Abstract
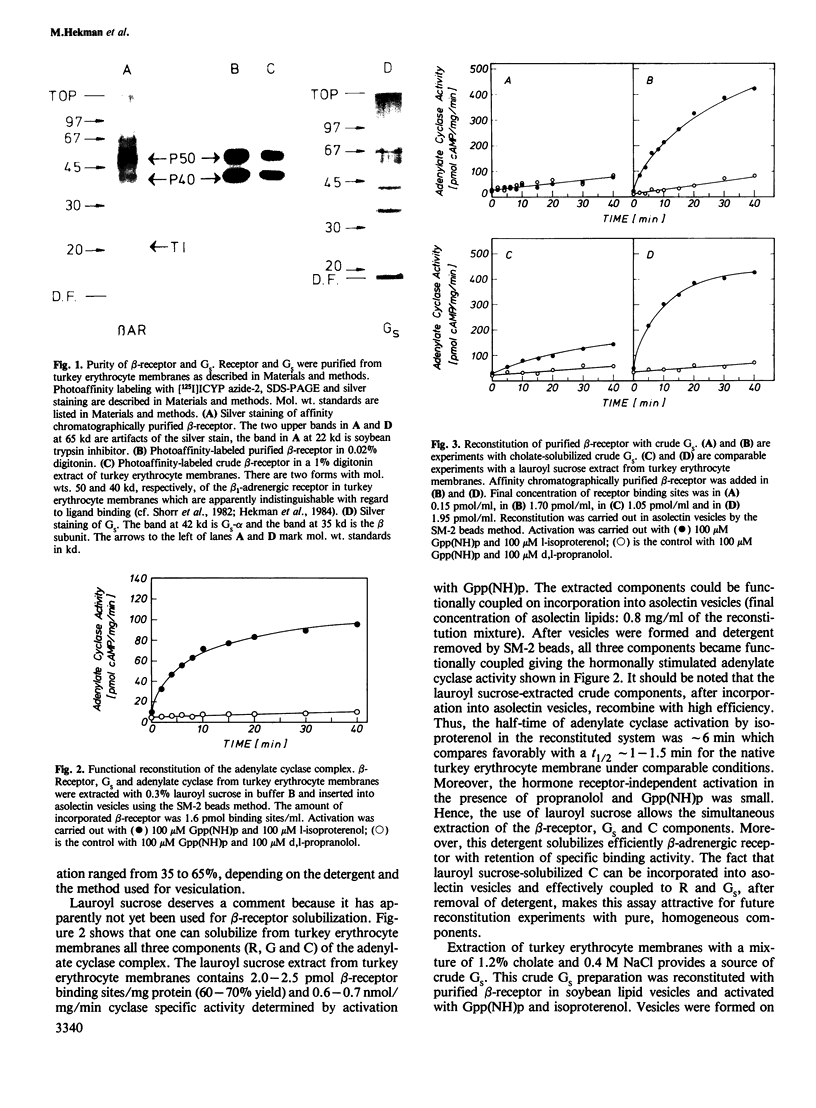
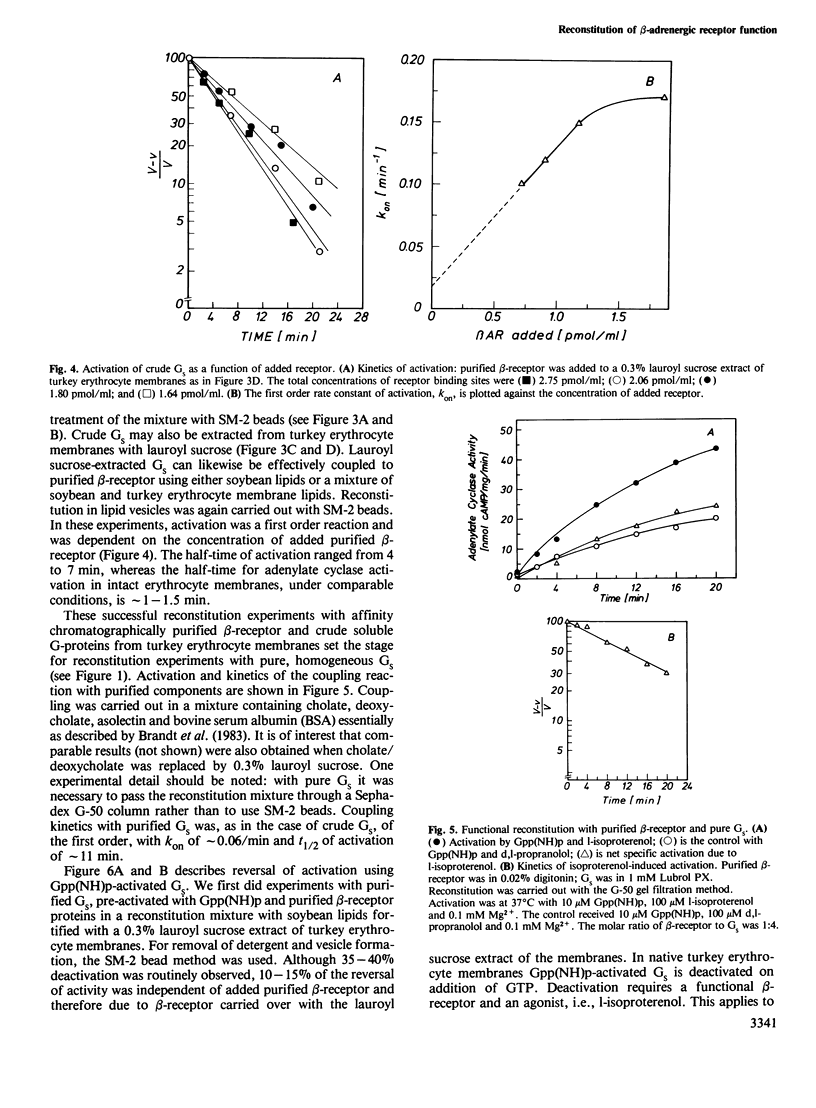
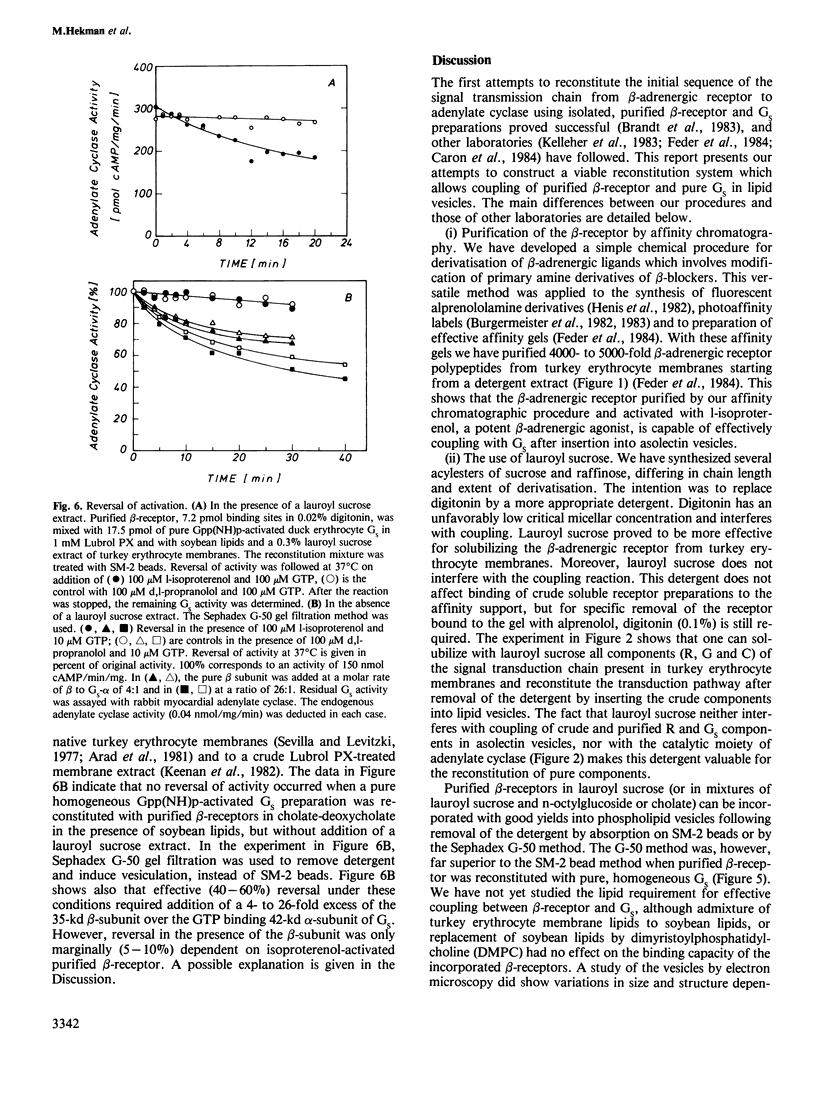
Beta 1-Adrenergic receptor proteins were extracted from turkey erythrocyte membranes with lauroyl sucrose and digitonin and purified by affinity chromatography on a column of alprenolol agarose Affi-gel 10 or 15. The 5000-fold purified receptor is able to couple functionally with the stimulatory GTP-binding protein (GS) from either turkey or duck erythrocytes. Functional coupling was achieved by three different approaches. (i) Purified beta-receptor polypeptides were coupled in phospholipid (asolectin) vesicles with GS from a crude cholate or lauroyl sucrose extract of turkey erythrocyte membranes. The detergent was removed and vesicles were formed with SM-2 beads. (ii) Purified beta-receptor was reconstituted with pure, homogeneous GS in asolectin vesicles. (iii) Purified beta-receptors were either coupled in asolectin vesicles with a mixture of pure, homogeneous Gpp(NH)p-activated GS and a lauroyl sucrose extract of turkey erythrocyte membranes, or with pure, homogeneous Gpp(NH)p-activated GS alone. The decay of activity was measured on addition of GTP and hormone. In (ii) and (iii), the detergent was removed and vesicles were formed by gel filtration on Sephadex G-50 columns. In each of the three different experimental conditions, the beta-receptor was activated with l-isoproterenol and activation was blocked with d,l-propranolol. Activated GS were measured separately by means of their capacity to activate a crude Lubrol PX-solubilized adenylate cyclase preparation from rabbit myocardial membrane. The kinetics of GS activation by purified beta-receptors occupied by l-isoproterenol was first order and activation was linearly dependent on receptor concentration.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arad H., Rimon G., Levitzki A. The reversal of the Gpp(NH)p-activated state of adenylate cyclase by GTP and hormone is by the "collision coupling" mechanism. J Biol Chem. 1981 Feb 25;256(4):1593–1597. [PubMed] [Google Scholar]
- Brandt D. R., Asano T., Pedersen S. E., Ross E. M. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983 Sep 13;22(19):4357–4362. doi: 10.1021/bi00288a002. [DOI] [PubMed] [Google Scholar]
- Burgermeister W., Hekman M., Helmreich E. J. Photoaffinity labeling of the beta-adrenergic receptor with azide derivatives of iodoccyanopindolol. J Biol Chem. 1982 May 10;257(9):5306–5311. [PubMed] [Google Scholar]
- Burgermeister W., Nassal M., Wieland T., Helmreich E. J. A carbene-generating photoaffinity probe for beta-adrenergic receptors. Biochim Biophys Acta. 1983 Apr 6;729(2):219–228. doi: 10.1016/0005-2736(83)90488-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Citri Y., Schramm M. Probing of the coupling site of the beta-adrenergic receptor. Competition between different forms of the guanyl nucleotide binding protein for interaction with the receptor. J Biol Chem. 1982 Nov 25;257(22):13257–13262. [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- Feder D., Arad H., Gal A., Hekman M., Helmreich E. J., Levitzki A. Resolution, reconstitution, and mode of action of the beta-adrenergic receptor-dependent adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:61–71. [PubMed] [Google Scholar]
- Gal A., Braun S., Feder D., Levitzki A. Reconstitution of a functional beta-adrenergic receptor using cholate and a novel method for its functional assay. Eur J Biochem. 1983 Aug 1;134(2):391–396. doi: 10.1111/j.1432-1033.1983.tb07580.x. [DOI] [PubMed] [Google Scholar]
- Hanski E., Sternweis P. C., Northup J. K., Dromerick A. W., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties of the turkey erythrocyte protein. J Biol Chem. 1981 Dec 25;256(24):12911–12919. [PubMed] [Google Scholar]
- Hekman M., Schiltz E., Henis Y. I., Elson E. L., Helmreich E. J. Mobility, localization, and heterogeneity of beta-adrenergic receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:47–60. [PubMed] [Google Scholar]
- Henis Y. I., Hekman M., Elson E. L., Helmreich E. J. Lateral motion of beta receptors in membranes of cultured liver cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2907–2911. doi: 10.1073/pnas.79.9.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homcy C. J., Rockson S. G., Countaway J., Egan D. A. Purification and characterization of the mammalian beta 2-adrenergic receptor. Biochemistry. 1983 Feb 1;22(3):660–668. doi: 10.1021/bi00272a021. [DOI] [PubMed] [Google Scholar]
- Keenan A. K., Gal A., Levitzki A. Reconstitution of the turkey erythrocyte adenylate cyclase sensitivity to 1-epinephrine upon re-insertion of the Lubrol solubilized components into phospholipid vesicles. Biochem Biophys Res Commun. 1982 Mar 30;105(2):615–623. doi: 10.1016/0006-291x(82)91479-6. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Rashidbaigi A., Ruoho A. E., Johnson G. L. Rapid vesicle reconstitution of alprenolol-Sepharose-purified beta 1-adrenergic receptors. Interaction of the purified receptor with N. J Biol Chem. 1983 Nov 10;258(21):12881–12885. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G., Stiles G. L. Mechanisms of membrane-receptor regulation. Biochemical, physiological, and clinical insights derived from studies of the adrenergic receptors. N Engl J Med. 1984 Jun 14;310(24):1570–1579. doi: 10.1056/NEJM198406143102406. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Helmreich E. J. Hormone-receptor--adenylate cyclase interactions. FEBS Lett. 1979 May 15;101(2):213–219. doi: 10.1016/0014-5793(79)81011-x. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution, activity, and properties of the 35,000-dalton (beta) subunit. J Biol Chem. 1983 Sep 25;258(18):11361–11368. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pedersen S. E., Ross E. M. Functional reconstitution of beta-adrenergic receptors and the stimulatory GTP-binding protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7228–7232. doi: 10.1073/pnas.79.23.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Gaugler B., Metzger H. Isolation of homologous and heterologous complexes between catalytic and regulatory components of adenylate cyclase by forskolin-Sepharose. FEBS Lett. 1983 Nov 28;164(1):154–160. doi: 10.1016/0014-5793(83)80040-4. [DOI] [PubMed] [Google Scholar]
- Puchwein G., Pfeuffer T., Helmreich E. J. Uncoupling of catecholamine activation of pigeon erythrocyte membrane adenylate cyclase by filipin. J Biol Chem. 1974 May 25;249(10):3232–3240. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Bleil J. D., Wassarman P. M. Quantitation of nanogram amounts of protein using [3H]dinitrofluorobenzene. Anal Biochem. 1978 Nov;91(1):354–356. doi: 10.1016/0003-2697(78)90850-3. [DOI] [PubMed] [Google Scholar]
- Sevilla N., Levitzki A. The activation of adenylate cyclase by 1-epinephrine and guanylylimidodiphosphate and its reversal by 1-epinephrine and GTP. FEBS Lett. 1977 Apr 1;76(1):129–134. doi: 10.1016/0014-5793(77)80136-1. [DOI] [PubMed] [Google Scholar]
- Shorr R. G., Strohsacker M. W., Lavin T. N., Lefkowitz R. J., Caron M. G. The beta 1-adrenergic receptor of the turkey erythrocyte. Molecular heterogeneity revealed by purification and photoaffinity labeling. J Biol Chem. 1982 Oct 25;257(20):12341–12350. [PubMed] [Google Scholar]
- Stewart J. C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980 May 1;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Vauquelin G., Geynet P., Hanoune J., Strosberg A. D. Affinity chromatography of the beta-adrenergic receptor from turkey erythrocytes. Eur J Biochem. 1979 Aug 1;98(2):543–556. doi: 10.1111/j.1432-1033.1979.tb13215.x. [DOI] [PubMed] [Google Scholar]



