Abstract
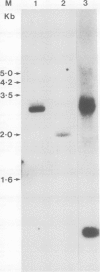
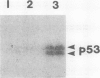
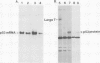
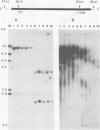
A cDNA clone for human p53 cellular tumor antigen has been isolated and characterized. This clone contains the complete 3'-untranslated region and most of the open reading frame for the protein. Nucleotide sequence analysis revealed that p53 mRNA contains an Alu repeat in the 3'-untranslated region. Hybridization selection experiments showed this clone was capable of selectively binding p53 mRNA. In vitro translation of SV80 mRNA resulted in the synthesis of two immunoreactive p53 polypeptide species. Northern blot analysis showed that human p53 mRNA was 2.8 kb in length and was present in cell lines containing high and low levels of p53 protein. There appears to be only a single p53 gene in human cells and Southern blot analysis demonstrated no major genomic rearrangements or amplification of the p53 gene in the transformed cell lines examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERSPERG N. LONG-TERM CULTIVATION OF HYPODIPLOID HUMAN TUMOR CELLS. J Natl Cancer Inst. 1964 Jan;32:135–163. [PubMed] [Google Scholar]
- Benchimol S., Pim D., Crawford L. Radioimmunoassay of the cellular protein p53 in mouse and human cell lines. EMBO J. 1982;1(9):1055–1062. doi: 10.1002/j.1460-2075.1982.tb01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Blanck G., Pollack R. E. Pre-crisis mouse cells show strain-specific covariation in the amount of 54-kilodalton phosphoprotein and in susceptibility to transformation by simian virus 40. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5670–5674. doi: 10.1073/pnas.80.18.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Bulbrook R. D. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982 Oct 15;30(4):403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Gurney E. G., Goodfellow P., Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. doi: 10.1073/pnas.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J. Different forms of p53 detected by monoclonal antibodies in non-dividing and dividing lymphocytes. Nature. 1984 Jul 12;310(5973):143–145. doi: 10.1038/310143a0. [DOI] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Bienz B., Givol D., Rechavi G., Zakut R. Analysis of recombinant DNA clones specific for the murine p53 cellular tumor antigen. EMBO J. 1983;2(10):1633–1639. doi: 10.1002/j.1460-2075.1983.tb01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Levine A. J. Molecular cloning of a cDNA specific for the murine p53 cellular tumor antigen. Proc Natl Acad Sci U S A. 1983 Jan;80(1):56–59. doi: 10.1073/pnas.80.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Goeddel D. V., Hayflick J. S., Reich N. C., Anderson C. W., Levine A. J. The amino acid sequence of murine p53 determined from a c-DNA clone. Virology. 1984 Apr 30;134(2):477–482. doi: 10.1016/0042-6822(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R., Kaplan L., Reich N., Lane D. P., Levine A. J. Characterization of human p53 antigens employing primate specific monoclonal antibodies. Virology. 1983 Dec;131(2):502–517. doi: 10.1016/0042-6822(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Oren M., Bienz B., Lavie V., Hazum S., Givol D. A single gene and a pseudogene for the cellular tumour antigen p53. Nature. 1983 Dec 8;306(5943):594–597. doi: 10.1038/306594a0. [DOI] [PubMed] [Google Scholar]