Abstract
Inflammatory bowel diseases (IBD) profoundly affect quality of life and have been gradually increasing in incidence, prevalence and severity in many areas of the world, and in children in particular. Patients with suspected IBD require careful history and clinical examination, while definitive diagnosis relies on endoscopic and histological findings. The aim of the present study was to investigate whether the alveolar air of pediatric patients with IBD presents a specific volatile organic compounds’ (VOCs) pattern when compared to controls. Patients 10–17 years of age, were divided into four groups: Crohn’s disease (CD), ulcerative colitis (UC), controls with gastrointestinal symptomatology, and surgical controls with no evidence of gastrointestinal problems. Alveolar breath was analyzed by ion molecule reaction mass spectrometry. Four models were built starting from 81 molecules plus the age of subjects as independent variables, adopting a penalizing LASSO logistic regression approach: 1) IBDs vs. controls, finally based on 18 VOCs plus age (sensitivity = 95%, specificity = 69%, AUC = 0.925); 2) CD vs. UC, finally based on 13 VOCs plus age (sensitivity = 94%, specificity = 76%, AUC = 0.934); 3) IBDs vs. gastroenterological controls, finally based on 15 VOCs plus age (sensitivity = 94%, specificity = 65%, AUC = 0.918); 4) IBDs vs. controls, built starting from the 21 directly or indirectly calibrated molecules only, and finally based on 12 VOCs plus age (sensitivity = 94%, specificity = 71%, AUC = 0.888). The molecules identified by the models were carefully studied in relation to the concerned outcomes. This study, with the creation of models based on VOCs profiles, precise instrumentation and advanced statistical methods, can contribute to the development of new non–invasive, fast and relatively inexpensive diagnostic tools, with high sensitivity and specificity. It also represents a crucial step towards gaining further insights on the etiology of IBD through the analysis of specific molecules which are the expression of the particular metabolism that characterizes these patients.
Introduction
Inflammatory bowel diseases (IBDs), which comprise Crohn’s disease (CD) and Ulcerative Colitis (UC), are chronic inflammatory conditions of the gastrointestinal tract that profoundly affect the quality of life and have been gradually increasing in incidence, prevalence and severity in many areas of the world. [1–5] Around 25% to 30% of all diagnoses are made in the first two decades of life. [1,6] Among childhood onset-IBD, there is an especially rising incidence of CD that is approximately 3/100,000. [3] The prevalence in the pediatric population (< 20 years of age) is reported to be 58/100,000 for CD and 34/100,000 for UC. [4]
Failure to diagnose and induce disease remission during the peri-pubertal period can have significant consequences such as missed pubertal growth spurt and reduced adult height, [7] or low bone mineral density leading to an increased long-term risk of fractures. [8]
Patients with suspected IBD require a careful history and clinical examination along with blood tests. However, normal laboratory investigations cannot exclude a diagnosis of IBD. [6,9] Definitive diagnosis relies on endoscopic and histological findings. [9] Gastrointestinal endoscopy and colonoscopy should be undertaken in any patient with suspected IBD. [9] Multiple mucosal biopsies should be obtained for histopathological examination. [6] Other ways of investigating the small bowel in CD are capsule endoscopy and magnetic resonance imaging. They can provide details about the extent of inflammatory changes in the mucosa and are also able to identify smaller superficial mucosal lesions without radiation. [2] The only non-invasive high sensitivity (73.5–100%) marker for gut inflammation is fecal calprotectin, which has, however, low specificity (65.9–97.9%). [10]
No simple, fast and cheap test for diagnosing and monitoring intestinal inflammation in IBD is available at present.
There is strong evidence to suggest that particular disorders that increase oxidative stress can be detected by molecular analysis of exhaled air. [11] Breath analysis represents a new diagnostic technique that started in the 1970s when Pauling et al. detected approximately 250 components in human breath using gas chromatography. [12] Various analytical techniques have been used to detect exhaled VOCs: the most commonly used are mass spectrometry (MS)-based techniques, [11] among which the leading is gas chromatography (GC-MS), which are followed by the use of nanoparticles sensor arrays. [13] Several studies have shown that VOCs profile can be helpful to diagnose several diseases, [14] including lung cancer, [15,16] breast cancer, [17,18] diabetes mellitus, [19] hepatic cirrhosis, [20] active tuberculosis, [21] cystic fibrosis [22] and preeclampsia. [23]
Metabolic derangement in IBDs was initially studied using the headspace of feces and urine. Probert compared the VOCs profile in the headspace gas emitted from fecal samples from IBD patients, healthy subjects and patients with infectious diarrhea. He found a specific pattern of compounds strongly associated with the alteration of intestinal homeostasis. [24] Another study demonstrated the potential application of fecal VOC analysis in diagnosing IBD in a pediatric cohort. [25] The headspace of urine in IBD patients showed a different VOC profile, with the suggestion that altered gut permeability is reflected in urinary profiles. [26]
A recent review investigated the role of VOCs breath analysis in the diagnosis of gastrointestinal diseases, including IBDs. [27] Lipid peroxidation appears to be the main mechanisms behind the changes in the VOCs profile in both CD and UC patients. Pentane, ethane, propane and isoprene appear to present consistently higher levels in patients with IBD compared to controls. Also fractional exhaled nitric oxide (FENO) measurements in patients with Crohn's disease has been investigated as a marker of active inflammation. Significantly higher levels of FENO were observed in CD patients with clinically active disease compared to CD patients in clinical remission. [28]
Hicks et al. has shown that exhaled breath VOCs profiling can distinguish IBDs adult patients from healthy controls. [29] VOCs belonging to the aldehyde group (butanal and nonanal) are elevated in both UC and CD, and are, especially in the latter, a marker of oxidative stress. Also volatile sulfur-containing compounds (dimethyl sulfide and hydrogen sulfide) were shown to be able to distinguish CD patients from UC and controls. Hydrogen sulfide was significantly lower in CD, while ammonia was significantly lower in UC compared to healthy controls. [29]
Only one study verified the presence of a specific VOCs pattern in the alveolar air of children with IBD, [30] and found that the values of three specific VOCs (1-octene, 1-decene, E-2-nonene) could discriminate between IBD and controls. However, no distinctive pattern could be identified for CD and UC.
The primary aim of our study is to investigate whether pediatric patients with IBD have specific VOCs patterns when compared to control subjects. Patients will be divided into four groups: CD, UC, controls with gastrointestinal symptomatology, and surgical controls with no evidence of gastrointestinal problems. Having identified specific VOCs patterns, the second aim of the study was to try to understand how discriminating molecules could be linked to the IBDs.
Methods
Cases and controls
The study was approved (RC 1/12) by the Technical Scientific Committee of the Institute for Maternal and Child Health—IRCCS “Burlo Garofolo” of Trieste, Italy. All enrolled patients and/or their parents or caregivers signed an informed consent form prior to their enrollment.
From June 2012 to June 2013, we enrolled patients aged 10–17 years affected by IBD (both ulcerative colitis and Crohn’s disease) (“cases”), other gastrointestinal diseases (“gastro controls”) and subjects without gastrointestinal problems (“healthy controls”). Diagnoses of ulcerative colitis and Crohn’s disease were made according to the ESPGHAN and NASPGHAN guidelines for the pediatric population. [31] All cases and gastro controls were enrolled at the outpatients service of the Gastroenterology Unit of the Institute for Maternal and Child Health—IRCCS Burlo Garofolo of Trieste, Italy. Subjects without gastroenterological problems were enrolled at the Day Surgery among patients hospitalized for issues not related to gastroenterology (orthopedic, otolaryngology, eye, dental, urology surgery): these patients were all carefully evaluated to exclude those with gastrointestinal symptoms. At the time of air sampling, which was carried out in the morning, all subjects has been fasting at least since midnight. Breath sampling in all day surgery controls was done pre-operatively. Additional information on their medical history and ongoing therapies was collected. Patients with IBD were also evaluated using the Pediatric Ulcerative Colitis Activity Index (PUCAI) [32], the Pediatric Crohn’s Disease Activity Index (PCDAI) [33]. Both indexes are reported in Tables A and B of S1 Text. The Paris disease classification has been used to classify IBD cases for localization and to capture the dynamic features of the disease phenotype. [34]
Alveolar air sampling
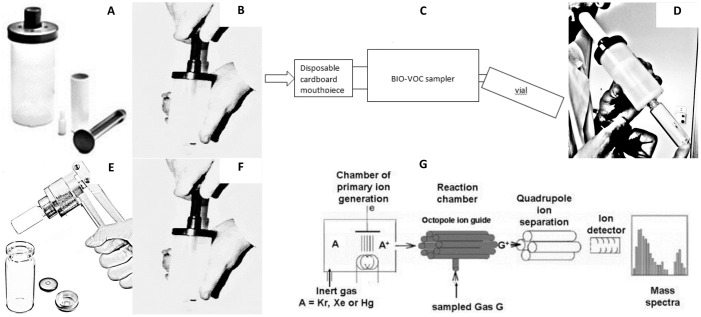
For breath sampling, subjects were asked to exhale once through a device called Bio–VOC™ breath sampler (Markes International Ltd., Llantrisant, UK) into a 20 ml volume glass vial (the Bio–VOC™ sampler avoids rebreathing). All glass vials had been previously sterilized and sealed individually. After completing exhalation, the glass vial was crimped airtight with the appropriate crimp cap. Two samples of expired air were collected for each subject to increase the possibility of obtaining at least one properly sealed sample. In addition, a glass vial was sealed with environmental air present at the same time and in the same place as each exhaled air sample. Vials were preserved at –20°C up to the moment of the Mass Spectrometry (MS) analysis. Fig 1 shows the steps of the sampling procedure and VOCs analysis.
Fig 1. Explanation of the sampling procedure and the VOCs measurement by IMR-MS.
A) breath sampler with disposable cardboard mouthpiece and the pushrod; B) connect the pushrod to the sampler and flush the sampler by pulling and pushing the rod in and out two or three times; C) remove to rod and connect the disposable mouthpiece to the sampler, placing the glass vial on the other side; D) have the patient breath normally and then keep exhaling trough the mouthpiece until their lungs are emptied; E) crimp airtight the glass vial with the appropriate crimp cap; F) throw away disposable mouthpiece and clean the breath sampler by flushing it two/three times using the pushrod; G) the glass vial, preserved at –20°C, is analyzed with the IMR-MS method, schematized here (reported from Defoort and colleagues [35]) and described in detail in Hornuss and colleagues [36].
Equipment
VOCs in alveolar breath and in environmental samples were analyzed using the “Airsense” Ion Molecule Reaction–Mass Spectrometry (IMR–MS) from V&F (medical development GmbH, Absam, Austria). The soft ionization process was performed via ion beams interacting with the gas sample, as already reported by Hormuss and colleagues. [36] The vials were placed in a V&F autosampler, heated up to 65°C and dynamically transferred to the V&F Airsense. The spectrometer measures the concentration of products in a sample. These products mainly represent molecules existing in traces in the sample but may, in some cases, also represent fragments of other molecules generated by the soft ionization occurring in the instrument.
The concentration of 97 volatile compounds (masses from 16 to 123) was measured in all samples. Thirty-one compounds had a known chemical structure (directly or indirectly calibrated with calibration gasses), while 66 groups of products were known only for their molecular weight (MW). A direct calibration was carried out for 23 chemical compounds: Acetylene, Ethane, Formaldehyde, Methanol, Acetonitrile (ACN), Formic Acid, Acetic Acid, Ethylene, Propene, Acetaldehyde, Butadiene, Butanol, Methyl Ethyl Ketone (MEK), Acetone, n–Propanol, Isoprene, n–Pentane, Benzene, n–Hexane, Toluene, n–Heptane, CO2 and O2. Our aim was to calibrate a panel of compounds between 16 and 123 Dalton, including petroleum-related products, micropollutants measured by various authors, molecules derived from human or animal metabolism (acetone, isoprene, n-pentane), molecules that are present in foods or their metabolites (acetic acid, formic acid, formaldehyde, methanol), and products found in alveolar air by various authors and that could have useful biological meanings. These products provided indications on the quality of the results obtained.
CO2 and O2 were measured by a specific detector with variation coefficients lower than 1%. For the reported compounds, the reproducibility of the assays was assessed by analyzing 30 environmental air samples collected in the same room, in six replicates on five different days over a three weeks period. The intra-assay (comparison of samples collected on the same day) coefficients of variation were less than 10% for Ethane, Formaldehyde, Methanol, ACN, Formic Acid, Ethylene, n-Butanol and n–Pentane. For the other products the intra-assay coefficients of variation were less than 20%, except for Acetone (24%) and Acetic Acid (35%). The inter-assay (comparison of samples collected on different days) coefficients of variation were lower than 10% for Formaldehyde, Methanol, ACN, Formic Acid and n-Butanol, and lower than 20% for the other products. The inter-assay coefficients of variation were 27% for Acetone and Ethane, and 40% for Acetic Acid. Coefficients of variation of ppb concentrations are considered to be highly satisfactory if below 20%. The values above 20% we obtained can be considered as acceptable.
Benzene was used for the indirect calibration of other eight molecules: Methane, HNO2, N2O, NO, H2S, H2O, Ammonia (NH3), Sulfur Dioxide (SO2). This model represents a semi–quantitative calibration procedure which is commonly used in (multicomponent) analytical devices.
The measured VOCs are given as absolute concentrations (ppm) and as volume percent for CO2 and O2, these latter gases being used to provide information on the quality of environmental or alveolar samples. CO2 values lower than 2% in alveolar air samples were presumed to be associated to missampling or to inadequate vial crimping: these samples were excluded.
Statistical analyses
We first described the sample of controls and patients with Crohn’s disease (CD) and with ulcerative colitis (UC). We then graphically represented, as Δ between medians of exhaled and environmental air, the comparison between the VOCs profile of all breath samples and that of all the environmental samples. We also graphically compared breath samples of CD and UC patients with controls values, again as Δ between breath samples of CD or UC patients and controls.
In order to establish if the VOCs were to be considered endogenous or exogenous, we ran a t–test for each of the 98 compounds, and verified whether the values in the environmental air samples were significantly higher than in the exhaled breath samples. If so, the “exogenous” compound was excluded from the regression models exposed below. Compounds with higher concentrations in the environment if compared to the exhaled breath have a partial pressure inducing pulmonary absorption, and their alveolar concentrations will be in constant equilibrium with the ones in the environment. Consequently they will substantially not be informative on the physiopathological conditions of the organism. The choice of focusing on compounds with alveolar concentrations higher than environmental ones was meant to restrict the panel to products primarily associated with specific metabolic (physiological or pathological) conditions, avoiding interferences attributable to “environmental pollutants”. The exclusion was also justified by the fact that in the environmental air samples few of these exogenous molecules had significantly different values in cases and controls, probably due to environmental differences in the outpatient clinic in which samples from the two groups were taken.
For the elaboration of a predictive model that might allow for future generation of a diagnostic tool, and considering the large number of independent variables involved in the analysis, we decided to adopt a Lasso (Least Absolute Shrinkage and Selection Operator) logistic regression (LLR) approach. [37,38] By shrinking the estimates of the regression coefficients towards zero relative to the maximum likelihood estimates, this penalizing estimation method prevents any overfitting that may arise as a consequence of either collinearity or high–dimensionality of independent variables. This method allows to shrink the regression coefficients adopting a tuning parameter λ, which controls the amount of shrinkage that is applied to the estimates. In addition, the shrinkage of some coefficients to zero reduces the number of covariates in the final model, allowing us to avoid using classical stepwise regression methods, which are strongly criticized for their lack of consistency. [39] Independent variables (molecules) were standardized to allow for optimal penalization.
In particular, we adopted an iterated LLR approach. [40] First, we used a 50–fold cross–validated LLR to reduce the number of variables in the model, eliminating all variables if coefficients were 0. Then we used this set of variables in a two–step iterated 50–fold cross–validated LLR, [40] in which the first LLR generated penalized weights to be used in a second adaptive LLR. [41]
LLR was used to generate four different models with the VOCs remaining after the exclusion of the exogenous ones, plus age as independent variables, diverging in terms of dependent variable: 1) IBD patients vs. controls (gastroenterological and healthy); 2) IBDs vs. gastroenterological controls; 3) Crohn’s disease (CD) patients vs. patients with ulcerative colitis (UC); 4) the first model was then replicated using only the molecules that had been directly or indirectly calibrated, with the intent of generating a model with unambiguously identified molecules. The intent of the first model is to try and separate IBDs from a “real population” mix, made of children with and without gastrointestinal problems. The second model aims at reproducing the situation that is found in a gastroenterology outpatient clinic. The third model represent a second step in diagnosis, moving from the identification of IBD to the separation between CD and UC, while also addressing the question of the differences in VOCs profiles between CD and UC patients. The fourth model has been deprived of the unknown molecules. This represents a limitation compared to the first model, but, being based on known molecules only, this latter model can be replicated more easily.
What varies from model to model is the way in which the λ value was selected in the first LASSO. In some cases, even when we had the possibility of selecting an optimal λ, based on the graph representing the penalization of the variables involved, we chose to adopt a less penalizing λ, obtaining as a result a larger number of variables to be included in the iterated LASSO procedure.
Analyses were carried out with Stata/IC 11.2 (StataCorp LP, College Station, TX, USA) and with R version 2.15.1 (The R Foundation for Statistical Computing, Vienna, Austria), and “penalized” (Goeman JJ. Penalized R package, version 0.9–42) and “polywog” (Kenkel B, Signorino CS. Bootstrapped Basis Regression with Oracle Model Selection, version 0.2–0) R packages.
Results
A total of 234 subjects was enrolled in the study over a one year period: 67 cases (33 UC and 34 CD patients), and 167 controls (65 gastrointestinal controls and 102 healthy controls) (Table 1). After receiving quick and simple instructions, all subjects carried out the air sampling without any difficulty. Cases and gastroenterological controls are described in Tables A to C in S2 Text.
Table 1. Description of the sample of inflammatory bowel disease cases and controls enrolled in the study (children 10 to 17 years of age).
| UC (33) | CD (34) | Gastro Ctrls (65) | Healthy Ctrls (102) | |
|---|---|---|---|---|
| Sex | F 15; M 18 | F 16; M 18 | F 27; M 38 | F 45; M 57 |
| Age | 14 (12–16) | 15 (14–16) | 12 (11–15) | 13 (11–14) |
F: Females; M: Males; UC: Ulcerative colitis; CD: Crohn’s disease; Gastro Ctrls: gastroenterological controls; Healthy Ctrls: Healthy controls. Age is expressed in years as median and interquartile range in parenthesis.
First, we analyzed the differences in concentration of the 97 molecules present in the environment and in the alveolar air of cases and controls, and excluded from further analyses 13 molecules with significantly lower values in the alveolar air compared to environmental air (M27, Ethane, Formaldehyde, Methanol, Formic Acid, SO2, NO, H2S, M31, M32, M48, M49, M80), which were thus defined as exogenous. Some of the excluded compounds, such as hydrogen-sulfide, could also have an endogenous nature. As explained above, however, the significantly higher presence of such compounds in environmental air if compared to exhaled breath would mean that most of the expired component would not be endogenous, and would thus be difficult to interpret.
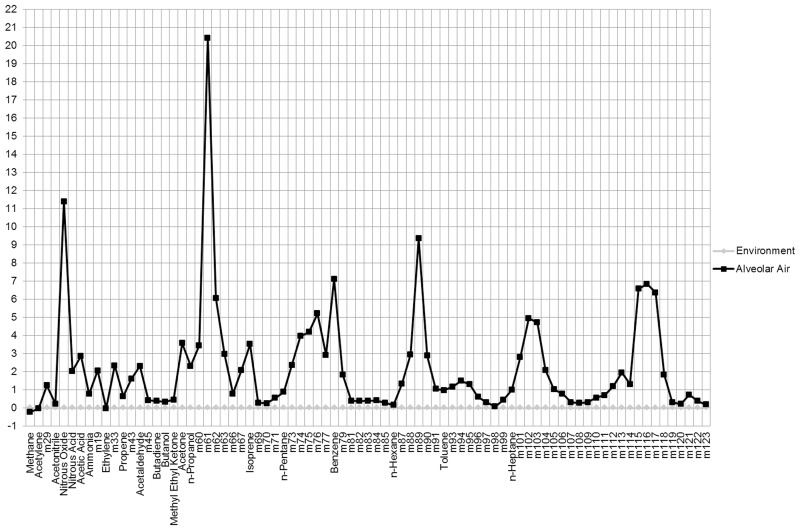
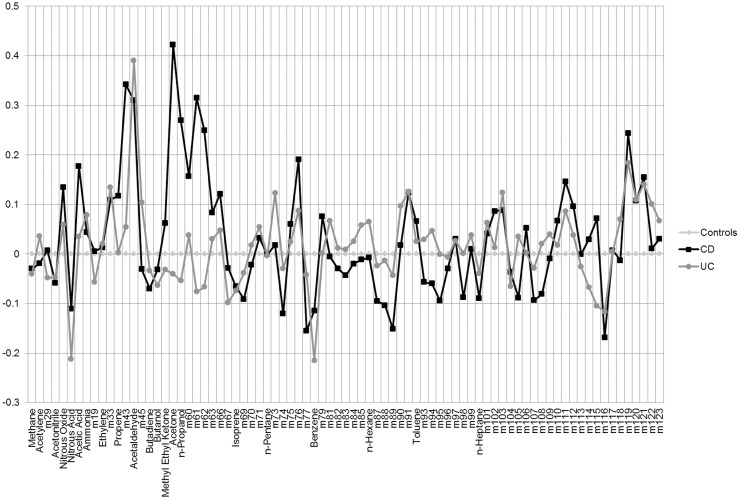
Data on H2O, O2 and CO2 concentrations were employed to assess whether the samples had been collected properly, but were excluded from the models because of their particularly cumbersome presence. We thus remained with the 81 molecules (Table 2) listed in Fig 2, which shows the difference between median values of alveolar and environmental air, with values standardized to environmental air. Fig 3 also shows the VOCs profiles of median values of CD and UC patients compared with control subjects, with values standardized to the median of control subjects. Mean values and 95% confidence intervals for these molecules, as identified in environmental air samples and in exhaled breath samples of cases and controls, are reported in S1 Table.
Table 2. Volatile organic compounds measured by ion-molecule reaction-mass spectrometry, with indications on whether molecules were directly or indirectly calibrated, and which molecules were included in the regression models after comparison between environmental air and exhaled air samples.
| Measured molecules (97) | Directly calibrated molecules (23) | Indirectly calibrated molecules* (8) | Molecules included in models (81) |
|---|---|---|---|
| CH4—Methane | x | x | |
| C2H2—Acetylene | x | x | |
| M27 | |||
| M29 | x | ||
| C2H6—Ethane | x | ||
| CH2O—Formaldehyde | x | ||
| CH4O—Methanol | x | ||
| C2H3N—Acetonitrile | x | x | |
| N2O—Nitrous Oxide | x | x | |
| CH2O2—Formic Acid | x | ||
| HNO2—Nitrous Acid | x | x | |
| SO2—Sulfur Dioxide | x | ||
| H2O—Water | x | ||
| O2—Oxygen | x | ||
| CO2—Carbon Dioxide | x | ||
| NH3—Ammonia | x | x | |
| M19 | x | ||
| C2H4—Ethylene | x | x | |
| NO–Nitric Oxide | x | ||
| M31 | |||
| M32 | |||
| M33 | x | ||
| H2S—Hydrogen Sulfide | x | ||
| C3H6—Propene | x | x | |
| M43 | x | ||
| C2H4O—Acetaldehyde | x | x | |
| M45 | x | ||
| M48 | |||
| M49 | |||
| C4H6—Butadiene | x | x | |
| C4H10O—Butanol | x | x | |
| C4H8O—Methyl Ethyl Ketone | x | x | |
| C3H6O—Acetone | x | x | |
| C3H8O—n-Propanol | x | x | |
| C2H4O2—Acetic Acid | x | x | |
| M60 | x | ||
| M61 | x | ||
| M62 | x | ||
| M63 | x | ||
| M66 | x | ||
| M67 | x | ||
| C5H8—Isoprene | x | x | |
| M69 | x | ||
| M70 | x | ||
| M71 | x | ||
| C5H12—n-Pentane | x | x | |
| M73 | x | ||
| M74 | x | ||
| M75 | x | ||
| M76 | x | ||
| M77 | x | ||
| C6H6—Benzene | x | x | |
| M79 | x | ||
| M80 | |||
| M81 | x | ||
| M82 | x | ||
| M83 | x | ||
| M84 | x | ||
| M85 | x | ||
| C6H14—n-Hexane | x | x | |
| M87 | x | ||
| M88 | x | ||
| M89 | x | ||
| M90 | x | ||
| M91 | x | ||
| C7H8—Toluene | x | x | |
| M93 | x | ||
| M94 | x | ||
| M95 | x | ||
| M96 | x | ||
| M97 | x | ||
| M98 | x | ||
| M99 | x | ||
| C7H16—n-Heptane | x | x | |
| M101 | x | ||
| M102 | x | ||
| M103 | x | ||
| M104 | x | ||
| M105 | x | ||
| M106 | x | ||
| M107 | x | ||
| M108 | x | ||
| M109 | x | ||
| M110 | x | ||
| M111 | x | ||
| M112 | x | ||
| M113 | x | ||
| M114 | x | ||
| M115 | x | ||
| M116 | x | ||
| M117 | x | ||
| M118 | x | ||
| M119 | x | ||
| M120 | x | ||
| M121 | x | ||
| M122 | x | ||
| M123 | x |
* Calibrated through Benzene
Fig 2. For the 81 molecules considered, difference between median values of alveolar and environmental air, with values standardized to environmental air.
Fig 3. For the 81 molecules considered, VOCs profiles of median values of CD and UC patients compared to control subjects, with values standardized to the median values of control subjects.
The 81 molecules plus the age of subjects were considered as independent variables in all four models. The results of the models are reported below.
IBD (CD + UC) vs. controls (surgical + gastroenterological)
The first model is based on the comparison between all IBDs without distinction and all controls. The final resulting model comprises 18 VOCs plus age in years, and has the following formula:
predicted probability = 1/(1+exp(– (–1.7610965 +0.4349465*Age –2.1312841*Methane –0.2097137*NitrousAcid +0.0011512*AceticAcid +0.0004002*Ammonia +0.0058942*Propene +0.0008888*Acetaldehyde –0.0232373*MethylEthylKetone –0.1222845*M69–0.0266032*M74 +0.1684388*M76–0.0489416*M79 +0.0425079*M81–0.0280041*M89 +0.0793372*M99–0.1427593*M105–0.0132398*M107–0.0539797*M115 +0.1597398*M118)))
Confidence intervals and standard error of the coefficients are reported in Table 3. The Area under the ROC curve (AUC) was 0.925 (95%CI: 0.889–0.961) (S1 Fig) and the performance of the model for sensitivity levels above 90% are reported in Table 4. The model could detect 96% of all IBD cases with a specificity of 69% (65% in gastro controls and 73% in surgical controls). In this case, 23 out of 65 gastro controls result as being false positives. No significant differences in the distribution of the diseases was found among these (Table 5). However, when the diseases are classified based on the presence (or plausibility) of an ongoing inflammatory process, while among the correctly classified two are found to be biliary duct atresias and one was a choledochal cyst, the false positives include a Behçet’s disease, a chronic intestinal pseudo-obstruction with ileostomy, and intestinal atresia, an infective ileitis, and a graft-versus-host disease.
Table 3. Variables and coefficients of the logistic regression model with outcome variables: Patients with inflammatory bowel disease vs. controls (surgical + gastroenterological).
| Variables | Coefficient | Std. Error | 95% CI |
|---|---|---|---|
| (Intercept) | -1.7610965 | 3.5213722 | -8.030–2.522 |
| Age (years) | 0.4349465 | 0.1115950 | 0.408–0.718 |
| Methane | -2.1312841 | 1.0351154 | -4.340 –-1.422 |
| Nitrous Acid | -0.2097137 | 0.1800298 | -0.489–0.000 |
| Acetic Acid | 0.0011512 | 0.0007470 | 0.000–0.002 |
| Ammonia | 0.0004002 | 0.0014281 | 0.000–0.003 |
| Propene | 0.0058942 | 0.0048373 | 0.000–0.013 |
| Acetaldehyde | 0.0008888 | 0.0013211 | 0.000–0.004 |
| Methyl Ethyl Ketone | -0.0232373 | 0.0353011 | -0.078–0.000 |
| M69 | -0.1222845 | 0.1114540 | -0.324–0.000 |
| M74 | -0.0266032 | 0.0190338 | -0.052–0.000 |
| M76 | 0.1684388 | 0.1444729 | 0.010–0.469 |
| M79 | -0.0489416 | 0.0624447 | -0.146–0.000 |
| M81 | 0.0425079 | 0.0313594 | 0.028–0.120 |
| M89 | -0.0280041 | 0.0444448 | -0.127–0.000 |
| M99 | 0.0793372 | 0.0365851 | 0.036–0.144 |
| M105 | -0.1427593 | 0.1024486 | -0.271–0.000 |
| M107 | -0.0132398 | 0.0956782 | -0.234–0.000 |
| M115 | -0.0539797 | 0.1593523 | -0.416–0.000 |
| M118 | 0.1597398 | 0.4747905 | 0.000–1.188 |
Table 4. Performance of model comparing inflammatory bowel disease patients to controls, for sensitivity levels above 90% (in parenthesis, the number of correctly classified cases).
| Predicted probability | Sensitivity in overall IBD (n.67) | Specificity in overall controls (n.167) | Specificity in gastro controls (n.65) | Specificity in surgical controls (n.102) |
|---|---|---|---|---|
| 0.0538911 | 100.00% (67) | 37.72% (63) | 34.31% (21) | 41.18% (42) |
| 0.0763976 | 98.51% (66) | 44.31% (74) | 38.46% (25) | 48.04% (49) |
| 0.1269103 | 97.01% (65) | 59.88% (100) | 56.92% (37) | 61.76% (63) |
| 0.1808475 | 95.52% (64) | 69.46% (116) | 64.62% (42) | 72.55% (74) |
| 0.1880790 | 92.54% (62) | 70.66% (118) | 66.15% (43) | 73.53% (75) |
| 0.1990687 | 91.04% (61) | 73.05% (122) | 67.69% (44) | 76.47% (78) |
Table 5. Diagnoses of the gastroenterological controls according to their classification in the model IBD vs. controls.
| Correctly classified (42) | False positives (23) | Total (65) | p* | |
|---|---|---|---|---|
| Celiac disease | 17 (40%) | 5 (22%) | 22 (34%) | 0.173 |
| Eosinophilic esophagitis | 5 (12%) | 1 (4%) | 6 (9%) | 0.411 |
| Recurrent abdominal pain | 1 (2%) | 3 (13%) | 4 (6%) | 0.123 |
| Constipation | 3 (7%) | 0 | 3 (5%) | 0.547 |
| Gastritis | 1 (2%) | 2 (9%) | 3 (3%) | 0.284 |
| Probable latent celiac disease | 1 (2%) | 1 (4%) | 2 (3%) | 1.000 |
| Functional dysphagia | 2 (5%) | 0 | 2 (3%) | 0.536 |
| Biliary duct atresia | 2 (5%) | 0 | 2 (3%) | 0.536 |
| Other | 10 (24%) | 11 (30%) | 21 (32%) | 0.058 |
* Fisher’s exact two-tailed test, considering one disease at the time vs. all others.
CD vs. UC
The model resulting from the attempt to separate CD from UC patients is based on 13 VOCs plus age, and has the following formula (Table 6):
Table 6. Variables and coefficients of the logistic regression model with outcome variables: Patients with Crohn’s disease vs. patients with ulcerative—Colitis.
| Variables | Coefficient | Std. Error | 95%CI |
|---|---|---|---|
| (Intercept) | -5.178 | 3.583 | -1.065e+01 –-0.223 |
| Age (years) | 3.444e-01 | 3.431e-01 | -4.371e-01–0.553 |
| Ammonia | 4.540e-03 | 5.657e-03 | 0.000–0.016 |
| M29 | 3.465e-03 | 4.852e-03 | 0.000–0.013 |
| Acetonitrile | -4.342e-02 | 5.218e-02 | -1.368e-01–0.002 |
| Nitrous Oxide | 2.776e-04 | 7.678e-04 | 0.000–0.002 |
| Acetaldehyde | -1.162e-03 | 2.567e-03 | -7.022e-03–0.000 |
| Methyl Ethyl Ketone | 3.496e-02 | 3.267e-02 | -3.278e-02–0.069 |
| M70 | -6.994e-04 | 2.479e-02 | -6.398e-02–0.000 |
| M74 | -1.175e-02 | 3.867e-02 | -9.873e-02–0.014 |
| M77 | -2.663e-02 | 3.456e-02 | -8.980e-02–0.000 |
| M79 | 1.301e-01 | 3.874e-01 | 0.000–1.049 |
| M89 | -9.094e-02 | 6.057e-01 | -1.108–0.906 |
| M90 | -3.621e-01 | 1.112 | -2.852–0.000 |
| M105 | -2.669e-01 | 6.501e-01 | -1.656–0.000 |
| M107 | -2.864e-01 | 2.272e-01 | -5.674e-01 –-0.011 |
| M114 | 2.873e-01 | 4.202e-01 | 1.758e-03–1.236 |
predicted probability = 1/(1+exp(–(–5.178+3.444e–01*Age +3.465e–03*M29–4.342e–02*Acetonitrile +2.776e–04*NitrousOxide +4.540e–03*Ammonia –1.162e–03*Acetaldehyde +3.496e–02* MethylEthylKetone –6.994e–04*M70–1.175e–02*M74–2.663e–02*M77 +1.301e–01*M79–9.094e–02*M89–3.621e–01*M90–2.669e–01*M105–2.864e–01*M107 +2.873e–01*M114)))
The model had an AUC of 0.934 (95%CI: 0.880–0.988) and yielded a percentage of correctly classified of 86.6% (S2 Fig). It had a sensitivity of 94% in detecting CD with a specificity of 76% (Table 7). Symmetrically, the model had a sensitivity of 94% in detecting UC with a specificity of 71%.
Table 7. Performance of model comparing Crohn’s disease patients to ulcerative colitis patients (in parenthesis, the number of correctly classified cases).
| Predicted probability | Sensitivity in Crohn Disease (n.34) | Specificity in UC controls (n.33) | Correctly classified |
|---|---|---|---|
| 0.3383077 | 100.00% (34) | 72.73% (24) | 86.57% |
| 0.3876584 | 94.12% (32) | 75.76% (25) | 85.07% |
| 0.5131218 | 88.24% (30) | 81.82% (27) | 85.07% |
| 0.5658435 | 79.41% (27) | 87.88% (29) | 83.58% |
| 0.6121589 | 76.47% (26) | 90.91% (30) | 83.58% |
| 0.6433669 | 70.59% (24) | 93.94% (31) | 82.09% |
| 0.7598559 | 52.94% (18) | 100.00% (33) | 76.12% |
IBD vs. Gastroenterological controls
The fourth model aims at replicating a “real life” situation, in which a patient with gastrointestinal symptoms needs to be diagnosed for IBD, and is therefore designed to distinguish IBD patients from gastroenterological controls. The model is based on 15 VOCs plus age (Table 8):
Table 8. Variables and coefficients of the logistic regression model with outcome variables: Patients with inflammatory bowel disease vs. gastroenterological controls.
| Variables | Coefficient | Std. Error | 95% CI |
|---|---|---|---|
| (Intercept) | -2.816 | 4.879 | -13.330–1.833 |
| Age (years) | 4.580e-01 | 2.140e-01 | 0.315–0.938 |
| Methane | -1.336 | 8.425e-01 | -2.651 –-0.107 |
| Acetonitrile | -3.995e-03 | 1.222e-02 | -0.032–0.000 |
| Nitrous Acid | -2.463e-01 | 1.360e-01 | -0.416 –-0.050 |
| Acetic Acid | 1.285e-03 | 8.897e-04 | 0.000–0.003 |
| Propene | 3.085e-03 | 4.451e-03 | 0.000–0.011 |
| Acetaldehyde | 8.199e-04 | 6.986e-04 | 0.000–0.002 |
| M67 | -2.030e-02 | 3.161e-02 | -0.084–0.000 |
| M74 | -3.135e-02 | 2.683e-02 | -0.073–0.000 |
| M75 | 7.298e-02 | 1.361e-01 | -0.216–0.206 |
| M79 | -8.453e-02 | 1.108e-01 | -0.312–0.000 |
| M81 | 4.231e-02 | 3.979e-02 | 0.004–0.122 |
| M89 | -5.543e-02 | 6.717e-02 | -0.151–0.000 |
| M91 | 2.516e-01 | 2.037e-01 | 0.000–0.572 |
| M94 | 5.698e-03 | 8.080e-03 | 0.000–0.021 |
| M105 | -1.073e-01 | 9.137e-02 | -0.236–0.000 |
predicted probability = 1/(1+exp(–(–2.816 +4.580e–01*Age –1.336*Methane –3.995e–03 *Acetonitrile –2.463e–01*NitrousAcid +1.285e–03*AceticAcid +3.085e–03*Propene +8.199e–04 *Acetaldehyde –2.030e–02*M67–3.135e–02*M74 +7.298e–02*M75–8.453e–02*M79 +4.231e–02 *M81–5.543e–02*M89 +2.516e–01*M91 +5.698e–03*M94–1.073e–01*M105)))
The model had an AUC of 0.918 (95%CI: 0.873–0.963) (S3 Fig), and was able to identify 94% of IBDs (94% both for CD and UC patients), with a specificity of 65% (Table 9). Taking the latter as the cut-off for sensitivity and specificity, we would still have 23 false positives out of 65 gastro controls (Table 10). Once again, if the diseases are classified based on the presence or plausibility of an ongoing inflammatory process, while among the correctly classified we find one intestinal atresia, the false positives include a Behçet’s disease, a chronic intestinal pseudo-obstruction with ileostomy, a graft-versus-host disease, and an infective ileitis.
Table 9. Performance of model comparing patients with inflammatory bowel disease vs. gastroenterological controls, for sensitivity levels above 90% (in parenthesis, the correctly classified).
| Predicted probability | Sensitivity in IBD (n.67) | Sensitivity in CD (n.34) | Sensitivity in UC (n.33) | Specificity in gastro controls (n.65) |
|---|---|---|---|---|
| 0.1870422 | 100.00% (67) | 100.00% (34) | 100.00% (33) | 46.15% (30) |
| 0.2633038 | 97.01% (65) | 97.06% (33) | 96.97% (32) | 55.38% (36) |
| 0.2931387 | 95.52% (64) | 94.12% (32) | 96.97% (32) | 58.46% (38) |
| 0.3613650 | 94.03% (63) | 94.12% (32) | 93.94% (31) | 64.62% (42) |
| 0.3761519 | 92.54% (62) | 94.12% (32) | 90.91% (30) | 66.15% (43) |
| 0.4073664 | 91.04% (61) | 94.12% (32) | 87.88% (29) | 69.23% (45) |
Table 10. Diagnoses of the gastroenterological controls according to their classification in the model IBD vs. gastro controls.
| Correctly classified (42) | False positives (23) | Total (65) | p* | |
|---|---|---|---|---|
| Celiac disease | 14 (33%) | 8 (35%) | 22 (34%) | 1.000 |
| Eosinophilic esophagitis | 5 (12%) | 1 (4%) | 6 (9%) | 0.411 |
| Recurrent abdominal pain | 2 (5%) | 2 (9%) | 4 (14%) | 0.610 |
| Constipation | 3 (7%) | 0 | 3 (5%) | 0.547 |
| Gastritis | 2 (5%) | 1 (4%) | 3 (5%) | 1.000 |
| Probable latent celiac disease | 1 (2%) | 1 (4%) | 2 (3%) | 1.000 |
| Functional dysphagia | 2 (5%) | 0 | 2 (3%) | 0.536 |
| Biliary duct atresia | 2 (5%) | 0 | 2 (3%) | 0.536 |
| Other | 11 (26%) | 10 (43%) | 21 (32%) | 0.175 |
* Fisher’s exact two-tailed test, considering one disease at the time vs. all others.
IBD (CD + UC) vs. controls (surgical + gastroenterological) only with directly or indirectly calibrated VOCs
Finally, the first model was replicated using only 21 unambiguously identified VOCs out of the 81 initial molecules. This version of the model was finally based on 12 VOCs plus age (Table 11):
Table 11. Variables and coefficients of the logistic regression model built with directly or indirectly calibrated VOCs, with outcome variable: Patients with inflammatory bowel disease vs. controls (surgical and gastroenterological).
| Variables | Estimate | Std. Error | 95% CI |
|---|---|---|---|
| (Intercept) | -2.616 | 1.848 | -4.499–0.702 |
| Age | 4.491e-01 | 8.989e-02 | 0.3.04–0.551 |
| Methane | -7.421e-01 | 1.380 | -3.952–0.000 |
| Acetonitrile | -4.356e-03 | 4.598e-03 | -0.012–0.000 |
| Nitrous Oxide | 1.589e-04 | 2.466e-04 | -0.000–0.000 |
| Nitrous Acid | -3.073e-01 | 5.877e-02 | -0.363 –-0.208 |
| Acetic Acid | 1.254e-03 | 4.640e-04 | 0.000–0.002 |
| Ammonia | 1.379e-03 | 9.499e-04 | 0.000–0.002 |
| Ethylene | -6.212e-03 | 9.546e-03 | -0.025–0.002 |
| Acetaldehyde | 1.480e-03 | 9.375e-04 | 0.000–0.003 |
| Acetone | 4.226e-04 | 5.043e-04 | 0.000–0.002 |
| Isoprene | -6.135e-03 | 2.162e-03 | -0.009 –-0.003 |
| Toluene | 1.207e-01 | 8.372e-02 | 0.000–0.225 |
| n-Heptane | 8.734e-03 | 2.090e-02 | 0.000–0.061 |
predicted probability = 1/(1+exp(–(–2.616+4.491e–01*Age –7.421e–01*Methane –4.356e–03*Acetonitrile +1.589e–04*NitrousOxide –3.073e–01*NitrousAcid +1.254e–03*AceticAcid +1.379e–03*Ammonia –6.212e–03*Ethylene +1.480e–03*Acetaldehyde +4.226e–04*Acetone –6.135e–03*Isopren+1.207e–01*Toluene +8.734e–03*n–Heptane)))
As expected, the AUC of this model was smaller than the one of the model comparing IBDs with controls (AUC = 0.888; 95%CI: 0.843–0.933), but still quite high. The model was able to detect 94% of IBDs with a specificity of 61% (54% for gastro controls and 66% for surgical controls) (Table 12). If we take the latter as the cut-off for sensitivity and specificity, 30 out of 65 gastro controls result as being false positives (Table 13). Looking at the inflammatory processes, while among the correctly classified we did not find any condition to report, the false positives include a Behçet’s disease, a chronic intestinal pseudo-obstruction with ileostomy, a graft-versus-host disease, and an infective ileitis.
Table 12. Performance of the model with calibrated VOCs comparing patients with inflammatory bowel disease vs. controls (surgical and gastroenterological), for sensitivity levels above 90% (in parenthesis, the correctly classified).
| Predicted probability | Sensitivity in overall IBD (n.67) | Specificity in overall controls (n.167) | Specificity in gastro controls (n.65) | Specificity in surgical controls (n.102) |
|---|---|---|---|---|
| 0.0325607 | 100.00% (67) | 24.55% (41) | 23.08% (15) | 25.49% (26) |
| 0.0713997 | 98.51% (66) | 41.92% (70) | 35.38% (23) | 46.08% (47) |
| 0.0984248 | 97.01% (65) | 48.50% (81) | 41.54% (27) | 52.94% (54) |
| 0.1314432 | 95.52% (64) | 55.09% (92) | 50.77% (33) | 57.84% (59) |
| 0.1516857 | 94.03% (63) | 61.08% (102) | 53.85% (35) | 65.69% (67) |
| 0.1624737 | 92.54% (62) | 63.47% (106) | 55.38% (36) | 68.63% (70) |
| 0.1762784 | 91.04% (61) | 65.27% (109) | 56.92% (37) | 70.59% (72) |
Table 13. Diagnoses of the gastroenterological controls according to their classification in the model IBD vs. controls, built with directly or indirectly calibrated VOCs.
| Correctly classified (35) | False positives (30) | Total (65) | p* | |
|---|---|---|---|---|
| Celiac disease | 14 (40%) | 8 (27%) | 22 (34%) | 0.301 |
| Eosinophilic esophagitis | 3 (9%) | 3 (10%) | 6 (9%) | 1.000 |
| Recurrent abdominal pain | 1 (3%) | 3 (10%) | 4 (14%) | 0.328 |
| Constipation | 3 (9%) | 0 | 3 (5%) | 0.241 |
| Gastritis | 1 (3%) | 2 (7%) | 3 (5%) | 0.591 |
| Probable latent celiac disease | 2 (6%) | 0 | 2 (3%) | 0.495 |
| Biliary duct atresia | 0 | 2 (7%) | 2 (3%) | 0.209 |
* Fisher’s exact two-tailed test, considering one disease at the time vs. all others.
Discussion
Among the VOCs identified by the first three models (based on 81 molecules listed in Table 2) as relevant for specific pathological conditions, five had been calibrated and quantified: Acetic Acid, Propene, Acetaldehyde, Acetonitrile and Methyl Ethyl Ketone (Table 14).
Table 14. Measured (the first 9 in the table) or hypothesized (the others)* VOCs that emerged as significant in our models**.
| IBD vs. Ctrls | CD vs. UC | IBD vs. Gastro Ctrls | |
|---|---|---|---|
| Age (years) | + | + | + |
| CH4: Methane (MW 16) | – | – | |
| NH3: Ammonia (MW 17) | + | + | |
| C2H3N: Acetonitrile (MW 41) | – | – | |
| C3H6: Propene (MW 42) | + | + | |
| N2O: Nitrous Oxide (MW 44) | + | ||
| C2H4O: Acetaldehyde (MW 44) | + | – | + |
| HNO2: Nitrous Acid (47) | – | – | |
| C2H4O2: Acetic Acid (MW 60) | + | + | |
| C4H8O: Methyl Ethyl Ketone (MW 72) | – | + | |
| M29: Methanimine (CH3N) | + | ||
| M67: Pyrrole (C4H5N) | – | ||
| M69: Isocyanatoethene (C3H3NO) or 2H–imidazolium (C3H5N2) | – | ||
| M70: Cyclopentane (C5H10) or crotonaldeyde (C4H6O) | – | ||
| M74: Propylhidrazyne (C3H10N2) or allyl mercaptane (C3H6S) | – | – | – |
| M75: Trimethylamine N–oxide (C3H9NO) | + | ||
| M76: Carbon disulfide (CS2) | + | ||
| M77: Methyl nitrate (CH3NO3) | – | ||
| M79: Pyridine (C5H5N) | – | + | – |
| M81: 1 or 3–Methylpyrrole (C5H7N) | + | + | |
| M89: 1–Nitropropane or 2–Nitropropane (C3H7NO2) or 2–(Dimethylamino)ethanol (C4H11NO) | – | – | – |
| M90: 2,2–Butanediol (C4H10O2) or ethoxyethanol (C4H10O2) | – | ||
| M91: 3–Aminopropanethiol (C3H9NS) | + | ||
| M94: Phenol (C6H6O) | + | ||
| M99: Ethyl cyanoformate (C4H5NO2) | + | ||
| M105: 2–(Ethylamino)ethanethiol or 2–(Dimethylamino)Ethanethiol (C4H11NS) | – | – | – |
| M107: 2,6–Dimethylpyridine (C7H9N) | – | – | |
| M114: 2,3,3–trimethylpentane (C8H18) | + | ||
| M115: 1–Pyrrolidineethanol (C6H13NO) or 2–Methoxythiazole (C4H5NOS) | – | ||
| M118: several molecules satisfy the inclusion criteria | + |
* In italics the compounds for which we could not find evidence in the literature.
** The last three columns show the molecules retained by each model in gray; the plus or minus sign designates the sign of the coefficient in the regression model.
Acetic Acid, systematically named ethanoic acid, is commonly used in animal IBD models to reproduce an IBD condition. [42–44] Recent literature suggests that Acetic Acid and similar compounds are produced from pyruvic acid via pyruvate dehydrogenase, and that acetone is also derived from the decarboxylation of pyruvic acid. [45] It is commonly assumed that anaerobic metabolism is characterized by the non–specific production of fatty acids, such as acetic acid which is the product of several pathogens including Staphylococcus aureus. [46] The identification of the distinct metabolism of a specific bacteria is an important marker to determine the best pharmacological treatment.
One of the products that are derived directly from acetic acid is acetaldehyde, systematic IUPAC name ethanal, that several reports identify as a significant marker of IBD. [30,47] Acetaldehyde is present in the intestinal colon and derives from an oxidative reaction caused by several pathogens. Its antimicrobial activity in this area has been fully ascertained. [46,48] Moreover, ethanal has already been described as a potential marker for the distinction between the diagnoses of CD and UC. [49]
Propene, also known as propylene or methyl ethylene, is a hydrocarbon compound. [49] Hydrocarbon compounds are known to be products of the metabolism of gram–positive and negative bacteria. [51,52] The specific origin of propene, and consequently its role in IBDs, is unknown, but it is likely that the degradation of propene occurs through the β–oxidation pathway, as with other hydrocarbons (i.e. isoprene, 1-undecene or 1,3-butadiene).
Acetonitrile, a chemical compound also called ethanenitrile or ethyl nitrile, is mentioned in a very interesting recent report as one of nine VOCs associated with the diagnosis of esophageal adenocarcinoma: [53] there are no data linking acetonitrile to the selective diagnosis of IBDs, but this recent evidence should encourage further investigations to verify whether this compound can be considered as a suitable marker of IBD. Moreover, acetonitrile was included in an innovative study that had the objective of evaluating the VOCs profile of patients depending on their body position (sitting, standing, supine, prone, left lateral and right lateral) and cardiac output, in order to identify specific VOCs or clusters of VOCs that could be considered as biomarkers. [54]
The last compound we found is Methyl Ethyl Ketone, also known as Butanone: it has never been identified as a specific ketone in VOCs studies, but like other methyl ketones, such as acetone, is produced during decarboxylation of fatty acid derives. [55] It is worth mentioning, however, that the production of ketones through non–fermenting enterobacteriaceae has different origins. In fact, Xiao and Xu showed that acetoin, also called 3-hydroxybutadone, was detected in non-fermenting Escherichia coli. [56] The synthesis of acetoin in Staphylococcus has been associated with catabolic aspects of the metabolism.
Four other molecules were indirectly quantified without specific calibration: Methane, Nitrous Oxide, Nitrous Acid, and Ammonia (Table 14).
Ammonia, a well investigated inorganic compound of nitrogen and hydrogen with formula NH3, has been shown to be produced in greater quantities by the microbiota of IBD patients compared to healthy individuals. [57–59] A possible hypothesis to explain this result is the pivotal role of ammonia and other short–chain fatty acids in determining the onset or chronicity of IBD, since the microbiota of IBD patients synthesizes large amounts of these compounds. [50,57]
It is worth noting that published studies report lower values of ammonia in UC patients compared to controls. [29] This evidence is in contrast with that reported by other studies, [57] and helps emphasize how results can vary in populations that differ in terms of average age and number of controls and patients. [60,61]
Moreover, recently published studies have shown that ammonia is involved in protein metabolism, with the consequent production of ammonium ions that can be converted to nitric oxide in the presence of nitric oxide synthase. [62,63] NO metabolites (nitrate/nitrite) are significantly increased in IBD, and NO levels have a great potential as biomarkers for the screening of IBD. [61,64–67]
The interest on NOX compounds is supported by several articles that agree in attributing to these compounds the role of biomarkers of bowel diseases. [68–70]
Finally, the production of methane in the distal colon is known to be due to endogenous (epithelial cells and dead bacteria) and exogenous (complex carbohydrates and non–digestible disaccharides) compounds. [71] Methane is an important biomarker of bacterial overgrowth typical of the IBD condition: several experimental studies tried to explain the mechanisms underlying the link between IBD and the abnormal biosynthesis of methane, [72,73] but to date this relation remains unclear. [74]
In conclusion, several VOCs show to be promising biomarkers for the non-invasive detection of IBD, thereby warranting further studies to assess whether the technical aspects of our experimental protocols on VOCs analysis can to be improved in the light of recent data in literature highlighting the importance of optimal sample collection. [75–77]
Some uncertainty remains for the other VOCs because more compounds—or fragments—may have the same MW. Following us on the hypothesis that these molecular weights refer to primary molecules, and not to fragments produced during the soft ionization and before MS detection, we compared our results with data from the literature on the composition of human alveolar air. MW 114 could correspond to 2,3,3–trimethylpentane, detected by Filipiak et al. [78] in the headspace of lung–cancer cells together with Acetaldehyde, MEK, Hexanal, Acrolein, and other aliphatic hydrocarbons. The same applies to MW 76, that could be identified as carbon disulphide, as detected by Navaneethan et al., [79] while MW 77 could be Methyl Nitrate, a product of oxidative stress reaction, as suggested by Minh et al. [80] MW 107 could correspond to 2,6–Dimethylpyridine, which is known to be a fragment of lysozyme [81]. MW 75 could be Trimethylamine N–oxide, a product of the microbiota, and the result of the conversion of phosphatidylcholine, a major component of cell membranes. [82–85]
For the molecules with MW 29, 67, 69, 70, 74, 79, 81, 89, 90, 91, 94, 99, 105, 115 and 118 Dalton, no data were available in literature. Thus, in order to better characterize these products, we looked at all the molecules with the above mentioned molecular weights reported by PubChem (http://pubchem.ncbi.nlm.nih.gov) or by the ChemSpider free–on–line database from the Royal Society of Chemistry (http://RSC.org; http://www.chemspider.com).
Among the reported molecules we excluded:
Molecules of clear industrial origin (for example products containing chlorine atoms or fluorine, bromine, etc.);
Molecules with ester linkage (as they are easily ionisable in the blood and they cannot be expelled with the alveolar air);
Highly reactive molecules, which show instability in the biological matrix (i.e. free radicals);
Molecules with a boiling point above 150°C, with high steam pressure (above 20 mm/Hg at 25°C) and low enthalpy of vaporization (>20 KJoule/mol): their concentration in the alveolar air should be so low that we should not be able to detect them with our equipment.
Reported in italics in Table 14 are molecules that, for their physical/chemical properties and based on the criteria identified above, could be associated with the molecular weights we identified.
We also evaluated if the performance of our models in correctly identifying CD and UC patients was affected by the level of activity of the diseases (by PCDAI and PUCAI respectively) and found no relevant relation (data not shown).
The main weakness of our study is the uncertainty in the definition of some of the molecules included in the final predictive models. Another weakness is the impossibility to recruit only CD and UC cases at onset, in the absence of an ongoing therapy that could partially affect the results of the regression models. We are aware that ideally suspect IBD cases should have been recruited at their first visit, and only subsequently divided into cases and controls, replicating a “real life” clinical situation. Selecting only suspicious cases at onset, however, would have required exceedingly long recruitment procedures. The positive aspect of our approach is that untreated cases (four CD and two UC) performed very well with all models, with predicted probabilities much higher than any possible cut–off we applied. This means that the effect of therapies, which are too complex and heterogeneous to be taken into account, is limited. The advantage of such a wide range of different therapies translates into models which are not directly affected in terms of outcome, although therapies almost certainly introduce some “noise”.
As specified in the Methods section, children had been fasting at least since midnight. Even if the prior evening meal did affect the colonic bacterial metabolism, and consequently alter the VOCs profile, we have no reason to believe meals were significantly different among the groups considered. Nevertheless, future studies might consider the possibility of standardizing the evening meal. We need to mention, however, that IBD patients might have different feeding patterns which could influence the composition of the microbiota, and consequently the VOCs pattern. In our study we did not correct for this aspect.
The main strength of the study lies in the use of a very precise instrument for the detection of VOCs. Our study clearly demonstrates that pediatric IBD patients (and CD patients in particular) have identifiable alveolar air VOCs patterns that differ from those of healthy subjects and gastroenterological controls. In addition, our models show that CD and UC present different patterns, emphasizing the different pathogenesis and clinical picture of the two diseases.
The results of the analysis of the false positives suggest that there might be something in common between IBDs and the false positives among the gastrointestinal controls, in terms of ongoing inflammatory processes. In fact, if we compare the false positives to the correctly classified in this group, we notice that the false positives have proportionally more severe and far more complex inflammatory clinical pictures. At this stage, however, this can only be a hypothesis, and certainly the intestinal microbiota, and/or the interaction between inflammation and the microbiota, could also play a role in determining the VOCs pattern.
In our opinion this study should be considered as a promising starting point. The creation of predictive models based on VOCs profiles, with the use of high precision instruments and advanced statistical methods, can contribute to the development of new non–invasive, fast and relatively inexpensive diagnostic tools, designed specifically for children, with very high sensitivity and specificity. It also represents a crucial step towards gaining further insights into the etiology of IBDs through the analysis of specific molecules which are the expression of the particular metabolism that characterizes these diseases. New prospective studies, following IBD patients from onset to post-treatment, should also be developed in order to study the relationship between VOCs profile and response to therapy.
Supporting information
(DTA)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank our colleague Alessandra Knowles for her invaluable help with the language revision of the present manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Rogers BH, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: an analysis of a computerized file of 1400 patients. J Chronic Dis. 1971;24(12):743–73. [DOI] [PubMed] [Google Scholar]
- 2.Albert JG, Martiny F, Krummenerl A, Stock K, Lesske J, Göbel CM,et al. Diagnosis of small bowel Crohn's disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54(12):1721–7. doi: 10.1136/gut.2005.069427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–39. doi: 10.1002/ibd.21349 [DOI] [PubMed] [Google Scholar]
- 4.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58(2):519–25. doi: 10.1007/s10620-012-2371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day AS, Ledder O, Leach ST, Lemberg DA. Crohn’s and colitis in children and adolescent. World J Gastroenterol. 2012; 18(41):5862–5869. doi: 10.3748/wjg.v18.i41.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory Bowel Disease in children and adolescents: recommendation for diagnosis—The Porto Criteria. J Pediatr Gastroenterol Nutr. 2005;41(1):1–7. [DOI] [PubMed] [Google Scholar]
- 7.Griffith AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18(3):509–23. doi: 10.1016/j.bpg.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Hill RJ, Brookes DS, Davies PS. Bones in pediatric Crohn's disease: a review of fracture risk in children and adults. Inflamm Bowel Dis. 2011;17(5):1223–8. doi: 10.1002/ibd.21471 [DOI] [PubMed] [Google Scholar]
- 9.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4(1):7–27. doi: 10.1016/j.crohns.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Kostakis ID, Cholidou KG, Vaiopoulos AG, Vlachos IS, Perrea D, Vaos G. Fecal Calprotectin in Pediatric Inflammatory Bowel Disease: A Systematic Review. Dig Dis Sci. 2013;58(2):309–19. doi: 10.1007/s10620-012-2347-5 [DOI] [PubMed] [Google Scholar]
- 11.Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res. 2012;6:027108 doi: 10.1088/1752-7155/6/2/027108 [DOI] [PubMed] [Google Scholar]
- 12.Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci USA. 1971;68(10):2374–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Brit J Cancer. 2010;103(4):542–51. doi: 10.1038/sj.bjc.6605810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Kant KD, van der Sande LJ, Jöbsis Q, van Schayck OC, Dompeling E. Clinical use of exhaled volatile organic compounds in pulmonary disease: a systematic review. Respir Res. 2012;13:117 doi: 10.1186/1465-9921-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer. 2009;9:348 doi: 10.1186/1471-2407-9-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999; 353(9168):1930–1933. doi: 10.1016/S0140-6736(98)07552-7 [DOI] [PubMed] [Google Scholar]
- 17.Phillips M, Cataneo RN, Ditkoff BA, Fisher P, Greenberg J, Gunawardena R, et al. Volatile markers of breast cancer in the breath. Breast J. 2003;9(3):184–91. [DOI] [PubMed] [Google Scholar]
- 18.Phillips M, Cataneo RN, Saunders C, Hope P, Schmitt P, Wai J. Volatile biomarkers in the breath of women with breast cancer. J Breath Res. 2010;4(2):026003 doi: 10.1088/1752-7155/4/2/026003 [DOI] [PubMed] [Google Scholar]
- 19.Phillips M, Cataneo RN, Cheema T, Greenberg J. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta. 2004;344(1–2):189–194. doi: 10.1016/j.cccn.2004.02.025 [DOI] [PubMed] [Google Scholar]
- 20.Netzer M, Millonig G, Osl M, Pfeifer B, Praun S, Villinger J, et al. A new ensemble-based algorithm for identifying breath gas marker candidates in liver disease using ion molecule reaction mass spectrometry (IMR-MS). Bioinformatics. 2009;25(7):941–7. doi: 10.1093/bioinformatics/btp093 [DOI] [PubMed] [Google Scholar]
- 21.Phillips M, Basa-Dalay M, Bothamley G, Cataneo RN, Lam PK, Natividad MP, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb). 2010; 90(2):145–51. [DOI] [PubMed] [Google Scholar]
- 22.Bennett L, Ciaffoni L, Denzer W, Hancock G, Lunn AD, Peverall R, et al. A chemometric study on human breath mass spectra for biomarker identification in cystic fibrosis. J. Breath Res. 2009; 3:046002 doi: 10.1088/1752-7155/3/4/046002 [DOI] [PubMed] [Google Scholar]
- 23.Moretti M, Phillips M, Abouzeid A, Cataneo RN, Greenberg J. Increased breath markers of oxidative stress in normal pregnancy and in preeclampsia. Am J Obstet Gynecol. 2004; 190(5):1184–90. doi: 10.1016/j.ajog.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 24.Probert CS. Role of faecal gas analysis for the diagnosis of IBD. Biochem Soc Trans. 2011;39(4):1079–80. doi: 10.1042/BST0391079 [DOI] [PubMed] [Google Scholar]
- 25.de Meij TG, de Boer NK, Benninga MA, Lentferink YE, de Groot EF, van de Velde ME, et al. Faecal gas analysis by electronic nose as novel, non-invasive method for assessment of active and quiescent paediatric inflammatory bowel disease: proof of principle study. J Crohns Colitis. 2014. doi: 10.1016/j.crohns.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 26.Arasaradnam RP, Ouaret N, Thomas MG, Quraishi N, Heatherington E, Nwokolo CU, et al. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(5):999–1003. doi: 10.1097/MIB.0b013e3182802b26 [DOI] [PubMed] [Google Scholar]
- 27.Markar SR, Wiggins T, Kumar S, Hanna GB. Exhaled Breath Analysis for the Diagnosis and Assessment of Endoluminal Gastrointestinal Diseases. J Clin Gastroenterol. 2015;49(1):1–8. doi: 10.1097/MCG.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 28.Quenon L, Hindryckx P, De Vos M, De Looze D, Joos G, Brusselle G, Peeters H. Hand-held fractional exhaled nitric oxide measurements as a non-invasive indicator of systemic inflammation in Crohn's disease. J Crohns Colitis. 2013;7(8):644–8. doi: 10.1016/j.crohns.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 29.Hicks LC, Huang J, Kumar S, Powles ST, Orchard TR, Hanna GB, Williams HR. Analysis of exhaled breath volatile organic compounds in inflammatory bowel disease: a pilot study. J Crohns Colitis. 2015;9(9):731–7. doi: 10.1093/ecco-jcc/jjv102 [DOI] [PubMed] [Google Scholar]
- 30.Patel N, Alkhouri N, Eng K, Cikach F, Mahajan L, Yan C, et al. Metabolomic analysis of breath volatile organic compounds reveals unique breathprints in children with inflammatory bowel disease: a pilot study. Aliment Pharmacol Ther. 2014;40(5):498–507. doi: 10.1111/apt.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 32.Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133: 423–432. doi: 10.1053/j.gastro.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 33.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447 [PubMed] [Google Scholar]
- 34.Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. doi: 10.1002/ibd.21493 [DOI] [PubMed] [Google Scholar]
- 35.Defoort F, Thiery S, Ravel S. A promising new on-line method of tar quantification by mass spectrometry during steam gasification of biomass. Biomass Bioenerg. 2014;65:64–71. [Google Scholar]
- 36.Hornuss C, Praun S, Villinger J, Dornauer A, Moehnle P, Dolch M, et al. Real-time monitoring of propofol in expired air in humans undergoing total intravenous anesthesia. Anesthesiology 2007;106:665–74. doi: 10.1097/01.anes.0000264746.01393.e0 [DOI] [PubMed] [Google Scholar]
- 37.Tibshirani R. Regression shrinkage and selection via the lasso. J R Statist Soc Ser B. 1996;58:267.–. [Google Scholar]
- 38.Goeman JJ. L1 penalized estimation in the Cox proportional hazards model. Biom J. 2010;52:70–84. doi: 10.1002/bimj.200900028 [DOI] [PubMed] [Google Scholar]
- 39.Judd CM, McClelland GH, Ryan CS. Data Analysis: A Model Comparison Approach. 2nd ed New York: Routledge; 2009. [Google Scholar]
- 40.Huang J, Ma S, Zhang CH. The Iterated Lasso for High-Dimensional Logistic Regression. Technical Report No. 392; Department of Statistics and Actuarial Science, The University of Iowa 2008.
- 41.Zou H. The Adaptive Lasso and Its Oracle Properties. J Am Stat Assoc. 2006;101:1418–29. [Google Scholar]
- 42.Esiringü F, Tuğcu-Demiröz F, Acartürk F, Coşkun Cevher Ş, Bircan F, Sarı Kılıçaslan SM. Investigation of the effect of intracolonic melatonin gel formulation on acetic acid-induced colitis. Drug Deliv. 2016; 23:2318–2326. doi: 10.3109/10717544.2014.982773 [DOI] [PubMed] [Google Scholar]
- 43.Sanei MH, Hadizadeh F, Adibi P, Alavi SA. Inflammatory cells' role in acetic acid–induced colitis. Adv Biomed Res. 2014;3:193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical–induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–88. doi: 10.4196/kjpp.2014.18.4.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jardine KJ, Sommer ED, Saleska SR, Huxman TE, Harley PC, Abrell L. Gas phase measurements of pyruvic acid and its volatile metabolites. Environ Sci Technol. 2010;44:2454–60. doi: 10.1021/es903544p [DOI] [PubMed] [Google Scholar]
- 46.Bos LD, Sterk PJ, Schultz MJ. Volatile metabolites of pathogens: a systematic review. PLoS Pathog. 2013;9:e1003311 doi: 10.1371/journal.ppat.1003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh S, Arcaroli J, Thompson DC, Messersmith W, Vasiliou V. Acetaldehyde and retinaldehyde–metabolizing enzymes in colon and pancreatic cancers. Adv Exp Med Biol. 2015;815:281–94. doi: 10.1007/978-3-319-09614-8_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen AG, Knechel S. Antimicrobial activity of food–related Penicillium sp. against pathogenic bacteria in laboratory media and a cheese model system. J Appl Microbiol. 1997;83:111–119. [DOI] [PubMed] [Google Scholar]
- 49.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71:483–499. doi: 10.1111/nure.12027 [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, Liu W, Zou H, Cheng T, Tian N, Xian M. Microbial production of short chain diols. Microb Cell Fact. 2014;13:165 doi: 10.1186/s12934-014-0165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner WP, Helmig D, Fall R. Isoprene Biosynthesis in Bacillus subtilis via the methylerythritol phosphate pathway. J Nat Prod. 2000;63: 37–40. [DOI] [PubMed] [Google Scholar]
- 52.Kuzma J, Nemecek–Marshall M, Pollock WH, Fall R. Bacteria produce the volatile hydrocarbon isoprene. Curr Microbiol. 1995;30: 97–103. [DOI] [PubMed] [Google Scholar]
- 53.Bhatt A, Parsi MA, Stevens T, Gabbard S, Kumaravel A, Jang S, et al. Volatile organic compounds in plasma for the diagnosis of esophageal adenocarcinoma: a pilot study. Gastrointest Endosc. 2015. [DOI] [PubMed] [Google Scholar]
- 54.Sukul P, Trefz P, Kamysek S, Schubert JK, Miekisch W. Instant effects of changing body positions on compositions of exhaled breath. J Breath Res. 2015;9:047105 doi: 10.1088/1752-7155/9/4/047105 [DOI] [PubMed] [Google Scholar]
- 55.Schulz S, Dickschat JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep. 2007;24: 814–842. doi: 10.1039/b507392h [DOI] [PubMed] [Google Scholar]
- 56.Xiao Z, Xu P. Acetoin metabolism in bacteria. Crit Rev Microbiol. 2007;33:127–140. doi: 10.1080/10408410701364604 [DOI] [PubMed] [Google Scholar]
- 57.van Nuenen MH, Venema K, van der Woude JC, Kuipers EJ. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:485–91. [DOI] [PubMed] [Google Scholar]
- 58.Roediger WE, Lawson MJ, Nance SH, Radcliffe BC. Detectable colonic nitrite levels in inflammatory bowel disease––mucosal or bacterial malfunction? Digestion. 1986;35:199–204. [DOI] [PubMed] [Google Scholar]
- 59.Hakansson A, Molin G. Gut Microbiota and Inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hibbard T, Killard AJ. Breath ammonia levels in a normal human population study as determined by photoacoustic laser spectroscopy. J Breath Res. 2011;5:037101 doi: 10.1088/1752-7155/5/3/037101 [DOI] [PubMed] [Google Scholar]
- 61.Brannelly NT, Hamilton–Shield JP, Killard AJ. The Measurement of Ammonia in Human Breath and its Potential in Clinical Diagnostics. Crit Rev Anal Chem. 2016;46:490–501. doi: 10.1080/10408347.2016.1153949 [DOI] [PubMed] [Google Scholar]
- 62.Kurada S, Alkhouri N, Fiocchi C, Dweik R, Rieder F. Review article: breath analysis in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;41:329–41. doi: 10.1111/apt.13050 [DOI] [PubMed] [Google Scholar]
- 63.Ajibola OA, Smith D, Spanel P, Ferns GA. Effects of dietary nutrients on volatile breath metabolites. J Nutr Sci. 2013;2:e34 doi: 10.1017/jns.2013.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reinders CA, Jonkers D, Janson EA, Stockbrügger RW, Stobberingh EE, Hellström PM, Lundberg JO. Rectal nitric oxide and fecal calprotectin in inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1151–7. doi: 10.1080/00365520701320505 [DOI] [PubMed] [Google Scholar]
- 65.Roediger WE. Review article: nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment Pharmacol Ther. 2008;27:531–41. doi: 10.1111/j.1365-2036.2008.03612.x [DOI] [PubMed] [Google Scholar]
- 66.Kearney DJ, Hubbard T, Putnam D. Breath ammonia measurement in Helicobacter pylori infection. Dig Dis Sci. 2002. November;47(11):2523–30. [DOI] [PubMed] [Google Scholar]
- 67.Bevc S, Mohorko E, Kolar M, Brglez P, Holobar A, Kniepeiss D, et al. Measurement of breath ammonia for detection of patients with chronic kidney disease. Clin Nephrol. 2017. June 9 doi: 10.5414/CNP88FX04 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Jang A, Kim WT, Kim IS. Simultaneous removal of volatile organic compounds (VOCs) and nitrogen: batch test. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38:2955–66. [DOI] [PubMed] [Google Scholar]
- 69.Buijck M, Berkhout DJ, de Groot EF, Benninga MA, van der Schee MP, Kneepkens CM, et al. Sniffing Out Paediatric Gastro–intestinal Diseases: the Potential of Volatile Organic Compounds as Biomarkers for Disease. J Pediatr Gastroenterol Nutr. 2016. April 21 doi: 10.1097/MPG.0000000000001250 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Arasaradnam RP, McFarlane M, Daulton E, Skinner J, O'Connell N, Wurie S, et al. Non–invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig Liver Dis. 2016;48:148–53. doi: 10.1016/j.dld.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 71.Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. 2010;55:2135–43. doi: 10.1007/s10620-009-1012-0 [DOI] [PubMed] [Google Scholar]
- 72.Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–18. doi: 10.1038/nrgastro.2012.85 [DOI] [PubMed] [Google Scholar]
- 73.Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu Rev Food Sci Technol. 2010;1:363–95. doi: 10.1146/annurev.food.102308.124101 [DOI] [PubMed] [Google Scholar]
- 74.Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short–chain fatty acids in gut inflammation and obesity. Dig Dis Sci. 2013;58:2756–66. doi: 10.1007/s10620-013-2744-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salvo P, Ferrari C, Persia R, Ghimenti S, Lomonaco T, Bellagambi F, Di Francesco F. A dual mode breath sampler for the collection of the end–tidal and dead space fractions. Med Eng Phys. 2015;37:539–44. doi: 10.1016/j.medengphy.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 76.Lourenço C, Turner C. Breath analysis in disease diagnosis: methodological considerations and applications. Metabolites. 2014;4:465–98. doi: 10.3390/metabo4020465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosias PP, Robroeks CM, Kester A, den Hartog GJ, Wodzig WK, Rijkers GT, et al. Biomarker reproducibility in exhaled breath condensate collected with different condensers. Eur Respir J. 2008;31:934–42. doi: 10.1183/09031936.00073207 [DOI] [PubMed] [Google Scholar]
- 78.Filipiak W, Sponring A, Mikoviny T, Ager C, Schubert J, Miekisch W, et al. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU–1 in vitro. Cancer Cell Int. 2008;8:17 doi: 10.1186/1475-2867-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Navaneethan U, Parsi MA, Lourdusamy V, Bhatt A, Gutierrez NG, Grove D, et al. Volatile organic compounds in bile for early diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis: a pilot study. Gastrointest Endosc. 2015;81:943–9.e1. doi: 10.1016/j.gie.2014.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minh TD, Oliver SR, Ngo J, Flores R, Midyett J, Meinardi S, et al. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am J Physiol Endocrinol Metab. 2011;300:E1166–75. doi: 10.1152/ajpendo.00634.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nonose S, Yamashita K, Okamura T, Fukase S, Kawashima M, Sudo A, Isono H. Conformations of disulfide–intact and–reduced lysozyme ions probed by proton–transfer reactions at various temperatures. J Phys Chem B. 2014;118:9651–61. doi: 10.1021/jp505621f [DOI] [PubMed] [Google Scholar]
- 82.Joyce SA, Gahan CG. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol. 2014;30:120–7. doi: 10.1097/MOG.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 83.Zechman JM, Aldinger S, Labows JN Jr. Characterization of pathogenic bacteria by automated headspace concentration-gas chromatography. J Chromatogr. 1986;377:49–57. [DOI] [PubMed] [Google Scholar]
- 84.DeLano FA, Chow J, Schmid-Schönbein GW. Volatile Decay Products in Breath During Peritonitis Shock are Attenuated by Enteral Blockade of Pancreatic Digestive Proteases. Shock. 2017. May 11 doi: 10.1097/SHK.0000000000000888 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mussap M, Antonucci R, Noto A, Fanos V. The role of metabolomics in neonatal and pediatric laboratory medicine. Clin Chim Acta. 2013;426:127–38. doi: 10.1016/j.cca.2013.08.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DTA)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.