Abstract
Aim
The glomerular filtration rate (GFR) falls progressively in chronic kidney disease (CKD) which is caused by a reduction in the number of functional nephrons. The dysfunctional nephron exhibits a lower glomerular capillary pressure that is induced by an unbalance between afferent and efferent arteriole. Therefore, we tested the hypothesis that oxidative stress induced by CKD differentially impairs the structure or function of efferent versus afferent arterioles.
Methods
C57BL/6 mice received sham operations (sham) or 5/6 nephrectomy (RRM) and 3 months of normal or high salt diet or tempol. GFR was assessed from the plasma inulin clearance, arteriolar remodeling from media/lumen area ratio, myogenic responses from changes in luminal diameter with increases in perfusion pressure and passive wall compliance from the wall stress/strain relationships.
Results
Mice with RRM fed a high salt (versus sham) had a lower GFR (553±25 versus 758±36μl/min/g kidney, p<0.01) and a larger efferent arteriolar diameter (9.6±0.8 versus 7.4±0.7μm, p<0.05) resulting in a lower media/lumen area ratio (1.4±0.1 versus 2.4±0.2, p<0.01). These alterations were corrected by tempol. The myogenic responses of efferent arterioles were about one-half that of afferent arterioles and were unaffected by RRM or salt. Passive wall compliance was reduced by high salt in both afferent and efferent arterioles.
Conclusion
A reduction in renal mass with a high salt diet induces oxidative stress that leads to an outward eutrophic remodeling in efferent arterioles and reduced wall compliance in both afferent and efferent arterioles. This may contribute to the lower GFR in this model of CKD.
Keywords: Efferent arteriole, myogenic response, reduced renal mass, vascular remodeling, wall compliance
INTRODUCTION
Renal autoregulation maintains glomerular filtration rate (GFR) and renal blood flow (RBF) independent of renal perfusion pressure over a physiological range. Autoregulation of RBF is mediated primarily by a rapid myogenic contraction of the afferent arteriole to increased perfusion pressure. This response is reinforced by tubuloglomerular feedback (TGF) and two additional mechanisms (Lai et al., 2010, Ito and Abe, 1997, Lai et al., 2011, Ito et al., 1992, Carlstrom et al., 2015). However, the afferent arteriole, glomerulus and efferent arteriole are arranged in series to regulate the pressure and flow through the glomerulus. The resistance offered by the efferent arteriole is responsible for the uniquely high glomerular capillary hydrostatic pressure and thereby contributes importantly to the regulation of the GFR. Moreover, a maintained or enhanced resistance in the efferent arteriole contributes to the glomerular capillary hypertension that results in both the adaptive increase in GFR and the barotrauma in reduced renal mass (RRM) models of chronic kidney disease (CKD) (Anderson et al., 1986). In those pathological conditions, the focus of therapy has been on angiotensin converting enzyme inhibitors to lower efferent arteriolar resistance. The tone of the efferent arteriole, and its regulation by angiotensin II, can contribute selectively to the autoregulation of the GFR (Carlstrom et al., 2015, Hall, 1986, Pelayo and Westcott, 1991). The role of afferent arterioles in the autoregulation of the RBF is well documented (Takenaka et al., 1994, Lai et al., 2012, Ren et al., 2010, Carlstrom et al., 2015). However, it is less investigated how efferent arteriolar resistance contributes to the precise autoregulation of the GFR and the glomerular capillary pressure, respectively. Thus, a rise in perfusion pressure inhibits renin release and angiotensin generation in the kidney that is considered necessary to reduce the tone in the efferent arteriole, thereby contributing to a maintained glomerular capillary pressure and to excellent autoregulation of the GFR (Hall et al., 1977). Whether the efferent arteriole also possesses a myogenic response remains controversial (Ito and Abe, 1997, Carlstrom et al., 2015). Thus, it is important to understand the normal adjustments to pressure of the efferent arteriole and its modification by salt diet or RRM since this contributes to the progression of CKD. However, less attention has been paid to the structural and functional alterations of efferent arterioles under physiological and pathological conditions (Ren et al., 2007).
Reactive oxygen species (ROS) that are overproduced during oxidative stress have been implicated in the increased contractility of renal afferent arterioles to angiotensin II (Carlstrom et al., 2010, Li et al., 2015) and to perfusion pressure (Lai et al., 2010, Carlstrom et al., 2010, Li et al., 2015, Ren et al., 2010). Afferent arterioles exposed to oxidative stress (Li et al., 2015) or prolonged hypertension show inward remodeling with a decrease in luminal diameter and an increase in the cross-sectional area of the media (Gattone et al., 1983). Dietary salt determines glomerulosclerosis and renal injury in rats with RRM (Cao et al., 2015), and to some extent also in patients with CKD (Lipkowitz and Wilcox, 2014). A high salt diet induces oxidative stress and impairs myogenic responses of afferent arterioles in mice with RRM (Lai et al., 2010). However, it is unknown whether oxidative stress induced by high salt diet and RRM affects myogenic responses and remodeling of efferent arterioles.
We selected the C57BL/6 mouse with RRM and high salt diet as the model of CKD. This is a convenient model to study the functional adaptation to a reduction in renal mass since this mouse strain retains a normal glomerular structure over 3 months and a normal blood pressure despite the severe oxidative stress (Lai et al., 2012). We compared structural remodeling and myogenic responses of afferent and efferent arterioles from sham-operated mice with those subjected to surgical RRM and fed a normal or a high salt diet. The role of ROS in sham-operated mice or mice with RRM fed a high salt diet was assessed in additional groups from the effects of three months oral application of tempol (Wilcox and Pearlman, 2008, Luo et al., 2010).
MATERIALS AND METHODS
Experimental animals
Male adult C57BL/6 mice weighting 24 to 30 g (SLAC laboratory animal company, Shanghai, China) were fed a 0.4% or 6% NaCl control test diet (TD92055, Harlan Teklad, CA, USA) and allowed free access to tap water. All the animal procedures and protocols were approved by the Zhejiang University Animal Care and Ethical Committee.
Animal preparation, surgery and protocols
As described previously (Lai et al., 2010, Lai et al., 2012), a two-step 5/6 surgical nephrectomy procedure was used to create the RRM model under inhalational anaesthesia with 2% isoflurane and oxygen mixed with room air in a vaporizer. Two-thirds of the left kidney was removed by stitching off each pole of the kidney. After one week, the right kidney was removed. Sham-operated control mice (sham) were subjected to a similar two stage surgical procedure without removal of renal mass. The mice were allowed to recover from the surgeries for one week and randomised to receive normal or high salt diets for 3 months. The mice with sham surgery or RRM fed a high salt diet were randomised further to receive vehicle or tempol (2 mmol/l in drinking water) throughout as previously (Lai et al., 2012). At 3 months, one set of mice (n=6–18 per group) were placed in metabolic cages for a 24 hours urine collection. Thereafter, a group of mice (n=5–6 per group) were euthanized for isolation and perfusion of individual renal afferent or efferent arterioles (Lai et al., 2010) and another group prepared for GFR measurements (Qi et al., 2004, Li et al., 2013).
Assessment of renal function
Three months after surgery, mice were placed in metabolic cages for 24 hours and urine was collected into containers with added antibiotics (2.6 mg streptomycin, 0.8 mg penicillin G and 5mg amphotericin B) to maintain sterility. Urinary albumin was assessed with a urine micro-albumin ELISA Kit (Albuwell M kit, Exocell, Philadelphia, PA, USA) (Lai et al., 2010).
Three months after sham or RRM procedures, mice were prepared for measurements of GFR from the plasma disappearance of FITC-inulin (Qi et al., 2004, Li et al., 2013). Five percent of FITC-inulin (3.74 μl /g body weight) was injected into the retro-orbital plexus under anaesthesia within 10 seconds. Thereafter, approximately 4 μl samples of blood were collected from a tail vein at 3, 7, 10, 15, 35, 55 and 75 min into heparinized capillary tubes and centrifuged (4,000RPM, 10min). The plasma fluorescence was assessed in a NanoDrop 3300 Fluorospectrometer (Thermo Scientific Company, Wilmington, DE, USA). GFR was calculated, as described (Qi et al., 2004, Li et al., 2013) from the slope of the plasma fluorescence versus time relationship.
Dissection and microperfusion of mouse efferent or afferent arterioles
A single efferent or afferent arteriole with attached glomeruli was dissected from each kidney, mounted and perfused in Dulbecco’s modified Eagle’s medium (DMEM) via a holding pipette containing an internal perfusion pipette as described previously (Lai et al., 2006, Patzak et al., 2004, Lai et al., 2009). Efferent arterioles were identified by their lack of an internal elastic lamina, their thinner and flattened endothelial cells and their smaller luminal diameter.
Assessment of arteriolar remodeling
Each arteriole was perfused at 60 mmHg and visualized by a confocal laser scanning microscope to measure the arteriolar outer diameter (D) and luminal diameter (d). Wall thickness (t) was calculated from: t= (D-d)/2; lumen area (L) from L=π (d/2)2 and media area (M) from M= π(D/2)2 - π(d/2)2. Remodeling was determined from the media/lumen area ratio.
Measurement of myogenic response
Myogenic responses of perfused afferent and efferent arterioles were assessed from the reductions in their luminal diameters at the period of two minutes after step 20 mmHg increases in perfusion pressure from 60 to 140 mmHg. The most active point in the perfused arteriole was selected to estimate its myogenic response. Only one afferent or efferent arteriole was studied from each mouse (Lai et al., 2010).
Determination of active wall tension
The wall tension (T) was calculated from: T= perfusion pressure × luminal radius. The tension was first assessed in arterioles in physiological solution (Tphysiol) and thereafter in Ca2+-free solution containing 5 × 10−3 mol·L−1 ethylene glycol-bis (2-aminoethylether) -N,N,N′,N′-tetraacetic acid (EGTA, Sigma-Aldrich, St. Louis, MO, USA) to abolish active tone (Tpassive). The active wall tension (AWT) was calculated from: AWT = (Tpassive) – (Tphysiol) (Lai et al., 2010).
Determination of passive arteriolar compliance
Arteriolar compliance was assessed from passive wall stress/strain relationships of arterioles in Ca2+-free solution. Passive wall stress (PWS) was calculated from passive wall tension per unit wall thickness (PWS = Tpassive/t) and passive wall strain from the ratio of change in luminal diameter to the diameter at maximum perfusion pressure of 160 mmHg (Strain = ΔD/D) (Li et al., 2015).
Chemicals and reagents
4-hydroxy-2, 2, 6, 6-tetramethylpiperidinyloxy (tempol) and FITC-Inulin were obtained from Sigma-Aldrich, St. Louis, MO, USA. Dulbecco’s modified Eagle’s medium (DMEM) with low glucose without Phenol Red indicator was purchased from Gibco, Waltham, MA, USA.
Statistics
Values were obtained from 5 to 14 mice per group and are presented as mean ± SEM. GraphPad Software Prism 6.0 was used for statistical analysis. Two-way ANOVA was used to analyse the difference of responses to graded perfusion pressure between groups and the effects of salt, RRM and tempol and their interaction. Curve fit and repeated two-way ANOVA were used to analyse the arteriolar compliance. Post hoc comparisons were performed using Bonferroni correction. A nonparametric test was used to analyse data in Table 1 and Table 2. The P-value < 0.05 was considered statistically significant and all P-values are two-sided.
Table 1.
Parameters of renal function
| Parameter | Normal Salt (NS) | High salt (HS) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Sham | RRM | Sham | Sham+tempol | RRM | RRM+tempol | |
| Urine albumin (μg/day) | 155±14 | 208±33 | 250±31 | 205±25 | 332±25‡ | 205±78* |
|
| ||||||
| Total GFR (μl/min/g kidney weight) | 758±36 | 754±74 | 756±98 | 620±105 | 553±25* | 725±47 |
Urine albumin and total GFR from sham-operated mice or RRM mice with NS or HS or from RRM mice with HS plus tempol.
Values are presented as mean ± SEM (n=6–14 per group for urine albumin, n=5–8 per group for GFR). By ANOVA test, P =0.002 in urine albumin, followed 4 comparisons from 6 groups were performed by Bonferroni multiple comparisons test: RRM with HS versus sham with NS: ‡, P<0.001, RRM with HS plus tempol versus RRM with HS: *, P <0.05. By ANOVA test, P =0.0443 in GFR, followed 4 comparisons from 6 groups were performed by Bonferroni multiple comparisons test: RRM with HS versus sham with HS: *, P <0.05.
Table 2.
Basal luminal diameters (μm) of afferent and efferent arterioles perfused ex vivo at 60mmHg
| Group | Afferent arterioles | Efferent arterioles | |||
|---|---|---|---|---|---|
|
|
|
||||
| Normal salt (NS) | High salt (HS) | NS | HS | HS + tempol | |
| Sham | 8.8 ± 0.8 | 10.1 ± 0.7 | 7.4 ± 0.7 | 6.8 ± 0.7 | 8.0 ± 0.4 |
| RRM | 10.9 ± 1.3 | 9.9 ± 0.7 | 7.4 ± 0.3 | 9.6 ± 0.8* | 7.1 ± 0.6* |
Basal luminal diameters of afferent and efferent arterioles from sham-operated mice or RRM mice with NS or HS or from RRM mice with HS and tempol. Values are presented as mean ± SEM (n=5–6 per group). By ANOVA test, P>0.05 in afferent arterioles, P =0.038 in efferent arterioles, followed 5 comparisons from 6 groups were performed by Bonferroni multiple comparisons test. RRM with HS versus sham with HS: *, P <0.05. RRM with HS plus tempol versus RRM with HS: *, P <0.05.
RESULTS
Compared to sham-operated mice fed a normal salt diet, those with RRM fed a high salt diet had an about 35% lower GFR (553 ± 25 versus 758 ± 36 μl/min/g kidney weight, p<0.05) and a doubling of albumin excretion (332 ± 25 versus 155 ± 14 μg/day, p< 0.001) (Table 1). There were no significant effects of high salt diet on sham-operated mice, and no significant effects of RRM on mice fed a normal salt diet. Mice with RRM fed a high salt diet plus tempol had a normal GFR and albumin excretion.
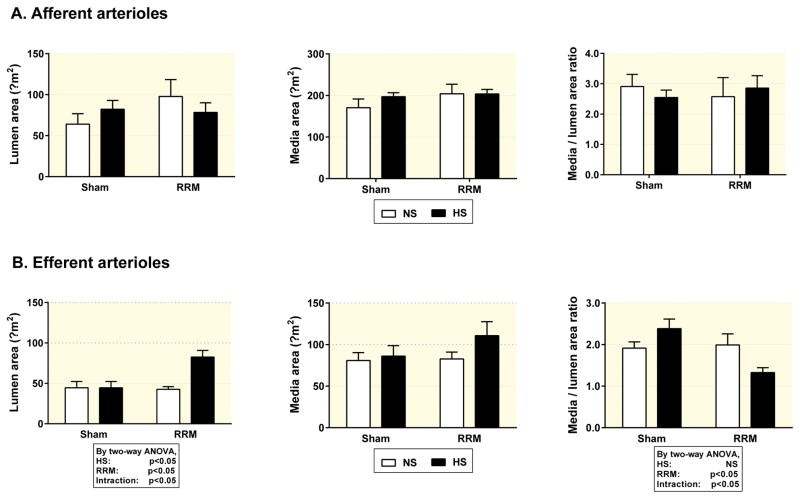
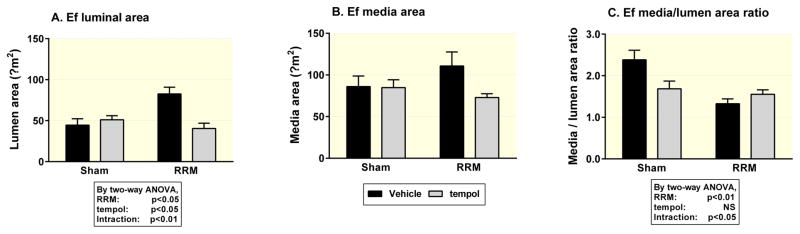
Compared to sham-operated mice fed a normal salt diet, efferent arterioles from mice with RRM fed a high salt diet had an about 30% enlargement in luminal diameter (9.6 ± 0.8 versus 7.4 ± 0.7 μm, p<0.05) (Table 2 and Figure 5), resulting in about 65% enlargement in lumen area (73.8 ± 10.8 versus 44.5 ± 7.8 μm2, p<0.05) (Figure 1) without a significant change in media area. However, there were no significant effects on any of these parameters of high salt diet in sham mice, or of RRM in mice with a normal salt diet (Figure 1 and Figure 2). By two-way ANOVA analysis, RRM combined with high salt diet led to an enlarged luminal area of efferent arterioles. The enlargement in efferent arteriolar luminal area in mice with RRM fed a high salt diet was prevented by tempol. Tempol also reduced the media area in the RRM with high salt diet group (Figure 2).
Figure 1.
Lumen area, media area and media/lumen area ratio in afferent arterioles (Panel A) and efferent arterioles (Panel B) from mice with sham operation fed a normal salt diet (NS, open boxes) or high salt diet (HS, solid boxes) or RRM fed a normal salt diet (NS, open boxes) or high salt diet (HS, solid boxes). Values are presented as mean ± SEM (n=6 per group). Data were analysed by two-way ANOVA.
Figure 2.
Lumen area, media area and media/lumen area ratio in efferent arterioles from mice with sham operation or RRM fed a high salt diet (HS) without (Vehicle, solid boxes) or with tempol (Gray filled boxes). Values are presented as mean ± SEM (n=6 per group). Data were analysed by two-way ANOVA.
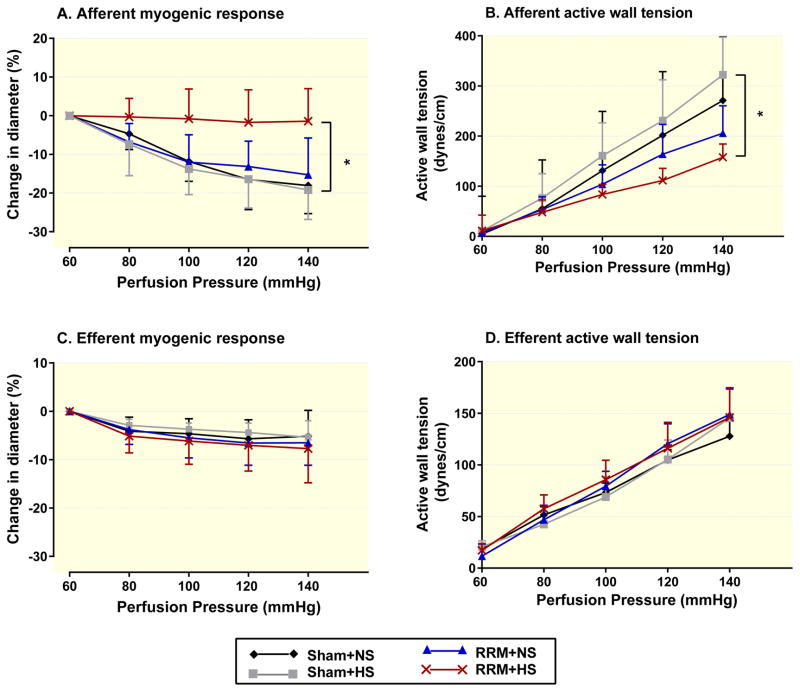
We confirmed our previous reports that normal mouse afferent arterioles have a marked reduction in luminal diameter with perfusion pressure above 60 mmHg accompanied by a linear increase in active wall tension but these myogenic responses are impaired in mice with RRM and further impaired by high salt diet (Lai et al., 2010). In contrast, the reduction in diameter or increase in active wall tension with perfusion pressure in efferent arterioles of sham-operated mice was only about one-half of corresponding afferent arterioles. Moreover, the myogenic responses of efferent arterioles were unchanged by RRM even in mice fed a high salt diet (Figure 3).
Figure 3.
Diameters (afferent, Panel A; efferent, Panel C) and active wall tension (afferent, Panel B; efferent, Panel D) of arterioles from shamed-operate mice with NS (black diamonds with black line) or HS (gray squares with gray line) or from RRM mice with NS (blue triangles with blue line) or HS (red crosses with red line). Values are presented as mean ± SEM (n=5 per group). By ANOVA test, P =0.0115 in Panel A, P =0.0313 in Panel B. Three comparisons from 4 groups were performed by Bonferroni multiple comparisons test. RRM+HS versus Sham + HS: *, P<0.05.
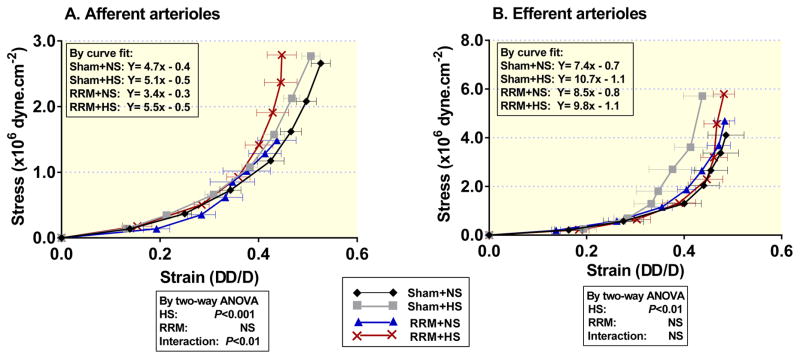
The passive wall compliance was reduced by high salt diet in both afferent and efferent arterioles from sham and RRM mice. Data were analysed by Curve fit to obtain the equation with slope and Y intercept (Figure 4) that was used to calculate the values of wall stress at wall strain (ΔD/D=0.45) for each group. The effects of HS and RRM on the wall stress were analysed by two-way ANOVA and Bonferroni multiple comparisons test: in afferent arterioles, RRM with NS versus sham with NS, P<0.05, RRM with HS versus RRM with NS, P<0.001 (Figure 4A); in efferent arterioles, sham with HS versus sham with NS, P<0.05 (Figure 4B).
Figure 4.
Passive wall stress/strain relationships in afferent arterioles (Panel A) and efferent arterioles (Panel B) from shamed-operated mice with NS (black diamonds with black line) or HS (gray squares with gray line) or from RRM mice with NS (blue triangles with blue line) or HS (red crosses with red line). Values are presented as mean ± SEM (n=5 per group). Data were analysed by Curve fit to obtain the equation with the slop and Y intercept that was used to calculate the values of wall stress at wall strain (ΔD/D=0.45) for each group. The effects of HS and RRM on the wall stress and their interaction are analysed by two-way ANOVA. Six comparisons from 4 groups were performed by Bonferroni multiple comparisons test. Panel A (afferent arterioles), RRM with NS versus sham with NS: P<0.05, RRM with HS versus RRM with NS: P< 0.001. Panel B (efferent arterioles), sham with HS versus sham with NS: P<0.05.
DISCUSSION
This study confirms our previous report that a high salt diet worsens the reduction in GFR, proteinuria and afferent arteriolar myogenic responses in mice with RRM (Lai et al., 2012). Unlike many human subjects with CKD (Weir et al., 1995) and the rat model of RRM (Smith et al., 1986), this C57BL/6 mouse model of RRM has normal blood pressure and little renal damage even after 3 months of high salt diet (Lai et al., 2012). The main new findings are that the efferent arterioles of sham-operated mice had only one-half of the myogenic response of corresponding afferent arterioles. Also unlike afferent arterioles (Lai et al., 2010), the myogenic responses of efferent arterioles were maintained in mice with RRM after 3 months of a high salt diet. In contrast to these functional preservation of efferent arterioles, they underwent remarkable structural changes that were not apparent in afferent arterioles. In response to the renal oxidative stress induced by high salt diet and RRM, efferent arterioles exhibited an outward eutrophic remodeling with an increase in luminal area. The effects of oxidative stress are to enhance the rapid myogenic reactivity to perfusion pressure (Lai et al., 2010, Lai et al., 2011) or angiotensin II in afferent arterioles (Li et al., 2015), whereas the efferent arteriole mounts a slow structural remodeling response. Nevertheless, the effects of oxidative stress to enhance the afferent arteriolar reactivity led to an increase in preglomerular vascular resistance during increased perfusion pressure. This effect should reinforce the delayed effects of ROS to increase the efferent arteriolar diameter that reduces the post-glomerular resistance to lower the GFR. A dilated efferent arteriole should limit the increase in glomerular capillary pressure in mice with RRM. Indeed, the absence of glomerulosclerosis is remarkable given the fact that RRM mice had a > 5-fold increase in parameters of oxidative stress (Lai et al., 2012) that are normally associated with rapid progression of CKD (Lever et al., 2016).
The mechanisms of vascular remodeling are not fully understood. There is evidence that hypertension leads to vascular remodeling (Gattone et al., 1983, Eftekhari et al., 2012). We reported that afferent arterioles from SOD-1, -2 and -3 gene deleted mice have greatly increased media area with maintained or reduced luminal diameters. This hypertrophic remodeling was attributed to superoxide which accumulates in the afferent arterioles of these SOD knockout mice (Li et al., 2015). However, vascular smooth muscle cell catalase transgenic mice fail to accumulate H2O2 in their blood vessels and fail to manifest vascular remodeling during infusions of angiotensin II, demonstrating an additional role for H2O2 (Zhang et al., 2005). The response to tempol does not discriminate between superoxide and H2O2 since tempol is a redox cycling nitroxide whose long-term use reduces both ROS (Wilcox and Pearlman, 2008). The efferent arterioles in our present study exhibited outward eutrophic remodeling with enlarged luminal diameter and decreased media/lumen area ratio without a change in media area.
The passive wall compliance was reduced by high salt diet in both afferent and efferent arterioles. Both high salt and RRM increase ROS (Lai et al., 2010, Lai et al., 2012). An increase in salt or damage to the kidney can increase ROS and decrease nitric oxide that are associated with renal and vascular fibrosis and sclerosis in afferent and efferent arterioles (Eftekhari et al., 2012). Thus, the decreased microvascular compliance observed with high salt diet may be a consequence of ROS-induced collagen synthesis and fibrosis.
The functional effects of remodeling on renal microvessels are still not fully understood. The arteriolar remodeling may alter the relative resistances of afferent and efferent arterioles that determine the RBF and GFR. The afferent arteriole is the important resistance vessel and the principal site for the regulation of the RBF. It is rapidly and strongly responsive to changes in perfusion pressure and neurohormones, thereby providing the principal acute response to physiological stimuli and stress. On the other hand, the efferent arteriole is the major post-glomerular resistance vessel and thereby can regulate the GFR somewhat independent of the RBF.
CONCLUSION
This study highlights some major differences in the adaptation of afferent and efferent arterioles to a reduction in renal mass combined with a high salt diet that together induce intense renal oxidative stress. The novel finding is that oxidative stress leads to an outward eutrophic remodeling of efferent arterioles and reduced wall compliance in both afferent and efferent arterioles. The adaptive increase in diameter of the efferent arteriole in this circumstance may prevent severe glomerular hypertension and barotrauma and thereby may account for the absence of much glomerular or renal damage or interstitial fibrosis in this C57BL/6 mouse model. The price paid may be a lower GFR.
Acknowledgments
This study was supported by research grants to En Yin Lai from the National Nature Science Foundation of China (Grant No. 31471100), to En Yin Lai, Lingli Li and Christopher S. Wilcox from The National Institute of Diabetes and Digestive and Kidney Diseases (DK-49870 and DK-36079), The National Heart, Lung, and Blood Institute (HL-68686), the George E. Schreiner Chair of Nephrology, the Smith-Kogod Family Foundation and the Georgetown University Hypertension Research Center. We would like to thank the Stiftelsen Nordisk Fysiologi, SNF and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) for their generous support for the AP Symposium on “RENOPROTECTION.”
Footnotes
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests in relation to the publication of this paper.
References
- Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, Hou FF. A Salt-Induced Reno-Cerebral Reflex Activates Renin-Angiotensin Systems and Promotes CKD Progression. J Am Soc Nephrol. 2015;26:1619–33. doi: 10.1681/ASN.2014050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension. 2010;56:907–13. doi: 10.1161/HYPERTENSIONAHA.110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL. Changes in blood pressure and systemic vascular resistance do not predict microvascular structure during treatment of mild essential hypertension. J Hypertens. 2012;30:794–801. doi: 10.1097/HJH.0b013e328350e4ff. [DOI] [PubMed] [Google Scholar]
- Gattone VH, 2nd, Evan AP, Willis LR, Luft FC. Renal afferent arteriole in the spontaneously hypertensive rat. Hypertension. 1983;5:8–16. doi: 10.1161/01.hyp.5.1.8. [DOI] [PubMed] [Google Scholar]
- Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol. 1986;250:R960–72. doi: 10.1152/ajpregu.1986.250.6.R960. [DOI] [PubMed] [Google Scholar]
- Hall JE, Guyton AC, Trippodo NC, Lohmeier TE, McCaa RE, Cowley AW., Jr Intrarenal control of electrolyte excretion by angiotensin II. Am J Physiol. 1977;232:F538–44. doi: 10.1152/ajprenal.1977.232.6.F538. [DOI] [PubMed] [Google Scholar]
- Ito S, Abe K. Contractile properties of afferent and efferent arterioles. Clin Exp Pharmacol Physiol. 1997;24:532–5. doi: 10.1111/j.1440-1681.1997.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Juncos LA, Carretero OA. Pressure-induced constriction of the afferent arteriole of spontaneously hypertensive rats. Hypertension. 1992;19:II164–7. doi: 10.1161/01.hyp.19.2_suppl.ii164. [DOI] [PubMed] [Google Scholar]
- Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, Le T, Li P, Palm F, Welch WJ, Wilcox CS. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am J Physiol Renal Physiol. 2012;303:F64–74. doi: 10.1152/ajprenal.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension. 2010;55:983–9. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EY, Patzak A, Persson AE, Carlstrom M. Angiotensin II enhances the afferent arteriolar response to adenosine through increases in cytosolic calcium. Acta Physiol (Oxf) 2009;196:435–45. doi: 10.1111/j.1748-1716.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int. 2006;70:690–8. doi: 10.1038/sj.ki.5001650. [DOI] [PubMed] [Google Scholar]
- Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–6. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Feng D, Luo Z, Welch WJ, Wilcox CS, Lai EY. Remodeling of Afferent Arterioles From Mice With Oxidative Stress Does Not Account for Increased Contractility but Does Limit Excessive Wall Stress. Hypertension. 2015;66:550–6. doi: 10.1161/HYPERTENSIONAHA.115.05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mizel D, Huang Y, Eisner C, Hoerl M, Thiel M, Schnermann J. Tubuloglomerular feedback and renal function in mice with targeted deletion of the type 1 equilibrative nucleoside transporter. Am J Physiol Renal Physiol. 2013;304:F382–9. doi: 10.1152/ajprenal.00581.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz MS, Wilcox CS. What level of sodium intake worsens renal outcomes? Am J Hypertens. 2014;27:1243–4. doi: 10.1093/ajh/hpu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Kleinmann F, Lai EY, Kupsch E, Skelweit A, Mrowka R. Nitric oxide counteracts angiotensin II induced contraction in efferent arterioles in mice. Acta Physiol Scand. 2004;181:439–44. doi: 10.1111/j.1365-201X.2004.01316.x. [DOI] [PubMed] [Google Scholar]
- Pelayo JC, Westcott JY. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest. 1991;88:101–5. doi: 10.1172/JCI115264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–6. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- Ren Y, D’Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;298:H1769–75. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Garvin JL, Liu R, Carretero OA. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int. 2007;71:861–6. doi: 10.1038/sj.ki.5002161. [DOI] [PubMed] [Google Scholar]
- Smith S, Anderson S, Ballermann BJ, Brenner BM. Role of atrial natriuretic peptide in adaptation of sodium excretion with reduced renal mass. J Clin Invest. 1986;77:1395–8. doi: 10.1172/JCI112447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–87. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- Weir MR, Dengel DR, Behrens MT, Goldberg AP. Salt-induced increases in systolic blood pressure affect renal hemodynamics and proteinuria. Hypertension. 1995;25:1339–44. doi: 10.1161/01.hyp.25.6.1339. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005;46:732–7. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]