Abstract
Phthalate exposure is ubiquitous and may affect child and adolescent health through both in utero exposure and direct exposure during childhood. Variability in exposure within women is not well documented. We analyzed 90 first-morning urine samples collected by ten reproductive-age women for phthalate metabolites and creatinine. Monoethyl [122 ng/mL (geometric mean concentration = 139 μg/g creatinine)], monobutyl [85.4 ng/mL (97.0 μg/g creatinine)], monobenzyl [37.2 ng/mL (42.2 μg/g creatinine)], and mono-2-ethylhexyl phthalate [9.4 ng/mL (10.7 μg/g creatinine)] were detected in most (94.4%) samples. The concentrations ranged from 23.8–1090 ng/mL, 43–437 ng/mL, 12.4–186 ng/mL, and 1.3–31.1 ng/mL, respectively. We observed considerable variation in phthalate concentrations by day for individual women. The intraclass correlation coefficient, indicating the proportion of variance explained by differences between subjects, ranged from 0.40 (monobutyl) to 0.68 (monoethyl). Monobenzyl and monoethyl phthalates showed higher levels on weekends as compared with weekdays (p = .01 for both). We found no significant difference between monoester levels from different menstrual cycles. Phthalate concentrations vary considerably for an individual and may require multiple samples for accurate assessment.
Keywords: Phthalate, women, metabolites, repeated measures, first morning void
INTRODUCTION
Phthalates are ubiquitous industrial chemicals that are used in pesticide formulations, plastics, and consumer products such as shampoos, soaps, and cosmetics. For example, dioctyl phthalate, dibutyl phthalate, diallyl phthalate, dimethyl phthalate, and diethyl phthalate have been used as active and as inert ingredients in pesticide formulations /1/. Diethyl phthalate and dibutyl phthalate are two phthalates commonly found in personal care products like deodorant and nail polish (2). Phthalates have well-established developmental toxicity in animals. Dibutyl and benzylbutyl phthalate exposure in utero and through lactation produce altered male repro-ductive development in male rat pups /3,4/. Furthermore, di(2-ethylhexyl)phthalate produces testicular toxicity in developing male rats /5/. Phthalates are also carcinogenic in rodents but via a mechanism not thought to be significant in humans /6/.
Humans generally metabolize the phthalate diesters (diethyl phthalate, dibutyl phthalate, and diethylhexyl phthalate) to the corresponding monoesters, monoethyl phthalate (MEP), mono-butyl phthalate (MBP), and monoethylhexyl phthalate (MEHP), which are glucuronidated and excreted in urine. Unpublished data suggest that benzyl butyl phthalate is metabolized pre-dominantly to monobenzyl (MBzP) phthalate /7/.
Very limited data exist regarding the potential human health effects of phthalates. Recent studies, however, found associations between urinary metabolite levels of certain phthalates and sperm concentration and motility /8/, sperm DNA damage /9/, and levels of follicle stimulating hormone /10/. In addition, male infants born to mothers with high phthalate exposure had decreased ano-genital distance and impaired testicular descent /11/.
A recent analysis of urine samples collected from participants in the Third National Health & Nutrition Examination Survey (NHANES III), conducted by the National Center for Health Statistics, demonstrated widespread exposure to phthalates among adults in the United States /12/. In this study, between 1988 and 1994, 289 subjects collected a single spot urine sample, which was analyzed for phthalate monoesters. Women of child-bearing age (20–40 years old) had statistically higher monobutyl phthalate levels than other age and gender groups. This difference may be related to phthalate content of personal care items (such as soaps, shampoos). An additional source of variation in urinary phthalate concentration may be the time since exposure. Phthalates are excreted quickly (on the order of hours) /7,13/, and the timing of sample collection can be a major source of variation. In this study, the availability of only one sample for each subject did not allow for estimation of variation within individuals.
Understanding the variability in phthalate levels across days within an individual is important for determining the sampling frequency necessary to assess phthalate exposure in epidemiologic studies of possible adverse health outcomes. Although the temporal variability of phthalate levels has been studied in other populations /14–16/, no published study has examined the variability of phthalate levels in women of reproductive age in the United States. Given the developmental toxicity observed in rodents and suggested in humans, exposure to reproductive age women is of concern. To examine the temporal variation in phthalate exposure, we measured phthalate monoesters in first morning void urine samples across several days in 10 women.
MATERIALS AND METHODS
Recruitment and Selection of Participants
The women in this study were originally recruited to participate in a study of the health of women office workers /17/. Briefly, participants were enrolled from large employers in the New York and Boston metropolitan areas between 1990 and 1993. Women who were 20–40 years of age, sexually active, and not consistently using birth control were asked to collect daily urine samples and keep daily diaries with information on menstrual bleeding. The urine specimens were stored at −20°C before analyses. For the current study, we selected 10 women from the 185 women who did not become pregnant during follow-up.
From the stored samples, for each woman, we selected 9 urine samples collected as first morning voids on the first 2 days of their first complete menstrual cycle in the study and the first 7 days of their second cycle. Using this design, we estimated the variability of phthalate metabolite concentrations within women and across women. Within women, we examined daily variation, differences between weekday and weekend phthalate levels, and variation across menstrual cycles. We compared variability within women with the variability across women. Emory University and the Centers for Disease Control and Prevention Human Subjects review boards approved this protocol.
Exposure Assessment
The phthalate assay used here is described in detail elsewhere /18/. Briefly, all samples were spiked with 13C4-labeled phthalate monoesters and 4-methylumbelliferone glucuronide and treated with a β-glucuronidase to release the phthalate monoesters from their conjugated form. The deconjugated urine samples were extracted twice with Oasis HLB SPE and suspended in the mobile phase. Chromatographic separation by HPLC was followed with tandem mass spectrometry on a triple-quadrupole instrument using atmospheric pressure chemical ionization (Finnigan Inc., San Jose, CA, USA). Levels of deconjugated 4-methylumbelliferone were monitored as quality control for the deconjugation step. Urine samples were also analyzed for creatinine levels and reported as micrograms phthalate monoester per gram urinary creatinine (ug/g creatinine). Method blanks, quality control samples (spiked human urine), and standards were analyzed along with the participants’ urine samples. Limited urine volume was available for some samples, and detection limits for those samples were slightly higher (0.9–9 ng/mL) than previously reported. (< 1.5 ng/mL). Urinary creatinine was measured using an ASTRA analyzer (Beckman Inc., Brea, CA, USA), based on a Jaffe rate reaction /19/. The intra-laboratory coefficients of variation assessed by repeated analyses of QC materials were: 4.9% for MEP, 6.1% for MBzP, 3.8% for MBP, and 8.4% for MEHP.
Precipitates from the urine samples were also analyzed for creatinine and phthalate monoester content. An aliquot of each urine sample was filtered through 5 mL Teflon-lined cartridges containing a HDPE filter (15–40 microns; Image Molding Inc., Denver, CO, USA). Water (500 μl) was added to each cartridge to loosen the precipitates from the cartridge bases, and then transferred by Pasteur pipette onto clean, pre-weighed watch glasses. The water was allowed to evaporate, and the weight of the precipitate obtained by difference. The dried precipitates were then dissolved by adding 400 μL of 1M NaOH followed by 430 μL of 1M acetic acid to neutralize the solution. Fifty microliters of each sample were analyzed for creatinine and phthalate monoesters. The creatinine in the precipitates accounted for 0%–37% of total creatinine.
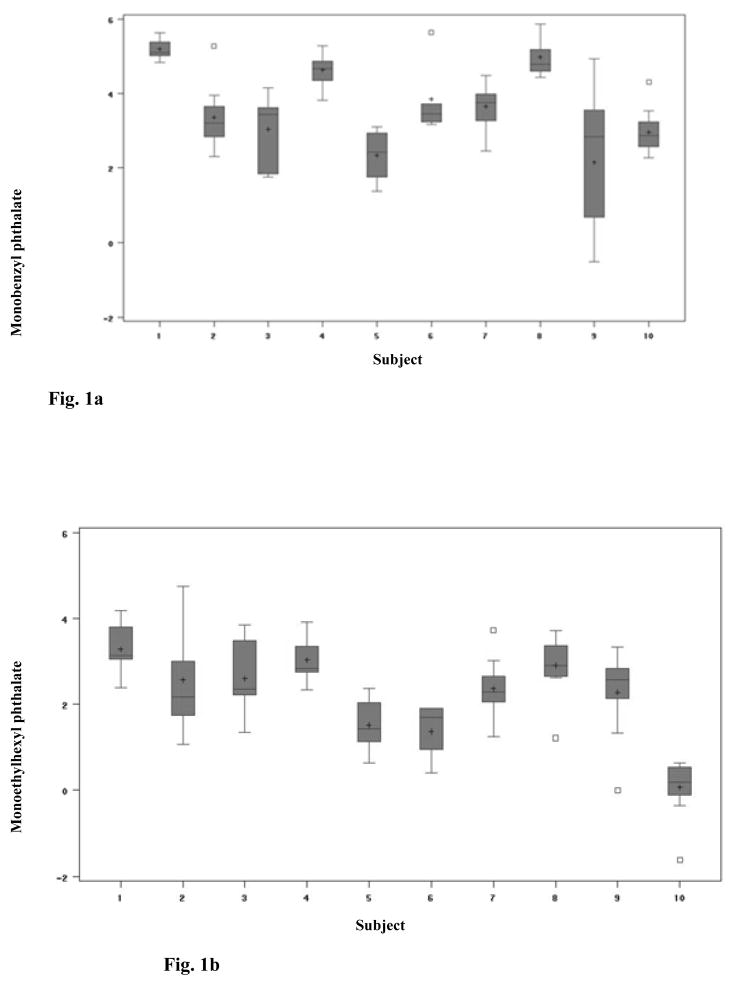
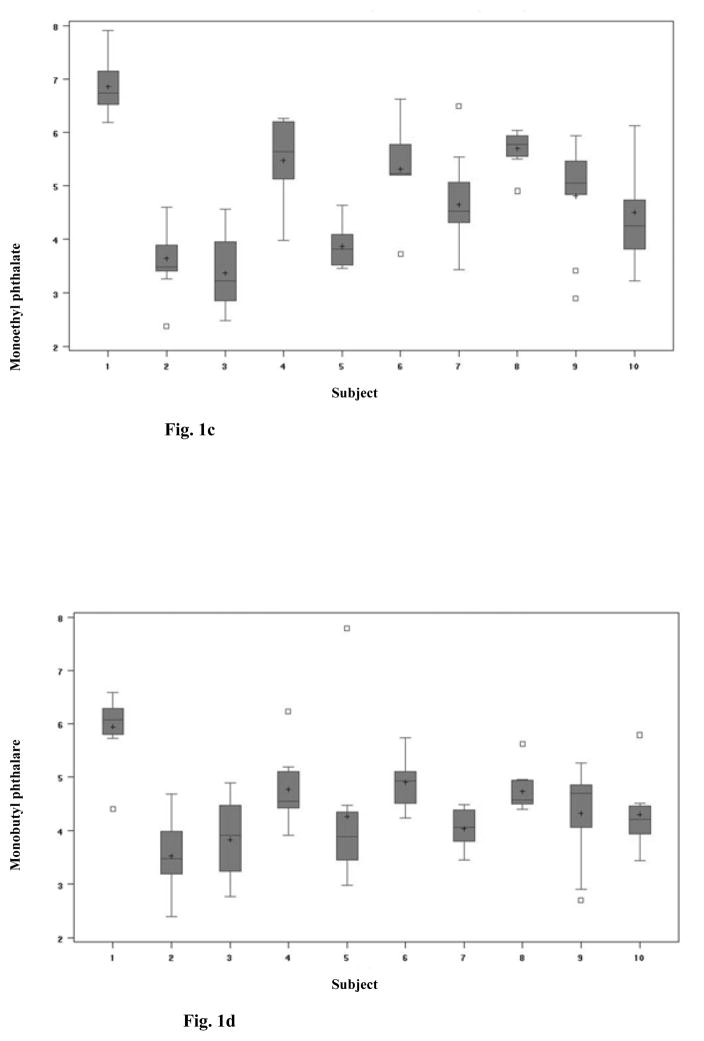
For each woman, box plots for the four urinary phthalate metabolites /MEP, MBP, MEHP, MBzP/ were created indicating the 10th, 25th, 75th, and 90th percentiles (Figure 1). Because the monoester measurements were not distributed normally, the data were transformed using a natural log scale before analyses of variance. To quantify the variation of phthalate levels between women (σb2) and within women (σw2), we fit a general linear mixed model. The model is: Yijk = β0 + αi + β1*Tk + β2*Cj +εijk, where Yijk is the log-urinary phthalate level for women i for the kth day type in the jth cycle, β0 is the mean level for the population, αi is the deviation from the population mean level for woman i. β1 and β2 represent the respective regression coefficients for the day type (weekend or weekday) effect (Tk) and the cycle effect (Cj) dummy variables. Each woman was considered to have her own effect of exposure, which represented a random effect αi with mean zero and variance σ b2. This model allows the estimation of average exposure difference among days as well as between cycles. The correlation between multiple measurements or intraclass correlation is calculated by σb2/( σb2+σw2). From this model we estimated the proportion of the total variation in phthalate monoesters that was within women and between (among) women. The variance of the average exposure was estimated by the generalized least squares estimator ((Σi (ni/(ni σb2 + σw2)) −1, where ni = number of measurements for the ith subject /20/.
Fig. 1.
Fig. 1a: Urinary Phthalate metabolite levels (Monobenzyl phthalate) (ng/mL) in individual women. Box plots represent 9 daily samples for all subjects except #5 (N = 8) and #6 (N = 5).
Fig. 1b: Monoethylhexyl phthalate (MEHP) phthalate levels
Fig. 1c: Monoethyl phthalate (MEP) phthalate levels
Fig. 1d: Monobutyl phthalate (MBP) phthalate levels
RESULTS
The 10 women had a mean age of 32 years (range: 23–36 years). Eight women were white, one was black, and one was Asian. Detectable levels of ethyl, butyl, benzyl and 2-ethylhexyl phthalate monoesters (levels of detection are 1.0, 0.6, 0.8, 1.2 ng/mL receptively) were found in most urine samples (94.4%). No urine sample contained detectable levels of monocyclohexyl (< 0.7–7 ng/mL), monoisononyl (< 0.8–8 ng/mL), or monooctyl phthalate (< 0.9–9 ng/mL). Summary statistics for the study population compared with a group of women of reproductive age (ages 18–40 years) from NHANES III are shown in Table 1. The geometric mean values for MEP, MBzP, MBP, and MEHP were 139 (SD=153.2), 97 (SD=88.1), 42 (SD=51.0) and 11 (SD=13.2) ug/g creatinine respectively. The distributions of phthalate monoester levels in daily urine samples for each woman showed considerable variability (Figure 1).
Table 1.
Phthalate levels in study sample compared with women in NHANES III
| Analyte | Study Population (N = 10) | NHANES III (N = 162) | ||||
|---|---|---|---|---|---|---|
| ng/mL | ug/g creatinine | ug/g creatinine | ||||
| Geometric mean (SD) | min/max | Geometric mean (SD) | min/max | Geometric mean | min/max | |
| MBzP | 37.2 (49.5) | < LOD/351 | 42 (51.0) | < LOD/415 | 23.1 | 2.3/189 |
| MEHP | 9.4 (11.2) | < LOD/115 | 11 (13.2) | < LOD/201 | 4.0 | < LOD/193 |
| MEP | 122 (154.6) | 10.8/2722 | 139 (153.2) | 21/2074 | 338 | < LOD/4540 |
| MBP | 85.5 (84.5) | 11/2418 | 97 (88.1) | 19/2231 | 43.7 | 4.7/2760 |
LOD, ‘limit of detection’; MBzP, monobenzyl phthalate; MEHP, monoethylhexyl phthalate ; MEP, monoethyl phthalate; MBP, monobutyl phthalate
In the analysis of variance, there was no significant difference in phthalate concentrations between cycles for any of the monoesters (Table 2). For monobenzyl and monoethyl phthalates, there was a significant effect of day type, with higher levels on weekends as compared to weekdays (geometric means of 56.7 and 31.8 ng/mL, and 170.6 and 108.1 ng/mL, respectively [p=0.01 for both]). The first two columns in Table 2 show between-women and within-women variations. The within-subject variation reflects the extent to which each participant’s daily level deviates from her mean level. The between-subject variation indicates the deviation of each participant’s mean level from the overall mean level. The between-women variation was significantly different than zero for all phthalates: MEP (1.15 ng/mL [p=0.02]), MEHP (0.87 ng/mL [p=0.02]), MBzP (1.07 ng/mL [p=0.02]), and MBP (0.41 ng/mL [p=0.03]). The proportion of variation due to between-subjects relative to total variation (intraclass correlation) was moderate for all phthalates: MEP (68%), MBzP (59%), MEHP (58%), and MBP (40%). Only MBP levels demonstrated higher within-subject variation as compared to the between-subject variation (Figure 1). MBP levels were fairly constant from one subject to another. We repeated this analysis using only the first two days of each cycle to simulate a balanced study design. The results were similar to the analysis using all cycle days, although MEP did not show significant variation by day type, and intraclass correlation decreased for all phthalates due to a relatively greater variance within women and decreased variance between women. In this analysis, variance between women was higher than variance within women only for MEP; for MBzP, MBP and MEHP, variance within women was greater.
Table 2.
Variance components for urinary phthalate monoester levels
| Analyte | Variance | Intraclass Correlation (%) | P-value | ||
|---|---|---|---|---|---|
| Among women (σb2) | Within women (σw2) | cycle | day type | ||
| MBzP | 1.07 | 0.76 | 0.59 | 0.18 | 0.01 |
| MEHP | 0.87 | 0.63 | 0.58 | 0.90 | 0.21 |
| MEP | 1.15 | 0.55 | 0.68 | 0.29 | 0.01 |
| MBP | 0.41 | 0.61 | 0.40 | 0.69 | 0.16 |
MBzP, monobenzyl phthalate; MEHP, monoethylhexyl phthalate ; MEP, monoethyl phthalate; MBP, monobutyl phthalate
To understand the increase in precision gained by multiple observations in estimating the true (geometric mean) exposure, we calculated the confidence interval around the geometric mean phthalate exposure for different numbers of repeated measurements (Table 3). The greatest increase in precision was observed when going from a single measurement to two measurements. When two measurements were available, the confidence intervals were relatively wide for all analytes (MBzP: 37.2 ng/mL, 95% CI [17.64, 78.32]; MEP: 122 ng/mL, 95% CI [58.33, 256.52]; MBP: 85.5 ng/mL, 95% CI: [50.54, 144.47]; MEHP: 9.4 ng/mL, 95% CI [4.81, 18.54]; Table 3). However, when 10 measurements per subject were included the precision of the estimates of the mean were moderately improved (MBzP: 95% CI [19.15, 72.11]; MEP: 95% CI [61.89, 241.75]; MBP: 95% CI: [55.80, 130.85]; MEHP: 95% CI [5.20, 17.18]).
Table 3.
Effect of varying sample number on precision of geometric mean estimation
| No. of samples | Geometric mean (95% CI) | |||
|---|---|---|---|---|
| MBzP | MEHP | MEP | MBP | |
| 1 | 37.2 (16.09, 85.85) | 9.4 (4.42, 20.18) | 122 (54.49, 274.58) | 85.5 (45.63, 160.01) |
| 2 | 37.2 (17.64, 78.32) | 9.4 (4.81, 18.54) | 122 (58.33, 256.52) | 85.5 (50.54, 144.47) |
| 4 | 37.2 (18.55, 74.47) | 9.4 (5.04, 17.70) | 122 (60.50, 247.33) | 85.5 (53.63, 136.14) |
| 6 | 37.2 (18.88, 73.16) | 9.4 (5.13, 17.41) | 122 (61.26, 244.24) | 85.5 (54.80, 133.24) |
| 8 | 37.2 (19.05, 72.51) | 9.4 (5.17, 17.26) | 122 (61.66, 242.69) | 85.5 (55.42, 131.75) |
| 10 | 37.2 (19.15, 72.11) | 9.4 (5.20, 17.18) | 122 (61.89, 241.75) | 85.5 (55.80, 130.85) |
Creatinine levels in these urine samples were 100.86 ± 48.8 mg/dL (mean ± SD) which are lower than the levels reported in adults in NHANES III, 137.9 ± 77.2.(12) The precipitates from some samples were found to contain a significant amount of creatinine but no detectable monoesters. The amount of creatinine in the precipitate was 0–37% of the total creatinine. After accounting for creatinine in any precipitates, the creatinine levels were 111.9 ± 74.3 mg/dL.
DISCUSSION
The phthalate monoester levels in the urine samples are comparable to the levels found in samples from selected women in the NHANES III (Table 1) /12/. The urine samples from the two studies were taken during similar time frames, for our study 1990–1993 and for NHANES III 1989–1994. More African-Americans (34%), however, were represented within the subjects from NHANES III than in our study (10%). The creatinine-adjusted and unadjusted levels of MBP, MBzP, and MEHP were higher in our sample than in the NHANES population, whereas the levels of MEP were lower (Table 1). In comparison with a study of United States (US) men of reproductive age /15/, the level of two phthalate monoesters (MBzP and MBP) were higher in this sample, whereas the levels of MEP and MEHP were similar. These differences may be due to the use of personal care products, as well as the different time periods of sample collection. A German study/14/ found levels of MBP, MBzP, and MEHP for adult women that were considerably lower than the levels found in this study (MEP not assessed).
For all but one phthalate monoester assessed(MBP), the proportion of variance from between/among-individual variability accounted for more than half the total variance. Repeated samples from the same women indicated considerable temporal variation in urinary phthalate monoester concentration. The intra-laboratory variation was small compared with the other sources of variation. The day type (weekend or weekday) was significant in predicting MBzP and MEP levels in our sample, with levels increased on weekends compared with weekdays. This finding may be due to a differing pattern of use of personal care products on those days. As increasing the number of samples analyzed would increase the precision of the estimation of phthalate monoester levels for a given individual, repeated measurements may be necessary to distinguish between/among women, depending on the analyte. This finding is consistent with earlier studies. Fromme et al /14/ reported significant variation between subjects, with ICCs ranging from 0.21 (5OH-MEHP) to 0.48 (MBzP). Similar results were found in a US study of men of reproductive age, in which MEHP showed the largest within-subject variability, and MEP showed the least /15/. Hoppin et al /16/ reported ICCs to range between 0.34 (MBzP) and 0.61 (MBP), and moderate correlation among repeated phthalate measurements [Pearson correlation coefficients ranging from 0.34 (MBzP) to 0.62 (MBP)].
The implications of this pilot study are,
Phthalate exposure in reproductive age women is common and detectible in the first morning urine sample.
The estimation of ‘usual’ phthalate exposure or exposure during critical windows of fetal development may require repeated samples from an individual.
A significant variation is found in phthalate exposure for certain analytes, according to weekend versus weekday.
Precipitates in stored urine samples contain significant amounts of creatinine. The creatinine in these precipitates should be quantified and included in the total creatinine to avoid overestimation of creatinine-adjusted phthalate levels.
Children are likely exposed to phthalates both in utero (from maternal exposure) and through everyday products in childhood. Although the human health effect of phthalate exposure is unclear, animal data suggest potential toxicity. Thus, additional studies are needed to assess phthalate exposure in childhood and early adolescence. Where possible, such studies should obtain multiple urine samples across both weekdays and weekends to fully explain the role of phthalate exposure in the health of children.
Acknowledgments
Funding for this research project was provided by an Emory University Research Committee Grant awarded to Michele Marcus. We acknowledge Elaine Gunter of the Nutritional Biochemistry Branch for creatinine measures, Nicole Malek, Jack Reidy, Manori Silva, and Carolyn Hodge of the Toxicology Branch from the Division of Laboratory Sciences of the National Center for Environmental Health within the Centers for Disease Control and Prevention for the monoester measures. We also acknowledge Kristin Rankin from the School of Public Health at the University of Illinois at Chicago for technical assistance.
References
- 1.Lists of inert pesticide ingredients. US Environmental Protection Agency, Office of Pesticide Programs; 1999. [accessed 1 July 1999]. Available from: http://www.epa.gov/opprd001/inerts/lists.html. [Google Scholar]
- 2.Houlihan J, Brody C, Schwan B. Not too pretty: phthalates, beauty products and the FDA: Environmental Working Group, Coming Clean, and Health Care Without Harm. 2002. [Google Scholar]
- 3.Ema M, Itami T, Kawasaki H. Effect of period of exposure on the developmental toxicity of butyl benzyl phthalate in rats. J Appl Toxicol. 1992;12(1):57–61. doi: 10.1002/jat.2550120112. [DOI] [PubMed] [Google Scholar]
- 4.Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43(1):47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- 5.Gray TJ, Gangolli SD. Aspects of the testicular toxicity of phthalate esters. Environ Health Perspect. 1986;65:229–35. doi: 10.1289/ehp.8665229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David RM, Moore MR, Cifone MA, Finney DC, Guest D. Chronic peroxisome proliferation and hepatomegaly associated with the hepatocellular tumorigenesis of di(2-ethylhexyl)phthalate and the effects of recovery. Toxicol Sci. 1999;50(2):195–205. doi: 10.1093/toxsci/50.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Eigenberg DA, Bozigian HP, Carter DE, Sipes IG. Distribution, excretion, and metabolism of butylbenzyl phthalate in the rat. J Toxicol Environ Health. 1986;17(4):445–56. doi: 10.1080/15287398609530839. [DOI] [PubMed] [Google Scholar]
- 8.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. Phthalate exposure and human semen parameters. 2003;14(3):269–77. [PubMed] [Google Scholar]
- 9.Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, et al. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ Health Perspect. 2003;111(9):1164–9. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Human Reprod. 2005;20(3):604–10. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- 11.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108(10):979–82. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int J Androl. 2006;29(1):155–65. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 81–5. [DOI] [PubMed] [Google Scholar]
- 14.Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health. 2007;210(1):21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110(5):515–8. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus M, McChesney R, Golden A, Landrigan P. Video display terminals and miscarriage. J Am Med Womens Assoc. 2000;55(2):84–8. 105. [PubMed] [Google Scholar]
- 18.Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72(17):4127–34. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- 19.Gunter EW, Lewis BL, Koncikowski SM. Laboratory methods used for the third National Health and Nutrition Examination Survey (1988–1994) Hyattsville, MD: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 20.Searle SR, Casella G, McCulloch CE. Variance components. USA: John Wiley & Sons, Inc; 1992. [Google Scholar]