Summary
Hearts of mice lacking Isl1, a LIM homeodomain transcription factor, are completely missing the outflow tract, right ventricle, and much of the atria. isl1 expression and lineage tracing of isl1-expressing progenitors demonstrate that Isl1 is a marker for a distinct population of undifferentiated cardiac progenitors that give rise to the cardiac segments missing in isl1 mutants. Isl1 function is required for these progenitors to contribute to the heart. In isl1 mutants, isl1-expressing progenitors are progressively reduced in number, and FGF and BMP growth factors are downregulated. Our studies define two sets of cardiogenic precursors, one of which expresses and requires Isl1 and the other of which does not. Our results have implications for the development of specific cardiac lineages, left-right asymmetry, cardiac evolution, and isolation of cardiac progenitor cells.
Introduction
Congenital heart disease is the most common of all birth defects (Hoffman and Kaplan, 2002). For successful prevention of or therapeutic intervention in congenital heart disease, it is of utmost importance to understand its etiology. Toward this goal, an understanding of the origin of specific cardiac lineages and their interactions with each other is critical. Understanding the origin and properties of cardiac progenitors is also important toward the development of cardiac stem cell therapies for both congenital and adult heart disease.
Recent work has defined two fields of cardiac progenitors, dubbed the primary and secondary, or anterior, heart fields (Kelly and Buckingham, 2002). The primary heart field is believed to give rise to the atria and ventricles of the heart, while the secondary or anterior field is believed to give rise to the outflow tract. The secondary/anterior field is believed to reside anterior and dorsal to the heart at the early linear heart tube stage. Initial evidence that the outflow tract of the heart was not present in the linear heart tube came from a series of in vivo lineage studies performed in chick embryos by de la Cruz and colleagues from 1977 onward (de la Cruz and Sanchez-Gomez, 2000). These studies demonstrated that the outflow tract is not present at the linear heart tube stage but did not indicate where the outflow tract came from at a later stage. In the last year, the source of the outflow tract has been addressed by studies from three different laboratories, two in chick embryos and one in mouse embryos (Kelly and Buckingham, 2002). Results of these studies demonstrated that at least some cells in the outflow tract originate from splanchnic mesoderm adjacent to the pharyngeal endoderm.
Lineage studies performed in chick with mitotracker, a fluorescent dye, or with a lacZ adenovirus demonstrated that lineage tracers injected into the aortic sac region at HH stage 8 or stage 16 were incorporated into the conal (proximal) and truncal (distal) regions of the outflow tract, respectively, demonstrating that outflow tract precursors are found in the aortic sac (Mjaatvedt et al., 2001). Mitotracker dye injected into the middle of the beating straight heart tube at HH stage 8 was found in the right ventricle at HH stage 12, indicating that at stage 8, right ventricular precursors are present in the linear heart tube. In experiments performed by other investigators, mitotracker was injected into the splanchnic mesoderm behind the outflow tract at stage 14 (Waldo et al., 2001). At stage 22, the dye was observed in the proximal conal region, not the truncal region of the heart. Together, these results in chick embryos demonstrate that splanchnic mesoderm adjacent to pharyngeal endoderm migrates in through the aortic sac to contribute cells to the outflow tract of the heart, and that the flow of progenitors along this path gives rise to distinct regions of the outflow tract at different times during development.
In mouse embryos, a lacZ transgene fortuitiously integrated into the fibroblast growth factor 10 (Fgf10) locus marked both the outflow tract and the right ventricle of the embryonic mouse heart (Kelly et al., 2001). Examination of earlier expression of this transgene indicated that at the cardiac crescent stage (ED7.5), lacZ was expressed in splanchnic mesoderm medial and adjacent to splanchnic mesoderm of the classical primary heart field. As development progressed, lacZ-positive cells were observed in anterior splanchnic mesoderm adjacent to pharyngeal endoderm and were subsequently observed in branchial arch mesoderm proximal to the heart, in the outflow tract, and in the right ventricle. This expression pattern largely mimicked that of the endogenous Fgf10 gene. DiI lineage tracers injected through foregut endoderm (ED8.25) or in the second branchial arch (ED9.5) were observed in cells within the proximal or distal part of the outflow tract, respectively, but were not observed in the right ventricle. These lineage studies in mouse embryos demonstrated that some cells in the region of the early foregut endoderm at ED8.25, or the second branchial arch at ED9.5, are incorporated into the outflow tract, and suggested that Fgf10 expression marked splanchnic mesodermal precursors of the anterior heart field.
Expression of Fgf10 and the Fgf10-lacZ transgene in both outflow tract and right ventricle raised the question as to whether both of these cardiac segments arose from a common developmental field. The lineage tracer studies in chick embryos did not address the origin of the right ventricle, although they demonstrated that right ventricular precursors are present in the linear heart tube at HH stage 8, a stage when outflow tract progenitors are still migrating into the heart.
Together, these studies in both chick and mouse embryos demonstrated that cells comprising the earliest fusing myocardium do not contain all progenitors of the outflow tract. Rather, this “primary” myocardium was supplemented by addition of cells from a secondary, or anterior, heart field, which contributed wholly or in part to the outflow tract (Kelly and Buckingham, 2002). The developmental history of the right ventricle relative to these two heart fields remained in question. How substantial the contribution of cells from the anterior heart field to the outflow tract was also unknown, as was the precise location of the anterior heart field.
Our analysis of mice homozygous null for the LIM homeodomain transcription factor isl1 offers a somewhat different perspective that might be brought to these outstanding questions concerning the primary and anterior heart fields. We suggest that it may be useful to define two cardiogenic fields, but in slightly different terms. One progenitor population expresses isl1 and will give rise to the outflow tract, right ventricle, a subset of left ventricular cells, and, surprisingly, a large number of atrial cells as well. The other does not express isl1 and will give rise to most of the left ventricle as well as atrial cells. We present evidence suggesting that progenitors of the outflow tract, right ventricle, and a majority of atrial progenitors express Isl1 and proliferate prior to the onset of differentiation. The specific expression of isl1 in undifferentiated precursors also allows a precise visualization of the isl1-expressing progenitor population and gives us an important handle for the isolation and characterization of a cardiac progenitor cell population. Isl1 not only defines this progenitor cell population, but is also required for these cells to contribute to the heart.
Results
Heart Development Is Severely Abnormal in Mice that Are Homozygous Null for Isl1
isl1 knockout mice have been examined for defects in both motor neuron and pancreatic development (Ahlgren et al., 1997; Pfaff et al., 1996). Mice that are homozygous null for isl1 exhibit growth retardation at approximately ED9.5 and die at approximately ED10.5. Heterozygous mutants survive and have no apparent phenotype. The cause of death in homozygous mutants has not previously been addressed, although vascular abnormalities were suspected (Pfaff et al., 1996). We therefore examined the cause of death in isl1−/− mice.
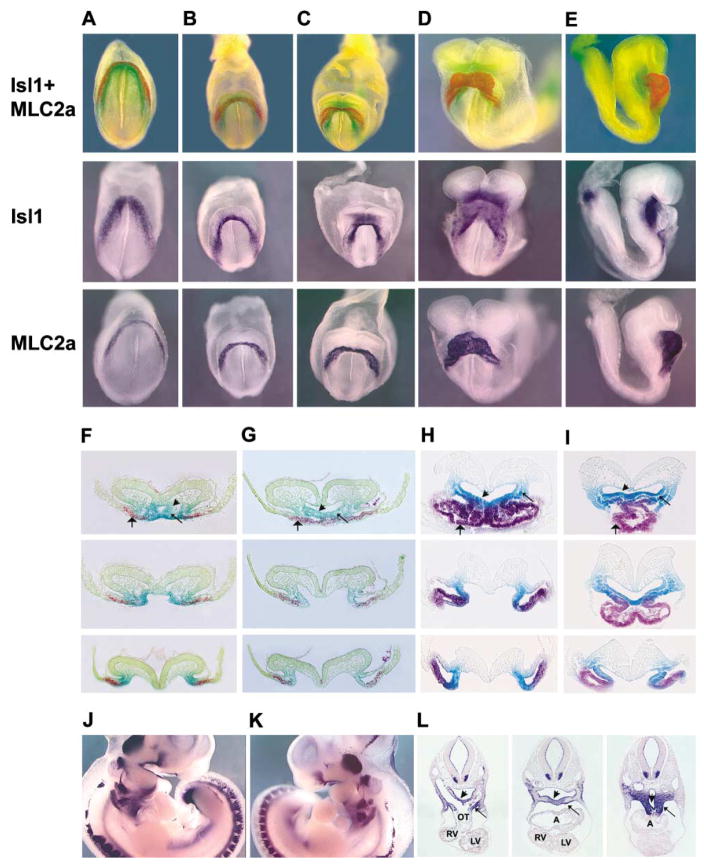
We examined homozygous null embryos between ED9.0 and ED9.5 and found that hearts were severely abnormal. At a gross morphological level, mutant hearts appeared misshapen and unlooped. Histological analysis confirmed this impression (Figure 1). As an initial attempt to characterize chamber identity of cells within mutant hearts, we performed whole-mount in situ hybridization analysis with markers for cardiac chambers. At these stages, atrial myosin light chain 2 (MLC2a) mRNA marks all myocardial cells (Kubalak et al., 1994). Ventricular myosin light chain 2 (MLC2v) mRNA specifically marks ventricular cells and cells of the A/V junction (Franco et al., 1999). Hybridization with probes for MLC2a (Figures 1A–1D) and MLC2v (Figures 1E–1H) mRNAs demonstrated that, in isl1 mutants, cells within the anterior part of the mutant heart had ventricular identity (Figure 1H), whereas cells in the posterior part did not (Figure 1H) and were therefore likely to have atrial identity, as they were positive for MLC2a staining (Figure 1D).
Figure 1. Whole-Mount mRNA In Situ, Histological, and Scanning EM Analyses of Wild-Type Littermates and isl1 Homozygous Mutants.
Wild-type littermates are indicated by +/+ and homozygous null isl1 mice are indicated by −/−.
(A–H) Embryos of ED9.5 (20–21 somite pairs) were whole-mount stained with digoxigenin-labeled riboprobes for MLC2a (A and C) or MLC2v
(E and G). Left, frontal, and right views of each embryo are shown in respective panels. Corresponding sections are also shown, progressively from anterior to posterior (B, D, F, and H). Results of this analysis demonstrated aberrant cardiac morphology in −/− mice and overall reduction in cardiac tissue.
(I–P). Specific probes utilized are indicated below each panel, and specific regions of interest are indicated by white arrows. (I and M) Front view of ED 9.5 (23 somite pairs) embryo hybridized with a probe for tbx5 mRNA. (J and N) Front view of ED8.5 (11 somite pairs) embryo hybridized with a probe for EHand. (K and O) Right side view of ED9.5 (23 somite pairs) embryo hybridized with a probe for fgf10. (L and P) Right side view of ED9.5 (23 somite pairs) embryo hybridized with a probe for wnt11.
(Q–T) Scanning electron microscopy analysis was consistent with these findings, as shown. Frontal views of embryos of ED8.75 (12 somite pairs) (Q and S) and ED9.5 (22 somite pairs) (R and T) are shown. Note that in isl1 mutants at 12 somite pairs (S), heart primordia closely resemble those of wild-type embryos of 5–6 somite pairs (Kaufman, 1999). At the 22 somite pairs stage, isl1 mutant hearts (T), when compared to those of wild-type littermates (R), appear to be lacking outflow tract and right ventricular segments, consistent with marker analysis. Atrial (A) and ventricular (V) segments of mutant heart shown in (T) have been labeled according to results of marker analysis.
Abbreviations: A, atria; LA, left atria; LV, left ventricle; OT, outflow tract; RA, right atria; RV, right ventricle; SV, sinus venosus; and V, ventricle.
A number of transcription factors are regionally expressed within the heart, and we utilized a panel of these to further explore cellular identity within isl1 mutant hearts. At stages examined, tbx5 is specifically expressed in the posterior pole of the heart, in atria and left ventricle (Bruneau et al., 1999). In isl1 mutants, both atrial and ventricular segments of the heart expressed tbx5, suggesting that ventricular portions of the mutant heart had left ventricular identity (Figures 1I and 1M). EHand is expressed strongly in left ventricle and very little in right ventricle (Cross et al., 1995; Cserjesi et al., 1995; Thomas et al., 1998). In isl1−/− embryos, EHand was strongly expressed throughout the ventricular tissue, suggesting that it had left ventricular identity, not right ventricular identity, consistent with results obtained with the tbx5 probe (Figures 1J and 1N). fgf10 is highly expressed in the outflow tract (Kelly et al., 2001) and in right ventricular precursors (Figure 1K). Hearts from isl1 mutants were missing Fgf10 expression, suggesting an absence of outflow tract and right ventricular identities in the mutant hearts (Figures 1K and 1O). Results consistent with this were obtained with a probe for wnt11, which marks the myocardium of the outflow tract of the heart at ED8.5 (Figure 1L). isl1 mutant hearts had no cardiac staining of wnt11 (Figures 1L and 1P). Together with the previous results from hybridization with probes for MLC2a and MLC2v, these data suggested that isl1 mutants were lacking an outflow tract and right ventricle, although cells with left ventricular, A/V canal, and atrial identities were present (Figure 1). Assessment by section analysis demonstrated a significant reduction in the amount of atrial tissue in isl1 mutants relative to somite-matched control littermates.
From this analysis, we inferred that isl1 mutants were missing complete segments of the heart. Additionally, mutant hearts had not undergone looping. This impression was strengthened by scanning electron microscopy analysis (Figures 1Q–1T). Intriguingly, cardiac primordia in isl1 mutants at ED8.75 (12 somite pairs) (Figure 1S) resembled cardiac primordia seen in wild-type embryos at earlier stages, at ED8.25 (5 somite pairs) (Kaufman, 1999), suggesting an interruption in heart development. A comparison of wild-type littermates to their mutant counterparts at ED9.5 (22 somite pairs) (Figures 1R and 1T) suggested an absence of outflow tract and right ventricle in mutants, consistent with marker analysis.
isl1 Expression during Heart Development
The severe cardiac phenotype of isl1−/− mice led us to investigate expression of isl1 during early stages of mouse heart development. We performed single and double whole-mount in situ hybridization on embryos from ED7.25 to ED8.75, utilizing probes for isl1 and MLC2a mRNAs. The latter is one of the earliest markers for differentiated cardiomyocytes. Results of this whole-mount in situ and subsequent section analysis demonstrated that isl1 is not co-expressed with MLC2a, but rather is expressed in an immediately adjacent population of cells (Figure 2). At the early cardiogenic crescent stages, isl1-expressing cells are contiguous with, but medial and dorsal to, MLC2a-expressing cells (Figures 2A, 2B, 2F, and 2G). As the heart tube forms, isl1-positive cells within splanchnic mesenchyme comprising the mesocardium and adjacent to foregut endoderm continue to be contiguous with MLC2a-positive cells throughout their extent, including anterior and posterior regions (Figures 2C–2E, 2H, and 2I). isl1 is expressed in both splanchnic mesoderm and in ventral foregut endoderm (Figures 2F–2I). At E10, isl1 continues to be expressed in ventral endoderm and splanchnic mesoderm but is not expressed in the myocardium of the heart (Figures 2J–2L).
Figure 2. Double and Single Whole-Mount In Situ Analyses of Embryos Stained for isl1 and MLC2a mRNAs.
(A–E) Embryos were doubly stained for isl1 mRNA (green) and MLC2a mRNA (red), or singly stained for Isl1 or MLC2a, as indicated to the left of each panel. (A) ED7; (B) ED7.5; (C) ED8.0 (2 somite pairs); (D) ED8.25 (5 somite pairs); (E) ED8.5 (8 somite pairs).
(F–I) Sections of embryos doubly stained for isl1 mRNA and MLC2a mRNA. (F and G) isl1 mRNA was detected with BCIP, which resulted in light green, and MLC2a mRNA was detected with fast red, which resulted in red. (H and I) isl1 mRNA was detected with magenta phos-tet red to give purple, and MLC2a was detected with BCIP/ferricyanide/ferrocyanide to give dark blue (MLC2a). For technical details, see Experimental Procedures. Stages of sectioned embryos correspond to those shown in whole mount. (F) ED 7.5; (G) ED8.0 (2 somite pairs); (H) ED8.25 (5 somite pairs); (I) ED8.5, (8 somite pairs). isl1 and MLC2a mRNAs are nonoverlapping, with isl1 mRNA being expressed in foregut endoderm (short arrow) and in splanchnic mesoderm (thin long arrow) and MLC2a mRNA being expressed in differentiated myocardium (wide long arrow).
(J–L) Staining for isl1 mRNA was performed on embryos of ED10 (26 somite pairs), shown in whole mount from the right (J) and from the left (K), and corresponding sections from anterior, mid, and posterior levels shown in (L). Isl1 mRNA continues to be expressed in foregut endoderm (short arrow) and in splanchnic mesoderm (thin long arrow) but is not observed in myocardium. Abbreviations: A, atria; LV, left ventricle; OT, outflow tract; RV, right ventricle.
Lineage Analysis of isl1-Expressing Cells
Although isl1 was not expressed in differentiated MLC2a-positive myocardial precursors, it was expressed in the region of the recently identified secondary or anterior heart field, that is, splanchnic mesodermal cells of the pharyngeal region. Recent evidence has suggested that the anterior heart field in mouse contributes to the outflow tract (Kelly and Buckingham, 2002). This observation, in concert with the cardiac phenotype in isl1 mutants, suggested that isl1-expressing cells might contribute to the outflow tract of the heart.
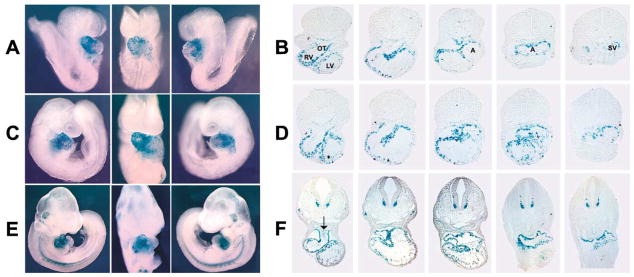
To investigate this question, we performed lineage analysis of isl1-expressing cells, by crossing an isl1-cre mouse (Srinivas et al., 2001) with a CMV β-actin-nlacZ indicator mouse (Zinyk et al., 1998). In progeny of this cross, Cre-mediated excision brings the nlacZ gene under the control of the ubiquitously expressed CMV β-actin-lacZ transgene, enabling the fate of isl1-expressing cells to be followed by staining for β-galactosidase activity, even when transcription from the endogenous isl1 locus has been repressed. Results of this analysis were startling and demonstrated that cells which previously expressed isl1 make a substantial contribution to the embryonic heart, comprising a majority of cells in the outflow tract, right ventricle, and atria, and also contribute to specific regions of the left ventricle (Figure 3). The β-galactosidase-positive cells were also observed within the endocardium (Figure 3F) and within a few endothelial cells lining the aortic arch arteries (data not shown). The majority of β-galactosidase-negative myocardial cells were observed within the ventral aspect of the left ventricle and the anterior ventral region of the left atria. Quantitative analysis of sections from ED9.75 hearts revealed that approximately 97% of cells within the outflow tract, 92% of cells within right ventricle, 65% of cells within left atria, 70% of cells within right atria, and less than 20% of cells within left ventricle stained positively for β-galactosidase.
Figure 3. Lineage Analysis of isl1-Expressing Cells during Early Heart Development.
CMV β-actin-lacZ indicator mice (Zinyk et al., 1998) were crossed to isl1-cre mice (Srinivas et al., 2001) to visualize the fate of isl1-expressing cells by X-gal staining. Progeny were harvested at different embryonic stages and stained with X-gal. Whole embryos are shown progressively in left, frontal, and right views. Corresponding sections are shown, progressing from anterior to posterior at (A and B) ED8.5 (10 somite pairs); (C and D) ED9.0 (13 somite pairs); and (E and F) ED10.25 (27 somite pairs). The arrow in (F) indicates X-gal-positive cells within the endocardium of the outflow tract. Abbreviations: A, atria; OT, outflow tract; LV, left ventricle; RV, right ventricle; SV, sinus venosus.
Reduction in the Number of isl1-Expressing Cells in isl1−/− Mutants
The observation that isl1-expressing cells contribute a majority of cells to the developing heart was consistent with our previous analysis of the cardiac phenotype in isl1 homozygous mutant mice, where whole segments of the heart were missing. The missing structures suggested that Isl1 might be required for proliferation, survival, and/or migration of isl1-expressing cardiogenic precursors. To address this question, we wanted to examine isl1-expressing cells within isl1 mutants and littermate controls. Although targeting of the isl1 gene deleted the third exon, containing the second LIM domain, the 5′ end of isl1 mRNA is still expressed in the mutant, enabling detection of isl1 message in mutant cells. Isl1 protein, however, is not detectable (Pfaff et al., 1996).
To track isl1-expressing cells in mutant and wild-type embryos, we performed whole-mount in situ hybridization analysis on embryos from ED8.5 to ED10 with a probe for isl1 mRNA. Results of this analysis demonstrated that there are progressively fewer isl1-expressing cells in the mutant, although some cells still remain (Figure 4). The reduced expression of isl1 mRNA is accompanied by an overall reduction of tissue in mutants (Figures 4G–4L). In conjunction with the cardiac phenotype of isl1 mutants, these results suggest that Isl1 is required for cell proliferation and/or cell survival.
Figure 4. Detection of isl1-Expressing Cells by Whole-Mount In Situ Analysis of isl1 mRNA in Wild-Type and isl1 Homozygous Mutants.
Wild-type littermates are indicated by +/+, and homozygous null isl1 mice are indicated by −/−. Whole-mount views are shown from left and right, with corresponding sections below, progressing from anterior to posterior. (A, D, G, and J) ED8.5 (8 somite pairs); (B, E, H, and K) ED9.0 (15 somite pairs); (C, F, I, and L) ED9.75 (25 somite pairs). The arrows in each figure indicate regions where, in the wild-type, a greater number of cells is present than in the mutants. Stars in (I) and (L) draw attention to the absence of isl1 expression in motor neurons in isl1 mutants, reflecting absence of motor neurons (Pfaff et al., 1996).
Isl1 Is Required for Proliferation and Survival of Cells within Pharyngeal Foregut Endoderm and Adjacent Splanchnic Mesoderm
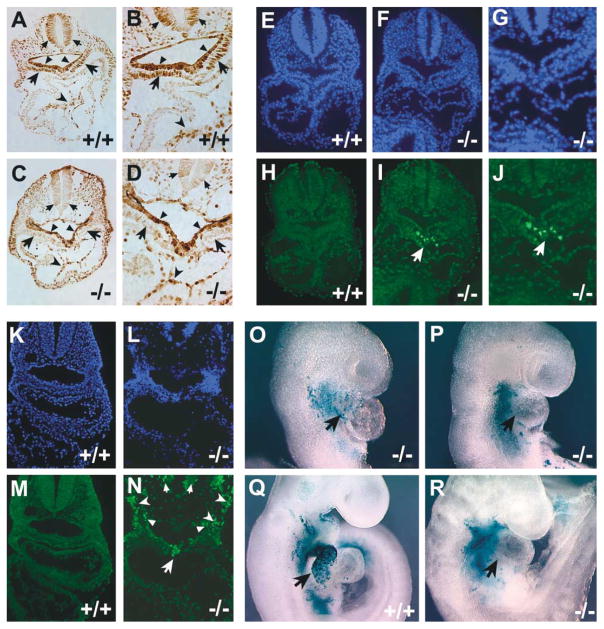
To further investigate the requirement for Isl1 in proliferation or survival of cardiac progenitors, we performed BrdU labeling studies and apoptosis analyses in isl1 mutant and control littermates at ED8.75 and ED9.5. BrdU labeling demonstrated that proliferative indices were significantly less in isl1 mutants relative to their controls in both pharyngeal endoderm and splanchnic mesoderm, where isl1 is normally expressed (Figures 5A–5D). Quantitative analyses indicated that the mesoderm was somewhat more affected, with an average 76% reduction in the percentage of BrdU-positive cells within the splanchnic mesoderm and a 39% reduction in the percentage of BrdU-positive cells within pharyngeal foregut endoderm of isl1 mutants relative to somite-matched wild-type littermate controls.
Figure 5. BrdU Labeling, TUNEL Analysis, and Lineage Labeling in the isl1 Mutant Background.
Wild-type littermates are indicated by +/+, and homozygous null isl1 mice are indicated by −/−.
(A–D) Sections from ED8.75 (12 somite pairs) BrdU-labeled embryos shown at low (A and C) and higher (B and D) magnification. Arrows indicate splanchic mesoderm, where proliferation indices were most strongly adversely affected in mutant embryos; unnotched arrowheads indicate foregut endoderm, which also exhibited lower proliferative activity in isl1 mutants relative to control littermates; notched arrowheads indicate endocardium, where proliferation was unaffected in isl1 mutants relative to control embryos.
(E–N) TUNEL analysis performed on sections from embryos of ED8.75 (12 somite pairs) (E–J) and ED9.5 (22 somite pairs) (K–N). (E–G, K, and L) DAPI staining to visualize nuclei. (H–J, M, and N) Fluorescein-labeled dUTP was utilized to visualize nicked DNA.
(O–R) Lineage analysis of isl1-expressing cells in an isl1 mutant background. Isl1+/−; ROSA26-lacZ indicator doubly heterozygous mice were crossed to isl1-cre mice to obtain mice which were either heterozygous mutant or homozygous mutant for isl1 and carried both the isl1-cre and ROSA26-lacZ indicator alleles.
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) was performed to examine whether apoptosis was also contributing to the reduction in cell number observed in isl1 mutants. TUNEL-positive cells were not observed in wild-type control embryos at ED8.75 or ED9.5 (Figures 5E, 5H, 5K and 5M). However, apoptotic cells were clearly observed in isl1 mutants (Figures 5F, 5G, 5I, 5J, 5L, and 5N). At ED8.75, TUNEL-positive staining was observed in an isl1-expressing population of splanchnic mesodermal cells immediately adjacent to pharyngeal endoderm (Figures 5I and 5J); at ED9.5, TUNEL labeling was more extensive but was still confined to cells that normally express isl1, including cells within endoderm and mesoderm (Figure 5N). No TUNEL staining was observed in myocardium of isl1 mutants.
Isl1 Is Required for Migration of Cardiac Progenitors into the Heart
To determine whether isl1-expressing progenitors were able to migrate into the heart in an isl1 mutant background, we performed lineage analysis of isl1-expressing cells in embryos that were homozygous null for Isl1. The isl1-cre mouse is effectively a null allele, with a phenotype indistinguishable from that of the original isl1 knockout, facilitating this analysis. Results demonstrated that isl1-expressing progenitors are not observed in the myocardium of isl1 mutants, in contrast to results with wild-type littermates (Figures 5O–5R). This observation demonstrated that isl1 mutant cells do not migrate into the heart.
Potential Downstream Targets of Isl1 Action
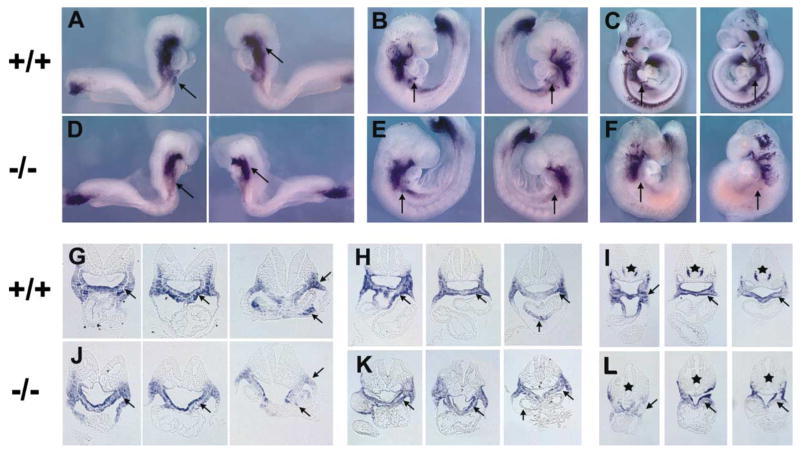
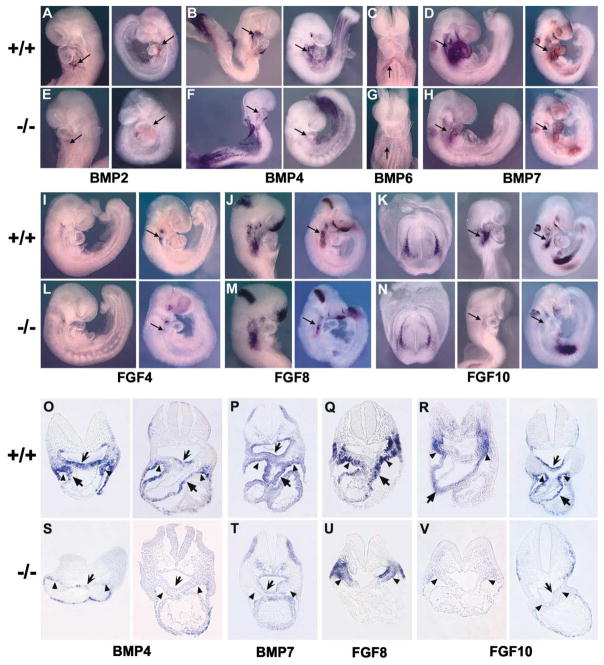
Our results suggest that the transcription factor Isl1 is required for cell proliferation and survival of cardiogenic precursors and that downstream targets of Isl1 are mediating this effect. Two growth factor pathways that have been implicated in growth and survival of cardiogenic precursors in both vertebrate and invertebrate heart development are bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) (Kirby, 2002; Yutzey and Kirby, 2002). A number of BMPs and FGFs have been described as being expressed in embryonic regions that overlap with and/or are adjacent to isl1-expressing cells, including BMPs 2, 4, 6, and 7 and Fgfs 4, 8, and 10 (Crossley and Martin, 1995; Dudley and Robertson, 1997; Kelly et al., 2001; Lyons et al., 1995; Niswander and Martin, 1992). To determine if any of these are targets of Isl1 action, we performed whole-mount in situ hybridization with probes for these growth factor genes, followed by section analyses (Figure 6). Results demonstrated a decrease in expression in each of these genes in isl1 null mice. Expression of some of these growth factors was severely downregulated or undetectable in pharyngeal endoderm and splanchnic mesodermal regions that are overlapping or adjacent to isl1 expression, including expression of BMP4, BMP7, and Fgf10. These genes are likely to be direct or indirect targets of Isl1. Expression of the other BMP and FGF genes was still present, but the domain of expression was decreased in regions overlapping or contiguous with isl1 expression, similar to results observed with isl1 mRNA in isl1 mutants (Figure 4). This decrease may reflect a decrease in the number of cells that express these growth factors, or a partial downregulation of expression.
Figure 6. Whole-Mount In Situ Analyses to Detect BMP and FGF mRNAs in Wild-Type Littermates and isl1 Homozygous Mutants.
Embryos of at least two stages were hybridized to probes for BMPs and FGFs. Wild-type littermates are indicated by +/+, and homozygous null isl1 mice are indicated by −/−. The mRNAs detected are shown below each relevant group of embryos. From left to right, the stages shown are (A and E) ED8.75 (11 somite pairs), ED9.5 (23 somite pairs); (B and F) ED8.5 (9 somite pairs), ED9.0 (16 somite pairs); (C and G) ED8.75 (12 somite pairs); (D and H) ED9.0 (15 somite pairs), ED9.5 (23 somite pairs); (I and L) ED9.0 (16 somite pairs); (J and M) ED8.5 (9 somite pairs), ED9.5 (21 somite pairs); (K and N) ED8.5 (8 somite pairs), ED9.5 (22 somite pairs). For each mRNA examined, there was a reduction in expression in isl1 mutants, as indicated by arrows (O–V). Section analyses of whole-mount embryos stained for BMP4, BMP7, fgf8, and fgf10. Notched arrows indicate pharyngeal endoderm, triangular arrowheads indicate splanchnic mesoderm, and solid arrows indicate outflow tract myocardium. (O and S) BMP4 mRNA expression at ED8.5 (left) and ED9.0 (right); (P and T) BMP7 mRNA expression at ED9.0; (Q and U) fgf8 mRNA expression at ED9.0; (R and V) fgf10 mRNA expression at ED8.5 (left) and ED9.5 (right).
Discussion
A Working Model of Early Heart Development
Our data demonstrate that Isl1-expressing progenitors give rise to the outflow tract as well as a majority of cells in both the right ventricle and the atria, and some cells within the left ventricle. Thus, the previously described secondary or anterior heart field appears to be a subset of this progenitor population. Isl1 function is required for these cells to contribute to the heart. In the absence of Isl1, hearts that form are missing segments normally contributed by isl1-expressing progenitors. In distinction, progenitors that will give rise to the majority of cells of the left ventricle and some atrial cells do not express isl1 and are capable of giving rise to cardiac cells of these identities in the absence of Isl1 function.
The appearance and characteristics of the heart in isl1 mutants, and our analysis of isl1 expression and fate mapping of isl1 progenitors, have led us to a working model of heart development, as shown in Figure 7. Previous studies had demonstrated that some cells from the anterior/secondary heart field migrate in to form the outflow tract following differentiation of the primary heart field. The extent of this contribution to the outflow tract, and exactly where the progenitors resided, were unknown. Our studies have demonstrated that undifferentiated Isl1 progenitors reside throughout the anterior posterior extent of splanchnic mesenchyme dorsal to the heart and are continuously migrating in not only to form anterior segments of the heart, the right ventricle, and outflow tract, but also to significantly contribute to more posterior segments, the atria.
Figure 7. Working Model of Early Heart Development.
isl1-expressing cells are shown in green; MLC2a-positive cells derived from non-isl1-expressing progenitors are shown in red; MLC2a-positive cells derived from isl1-expressing progenitors are shown in purple. (A) Embryo of 3–4 somite pairs; (B) embryo of 6–8 somite pairs; (C) embryo of 11–13 somite pairs. From left to right, embryos are shown in frontal, then lateral views, followed by two cross-section views. Lines drawn through the frontal views indicate the anterior-posterior position of the corresponding sections (a–f). Arrows indicate the direction of migration of isl1-expressing progenitors. (D) Flow chart diagramming the role of Isl1 in heart formation.
In this model, the first protruding segments of cardiogenic mesoderm at the midline, at 3–4 somite pairs, are the first to differentiate, do not derive from isl1-expressing cells, and will give rise to a majority of cells within the left ventricle and some of the adjacent atrium. isl1-expressing progenitors migrate in, progressively differentiating and downregulating isl1 expression as they join the “first wave” of heart progenitors, to form the majority of cells of the right ventricle, outflow tract, and remainder of the atria. It should be noted that a substantial number of descendents of isl1-expressing progenitors were also found within the left ventricle, at the junctional region with the right ventricle, within trabeculae, and along the wall of the inner curvature, descending slightly into the dorsal wall of the left ventricle.
During earliest stages of heart development, from presomite through to the 9–11 somite pairs stage, Isl1 cells migrate in throughout the anterior-posterior extent of the myocardium, when adjacent splanchnic mesenchyme is contiguous with differentiating myocardium (Figures 7A and 7B). From 11 to 13 somite pairs, isl1 progenitors migrate into the heart through the two regions that remain connected to the splanchnic mesenchyme of the dorsal body wall. Anteriorly, this is the region of the aortic sac, and posteriorly, the dorsal mesocardium (Figure 7C).
Previous anatomical analysis of human heart development utilizing molecular markers has suggested that extracardiac mesenchyme, which migrates in through the dorsal mesocardium, contributes to both atrial and atrioventricular septation (Lamers and Moorman, 2002). There is controversy as to whether the mesenchymal cap on the leading edge of the primary atrial septum originates from this extracardiac mesoderm or derives from cushion endothelium. This question can now be addressed by isl1 lineage analysis. Furthermore, it will be of interest to investigate the role of isl1-derived myocardial cells in cardiac septation generally.
The Role of Isl1 in Heart Development
In isl1 mutants, there is a severe reduction in cardiac tissues, with some entire segments missing. This could be owing to defects in proliferation, survival, or migration. We have performed experiments to address each of these possibilities, and from our results, we conclude that each of these aspects is affected in isl1 mutants. In contrast to results with isl1-expressing cells in isl1 mutants, myocardium in isl1 mutants, derived from non-isl1-expressing lineages, evidenced no defects in proliferation and no cell death.
isl1 expression is downregulated and extinguished as precursors differentiate, indicating that in cardiogenic precursors, Isl1 function may be required to maintain an undifferentiated state and/or may be incompatible with a differentiated state. Isl1 is required for generation of motor neurons but continues to be expressed and functions in a subset of differentiated motor neurons (Pfaff et al., 1996). In pancreatic development, Isl1 function is required in both pancreatic mesenchyme and in differentiated islet cells (Ahlgren et al., 1997).
Growth of the heart following myocyte differentiation has led to the belief that extensive proliferation of differentiated myocytes occurs to account for myocardial growth. The migration of isl1-expressing precursors into the heart suggests that some growth of the heart can also be accounted for by this migration. Additionally, our BrdU-labeling studies demonstrated higher proliferative rates within isl1-positive splanchnic mesenchyme than within MLC2a-positive myocardium at early stages of heart development. However, in isl1 mutants, non-isl1-expressing progenitors differentiate and undergo an expansion, suggesting that proliferation and migration of undifferentiated progenitors and proliferation of differentiated myocytes both play a role in cardiac growth.
Our studies demonstrate that Isl1 defines and is required for one pool of cardiogenic precursors. It is of interest to understand other genes that may be similarly required in non-isl1-expressing cardiac progenitors. In this context, it is intriguing to note that the nkx2.5 knockout mouse has a mutant heart that has an outflow tract, right ventricular cells, and atrial cells (Harvey et al., 1999; Tanaka et al., 1999). A number of markers for left ventricular identity are absent, suggesting absence of left ventricular identity. These observations raise the possibility that Nkx2.5 is required for formation of cardiac tissue from non-isl1-expressing progenitors. Nkx2.5 may also play a role in the isl1-expressing heart field, although it clearly is not required in the manner that Isl1 is, given the contrasting phenotypes of isl1 and nkx2.5 null mice. We are currently creating mice that are doubly mutant for isl1 and nkx2.5 to assess these possibilities.
We have demonstrated that both pharyngeal endoderm and splanchnic mesoderm are adversely affected in isl1 mutants, in terms of proliferation, cell survival, and expression of growth factors. The effects on foregut endoderm are interesting in that this tissue has been shown to play an important role in heart development (Lough and Sugi, 2000). However, our studies do not directly address the cell autonomous requirement for Isl1 with regard to heart development. We are currently performing studies to address this question.
Left-Right Cardiac Asymmetry
Looping of the heart occurs in a characteristic left-right orientation, with the outflow tract and right ventricle looping rightward. Perturbation of left-right axis information can result in situs inversus of the heart, a leftward looping of the outflow tract and right ventricle. Atrial isomerism can also result. Our data suggest that left-right information imparted to isl1-expressing precursors will be a critical determinant of left-right cardiac asymmetry. Previous analysis of genetic markers has suggested that initial left-right axis information is preserved in the arrangement of the atria but is rotated in the ventricles. That is, the “left” and “right” ventricles do not strictly correspond to the left and right body axis (Campione et al., 2001; Franco et al., 2000). Our finding that the left and right ventricles derive from distinct cardiogenic progenitor populations gives further insight into this observation.
Downstream Targets of Isl1
Absence of Isl1 resulted in reduction of the number of isl1-expressing cardiogenic precursors, suggesting that growth factor signaling may be perturbed. Our results with FGF and BMP growth factors suggest that these genes are likely to be direct or indirect targets of Isl1 action.
isl1 mutants exhibited an overall reduction in the domain of fgf8 expression, but their phenotype was more severe than that seen with fgf8 hypomorphs. Mouse knockouts of fgf4 or fgf8 are very early embryonic lethal, but mice that are hypomorphic for fgf8 die neonatally due to cardiovascular defects, including malformations of the outflow tract (Abu-Issa et al., 2002; Feldman et al., 1995; Frank et al., 2002; Sun et al., 1999). In isl1 mutants, fgf10 expression was present at ED8.5, but by ED9.5 was absent in regions overlapping or adjacent to isl1-expressing cardiogenic precursors. Mouse knockouts of fgf10 die neonatally, which has been ascribed to their lung phenotype (Sekine et al., 1999). However, there may be an as yet undescribed cardiac phenotype, albeit clearly not as severe as the isl1 cardiac phenotype.
BMP4 and BMP7 are co-expressed in a highly overlapping manner with isl1-expressing cardiogenic precursors. BMP2 and BMP6 are expressed in a distinctive manner from BMP4, BMP7, or each other, but their expression also overlaps with that of isl1. Knockouts of each of these BMPs have been made, and double knockouts of BMP6/7 have been made (Kim et al., 2001; Winnier et al., 1995; Zhang and Bradley, 1996). None of these mutants recapitulates the cardiac phenotype of the isl1 mutant. In some cases, mutants exhibit defects in implantation or gastrulation, precluding analysis of their specific effects on heart formation. Several BMP mutants that survive the earlier defects exhibit cardiac defects, but none comparable to those observed in isl1 mutants, perhaps due to redundancy with extant BMPs.
Our results suggest that the cardiac phenotype in isl1 mutants may be all or in part due to defects in either FGF or BMP signaling or both. Discriminating between these possibilities will require future experiments to ablate these signaling pathways in isl1-expressing progenitors. Additionally, other growth factor pathways may be affected in isl1 mutants. We are also investigating this possibility.
Evolution of the Heart
Expression of isl1 in the splanchnic mesoderm of the pharyngeal and foregut region is intriguing in view of a number of experiments which have suggested that the vertebrate heart has evolved from pharyngeal or gut mesoderm (Haun et al., 1998; Park et al., 1998; Ranganayakulu et al., 1998). Previous data has demonstrated that isl1 is expressed in cardiogenic precursors in chick (Yuan and Schoenwolf, 2000). There is a Drosophila homolog of isl1 that, as for mouse isl1, has a role in motor neuron development, and, intriguingly, is expressed in the dorsal vessel (Thor and Thomas, 1997). It will be of great interest to examine the role of isl1 in cardiogenesis in other species, to gain further insight into cardiac evolution. In this respect, it will be of interest to determine whether formation of the single ventricle in zebrafish is isl1 dependent or independent.
Cardiac Progenitor Cells
Perhaps one of the most exciting aspects of our discovery of the role of Isl1 in cardiogenesis is the prospect of utilizing Isl1 as a marker for an undifferentiated cardiac progenitor state. Cell sorting on the basis of Isl1 expression will allow new characterization of these progenitors. Additionally, Isl1’s role in dictating the proliferation and/or survival of these cells suggest that Isl1, in concert with other factors, may be utilized to drive a cardiac progenitor cell state.
Experimental Procedures
Mouse Mutants
The generation of isl1 null mutants has been previously described (Pfaff et al., 1996). The knockout construct deleted the exon encoding the second LIM domain of Isl1. isl1-cre mice were generously provided by Thomas M. Jessell and have been previously described (Srinivas et al., 2001). An IRES-cre cassette was inserted into the exon encoding the second LIM domain of Isl1, disrupting isl1 gene expression. ROSA26-lacZ indicator mice were generated by Phil Soriano (Soriano, 1999).
Whole-Mount RNA In Situ Hybridization
Whole-mount RNA in situ hybridization was carried out as previously described (Wilkinson, 1992). References for specific RNA probes that were used are as follows: MLC2a (Kubalak et al., 1994); MLC2v (O’Brien et al., 1993); tbx5 (Bruneau et al., 1999); tbx20 (our own unpublished results); BMP2, BMP6, BMP7, BMP4, and BMP5 (Kim et al., 2001; Lyons et al., 1995); FGF4, FGF8, and FGF10 (Feldman et al., 1995; Sun et al., 1999); EHand (Cross et al., 1995); and isl1 (EST, GenBank Accession No.: AA198791). Double RNA in situ hybridization was performed utilizing digoxigenin- and fluorescein-labeled probes that were conjugated with alkaline phosphatase (Roche Cat. #1277073, 1685619). Staining reactions were performed with BCIP/ferricyanide/ferrocyanide according to Janet Rossant’s lab protocols website (http://www.mshri.on.ca/rossant/protocols/doubleINsitu.html) and magenta phos-tet red according to Claudio Stern’s lab protocols website (http://sternlab.anat.ucl.ac.uk/INSITU.htm) or with fast red (Roche Cat. #1496549) and BCIP alone. After incubation with and staining to detect the first antibody (Anti-Fluorescein-AP, Roche Cat. #1426338), embryos were incubated at 65°C for 1 hr to inactivate alkaline phosphatase activity and washed before incubating with and staining to detect the second antibody (Anti-Digoxigenin-AP, Roche Cat. #1093274). For embryos stained with fast red, which is soluble in alcohol, cryosections were prepared. For embryos stained with BCIP/Ferricyanide/Ferrocyanide and MagentaPhos-tet Red, paraffin sections were prepared, with brief washes in alcohol to minimize loss of signal.
Scanning Electron Microscopy
A standardized procedure for scanning electron microscopy (SEM) of the heart was utilized (Pexieder, 1986). Briefly, embryos were submitted to ethanol dehydration and critical point drying from Freon 113 to Freon 23. Dried specimens were mounted on SEM tubes, ion sputtered with 300 nm gold, and examined in the scanning electron microscope. SEM photomicrographs were taken in standard orientations and magnifications.
Cell Proliferation Analysis
Timed pregnant mice were injected intraperitoneally with BrdU (Amersham-Pharmacia, Buckinghamshire, UK) and killed 2 hr after injection. PFA-fixed paraffin sections were denatured with 2N HCl, trypsinized, and incubated with a mouse monoclonal antibody to BrdU (Sigma, B 2531). Detection was performed utilizing a peroxidase ABC kit (Vector Laboratories) and 3-3 diaminobenzidine. BrdU-labeled cells were counted from sections of foregut endoderm and splanchnic mesenchyme dorsal to the heart where isl1 is expressed at stages between ED8.5 and ED9.5. Six mutant embryos and six wild-type littermates at each stage were included in the analysis. For quantitative analyses, representative sections from anterior, mid, and posterior levels of each embryo were utilized to count the total number of cells within endoderm or within splanchnic mesenchyme and the number that were labeled with BrdU, to give a proliferative index.
Apoptosis Assays
Mutant and wild-type embryos (from E8.5 to E9.25) were collected for apoptosis studies. To detect apoptotic cells, the terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) assay was performed on paraffin sections according to the manufacturer’s instructions (Fluorescein In Situ Cell Death Detection Kit, Boehringer Mannheim, Mannheim, Germany). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Vector Laboratories). In addition to TUNEL staining, DNA fragmentation was verified under UV illumination using DAPI counter-stain.
Acknowledgments
We would like to thank Phil Soriano, Eric Mercer, and David Anderson for their generosity in providing lacZ indicator mice and Tom Jessell, Yasuto Tanabe, and Chris William for their generosity in providing isl1-cre mice. Thank you to Xiaoxue Zhang for critical technical assistance. We would like to thank Karen Lettieri, in the Pfaff lab, for her expert technical assistance and generosity. We would also like to thank Julie Anderson and Kim Weldy for their tremendous work with our mouse colony. Thanks to a number of people who sent us cDNAs to make probes for RNA in situ, including Karen Lyons, James Cross, Andy McMahon, Gail Martin, Robert Maxson, and Benoit Bruneau. Thank you to Janet Rossant and Claudio Stern for technical advice on double in situ staining. Thanks to Ken Chien for his continued encouragement and critical reading of the manuscript. This work was supported by grants to S.E. from AHA and NIH, and an AHA fellowship awarded to C.-L.C.
References
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and isl cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Logan M, Davis N, Levi T, Tabin CJ, Seidman JG, Seidman CE. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211:100–108. doi: 10.1006/dbio.1999.9298. [DOI] [PubMed] [Google Scholar]
- Campione M, Ros MA, Icardo JM, Piedra E, Christoffels VM, Schweickert A, Blum M, Franco D, Moorman AF. Pitx2 expression defines a left cardiac lineage of cells: evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev Biol. 2001;231:252–264. doi: 10.1006/dbio.2000.0133. [DOI] [PubMed] [Google Scholar]
- Cross JC, Flannery ML, Blanar MA, Steingrimsson E, Jenkins NA, Copeland NG, Rutter WJ, Werb Z. Hxt encodes a basic helix-loop-helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- de la Cruz MV, Sanchez-Gomez C. Straight heart tube. Primitive cardiac cavities vs. primitive cardiac segments. In: de La Cruz MV, Markwald RR, editors. Living Morphogenesis of the Heart. Boston: Birkhauser; 2000. pp. 85–99. [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- Franco D, Markman MM, Wagenaar GT, Ya J, Lamers WH, Moorman AF. Myosin light chain 2a and 2v identifies the embryonic outflow tract myocardium in the developing rodent heart. Anat Rec. 1999;254:135–146. doi: 10.1002/(SICI)1097-0185(19990101)254:1<135::AID-AR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Franco D, Campione M, Kelly R, Zammit PS, Buckingham M, Lamers WH, Moorman AF. Multiple transcriptional domains, with distinct left and right components, in the atrial chambers of the developing heart. Circ Res. 2000;87:984–991. doi: 10.1161/01.res.87.11.984. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RP, Biben C, Elliott DA. Transcriptional control and pattern formation in the developing vertebrate heart: studies on Nk-2 class homeodomain factors. In: Harvey RP, Rosenthal N, editors. Heart Development. San Diego, CA: Academic Press; 1999. pp. 111–129. [Google Scholar]
- Haun C, Alexander J, Stainier DY, Okkema PG. Rescue of Caenorhabditis elegans pharyngeal development by a vertebrate heart specification gene. Proc Natl Acad Sci USA. 1998;95:5072–5075. doi: 10.1073/pnas.95.9.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego, CA: Academic Press; 1999. [Google Scholar]
- Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–216. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Molecular embryogenesis of the heart. Pediatr Dev Pathol. 2002;5:516–543. doi: 10.1007/s10024-002-0004-2. [DOI] [PubMed] [Google Scholar]
- Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E, Chien KR. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J Biol Chem. 1994;269:16961–16970. [PubMed] [Google Scholar]
- Lamers WH, Moorman AF. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res. 2002;91:93–103. doi: 10.1161/01.res.0000027135.63141.89. [DOI] [PubMed] [Google Scholar]
- Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- O’Brien TX, Lee KJ, Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci USA. 1993;90:5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Lewis C, Turbay D, Chung A, Chen JN, Evans S, Breitbart RE, Fishman MC, Izumo S, Bodmer R. Differential rescue of visceral and cardiac defects in Drosophila by vertebrate tinman-related genes. Proc Natl Acad Sci USA. 1998;95:9366–9371. doi: 10.1073/pnas.95.16.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pexieder T. Standardized method for study of normal and abnormal cardiac development in chick, rat, mouse, dog, and human embryos. Teratology. 1986;33:91C–92C. [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G, Elliott DA, Harvey RP, Olson EN. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development. 1998;125:3037–3048. doi: 10.1242/dev.125.16.3037. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- Thomas T, Yamagishi H, Overbeek PA, Olson EN, Srivastava D. The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev Biol. 1998;196:228–236. doi: 10.1006/dbio.1998.8849. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. The Drosophila isl gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yuan S, Schoenwolf GC. Isl-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat Rec. 2000;260:204–207. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Kirby ML. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev Dyn. 2002;223:307–320. doi: 10.1002/dvdy.10068. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zinyk DL, Mercer EH, Harris E, Anderson DJ, Joyner AL. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr Biol. 1998;8:665–668. doi: 10.1016/s0960-9822(98)70255-6. [DOI] [PubMed] [Google Scholar]