Abstract
Background
Extended criteria donor lungs deemed unsuitable for immediate transplantation can be reconditioned using ex vivo lung perfusion (EVLP). Objective identification of which donor lungs can be successfully reconditioned and will function well post-operatively has not been established. This study assessed the predictive value of markers of inflammation and tissue injury in donor lungs undergoing EVLP as part of the DEVELOP-UK study.
Methods
Longitudinal samples of perfusate, bronchoalveolar lavage, and tissue from 42 human donor lungs undergoing clinical EVLP assessments were analyzed for markers of inflammation and tissue injury. Levels were compared according to EVLP success and post-transplant outcomes. Neutrophil adhesion to human pulmonary microvascular endothelial cells (HPMECs) conditioned with perfusates from EVLP assessments was investigated on a microfluidic platform.
Results
The most effective markers to differentiate between in-hospital survival and non-survival post-transplant were perfusate interleukin (IL)-1β (area under the curve = 1.00, p = 0.002) and tumor necrosis factor-α (area under the curve = 0.95, p = 0.006) after 30 minutes of EVLP. IL-1β levels in perfusate correlated with upregulation of intracellular adhesion molecule-1 in donor lung vasculature (R2 = 0.68, p < 0.001) and to a lesser degree upregulation of intracellular adhesion molecule-1 (R2 = 0.30, p = 0.001) and E-selectin (R2 = 0.29, p = 0.001) in conditioned HPMECs and neutrophil adhesion to conditioned HPMECs (R2 = 0.33, p < 0.001). Neutralization of IL-1β in perfusate effectively inhibited neutrophil adhesion to conditioned HPMECs (91% reduction, p = 0.002).
Conclusions
Donor lungs develop a detectable and discriminatory pro-inflammatory signature in perfusate during EVLP. Blocking the IL-1β pathway during EVLP may reduce endothelial activation and subsequent neutrophil adhesion on reperfusion; this requires further investigation in vivo.
Keywords: lung transplantation, ex vivo lung perfusion, lung injury, biomarker, interleukin-1β
The availability of suitable donor lungs for transplantation falls short of demand and contributes to a substantial waiting list mortality.1 Ex vivo lung perfusion (EVLP) is a promising technique to objectively assess and potentially recondition organs unsuitable for immediate use and accounts for 20% of transplants in some established centers.2, 3, 4, 5 However, predicting which “higher risk” donor lungs can be reconditioned effectively and function well after transplant remains challenging, and the ability to stratify such organs will increase safe access to lung transplantation.6 In addition to being an effective donor lung assessment platform, EVLP could potentially offer an opportunity to attenuate inflammation and protect vascular integrity in the lung before implantation.
We have previously demonstrated the feasibility of identifying pro-inflammatory signals in perfusate during EVLP of unsuitable donor lungs.7 In this study, we used samples collected from a large cohort of clinical EVLP procedures performed as part of the DEVELOP-UK multicenter trial.8 Our aim was to examine the potential of markers of inflammation and tissue injury early during clinical EVLP to distinguish donor lungs that can be reconditioned and to predict post-transplant outcomes. In addition, we investigated whether any of these perfusate markers might have a mechanistic role that would be amenable to therapeutic modulation on an accessible flow-based in vitro therapy testing platform for EVLP.
Methods
Study design
This is the prospective mechanistic arm of the DEVELOP-UK trial (ISRCTN 44922411). Ethics approval was granted, and informed consent for research was obtained from donor families and lung transplant recipients (REC 11/NE/0342).8
EVLP protocol
The study included adult donor lungs deemed unsuitable for lung transplantation by all centers in the United Kingdom but meeting pre-defined EVLP criteria (Tables S1 and S2 [available in the online version of this article at www.jhltonline.org]). EVLP assessments were performed using a Vivoline LS1 EVLP circuit (Vivoline Medical AB, Lund, Sweden) following 1 of 2 standardized perfusion protocols previously described in detail.8 Briefly, an initial Hybrid protocol featured an open left atrium, acellular perfusate, and perfusate flow limited to 40%–60% of donor calculated cardiac output (n = 22). Subsequently, the perfusion strategy was changed to a Lund protocol with cellular perfusate (hematocrit 10%–15%) and full flow perfusion (n = 31). Assessment methods, including sampling procedures, remained unchanged.
Sample collection
Bronchoalveolar lavage (BAL) was performed in a subsegmental bronchus of the right or left lower lobes.9 Saline (120 ml) was instilled via bronchoscopy and suctioned back before starting ventilation at the beginning of EVLP and repeated in the same lobe but different subsegmental bronchus at the end of perfusion.
Tissue biopsy samples were taken from either the right middle lobe or the lingula before and after EVLP assessment using a stapler. These specimens were snap frozen in liquid nitrogen for protein and RNA isolation and fixed in formaldehyde and paraffin embedded for immunohistochemistry. A control sample was collected from the primed EVLP circuit before starting donor lung perfusion. Repeated perfusate samples (5 ml) were then collected at 15 and 30 minutes after starting perfusion and every 30 minutes thereafter.
Immunoassays
All protein concentrations measured in perfusate were adjusted to the donor predicted total lung capacity as an estimate of perfused donor lung volume and reported as corrected perfusate concentrations (pg/ml), as previously described.7 Lactate dehydrogenase levels were measured in perfusate, BAL, and tissue lysate as per manufacturer’s instructions (Thermo Fisher Scientific, Rockford, IL). Perfusate and tissue lysates were analyzed with a V-PLEX Human Biomarker 40-Plex Kit and a Human MMP 3-Plex Ultra-Sensitive Kit (both Meso Scale Diagnostics, LLC, Rockville, MD). BAL was analyzed using a V-PLEX human pro-inflammatory Panel 1 Kit and a Human MMP 3-Plex Ultra-Sensitive Kit (both Meso Scale Diagnostics, LLC). Human Syndecan-1 and endothelin-1 were measured using sandwich enzyme-linked immunosorbent assays (R&D Systems, Inc., Minneapolis, MN).
Cell culture
Human pulmonary microvascular endothelial cells (HPMECs) were obtained from PromoCell (Heidelberg, Germany). Peripheral blood neutrophils were isolated in-house 2 hours before usage from healthy volunteers through a standard protocol.10
Neutrophil adhesion assay
A microfluidic platform (Cellix Ltd., Dublin, Ireland) was used for modeling leukocyte adhesion, as previously described.11 Endothelial cell substrates (Vena8; Cellix Ltd.) were pre-coated overnight with 100 μg/ml fibronectin (Sigma-Aldrich, St Louis, MO) and HPMECs seeded to a confluent monolayer (1 × 105 cells/channel). HPMECs were stimulated with blank perfusate (negative control), perfusate with 1 ng/ml interleukin (IL)-1β (positive control) (R&D Systems, Inc.), or clinical EVLP perfusates for 4 hours. For blocking experiments, perfusates were pre-incubated for 1 hour with 4 μg/ml anti–IL-1β neutralizing antibody (NAb) (R&D Systems, Inc.). Primary neutrophils (5 × 106 cells/ml) were passed over the HPMEC monolayer at 0.5 dyne/cm2 for 5 minutes. Images (5 images/channel) were captured in the last 2 minutes, and average neutrophil adhesion was determined using DucoCell software (Cellix Ltd.).
Flow cytometry
HPMECs conditioned for 4 hours with perfusates were stained with conjugated antibodies specific for CD31 (WM59), intracellular adhesion molecule-1 (ICAM-1) (HA58), E-selectin (CL2), and isotype controls (all eBioscience, San Diego, CA). Samples were acquired on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Inc., San Carlos, CA).
Immunohistochemistry
Lung tissue taken pre-EVLP and post-EVLP from 4 Declined lungs (lungs declined from transplantation after EVLP assessment) and 4 Survival lungs (lungs with recipients surviving to hospital discharge post-transplant) with highest levels of IL-1β in the perfusate were examined (n = 16 sections). Immunolocalization of ICAM-1 (EP1442Y), E-selectin (polyclonal), and neutrophil elastase (EPR7479) (all Abcam, Inc., Cambridge, MA) was by the avidin-biotin method with biotinylated secondary antibodies and subsequent DAB Chromogen staining (Vector Laboratories, Inc., Burlingame, CA). Five fields (20× magnification) from each section were scored by 4 blinded arbiters using a semi-quantitative scoring system (0 = no staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining).
Statistical analysis
A logistic regression approach was used to examine the association between EVLP success and a number of potential predictors based on donor characteristics, EVLP protocol, and indices measured during EVLP using the software package R (R Foundation for Statistical Computing, Vienna, Austria). Logged protein levels were analyzed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA). The ability of each biomarker to discriminate between outcome groups (Survival, Non-survival, and Decline) was assessed with Mann-Whitney U test and a receiver operating characteristics (ROC) curve with calculation of the area under the curve (AUC) followed by Benjamini-Hochberg false discovery rate controlling procedure at p-value < 0.05.12 In vitro experiments were analyzed with paired or unpaired t-tests and goodness of fit by linear regression.
Results
Between April 2012 and July 2014, 53 donor lungs deemed unsuitable for immediate transplantation by UK lung transplant programs were evaluated with EVLP in the DEVELOP-UK study. A detailed description of the study population can be found in the final study report.8
Study group
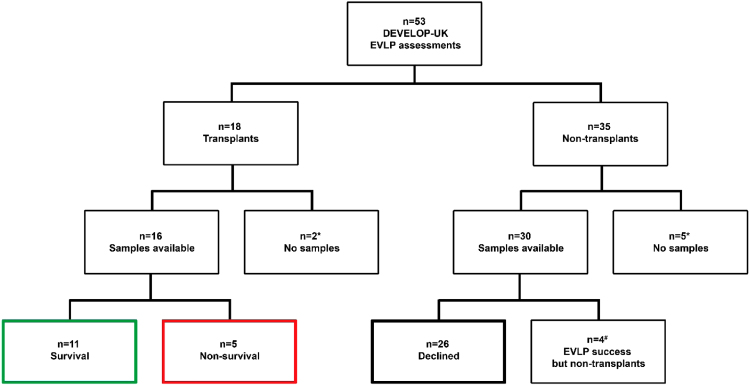
Samples were available from 42 of 53 donor lungs forming the study group. There were 11 donor lungs with incomplete sampling excluded from this study (Figure 1). From the 42 donor lungs included, 16 (38%) were transplanted, and 26 did not satisfy criteria for transplant after EVLP.
Figure 1.
Flow chart of outcome after EVLP in the DEVELOP-UK study. *Seven assessments lacked samples owing to either early cases or lack of consent from donor families. #Four donor lungs were excluded, as they were accepted for transplantation on the basis of the EVLP assessment but were later declined owing to unforeseen logistical reasons not related to lung performance, as follows: (1) accepted lung pair—recipient infected and too unwell when arriving for transplant; (2) accepted lung pair—successful selective right lung perfusion owing unknown left pulmonary artery laceration at procurement, no available single lung recipient; (3) accepted left single lung—emergency in operating room and lack of additional surgical capacity; and (4) accepted lung pair successfully preserved on EVLP while awaiting histology—decline owing to chronic lymphocytic leukemia on donor liver nodule histology.
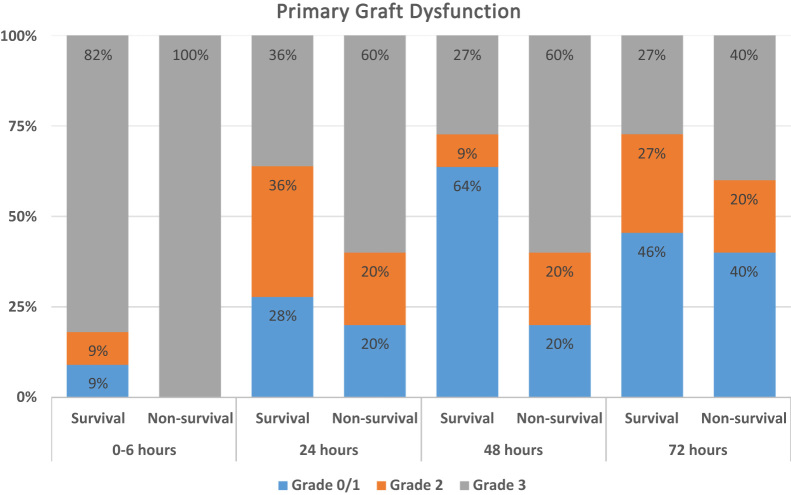
The results were compared between 3 subgroups depending on outcome: transplanted lungs with recipient survival to hospital discharge (Survival, n = 11), transplanted lungs with recipient in-hospital mortality before discharge (Non-survival, n = 5), and donor lungs declined from transplantation after EVLP (Declined, n = 26). There was no evidence to indicate that specific donor characteristics or organ assessment indices independently influenced EVLP outcome, including perfusion protocol (Table 1). The median time of EVLP was 175 minutes (range, 73–383 minutes) with no significant difference between the 3 outcome subgroups. Detailed donor and recipient characteristics are summarized in Table S3 (available in the online version of this article at www.jhltonline.org). Details of perioperative and post-transplant outcomes are listed in Table 2. There was a trend toward worse early allograft function (primary graft dysfunction [PGD] severity, need for extracorporeal mechanical oxygenation, time of invasive ventilation, and intensive care stay) in the Non-survival group compared with recipients in the Survival group. Figure 2 shows PGD scores by outcome group and time post-transplant. There was an increased rate of early PGD grade 3 in the Non-survival group (60% PGD grade 3 in Non-survival group compared with 27% PGD grade 3 in Survival group at 48 hours).
Table 1.
Univariate Logistic Regression Analysis of Predictors of Successful EVLP Reconditioning (EVLP Success)
| Variable | Category or units | Number | Number of successes | OR (95% CI) |
|---|---|---|---|---|
| EVLP protocol | Lund | 31 | 14 | 1 |
| Hybrid | 22 | 8 | 0.69 (0.23, 2.13) | |
| Donor age | Based on a 10-year increase | 53 | 22 | 1.47 (0.89, 2.44) |
| Sex (reference: male) | Female | 26 | 9 | 0.57 (0.19, 1.72) |
| Male | 27 | 13 | 1 | |
| Smoking (reference: non-smoker) | Non-smoker | 29 | 13 | 1 |
| Smoker | 24 | 9 | 0.74 (0.25, 2.23) | |
| Ischemic time | Based on 1-hour increase | 48a | 21 | 1.00 (0.61, 1.64) |
| Duration of ventilation | Based on 1-day increase | 53 | 22 | 1.18 (0.86, 1.60) |
| Optimized donor Pao2/Fio2 ratio before EVLP | Based on 100-mm Hg increase | 53 | 22 | 0.80 (0.50, 1.28) |
| Donor type (reference: DBD) | DBD | 39 | 17 | 1 |
| DCD | 14 | 5 | 0.72 (0.20, 2.54) | |
| Pao2/Fio2 ratio after EVLP | Based on 100-mm-Hg increase | 48a | 22 | 1.35 (0.85, 2.13) |
| Compliance start | Based on 10-ml/mbar increase | 28a | 15 | 1.26 (0.93, 1.71) |
| Change in complianceb | Based on 10-ml/mbar increase | 15a | 7 | 0.98 (0.52, 1.86) |
| Airway resistance start | Based on 1-mbar/liter/sec increase | 24a | 11 | 0.93 (0.78, 1.10) |
| Change in airway resistanceb | Based on 1-mbar/liter/sec increase | 12a | 4 | 2.28 (0.44, 11.77) |
| Peak airway pressure start | Based on 1-cm H2O increase | 41a | 18 | 0.88 (0.74, 1.04) |
| Change in peak airway pressureb | Based on a 1-cm H2O increase | 24a | 9 | 1.06 (0.85, 1.32) |
| EVLP time | Based on 1-hour increase | 49a | 20 | 0.95 (0.54, 1.65) |
CI, confidence interval; DBD, donation after brain death; DCD, donation after circulatory death; EVLP, ex vivo lung perfusion; OR, odds ratio; Pao2/Fio2, arterial oxygen partial pressure/fraction of inspired oxygen.
A logistic regression analysis was used to examine the association between successful reconditioning and a number of potential predictors based on donor characteristics and indices measured during EVLP. ORs are presented for different categories or, in the case of continuous variables, based on a unit increase in the variable. Although some of the point estimates for ORs varied from 1, the associated 95% CI included 1 in all instances.
Excluding patients with missing data.
Changes defined as start − end.
Table 2.
Recipient Post–Lung Transplant Outcomes in EVLP Arm
| EVLP number | Age (years)/sex | Diagnosis | High risk | Tx | CPB | Pao2/Fio2 ratio at 24 hours post-Tx (mm Hg) | PGD score at 72 hours | Post-operative ECMO | Invasive ventilation, days | ICU stay (days) | Hospital stay (days) | Survival to discharge | 1-year survival | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27 | 59/M | IPF | No | Yes—DL | Yes | 378 | 0 | No | 2 | 3.5 | 46 | Yes | Yes | |
| 28 | 32/F | CF | NIV | Yes—DL | No | 217 | 3 | No | 1.5 | 7.5 | 21 | Yes | Yes | |
| 29 | 56/M | COPD | NIV | Yes—DL | Yes | 335 | 1 | Yes | 15 | 15 | 25 | Yes | No | Pneumonia |
| 30 | 62/F | IPF | NIV | Yes—SL | Yes | 428 (ECMO) | 3 | Yes | 65 | 65.5 | 93 | Yes | Yes | |
| 31 | 44/M | PAH | PAH | Yes—DL | Yes | 221 | 3 | Yes | 0.33 | 21 | 44 | Yes | Yes | |
| 32 | 48/M | IPF | RV↓ | Yes—DL | Yes | 286 | 1 | No | 0.5 | 1.5 | 16 | Yes | Yes | |
| 33 | 49/M | IPF | No | Yes—DL | Yes | 326 (ECMO) | 2 | Yes | 70 | 68 | 87 | Yes | Yes | |
| 34 | 59/F | COPD | No | Yes—DL | Yes | 146 | 1 | No | 1.5 | 5 | 25 | Yes | Yes | |
| 35 | 64/M | COPD | NIV | Yes—DL | No | 278 | 2 | No | 0.5 | 2.5 | 16 | Yes | Yes | |
| 36 | 20/M | CF | NIV | Yes—DL | Yes | 203 | 2 | No | 2.5 | 6.5 | 17 | Yes | Yes | |
| 37 | 60/M | COPD | No | Yes—DL | Yes | 345 | 1 | No | 1.5 | 5.5 | 25 | Yes | Yes | |
| 38 | 56/M | IPF | No | Yes—DL | Yes | 113 (ECMO) | 3 | Yes | 100a | 98 | 100a | No | No | PGDb and sepsis |
| 39 | 56/F | BE | No | Yes—DL | Yes | 257 | 1 | No | 3 | 14 | 31a | No | No | Pneumonia and sepsis |
| 40 | 56F | COPD | No | Yes—DL | Yes | 401 | 2 | No | 18a | 18a | 18a | No | No | PGDb and hypoxic brain injury |
| 41 | 23/M | CF | NIV + RV↓ | Yes—DL | Yes | 368 (ECMO) | 1 | Yes | 3 | 15 | 63a | No | No | PGDb and pneumonia |
| 42 | 58/M | IPF | NIV | Yes—SL | Yes | 71 | 3 | No | 31a | 31a | 31a | No | No | PGDb and respiratory arrest |
BE, bronchiectasis; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; DL, double-lung; ECMO, extracorporeal membrane oxygenation; EVLP, ex vivo lung perfusion; F, female; ICU, intensive care unit; IPF, idiopathic pulmonary fibrosis; M, male; NIV, non-invasive ventilation; PAH, pulmonary artery hypertension; Pao2/Fio2, arterial oxygen partial pressure/fraction of inspired oxygen; PGD, primary graft dysfunction; RV↓, right ventricular failure; SL, single-lung; Tx, transplantation.
Death before hospital discharge.
Of 4 recipients in whom we believe PGD played a role in the cause of death, 2 had severe PGD at all time points up to 72 hours. One patient had very severe graft failure on arrival in the ICU and had to be salvaged with emergency ECMO. ECMO was weaned just before 72 hours and the patient had at this time point a saturation reflecting mild PGD, even though he never recovered and continued to need hospital treatment for failing graft function until his death at 63 days post-Tx. The fourth recipient had moderately severe PGD up to 72 hours, which never recovered. The patient was never weaned off invasive ventilation and died 18 days post-transplant.
Figure 2.
PGD score by outcome subgroup and time post-transplant. PGD is the clinical syndrome of chest radiographic changes and poor oxygenation that represents early acute injury to the transplanted lung. The PGD scores used in the study were as defined in the International Society for Heart and Lung Transplantation consensus definition.28 The distribution of the PGD score by study group, measured at baseline and 24, 48, and 72 hours after the transplant, is shown. A score of 0 represents no evidence of PGD, and a score of 3 represents the most severe form of PGD.
Biomarker analysis
Bronchoalveolar lavage
No significant differences in markers of inflammation or tissue injury were found in BAL samples when comparing samples from pre-EVLP and post-EVLP. Similarly, BAL levels of analyzed markers did not differ significantly between outcome groups pre-EVLP or post-EVLP.
Donor lung tissue
In lung tissue lysates, the levels of a large number of inflammatory markers (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, interferon-γ, monocyte chemotactic protein-1, macrophage inflammatory protein-1α, tumor necrosis factor [TNF]-α, and TNF-β) increased significantly between the start and end of perfusion. However, none of the proteins measured in tissue differed significantly between the individual outcome groups.
Perfusate
In perfusate, 40 of 45 markers were detectable and increased significantly in serial measurements during EVLP. Only matrix metalloproteinase-9, placental growth factor (PlGF) PIGF, TNF-β, serum amyloid A, and vascular endothelial growth factor A remained unchanged over the course of perfusion. No marker was significantly different between outcome groups at the 15-minute sampling time point, but at 30 minutes of EVLP, differences were established between groups in 2 biomarkers. These separations were maintained but were not increased at later time points.
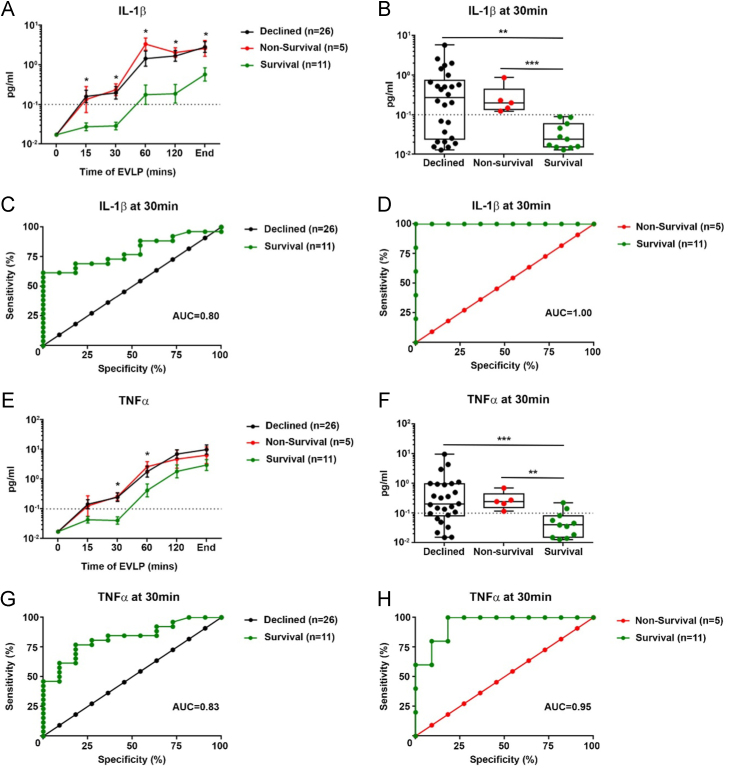
The protein markers with ability to differentiate Declined lungs from Survival lungs were IL-1β and TNF-α in perfusate after 30 minutes of perfusion (Mann-Whitney U = 58.8, Z = −2.81, p = 0.004 and Mann-Whitney U = 49.5, Z = −3.11, p = 0.001, respectively) (Figure 3B and F). The AUC for ROC curve of 30 minutes perfusate levels for Declined vs. Survival was 0.80 (p = 0.005) for IL-1β and 0.83 (p = 0.002) for TNF-α (Figure 3C and G). The most effective markers to differentiate recipient in-hospital mortality (Non-survival group) from mortality after successful discharge (Survival group) were similarly perfusate IL-1β (Mann-Whitney U = 0.0, Z = −3.12, p < 0.001) and TNF-α (Mann-Whitney U = 3.0, Z = −2.78, p = 0.003) after 30 minutes of perfusion (Figure 3B and F). ROC curve analysis showed high precision for both IL-1β (AUC = 1.00, p = 0.002) and TNF-α (AUC = 0.95, p = 0.006) to differentiate between the Non-survival and Survival groups. With a total lung capacity scaled cutoff value of 0.1 pg/ml, perfusate IL-1β after 30 minutes of EVLP had a sensitivity and specificity of 100% to diagnose Non-survival lungs.
Figure 3.
(A–H) Perfusate biomarkers after 30 minutes of EVLP—association with EVLP performance and recipient in-hospital mortality. Perfusate levels of IL-1β (A) and TNF-α (E) over the course of the EVLP assessment separated into 3 outcome groups (mean ± SEM): Survival, Non-survival, and Declined. Box and whisker plots show perfusate levels of IL-1β (B) and TNF-α (F) for the respective groups at the 30-minute sampling time point with a total lung capacity adjusted cutoff at 0.1 pg/ml (dotted line). ROC curves for potential of IL-1β (C) and TNF-α (G) as biomarkers of EVLP performance (Survival vs Declined groups) in perfusate at 30 minutes of EVLP. (D) and (H) show ROC curves for the potential of perfusate IL-1β and TNF-α as biomarkers of in-hospital post-transplant mortality (Survival vs Non-survival groups) at the 30-minute sampling time point. As early as 30 minutes into the EVLP assessment, both cytokines were efficiently discriminatory between the 3 groups in samples from the circulating perfusate fluid. **p < 0.01, ***p < 0.001.
The predictive value of perfusate IL-1β and TNF-α after 30 minutes of EVLP remained robust when applied to the primary study end-point of 1-year post-transplant survival; the AUC for ROC curve of 1-year Non-survival vs Survival was 0.93 (p = 0.005) for IL-1β and 0.95 (p = 0.003) for TNF-α (Figure S3 [available in the online version of this article at www.jhltonline.org]). With a total lung capacity scaled cutoff value of 0.1 pg/ml, perfusate IL-1β after 30 minutes of EVLP had a diagnostic sensitivity of 83% and specificity of 100% for recipient 1-year post-transplant mortality.
Subanalysis with adjustment for EVLP protocol showed no significant impact on protein levels. Detailed protein levels and fold changes during EVLP in the 3 lung compartments are illustrated in Figures S1 and S2 (available in the online version of this article at www.jhltonline.org).
Molecular and cellular staining in EVLP donor lung tissue
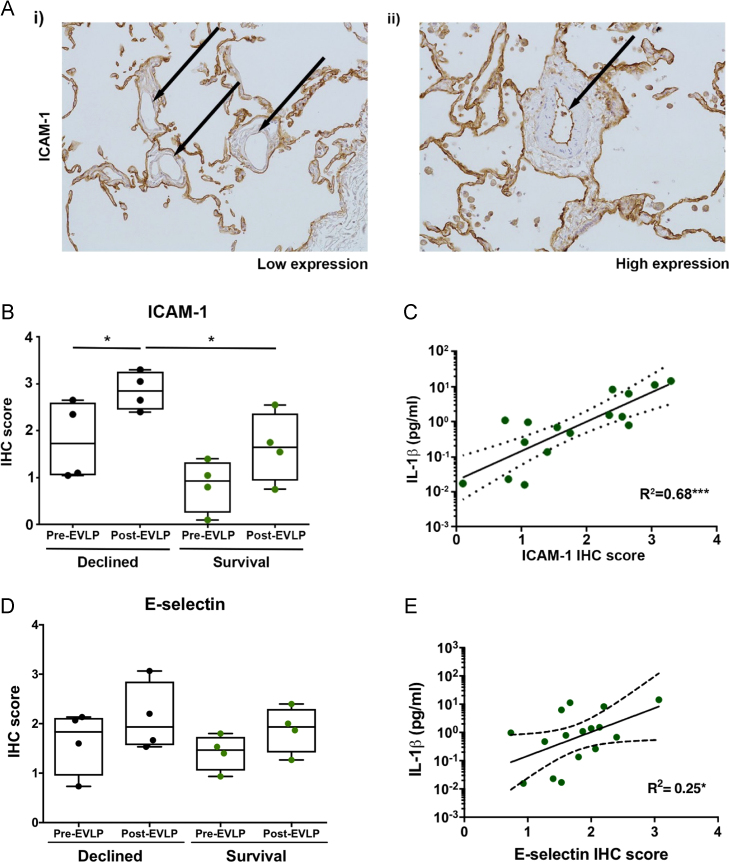
ICAM-1 staining intensity was elevated in the Declined group post-EVLP (mean 2.85, SEM 0.20) compared with pre-EVLP (mean 1.79, SEM 0.42; t3 = 4.67, p = 0.019) and with post-EVLP in the Survival group (mean 1.65, SEM 0.37; t6 = 2.85, p = 0.029) (Figure 4B). Endothelial ICAM-1 expression in lung tissue was strongly correlated with perfusate levels of IL-1β from the same lung (R2 = 0.68, p < 0.001) (Figure 4C). E-selectin staining followed a similar trend but was not significantly different between groups, and the correlation with perfusate IL-1β levels was weaker (R2 = 0.25, p < 0.047). Survival lungs contained significantly fewer neutrophils after EVLP (mean 7.0, SEM 2.04) compared with Declined lungs (mean 22.8, SEM 2.90; t6 = 4.44, p = 0.004). EVLP reduced the numbers of neutrophils in both Survival and Declined lungs but not significantly (data not shown).
Figure 4.
(A–E) Expressions of ICAM-1 and E-selectin in immunohistochemistry (IHC) stained clinical EVLP donor lung tissue and association with perfusate IL-1β levels. (A) Paraffin-embedded donor lung sections DAB stained for ICAM-1 expression. Arrows mark the low-intensity stained (i) or high-intensity stained (ii) pulmonary vascular endothelium. Unselective background staining seen on adhesive alveolar epithelium. Box and whisker plots show average ICAM-1 (B) and E-selectin (D) staining intensity scores for pre-EVLP and post-EVLP biopsy specimens from 4 Declined lungs and 4 Survival lungs. Scatter plots show the correlation between average ICAM-1 (C) and E-selectin (E) intensity scores in the same experiment and corresponding perfusate IL-1β levels (linear regression line with 95% confidence intervals). *p < 0.05, ***p < 0.001.
Neutrophil adhesion assay
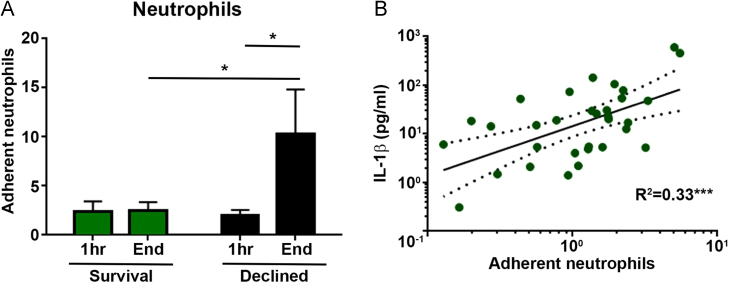
Adhesion of primary human neutrophils to a monolayer of HPMECs conditioned with perfusate samples from 16 individual clinical EVLP assessments, including Survival (n = 7) and Declined (n = 9) lungs, was quantified using a flow-based in vitro platform with 3 neutrophil donor repeats. Neutrophil adhesion to HPMECs conditioned with perfusate from the beginning of EVLP was similar between Survival and Declined groups but significantly higher in the group of HPMECs conditioned with end of EVLP perfusate from Declined lungs (mean 10.3, SEM 4.5) compared with Survival lungs (mean 2.5, SEM 0.9; t92 = 2.10, p = 0.038) (Figure 5A). Neutrophil adhesion to conditioned endothelium was positively correlated with perfusate IL-1β levels (R2 = 0.33, p < 0.001) (Figure 5B).
Figure 5.
Neutrophil adhesion to conditioned human pulmonary microvascular endothelial cells and association with perfusate IL-1β levels. (A) In vitro neutrophil adhesion comparison between HPMECs conditioned with start or end EVLP perfusates from 7 Survival lungs and 9 Declined lungs (n = 3 neutrophil donor repeats with each donor lung perfusate sample; mean ± SEM). (B) Correlation between average neutrophil adhesions in the same experiment and corresponding perfusate IL-1β levels (n = 3 neutrophil donor repeats with each donor lung perfusate sample; linear regression line with dotted 95% confidence intervals). The neutrophil adhesion was significantly higher to cells conditioned with end perfusates from Declined lungs compared with start perfusates from the same lungs and end perfusates from Survival lungs. Neutrophil adhesion was positively correlated to IL-1β levels in the perfusate used to condition the endothelial cells. *p < 0.05, ***p < 0.001.
Adhesion molecule expression
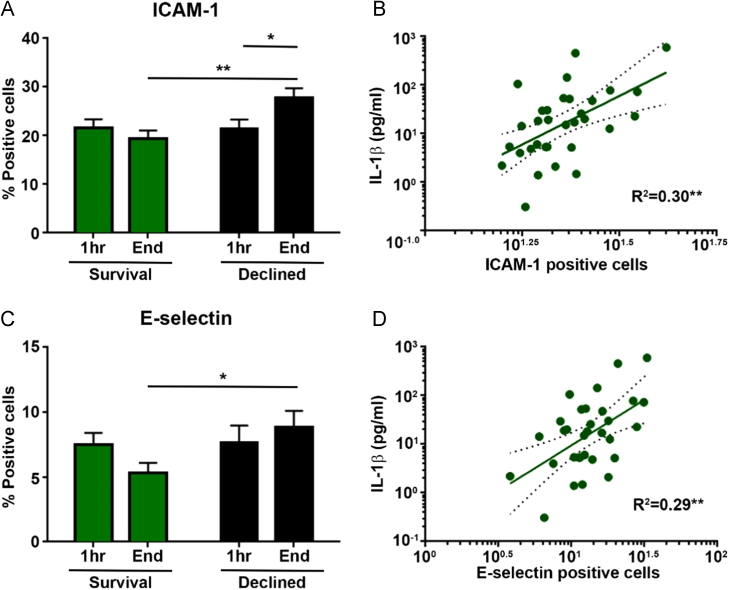
HPMECs conditioned with perfusate from end of EVLP of Declined donor lungs showed significantly elevated E-selectin and ICAM-1 expression compared with perfusate from Survival lungs (ICAM-1: meanDeclined 27.9, SEM 1.9 vs meanSurvival 19.4, SEM 1.7; t22 = 3.31, p = 0.003; E-selectin: meanDeclined 8.8, SEM 1.3 vs meanSurvival 5.3, SEM 0.8; t22 = 2.21, p = 0.038) (Figure 6A and B). IL-1β levels in perfusate were positively correlated with expression of ICAM-1 (R2 = 0.30, p = 0.001) and E-selectin (R2 = 0.29, p = 0.001) (Figure 6C and D).
Figure 6.
(A–D) Expression of cell surface adhesion molecules on conditioned HPMECs and association with perfusate IL-1β levels. Flow cytometry investigation of ICAM-1 (A) and E-selectin (C) expression on conditioned HPMECs compared between cells stimulated with start or end EVLP perfusates from 7 Survival lungs and 9 Declined lungs (mean ± SEM). Scatter plots show the association between the fraction of conditioned endothelial cells positive for ICAM-1 (B) and E-selectin (D) in the same experiment and corresponding perfusate IL-1β levels (linear regression line with dotted 95% confidence intervals). Endothelial adhesion molecule expressions were significantly higher on cells conditioned with end perfusates from Declined lungs compared with start perfusates from the same lungs (only ICAM-1) and end perfusates from Survival lungs. ICAM-1 and E-selectin expressions were positively correlated to IL-1β levels in the perfusate used to condition the endothelial cells. *p < 0.05, **p < 0.01.
Inhibition of neutrophil adhesion
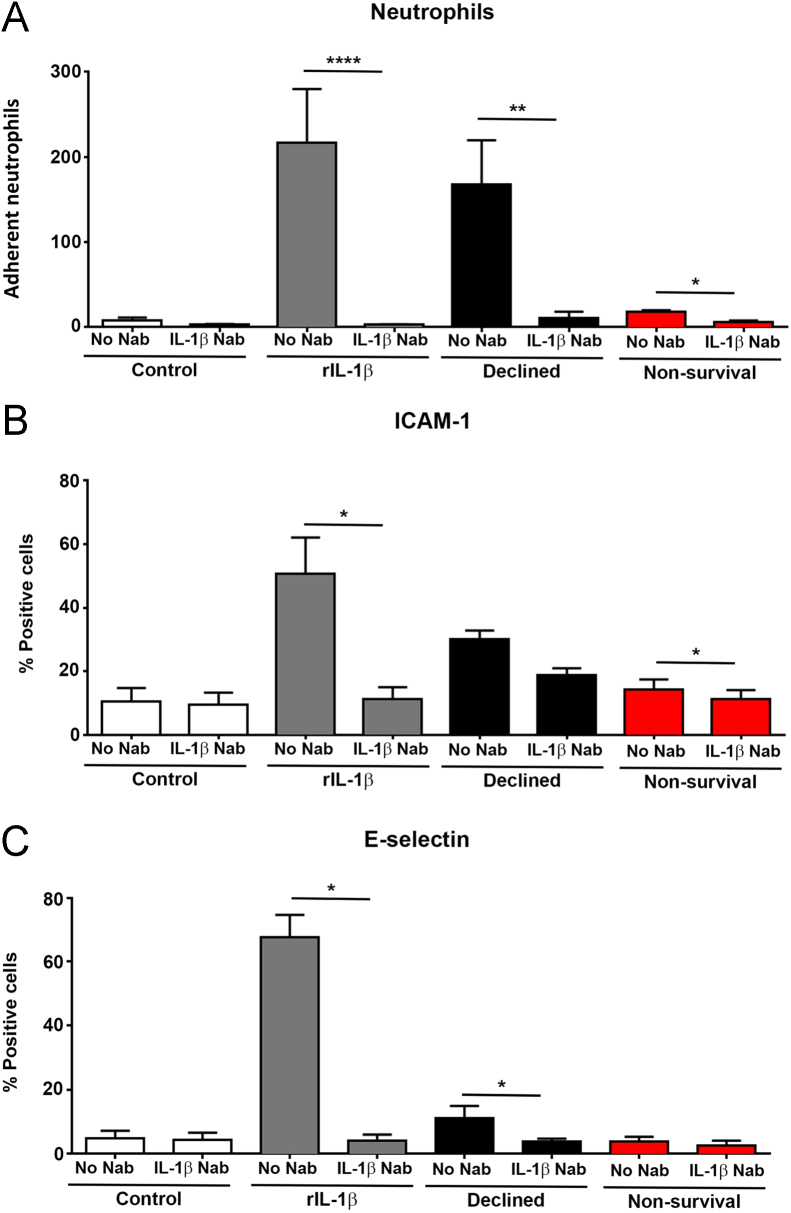
Pre-treatment of perfusates with an anti–IL-1β NAb before HPMEC conditioning effectively reduced neutrophil adhesion (n = 5 neutrophil donor repeats) induced by perfusate from a highly inflamed Declined lung (IL-1β 592 pg/ml) (mean 168.1, SEM 51.7 vs meanNAb 10.5, SEM 7.8; t4 = 4.64, p = 0.009) and from a transplanted Non-survival lung with PGD and early in-hospital mortality (IL-1β 49 pg/ml) (mean 18.0, SEM 1.9 vs meanNAb 6.4, SEM 1.3; t4 = 4.12, p = 0.015) (Figure 7A). A similar pattern of reduced ICAM-1 and E-selectin expressions was seen when perfusates from 4 representative Declined EVLP lungs and 4 representative Non-survival EVLP lungs were pre-treated with anti–IL-1β NAb. A 32% reduction of ICAM-1 (mean 22.2, SEM 3.5 vs meanNAb 15.0, SEM 2.2; t7 = 2.67, p = 0.032) and 57% reduction of E-selectin expression (mean 7.5, SEM 2.3 vs meanNAb 3.2, SEM 0.9; t7 = 5.25, p = 0.001) was observed on treated cells (Figure 7B and C).
Figure 7.
Effect of blocking perfusate IL-1β with an anti–IL-1β neutralizing antibody. In vitro effect of anti–IL-1β NAb pre-treatment on neutrophil adhesion (A) to HPMECs conditioned with perfusate from either a Declined lung or a Non-survival lung (n = 5 neutrophil donor repeats; mean ± SEM). Effect of IL-1β NAb pre-treatment on ICAM-1 (B) and E-selectin (C) expressions on HPMECs conditioned with perfusate from either Declined lungs or Non-survival lungs (n = 4 donor lung perfusates from each group). Pre-treatment of perfusates with an IL-1β NAb efficiently reduced neutrophil adhesion and adhesion molecule expressions on conditioned pulmonary endothelial cells in vitro. *p < 0.05, **p < 0.01, ****p < 0.0001.
Discussion
In this study, we demonstrate that an early pro-inflammatory signal in perfusate during clinical EVLP of extended criteria donor lungs predicts the likelihood of successful EVLP as well as early and mid-term post-transplant outcomes. Donor lungs are highly susceptible to injury in the critical care environment, and the extent of injury is difficult to assess at the time of organ procurement, making current donor lung acceptance criteria poor discriminators of lung injury.13, 14, 15, 16 Correspondingly, we found no evidence that specific donor characteristics or physiologic parameters independently influenced the EVLP outcome in this cohort. However, post-transplant non-surviving recipients exhibited inferior allograft function (shown as higher rates of severe PGD, need for extracorporeal mechanical oxygenation, and need for long-term invasive ventilation), which contributed to the very high incidence of adverse outcomes among EVLP lung recipients in this study. We demonstrated a measurable increase in perfusate IL-1β and TNF-α at 30 minutes in declined donor lungs compared with transplanted lungs. Furthermore, we found clear differences in donor lungs transplanted with good outcome—survival to hospital discharge and 1-year survival—compared with lungs transplanted with early recipient mortality, hence providing unique information that standard criteria did not. Perfusate levels of IL-1β measured 30 minutes into EVLP could distinguish subsequent in-hospital mortality with a sensitivity and specificity of 100% and 1-year survival with similar precision. BAL appears to show a comparable but weaker signal than seen in perfusate. With bronchoscopy being more invasive and the quality of BAL more user dependent than perfusate sampling, the benefit of performing BAL for biomarker identification during EVLP is questionable.
Acute donor lung injury is characterized by acute inflammation, increased vascular permeability, and neutrophil infiltration.17, 18 Therefore, we assessed the effect of the perfusate milieu from clinical EVLP runs on neutrophil–endothelial cell interactions in an in vitro model. We found perfusate IL-1β to be the most potent activator of pulmonary endothelium with concentrations strongly correlated with neutrophil adhesion and adhesion molecule expressions in lung tissue, which supports previous studies outside EVLP linking IL-1β with acute lung injury and ischemia-reperfusion injury.19, 20, 21, 22 An activated endothelium during EVLP will promote recipient neutrophil adherence on reperfusion and may explain the increased rate of PGD seen in the Non-survival group. A similar picture and correlation to perfusate IL-1β levels was seen on conditioned endothelial cells in vitro where both neutrophil adhesion and adhesion molecule expressions could be significantly reduced by specifically blocking IL-1β in the perfusate. The therapeutic potential of IL-1β neutralization during clinical EVLP of extended criteria donor lungs has not been examined and warrants further investigation.
Our findings support recent observations made by Machuca et al23 demonstrating the feasibility of detecting perfusate biomarkers during clinical EVLP that might improve donor lung assessment. IL-8, proposed as one of the optimum markers of EVLP transplant outcome in their study, has consistently shown potential in previous studies of donor lung injury from our group.24, 25, 26 The potential of IL-8 to discriminate successful EVLP in our pilot study was enhanced by combining IL-8 with IL-1β. In this cohort, perfusate IL-8 levels were higher in Declined lungs compared with transplanted lungs but could not significantly differentiate between groups. As perfusate IL-1β was a marker on its own, the combination with IL-8 did not add any significant value. Similar to findings in our single-center pilot study, there was a trend toward an association between levels of several pro-inflammatory markers in perfusate and BAL and allograft function (Pao2/Fio2 ratio at 72 hours and lung function tests at follow-up), but none reached statistical significance.
The role of the endothelin-1 pathway as a biomarker for donor lung assessment during EVLP described by Machuca et al27 could not be replicated in our study. Perfusate endothelin-1 levels were similar in all groups, whether assessing survival, PGD, or need for extracorporeal mechanical oxygenation post-transplant.
A limitation of our study is its relative sample size with 42 human EVLP assessments included, yet this number is one of the largest cohorts reported to date and sufficient to demonstrate the feasibility of using perfusate markers to classify successful EVLP. All 42 donor lungs in this study were assessed with EVLP with intent for transplantation following pre-defined criteria for transplant suitability to reduce center bias in decision making. All protein analyses on collected samples were performed after completion of the EVLP procedure and had no impact on decisions at the time. Future studies will need to investigate emerging real-time cytokine tests, as time-sensitive assays with high sensitivity will be essential for the utility of any biomarker during clinical EVLP.
In conclusion, the results of this study validate our previous findings in a pilot study and clearly demonstrate the potential role of IL-1β as a biomarker of EVLP reconditioning and, more importantly, post-transplant survival. Therapeutic targeting of IL-1β provides an exciting opportunity to decrease endothelial activation and potentially reduce the incidence of early graft injury post-transplant. If successful, these principles may expand to other organs with ischemia-reperfusion injury.
Disclosure statement
This was a mechanistic substudy of the multicenter trial DEVELOP-UK supported by the National Institute for Health Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation. The main study was funded by the NIHR Health Technology Assessment Programme and was led by chief investigator Andrew Fisher (Newcastle) and local principal investigators John Dark (Newcastle), Stephen Tsui (Papworth), Nizar Yonan (Manchester), Andre Simon (Harefield), Nandor Marczin (Harefield), and Jorge Mascaro (Birmingham). This substudy was supported by a research grant awarded by the United Kingdom Cystic Fibrosis Trust as a result of a donation made by the Robert Luff Foundation for research into lung transplantation. The funding organization had no role in the collection of data, its analysis, or interpretation and had no influence on the manuscript content.
We acknowledge the contribution of a large number of DEVELOP-UK investigators from all adult lung transplant centers in the United Kingdom.
Footnotes
Supplementary data associated with this article can be found in the online version at www.jhltonline.org.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.NHSBT Annual report on transplant activity. 2015 Available from http://www.odt.nhs.uk/pdf/activity-report/activity_report_2015_16.pdf, accessed May 20, 2017. [Google Scholar]
- 2.Cypel M., Yeung J.C., Liu M. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 3.Boffini M., Ricci D., Barbero C. Ex vivo lung perfusion increases the pool of lung grafts: analysis of its potential and real impact on a lung transplant program. Transplant Proc. 2013;45:2624–2626. doi: 10.1016/j.transproceed.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen I.S., Moller-Sorensen H., Moller C.H. First Danish experience with ex vivo lung perfusion of donor lungs before transplantation. Dan Med J. 2014;61:A4809. [PubMed] [Google Scholar]
- 5.Sage E., Mussot S., Trebbia G. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experiencedagger. Eur J Cardiothorac Surg. 2014;46:794–799. doi: 10.1093/ejcts/ezu245. [DOI] [PubMed] [Google Scholar]
- 6.Andreasson A.S., Dark J.H., Fisher A.J. Ex vivo lung perfusion in clinical lung transplantation—state of the art. Eur J Cardiothorac Surg. 2014;46:779–788. doi: 10.1093/ejcts/ezu228. [DOI] [PubMed] [Google Scholar]
- 7.Andreasson A.S., Karamanou D.M., Gillespie C.S. Profiling inflammation and tissue injury markers in perfusate and bronchoalveolar lavage fluid during human ex vivo lung perfusion. Eur J Cardiothorac Surg. 2017;51:577–586. doi: 10.1093/ejcts/ezw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher A., Andreasson A., Chrysos A. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 2016;20:1–276. doi: 10.3310/hta20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haslam P.L., Baughman R.P. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–248. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott J., Harris G.J., Pinder E.M. Exchange protein directly activated by cyclic AMP (EPAC) activation reverses neutrophil dysfunction induced by beta2-agonists, corticosteroids, and critical illness. J Allergy Clin Immunol. 2016;137:535–544. doi: 10.1016/j.jaci.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Naemi F.M., Carter V., Kirby J.A., Ali S. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;96:258–266. doi: 10.1097/TP.0b013e3182985504. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 13.Avlonitis V.S., Fisher A.J., Kirby J.A., Dark J.H. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003;75:1928–1933. doi: 10.1097/01.TP.0000066351.87480.9E. [DOI] [PubMed] [Google Scholar]
- 14.Cypel M., Yeung J.C., Hirayama S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Reyes K.G., Mason D.P., Thuita L. Guidelines for donor lung selection: time for revision? Ann Thorac Surg. 2010;89:1756–1764. doi: 10.1016/j.athoracsur.2010.02.056. discussion 1764-5. [DOI] [PubMed] [Google Scholar]
- 16.Fisher A.J., Donnelly S.C., Pritchard G., Dark J.H., Corris P.A. Objective assessment of criteria for selection of donor lungs suitable for transplantation. Thorax. 2004;59:434–437. doi: 10.1136/thx.2003.007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers C., Rankin S.M., Condliffe A.M., Singh N., Peters A.M., Chilvers E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnadasan B., Naidu B.V., Byrne K., Fraga C., Verrier E.D., Mulligan M.S. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;125:261–272. doi: 10.1067/mtc.2003.16. [DOI] [PubMed] [Google Scholar]
- 20.Chang D.M., Hsu K., Ding Y.A., Chiang C.H. Interleukin-1 in ischemia-reperfusion acute lung injury. Am J Respir Crit Care Med. 1997;156:1230–1234. doi: 10.1164/ajrccm.156.4.9702095. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow S.C., Ramachandran S., Csontos K.A., Jia J., Mohanakumar T., Chapman W.C. Interleukin-1beta is prominent in the early pulmonary inflammatory response after hepatic injury. Surgery. 2005;138:64–70. doi: 10.1016/j.surg.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Merry H.E., Phelan P., Doaks M., Zhao M., Mulligan M.S. Functional roles of tumor necrosis factor-alpha and interleukin 1-beta in hypoxia and reoxygenation. Ann Thorac Surg. 2015;99:1200–1205. doi: 10.1016/j.athoracsur.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Machuca T.N., Cypel M., Yeung J.C. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann Surg. 2015;261:591–597. doi: 10.1097/SLA.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 24.Fisher A.J., Donnelly S.C., Hirani N. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163:259–265. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 25.Fisher A.J., Donnelly S.C., Hirani N. Enhanced pulmonary inflammation in organ donors following fatal non-traumatic brain injury. Lancet. 1999;353:1412–1413. doi: 10.1016/S0140-6736(99)00494-8. [DOI] [PubMed] [Google Scholar]
- 26.De Perrot M., Sekine Y., Fischer S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 27.Machuca T.N., Cypel M., Zhao Y. The role of the endothelin-1 pathway as a biomarker for donor lung assessment in clinical ex vivo lung perfusion. J Heart Lung Transplant. 2015;34:849–857. doi: 10.1016/j.healun.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Christie J.D., Van Raemdonck D., de Perrot M. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24:1451–1453. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material