Abstract
Introduction
Polyfluoroalkyl chemicals (PFCs) are commercially synthesized chemicals used in consumer products. Exposure to certain PFCs is widespread, and some PFCs may act as endocrine disruptors. We used data from the Avon Longitudinal Study of Parents and Children (ALSPAC) in the United Kingdom to conduct a nested case-control study examining the association between age at menarche, and exposure to PFCs during pregnancy.
Methods
Cases were selected from female offspring in the ALSPAC who reported menarche before the age of 11.5 years (n=218), and controls were a random sample of remaining girls (n=230). Serum samples taken from the girls’ mothers during pregnancy (1991–1992) were analyzed using on-line solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry for 8 PFCs. Logistic regression was used to determine association between maternal serum PFC concentrations, and odds of earlier age at menarche.
Results
PFOS and PFOA were the predominant PFCs (median serum concentrations of 19.8 ng/mL and 3.7 ng/mL). All but one PFC were detectable in most samples. Total PFC concentration varied by number of births (inverse association with birth order; p-value <0.0001) and race of the child (higher among whites; p-value=0.03). The serum concentrations of carboxylates were associated with increased odds of earlier age at menarche; concentrations of perfluorooctane sulfonamide, the sulfonamide esters and sulfonates were all associated with decreased odds of earlier age at menarche. However, all confidence intervals included the null value of 1.0.
Conclusions
ALSPAC study participants had nearly ubiquitous exposure to most PFCs examined, but PFC exposure did not appear to be associated with altered age at menarche of their offspring.
Keywords: ALSPAC, Polyfluoroalkyl chemicals, Menarche
1. Introduction
Puberty is a critical time of growth and development. Timing and pattern of developmental milestones yield information on overall health status, past exposures, and may predict future health out-comes.(Biro et al., 2001; Golub et al., 2008) Age at menarche has decreased from the late 19th century to present,(Wyshak and Frisch, 1982; Zacharias, 1969) and a secular trend towards earlier development of secondary sexual characteristics has been reported among girls in the United Kingdom.(Rubin et al., 2009) While improvements in nutritional status may be responsible in part, exposure to environmental chemicals may also contribute to altered timing and patterns of pubertal development.
Polyfluoroalkyl chemicals (PFCs) are a class of commercially synthesized chemicals, used as surfactants, surface coatings, and in other applications to decrease staining and sticking. The manufacture of PFCs began in the 1950’s, and although there are many different PFCs, the two most studied compounds are perfluorooctanoate (PFOA; also known as C8) and perfluorooctane sulfonate (PFOS). Exposure to PFCs can occur through inhalation, ingestion, and dermal absorption; in addition, PFCs are able to cross the placental barrier in both humans(Inoue et al., 2004) and animals,(Lau et al., 2003; Thibodeaux et al., 2003) leading to potential fetal exposure. PFCs act by a variety of mechanisms including alteration of endogenous hormone production,(Shi et al., 2009; Biegel et al., 1995; Bookstaff et al., 1990; Cook et al., 1992; Liu et al., 1996; Austin et al., 2003) mammary gland development,(Wolf et al., 2007) and expression of estrogen responsive genes,(Du et al., 2009; Liu et al., 2007; Tilton et al., 2008; Wei et al., 2007; Wei et al., 2008) each of which could impact timing and progression of pubertal development, and reproductive function. However, there have been no studies published which investigate the association between PFC exposure and age at menarche. We used data from a prospective cohort study conducted in the United Kingdom to perform a nested case-control study examining the association between age at menarche and gestational exposure to PFCs.
2. Materials and methods
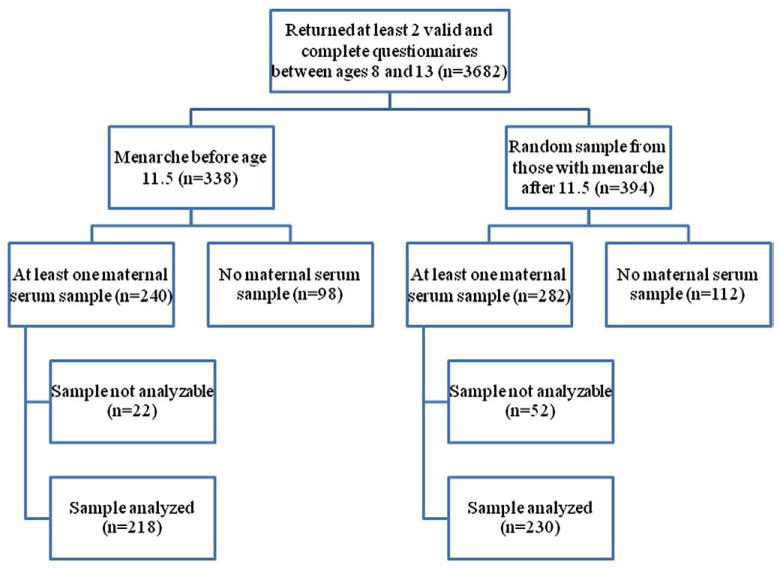
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective cohort study of approximately 14,000 pregnant women residing in Avon (UK) who had an expected delivery date between April 1, 1991 and December 31, 1992.(Golding et al., 2001) Information has been collected on these women and their offspring through interviews, mailed questionnaires, and clinic visits; details on recruitment and study methods have been described elsewhere. (Golding et al., 2001; 22,) A ‘Growing and Changing’ questionnaire was developed to collect information on the offspring’s pubertal development and distributed to participants at the ages of 8, 9, 10, 11, and 13 years (1999–2004).(Rubin et al., 2009) Menarche was determined via self-report of menarche status and, if appropriate, age at menarche. From the original base of 14,610 live births, case and control series were selected from singleton (n=11,820) female subjects (n=5756) who had completed at least two puberty staging questionnaires (including self-assessed Tanner stage of pubic hair and breast development, and reported age at menarche) between the ages of 8 and 13 (5 possible questionnaires returned; n=3682; Fig. 1). Girls meeting eligibility criteria were ordered according to reported age at menarche, at the time the 13 year old data became available. A cut-off of 11.5 years was established as defining ‘earlier’ menarche. Eligible case subjects could complete any 2 questionnaires in the series provided one was completed after menarche, while for control subjects the 13-year questionnaire had to be completed in order to ascertain that menarche had not occurred by our cut-off of 11.5 years. Of girls reporting menarche before the age of 11.5 (n=338), 71% (n=240) of these had at least one prenatal maternal serum sample available, and were considered potential cases. Among girls who reported menarche at or after the age of 11.5, a random sample of 394 was chosen; of these, 71.6% (n=282) had at least one maternal serum sample available, and were considered potential controls. After evaluating the integrity of the maternal serum samples, 90.8% (n=218) of potential cases and 81.6% (n=230) of potential controls had analyzable samples. Serum samples were collected from mothers during pregnancy; because serum concentrations of PFCs are relatively stable throughout pregnancy, (Fei et al., 2007) the earliest available serum sample was chosen in the event that multiple samples were available. These analyses were designed to detect an odds ratio of 1.7 or greater with a power of 0.80 (based on 225 cases and 225 controls).
Fig. 1.
Flowchart of eligibility and exclusions.
The following PFCs were included in these analyses: perfluor-ooctane sulfonamide (PFOSA); 2-(N-ethyl-perfluorooctane sulfona-mido) acetate (Et-PFOSA-AcOH); 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH); PFOS; PFOA; perfluorohex-ane sulfonate (PFHxS); perfluorononanoate (PFNA); and perfluor-odecanoate (PFDeA). Maternal serum samples were collected from storage facilities at the University of Bristol, and sent to the National Center for Environmental Health at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, where they were analyzed by on-line solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry. (Kuklenyik et al., 2005)
For analytes which were detectable in at least 30% of samples, values below the limit of detection (LOD) were replaced with √LOD/2. For analytes detectable in fewer than 30% of samples, no substitution was made for values below the LOD (value set to missing). PFCs concentrations were assessed both individually, and in summation. The following categories were used to group PFCs, based on chemical classes: sulfonamides (PFOSA), sulfonamide esters (Et-PFOSA-AcOH, Me-PFOSA-AcOH), sulfonates (PFOS, PFHxS), and carboxylates (PFDeA, PFNA, PFOA). As with many environmental exposures, distribution of the PFC concentrations was skewed, and a natural log transformation was used to approximate normality in continuous analyses. In addition, each PFC concentration was treated as a binary exposure, with values categorized as being either at or above the median of values among cases, or below the median.
Potential confounders were identified from the literature on characteristics associated with pubertal development. These included mother’s pre-pregnancy BMI (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], and ≥ 30 [obese]), mother’s age at delivery (<20, 20–29, and ≥ 30 years), mother’s age at menarche (8–11, 12–14, and 15+ years), mother’s educational level (certificate of secondary education [CSE]/none, vocational, O-level, A-level, degree), mother’s social class (lower, middle, upper), child’s ethnic background (white, non-white), and child’s birth order (first born, second born, third born or later). Social class was derived using the United Kingdom’s 1991 Office of Population Censuses and Surveys.(OPCS, 1991) Upper class consisted of classes I (professional occupations) or II (managerial and technical occupations); middle class of classes IIINM (non-manual skilled occupations) or IIIM (manual skilled occupations); and lower class of classes IV (partly skilled occupations) or V (unskilled occupations).
To assess the association between potential confounders and earlier age at menarche, logistic regression models were used, with an inclusion criterion of p ≤ 0.30. Next, association of potential confounders with total PFC concentration (after natural log transformation) was assessed by a linear model, again using a criterion of p ≤ 0.30. Those variables associated with both outcome (earlier age at menarche) and exposure (PFC concentration) using these guidelines were considered potential confounders, and included in multivariate logistic models to determine association of maternal PFC concentration with earlier age at menarche. Human subject protection was assessed and approved by the ALSPAC Law and Ethics Committee, the Local Research Ethics Committees, and CDC Institutional Review Board.
3. Results
In the ALSPAC cohort, girls were born to mothers who had relatively high education and social class (Table 1). Cases were more likely to have mothers with an earlier age at menarche (32.5% reporting menarche between 8 and 11 years of age, compared to 15.2% of controls; p=0.0004). They were also more likely to have mothers who had an overweight or obese pre-pregnancy BMI (29.4% compared to 14.9% of controls; p=0.01), and less likely to have mothers in either the youngest or the oldest age groups at delivery (p=0.29). Cases were more likely to be the first born child (38.9% compared to 33.0% of controls; p=0.23). Although there was little racial/ethnic diversity in the overall population, there were more non-white girls among the cases (5.7% compared to 1.4% of controls; p=0.03). At the time of selection, 51.3% of controls had achieved menarche (median age of 12.42 years); the median age at menarche among cases was 11.08 years. After adding the 14-year data, which had not been available at the time of subject selection, an additional 39 controls (14.2%) had achieved menarche.
Table 1.
Characteristics of study population.
| Early menarche (N=218) | Not early menarche (N=230) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristica | N | % | N | % | p-value for differenceb |
| Mother’s highest education | 0.88 | ||||
| CSE/none | 31 | 14.8 | 26 | 11.9 | |
| Vocational | 17 | 8.1 | 15 | 6.9 | |
| O-level | 67 | 31.9 | 73 | 33.3 | |
| A-level | 61 | 29.1 | 66 | 30.1 | |
| Degree | 34 | 16.2 | 39 | 17.8 | |
| Mother’s social class | 0.99 | ||||
| Lower | 18 | 10.5 | 19 | 10.0 | |
| Middle | 75 | 43.6 | 84 | 44.0 | |
| Upper | 79 | 45.9 | 88 | 46.1 | |
| Mother’s age at menarche | 0.0004 | ||||
| 8–11 years | 63 | 32.5 | 30 | 15.2 | |
| 12–14 years | 119 | 61.3 | 153 | 77.3 | |
| ≥ 15 years | 12 | 6.2 | 15 | 7.6 | |
| Mother’s pre-pregnancy BMI | 0.01 | ||||
| <18.5 | 7 | 3.6 | 11 | 5.4 | |
| 18.5–24.9 | 132 | 67.0 | 163 | 79.5 | |
| 25.0–29.9 | 39 | 19.8 | 19 | 9.3 | |
| ≥ 30.0 | 19 | 9.6 | 12 | 5.6 | |
| Mother’s age at delivery | 0.29 | ||||
| <20 years | 1 | 0.2 | 7 | 3.1 | |
| 20–24 years | 43 | 19.9 | 41 | 17.9 | |
| 25–29 years | 83 | 38.4 | 81 | 35.4 | |
| ≥ 30 years | 89 | 41.2 | 100 | 43.7 | |
| Child birth order | 0.23 | ||||
| First born | 81 | 38.9 | 73 | 33.0 | |
| Second born | 66 | 31.7 | 87 | 39.4 | |
| Third born or later | 61 | 29.3 | 61 | 27.6 | |
| Child ethnic background | 0.03 | ||||
| White | 199 | 94.3 | 214 | 98.6 | |
| Non-white | 12 | 5.7 | 3 | 1.4 | |
| Child menarche status and age at menarche | |||||
| Number achieving menarche by age 13 (%) | 218 | 100 | 118 | 51.3 | – |
| Median | Quartiles | Median | Quartiles | – | |
| Age at menarche | 11.08 | 10.75–11.33 | 12.58 | 12.17–(NA) | – |
Information was missing for some girls, including information on mother’s education (n=19, 4.2%), mother’s social class (n=85, 18.9%), mother’s age at menarche (n=56, 12.5%), mother’s pre-pregnancy BMI (n=46, 10.3%), mother’s age at delivery (n=3, 0.7%), girl’s birth order (n=19, 4.2%), and girl’s race/ethnicity (n=20, 4.5%).
Compared using logistic regression.
PFOS and PFOA were found at the highest median concentrations of 19.8 ng/mL and 3.7 ng/mL, respectively (Table 2). All PFCs were detectable in most samples, with the exception of PFDeA, which was only detected in 2.7% of samples. The median total PFC concentration was 27.3 ng/mL, but this varied somewhat by maternal and child characteristics (Table 3). There was an inverse relationship of total PFC concentration with birth order (p-value<0.0001), with the highest concentrations among first-time mothers (median of 30.8 ng/mL) and the lowest among women who had three or more children (median of 24.4 ng/mL). Also, white girls’ mothers had higher total PFC concentrations compared to non-white girls’ mothers (medians of 27.3 ng/mL and 23.4 ng/mL, respectively; p-value=0.03). However, due to the small number of non-white children, race/ethnicity was not included in further analyses. Median total PFC concentrations was higher for older compared to younger mothers (median of 28.6 ng/mL for mothers aged 25–29 years, compared to 23.3 ng/mL for mothers aged <20 years; p-value =0.27), and among mothers with CSE or no education (24.7 ng/mL), compared to mothers with an A-level education (29.6 ng/mL, p-value=0.31). There was little variation in PFC concentrations by maternal age at menarche (p-value=0.40), pre-pregnancy BMI (p-value=0.46), or social class (p-value=0.92).
Table 2.
Gestational serum concentrations among mothers of girls with and without earlier age at menarche.
| Analytea | Total | Early menarche (N=218) | Not early menarche (N=230) | p-valueb | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N (%) above LOD | Median (quartiles) | N (%) above LOD | Median (quartiles) | N (%) above LOD | Median (quartiles) | ||
| PFOSA | 311 (69.4) | 0.2 (0.2–0.3) | 153 (70.2) | 0.2 (0.2–0.3) | 158 (68.7) | 0.2 (0.2–0.3) | 0.23 |
| Sulfonamide esters | – | 1.1 (0.8–1.8) | – | 1.1 (0.8–1.7) | – | 1.2 (0.8–1.9) | 0.53 |
| Et-PFOSA-AcOH | 437 (97.5) | 0.6 (0.4–0.9) | 212 (97.3) | 0.7 (0.4–1.0) | 225 (97.8) | 0.6 (0.4–0.9) | 0.75 |
| Me-PFOSA-AcOH | 383 (85.5) | 0.4 (0.3–0.8) | 179 (82.1) | 0.4 (0.3–0.7) | 204 (88.7) | 0.4 (0.3–0.8) | 0.26 |
| Sulfonates | – | 21.7 (16.6–27.2) | – | 21.0 (17.0–27.2) | – | 21.9 (16.1–26.8) | 0.95 |
| PFOS | 448 (100) | 19.8 (15.1–24.9) | 218 (100) | 19.5 (15.4–24.8) | 230 (100) | 20.0 (14.6–24.9) | 0.99 |
| PFHxS | 447 (99.8) | 1.6 (1.2–2.2) | 218 (100) | 1.7 (1.3–2.2) | 229 (99.6) | 1.6 (1.2–2.2) | 0.47 |
| Carboxylates | – | 4.4 (3.4–5.7) | – | 4.6 (3.4–5.8) | – | 4.3 (3.3–5.4) | 0.15 |
| PFOA | 448 (100) | 3.7 (2.8–4.8) | 218 (100) | 3.9 (2.9–5.0) | 230 (100) | 3.6 (2.7–4.7) | 0.15 |
| PFNA | 447 (99.8) | 0.6 (0.5–0.8) | 217 (99.5) | 0.7 (0.5–0.8) | 230 (100) | 0.6 (0.5–0.8) | 0.67 |
| PFDeA | 12 (2.7) | –– | 5 (2.3) | –– | 7 (3.0) | – | – |
| ΣPFC | – | 27.3 (21.6–34.5) | – | 27.3 (22.3–34.8) | – | 27.3 (20.9–34.2) | 0.78 |
The limits of detection (LOD) were 0.1 ng/mL (PFOSA, PFHxS, PFOA, PFNA) and 0.2 ng/mL (Et-PFOSA-AcOH, Me-PFOSA-AcOH, PFOS and PFDeA).
p-value for difference between cases and controls.
Table 3.
Median and quartiles of total maternal PFC serum concentrationsa by maternal and child characteristics.
| Group | Median (quartiles) | p-valueb |
|---|---|---|
| Mother’s highest education | 0.31 | |
| CSE/none | 24.7 (21.6–30.8) | |
| Vocational | 28.1 (23.5–35.8) | |
| O-level | 27.0 (21.3–35.2) | |
| A-level | 29.6 (23.0–35.0) | |
| Degree | 26.6 (20.4–35.4) | |
| Mother’s social class | 0.92 | |
| Lower | 27.4 (21.1–33.1) | |
| Middle | 28.7 (23.0–36.2) | |
| Upper | 28.7 (22.0–36.8) | |
| Mother’s age at menarche | 0.40 | |
| 8–11 years | 27.5 (22.7–35.7) | |
| 12–14 years | 27.1 (21.4–34.4) | |
| ≥ 15 years | 29.0 (24.2–35.2) | |
| Mother’s pre-pregnancy BMI | 0.46 | |
| <18.5 | 24.6 (21.2–31.6) | |
| 18.5–24.9 | 27.5 (21.6–35.5) | |
| 25.0–29.9 | 29.5 (24.5–35.0) | |
| ≥ 30.0 | 25.6 (21.1–33.2) | |
| Mother’s age at delivery | 0.27 | |
| <20 years | 23.3 (20.2–28.2) | |
| 20–24 years | 26.8 (21.4–33.5) | |
| 25–29 years | 28.6 (22.0–35.2) | |
| ≥ 30 years | 27.1 (21.4–35.7) | |
| Child birth order | <0.0001 | |
| First born | 30.8 (25.0–37.7) | |
| Second born | 27.1 (21.9–34.0) | |
| Third born or later | 24.4 (19.2–30.6) | |
| Child ethnic background | 0.03 | |
| White | 27.3 (21.8–35.2) | |
| Non-white | 23.4 (20.3–32.5) |
The limits of detection (LOD) were 0.1 ng/mL (PFOSA, PFHxS, PFOA, PFNA) and 0.2 ng/mL (Et-PFOSA-AcOH, Me-PFOSA-AcOH, PFOS and PFDeA).
p-value for difference.
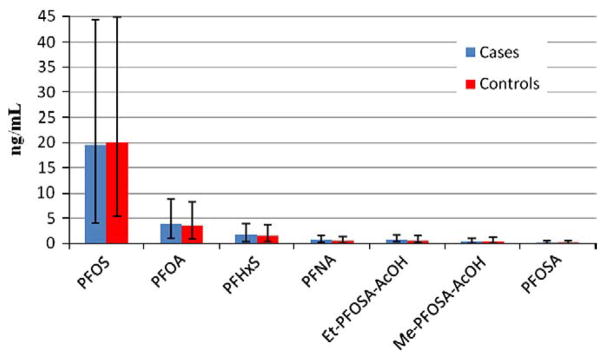
In the unadjusted analysis, there was no difference between cases and controls with respect to the mothers’ total PFC concentrations, whether as a continuous or as a binary variable (Table 4, Fig. 2). Only the mothers’ total serum concentrations of carboxylates were associated with increased odds of earlier age at menarche of their offspring; mothers’ serum concentrations of PFOSA, the sulfonamide esters and sulfonates were all associated with decreased odds of earlier age at menarche. In multivariate analysis, birth order and maternal age at delivery were included as potential confounders based on association with both outcome and PFC serum concentrations (Table 4). Results were similar to the unadjusted analysis, with most effect measures attenuated by the inclusion of these covariates. However, the association of mothers’ total sulfonates serum concentrations with their girls’ age at menarche was accentuated in the multivariate model, with an OR=0.66 (95% CI: 0.40–1.08) when treated as a continuous, and an OR=0.74 (95% CI: 0.50–1.09) when treated as a binary outcome.
Table 4.
Regression analysis association between maternal PFC serum concentrations and earlier age at menarche, unadjusted and controlling for birth order and maternal age at delivery.
| Analyte | Continuous | Binary | ||
|---|---|---|---|---|
|
|
|
|||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
|
|
|
|
|
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PFOSA | 0.89 (0.66–1.20) | 0.91 (0.67–1.24) | 0.82 (0.57–1.19) | 0.85 (0.58–1.25) |
| Sulfonamide esters | 0.90 (0.64–1.25) | 0.91 (0.65–1.28) | 0.76 (0.52–1.11) | 0.78 (0.53–1.14) |
| Et-PFOSA-AcOH | 1.02 (0.75–1.40) | 1.03 (0.75–1.43) | 1.17 (0.81–1.70) | 1.17 (0.80–1.72) |
| Me-PFOSA-AcOH | 0.86 (0.67–1.11) | 0.86 (0.66–1.12) | 0.85 (0.58–1.24) | 0.85 (0.57–1.25) |
| Sulfonates | 0.82 (0.52–1.30) | 0.66 (0.40–1.08) | 0.83 (0.57–1.20) | 0.74 (0.50–1.09) |
| PFOS | 0.85 (0.53–1.36) | 0.68 (0.40–1.13) | 0.92 (0.63–1.33) | 0.83 (0.56–1.23) |
| PFHxS | 1.00 (0.74–1.33) | 0.89 (0.65–1.22) | 1.17 (0.81–1.70) | 1.11 (0.76–1.64) |
| Carboxylates | 1.21 (0.76–1.95) | 0.98 (0.58–1.66) | 1.35 (0.93–1.96) | 1.25 (0.84–1.88) |
| PFOA | 1.25 (0.79–1.95) | 1.01 (0.61–1.68) | 1.35 (0.93–1.96) | 1.29 (0.86–1.93) |
| PFNA | 1.00 (0.66–1.52) | 0.91 (0.59–1.40) | 1.28 (0.88–1.86) | 1.15 (0.78–1.69) |
Continuous represents natural log transformed values (values below the LOD are substituted with √LOD/2), while binary represents at or above the median value among cases, versus below the median value among cases. PFDeA is not included in the summed value if it was below the LOD.
Fig. 2.
Median and quartiles of maternal PFC serum concentration for cases and controls.
In sensitivity analyses, we adjusted for girl’s race/ethnicity, and also estimated associations excluding girls of non-white race/ethnicity (n=15). These analyses did not substantially change the results found in the original multivariate models. As a second sensitivity analysis, exposure was redefined using the total PFC maternal concentration quartiles among cases; that is, girls born from mothers with serum concentrations during pregnancy at or above the 75th percentile were considered ‘exposed’ while girls born from mothers with serum concentrations at or below the 25th percentile were considered ‘unexposed.’ Again, this did not substantially alter the original results. The direction of association did switch from below to above the null for the sulfonates (adjusted OR=1.17, 95% CI: 0.67–2.04) and the odds ratio was increased for the carboxylates (adjusted OR=1.41, 95% CI: 0.76–2.62) using these more extreme cut-offs, but all of the confidence intervals included the null value of 1.0. In the case of the sulfonates, the change in the direction of association was due to a larger proportion of cases (57%) in the second quartile compared to quartiles 1 (43%), 3 (45.5%) and 4 (49%). In analyses of PFC maternal serum concentration, we chose as our cutoff the median among cases a priori, since this was the smaller group (compared to controls). However, we also performed the analyses using the median value among controls, and results were not substantially different (data not shown). Finally, the analysis was performed using ordinal logistic regression, where the girls’ age at menarche was categorized as 8–10 years, 11–12 years, or ≥ 13 years. Using this ordinal outcome, all associations were in the direction of higher maternal PFC concentrations associated with later age at menarche of the girls, although again all the confidence intervals included the null.
4. Discussion
We used data from the ALSPAC cohort to explore the association between gestational PFC exposure and age at menarche. Although study participants had nearly ubiquitous exposure to all PFCs except PFDeA, gestational PFC exposure as estimated from the pregnant women’s PFCs serum concentrations, did not appear to be associated with age at menarche. The strongest effect was seen for the sulfonates, which were associated with reduced odds of earlier age at menarche (i.e. higher maternal serum concentrations of sulfonates were associated with the girls’ later age at menarche). The direction of effect did depend on the class of PFC examined, with differing results seen for the sulfonates compared to PFOSA, sulfonamido esters, and carboxylates. This may be due to differences in the mechanism of action resulting from the physicochemical properties of the PFCs studied, or may be due to chance given the low level of effect. At the 2009 annual meeting for the International Society for Environmental Epidemiology, Pinney et al reported that among US girls, there was an association between serum PFOA and probability of being in breast stage 2 or higher.(Pinney et al., 2009) In our cohort, there was a modest, non-significant association between odds of earlier menarche and mother’s PFOA above the median; it is possible that PFOA may affect both secondary sexual characteristic development and menarche through direct or indirect action on the hypothalamic pituitary gonadal axis. One possibility is that PFOA alters circulating hormone levels; PFOA exposure has been shown to increase serum estradiol in rats,(Liu et al., 1996; Biegel et al., 2001) but there is as yet little evidence for such an effect in humans, aside from a report of modestly increased estradiol among male workers with high occupational exposure.(Olsen et al., 1998) There was also variability for certain compounds depending on how ‘earlier’ menarche was defined. In the binary outcome model, higher maternal carboxylate concentrations were associated with earlier age at menarche, while in the ordinal outcome model, higher carboxylate levels were associated with later age at menarche. Although neither effect measure was statistically significant, the nature of the relationship between PFC exposure and pubertal development warrants further research.
Serum concentrations of PFCs during 1991–1992 among mothers of girls participating in the ALSPAC were similar to or slightly lower compared to other studies.(Houde et al., 2006) A Danish study of pregnant women participating in the Danish National Birth Cohort from March 1996 to November 2002 reported mean PFOS serum concentrations of 35.3 (+/−13.0) ng/mL during the first trimester, and mean PFOA concentrations of 5.6 (+/−2.5) ng/mL.(Fei et al., 2007) Among female participants in the 2003–2004 National Health and Nutrition and Examination Survey (NHANES; a nationally representative sample of the general US population) aged 12 years and over, the geometric mean serum concentration of PFOS was 18.4 μg/L, and was higher for the older age groups, and for non-Hispanic white and black participants compared to Mexican American participants.(Calafat et al., 2007) Serum concentrations of PFOA were lower compared to PFOS (geometric mean of 3.5 μg/L), and there was less variability by age. However, serum concentrations were still higher among non-Hispanic whites compared to non-Hispanic blacks and Mexican-Americans. The composition of the ALSPAC cohort limited our ability to compare PFC exposure by race, but similarly to the United States findings, white girls had a higher serum concentration of PFCs compared to non-white girls. Variation was also seen by socio-economic indicators, maternal pre-pregnancy BMI and age at delivery, and birth order. Specifically, PFC concentrations during pregnancy were higher for older moms, and who were giving birth to their first-born child. These differences may reflect decreasing exposure to PFCs over time, as well as decreased maternal body burden with successive pregnancies.
To our knowledge, this is the first published study to examine the association between PFC exposure in utero, and age at menarche. Although we did not find a significant relationship in this cohort, there is biological plausibility for such an association. Exposures during pregnancy are extremely relevant to pubertal development, since this represents the period of organ and brain development, including the brain, endocrine system and reproductive tract. Further, the fetus is more susceptible to such exposures due to smaller size, lack of a complete blood-brain barrier, and absence of metabolizing enzymes. Based on evidence from animal studies, the mechanisms of action for PFCs may be particularly relevant for in utero exposure. PFCs may alter the expression of estrogen-responsive genes,(Du et al., 2009; Liu et al., 2007; Tilton et al., 2008; Wei et al., 2007; Wei et al., 2008) as well as decreasing serum testosterone and increasing serum estradiol.(Shi et al., 2009; Biegel et al., 1995; Bookstaff et al., 1990; Cook et al., 1992; Liu et al., 1996) Studies in mice and rats have also shown that PFOA exposure causes stunted mammary epithelial branching and growth,(Wolf et al., 2007) and increased incidence of mammary fibroadenomas. (Sibinski, 1987) Less directly, PFCs may disrupt fatty acid metabolism and thyroid hormone production.(Thibodeaux et al., 2003; 32,) Human studies have shown that an undernourished fetal environment can affect the development of the reproductive axis, (Rhind et al., 2001) as well as impact future risk of developing cardiovascular disease, diabetes, stroke, and obesity.(Remacle et al., 2004; Barker, 1997) This has implications for pubertal development, as overweight and obese girls experience accelerated puberty compared to their peers,(Biro et al., 2006a; Biro et al., 2003; Wang, 2002) possibly due to increased estrogenic compounds by adipose tissue.
Limitations of this study include a single measurement of PFC exposure, lack of complete information on age at menarche among controls, and some missing information on covariates. Although we only measured PFC exposure in utero at a single time point, the estimated half-lives for some PFCs are on the order of several years; (Andersen et al., 2008; Olsen et al., 2009) thus our measurements should represent maternal PFC body burden throughout gestation. It is possible that the cases and controls selected were not representative of the cohort. When comparing female study participants who returned at least two Growing and Changing questionnaire, to those who did not return any questionnaires, (parents of) non-respondents tended to be of somewhat lower educational attainment (86.8% of respondents had CSE or higher, compared to 72.1% of non-respondents) and social class (8.6% of respondents in the lower social class, compared to 16.4% of non-respondents). In addition, mothers of respondents were generally older at time of index birth (85.1% aged 25 or older) compared to non-respondents (69.9% aged 25 or older). Finally, non-respondents were more likely to be of non-white race/ethnicity (6.4% compared to 3.8% of respondents). This could have affected our findings since socio-economic status is related to age at menarche;(Braithwaite et al., 2009) however, there is no evidence that these demographic characteristics are associated with PFC serum concentrations. Although race/ethnicity is also related to age at menarche(Biro et al., 2006b; Freedman et al., 2002; Wu et al., 2002; Britton et al., 2004; McDowell et al., 2007) and was associated with maternal PFC serum concentrations in this study, we were not able to more closely examine the effect of race/ethnicity due to the small number of non-white girls. Finally, due to a relatively small sample size, the study may have been underpowered to detect an association between gestational PFC exposure and age at menarche. Strengths of this study are the inclusion of multiple PFC biomarkers, inclusion of information on important maternal and child characteristics, and the representative nature of the cohort.
5. Conclusions
We compared exposure to PFCs during pregnancy among mothers of girls who did and did not have earlier age at menarche in the ALSPAC cohort. PFC serum concentration, both total and for individual compounds, varied by maternal characteristics. However, gestational PFC exposure during pregnancy did not appear to be associated with age at menarche in this cohort.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the entire ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We also thank Larry Needham, Amal Wanigatunga, Brian Basden and Tao Jia for their technical assistance with sample analysis.
This work was supported by the Centers for Disease Control and Prevention. The UK Medical Research Council; the Wellcome Trust; and the University of Bristol provide core support for ALSPAC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Andersen ME, Butenhoff JL, Chang SC, et al. Perfluoroalkyl acids and related chemistries–toxicokinetics and modes of action. Toxicol Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SM. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–9. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avon Longitudinal Study of Parents and Children. University of Bristol, Department of Social Medicine; [Accessed September 22, 2008]. at http://www.bristol.ac.uk/alspac/ [Google Scholar]
- Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–13. doi: 10.1016/s0899-9007(97)00193-7. (Burbank, Los Angeles County, Calif) [DOI] [PubMed] [Google Scholar]
- Biegel LB, Liu RC, Hurtt ME, Cook JC. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol. 1995;134:18–25. doi: 10.1006/taap.1995.1164. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci. 2001;60:44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- Biro FM, McMahon RP, Striegel-Moore R, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–43. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- Biro FM, Lucky AW, Simbartl LA, et al. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J Pediatr. 2003;142:643–6. doi: 10.1067/mpd.2003.244. [DOI] [PubMed] [Google Scholar]
- Biro FM, Khoury P, Morrison JA. Influence of obesity on timing of puberty. Int J Androl. 2006a;29:272–7. doi: 10.1111/j.1365-2605.2005.00602.x. (discussion 86–90) [DOI] [PubMed] [Google Scholar]
- Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr. 2006b;148:234–40. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Bookstaff RC, Moore RW, Ingall GB, Peterson RE. Androgenic deficiency in male rats treated with perfluorodecanoic acid. Toxicol Appl Pharmacol. 1990;104:322–33. doi: 10.1016/0041-008x(90)90306-f. [DOI] [PubMed] [Google Scholar]
- Braithwaite D, Moore DH, Lustig RH, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20:713–20. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- Britton JA, Wolff MS, Lapinski R, et al. Characteristics of pubertal development in a multi-ethnic population of nine-year-old girls. Ann Epidemiol. 2004;14:179–87. doi: 10.1016/j.annepidem.2002.08.001. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JC, Murray SM, Frame SR, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol. 1992;113:209–17. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- Du YB, Shi XJ, Liu CS, Yu K, Zhou BS. Chronic effects of water-borne PFOS exposure on growth, survival and hepatotoxicity in zebrafish: a partial life-cycle test. Chemo-sphere. 2009;74:723–9. doi: 10.1016/j.chemosphere.2008.09.075. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–82. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PMD, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121:S218–30. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- November 21, 2002 report: Hazard Assessment of Perfluorooctane sulfonate (PFOS) and its salts. Organisation for Economic Co-operation and Development. ENV/JM/RD(2002) 17/FINAL.
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol. 2006;40:3463–73. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–7. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem. 2005;77:6085–91. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003;74:382–92. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Liu RC, Hahn C, Hurtt ME. The direct effect of hepatic peroxisome proliferators on rat Leydig cell function in vitro. Fundam Appl Toxicol. 1996;30:102–8. doi: 10.1006/faat.1996.0047. [DOI] [PubMed] [Google Scholar]
- Liu CS, Du YB, Zhou BS. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat Toxicol. 2007;85:267–77. doi: 10.1016/j.aquatox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Adolesc Health. 2007;40:227–31. doi: 10.1016/j.jadohealth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med. 1998;40:614–22. doi: 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, et al. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology. 2009;256:65–74. doi: 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- OPCS. Standard Occupational Classification. London: HMSO; 1991. [Google Scholar]
- Pinney SM, Windham GC, Biro FM, et al. Perfluorooctanoic acid (PFOA) and pubertal maturation in young girls. The International Society for Environmental Epidemiology; Dublin, Ireland: 2009. [Google Scholar]
- Remacle C, Bieswal F, Reusens B. Programming of obesity and cardiovascular disease. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S46–53. doi: 10.1038/sj.ijo.0802800. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Rae MT, Brooks AN. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction. 2001;122:205–14. doi: 10.1530/rep.0.1220205. [DOI] [PubMed] [Google Scholar]
- Rubin C, Maisonet M, Kieszak S, et al. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2009;23(5 Sep):492–504. doi: 10.1111/j.1365-3016.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol. 2009;27:352–9. doi: 10.1016/j.reprotox.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Sibinski LJ. Two-year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 (perfluorooctane ammonium carboxylate) in rats. 1987. Report prepared for 3M, St. Paul, Minnesota by Riker Laboratories Inc. Study No. 0281CR0012; 8EHQ-1087-0394, October 16, 1987. [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 2003;74:369–81. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Orner GA, Benninghoff AD, et al. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ Health Perspect. 2008;116:1047–55. doi: 10.1289/ehp.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–10. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- Wei Y, Dai J, Liu M, et al. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus) Environ Toxicol Chem. 2007;26:2440–7. doi: 10.1897/07-008R1.1. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liu Y, Wang J, Tao Y, Dai J. Toxicogenomic analysis of the hepatic effects of perfluorooctanoic acid on rare minnows (Gobiocypris rarus) Toxicol Appl Pharmacol. 2008;226:285–97. doi: 10.1016/j.taap.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Fenton SE, Schmid JE, et al. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95:462–73. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110:752–7. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med. 1982;306:1033–5. doi: 10.1056/NEJM198204293061707. [DOI] [PubMed] [Google Scholar]
- Zacharias L, Wurtman RJ. Age at menarche. Genetic and environmental influences. New Engl j med. 1969;280:868–75. doi: 10.1056/NEJM196904172801606. [DOI] [PubMed] [Google Scholar]