leroux JC. The interactions of amphiphilic antisense oligonucleotides with serum proteins and their effects on in vitro silencing activity. Biomaterials. 2012;33:5955–65. doi: 10.1016/j.biomaterials.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Alterman JF, Hall LM, Coles AH, Hassler MR, Didiot MC, Chase K, Abraham J, Sottosanti E, Johnson E, Sapp E, et al. Hydrophobically Modified siRNAs Silence Huntingtin mRNA in Primary Neurons and Mouse Brain. Mol Ther Nucleic Acids. 2015;4:e266. doi: 10.1038/mtna.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 30.Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trepanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–6. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 32.Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci. 2001;16:201–4. doi: 10.1385/JMN:16:2-3:201. discussion 215–21. [DOI] [PubMed] [Google Scholar]
- 33.Wang JZ, Xiao N, Zhang YZ, Zhao CX, Guo XH, Lu LM. Mfsd2a-based pharmacological strategies for drug delivery across the blood-brain barrier. Pharmacol Res. 2016;104:124–31. doi: 10.1016/j.phrs.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Chillemi R, Greco V, Nicoletti VG, Sciuto S. Oligonucleotides conjugated to natural lipids: synthesis of phosphatidyl-anchored antisense oligonucleotides. Bioconjug Chem. 2013;24:648–57. doi: 10.1021/bc300602g. [DOI] [PubMed] [Google Scholar]
- 35.Musacchio T, Vaze O, D'Souza G, Torchilin VP. Effective stabilization and delivery of siRNA: reversible siRNA-phospholipid conjugate in nanosized mixed polymeric micelles. Bioconjug Chem. 2010;21:1530–6. doi: 10.1021/bc100199c. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Li Y, Mozhi A, Zhang L, Liu Y, Xu X, Xing J, Liang X, Ma G, Yang J, et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials. 2014;35:6519–33. doi: 10.1016/j.biomaterials.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 37.Wichmann O, Schultz C. FRET probes to monitor phospholipase A2 activity. Chem Commun (Camb) 2001:2500–1. doi: 10.1039/b107670c. [DOI] [PubMed] [Google Scholar]
- 38.Wakamiaya T, Saruta K, Yasuoka J, Kusumoto S. an efficient procedure for solid-phase synthesis of phosphopeptides by the Fmoc strategy. Chem. Lett. 1994:1099–1102. [Google Scholar]
- 39.Albers MF, Hedberg C. Amino acid building blocks for Fmoc solid-phase synthesis of peptides phosphocholinated at serine, threonine, and tyrosine. J Org Chem. 2013;78:2715–9. doi: 10.1021/jo302587g. [DOI] [PubMed] [Google Scholar]
- 40.Nelson PS, Kent M, Muthini S. Oligonucleotide labeling methods. 3. Direct labeling of oligonucleotides employing a novel, non-nucleosidic, 2-aminobutyl-1,3-propanediol backbone. Nucleic Acids Res. 1992;20:6253–9. doi: 10.1093/nar/20.23.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, Wanders L, Griffey RH, Swayze EE, Bhat B. Fully 2'-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48:901–4. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 42.Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T. Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev. 2003;13:83–105. doi: 10.1089/108729003321629638. [DOI] [PubMed] [Google Scholar]
- 43.Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11:1165–75. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Berezhna SY, Supekova L, Supek F, Schultz PG, Deniz AA. siRNA in human cells selectively localizes to target RNA sites. Proc Natl Acad Sci U S A. 2006;103:7682–7. doi: 10.1073/pnas.0600148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalantari R, Chiang CM, Corey DR. Regulation of mammalian transcription and splicing by Nuclear RNAi. Nucleic Acids Res. 2016;44:524–37. doi: 10.1093/nar/gkv1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalantari R, Hicks JA, Li L, Gagnon KT, Sridhara V, Lemoff A, Mirzaei H, Corey DR. Stable association of RNAi machinery is conserved between the cytoplasm and nucleus of human cells. RNA. 2016;22:1085–98. doi: 10.1261/rna.056499.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikan M, Osborn MF, Coles AH, Godinho BMDC, Hall LM, Haraszti RA, Hassler MR, Echeverria D, Aronin N, Khvorova A. Docosahexaenoic Acid Conjugation Enhances Distribution and Safety of siRNA upon Local Administration in Mouse Brain. Mol Ther Nucleic Acids. 2016 doi: 10.1038/mtna.2016.50. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coles AH, Osborn MF, Alterman JF, Turanov AA, Godinho BM, Kennington L, Chase K, Aronin N, Khvorova A. A High-Throughput Method for Direct Detection of Therapeutic Oligonucleotide-Induced Gene Silencing In Vivo. Nucleic Acid Ther. 2016;26:86–92. doi: 10.1089/nat.2015.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemmings HC, Jr, Williams KR, Konigsberg WH, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. I. Amino acid sequence around the phosphorylated threonine. J Biol Chem. 1984;259:14486–90. [PubMed] [Google Scholar]
- 50.O'Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–42. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- 51.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–57. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou LL, Ma JL, Wang T, Yang TB, Liu CB. Cell-penetrating Peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen W, Liang XH, Sun H, Crooke ST. 2'-Fluoro-modified phosphorothioate oligonucleotide can cause rapid degradation of P54nrb and PSF. Nucleic Acids Res. 2015;43:4569–78. doi: 10.1093/nar/gkv298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janas MM, Jiang Y, Schlegel MK, Waldron S, Kuchimanchi S, Barros SA. Impact of Oligonucleotide Structure, Chemistry, and Delivery Method on In Vitro Cytotoxicity. Nucleic Acid Ther. 2016 doi: 10.1089/nat.2016.0639. [DOI] [PubMed] [Google Scholar]
- 56.Jirka SM, Tanganyika-de Winter CL, Boertje-van der Meulen JW, van Putten M, Hiller M, Vermue R, de Visser PC, Aartsma-Rus A. Evaluation of 2'-Deoxy-2'-fluoro Antisense Oligonucleotides for Exon Skipping in Duchenne Muscular Dystrophy. Mol Ther Nucleic Acids. 2015;4:e265. doi: 10.1038/mtna.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muschiol C, Berger MR, Schuler B, Scherf HR, Garzon FT, Zeller WJ, Unger C, Eibl HJ, Schmahl D. Alkyl phosphocholines: toxicity and anticancer properties. Lipids. 1987;22:930–4. doi: 10.1007/BF02535558. [DOI] [PubMed] [Google Scholar]
- 58.Benson MD, Smith RA, Hung G, Kluve-Beckerman B, Showalter AD, Sloop KW, Monia BP. Suppression of choroid plexus transthyretin levels by antisense oligonucleotide treatment. Amyloid. 2010;17:43–9. doi: 10.3109/13506129.2010.483121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.