Abstract
Introduction
T-cell acute lymphoblastic leukemia (ALL) and lymphoma (LBL) are aggressive hematologic neoplasms that are treated with combination chemotherapy in the frontline, but have limited options in the relapsed or refractory setting. Based on observations in patients with purine nucleoside phosphorylase (PNP) deficiency, a guanosine nucleoside analogue, arabinosylguanine (ara-G) was developed that provided T-cell specificity. Nelarabine was developed as the water-soluble, clinically useful-prodrug of ara-G and based on its activity was approved for the treatment of relapsed or refractory T-ALL/LBL.
Areas covered
In this narrative review, we will summarize the preclinical studies, early dose-finding studies, and efficacy studies that led to approval of nelarabine. The review will succinctly cover response rates and safety signals reported during clinical development. We will also cover more recent work with nelarabine, including combination studies, modified dosing schedules, and frontline treatment approaches.
Expert commentary
Based on evidence from the literature review and our own experience with nelarabine, we conclude that it is an effective agent in the treatment of T-cell malignancies. Understanding the factors that modulate the risk of dose-limiting neurotoxicity, how to mitigate this toxicity, and how to safely combine it with other active agents will continue to broaden its use.
Keywords: Nucleoside analogue, T-cell, pediatric ALL, arabinosylguanine, GW506U78, compound 506, nelarabine, purine nucleoside phosphorylase, ara-G
1.0 Introduction
While therapeutic options for B-cell acute lymphoblastic leukemia (ALL) have expanded recently with the development of monoclonal antibodies1–3, tyrosine kinase inhibitors4,5, and chimeric-antigen receptor (CAR) engineered T-cells6, therapies for T-cell ALL (T-ALL) are more limited and remain unsatisfactory. One bright spot in the development of specific T-cell directed therapies in ALL has been the discovery and approval of nelarabine.
The initial impetus towards development of nelarabine began with the observation that intracellular deoxyguanosine (dGuo) triphosphate (dGTP) accumulation was specifically toxic to T-cells in patients with the inherited immunodeficiency syndrome purine nucleoside phosphorylase (PNP) deficiency.7–10 Purine nucleoside phosphorylase (enzyme) acts on the glycosidic bond of deoxyguanosine resulting in its catabolism to free base. Lack of this enzyme results in accumulation of dGuo in plasma and selective accumulation of dGTP in T-cells which then acts as a cytotoxic agent.10,11 This inspired the notion that dGTP could be therapeutically used to target T-cell neoplasms, leading to the development of the PNP-resistant dGuo analogue arabinofuranosylguanine (ara-G) or an inhibitor of PNP enzyme such as forodesine. Preclinical studies confirmed the T-cell selective cytotoxicity of ara-G12,13 and provided the rationale for the clinical development of this analogue in T-ALL.14,15 Challenges with the solubility of ara-G significantly hindered its initial development. This eventually prompted the synthesis of nelarabine, a water-soluble prodrug of ara-G that could be administered intravenously and which, upon infusion, is rapidly converted to ara-G in the plasma by adenosine deaminase (Figure 1).16
Figure 1.
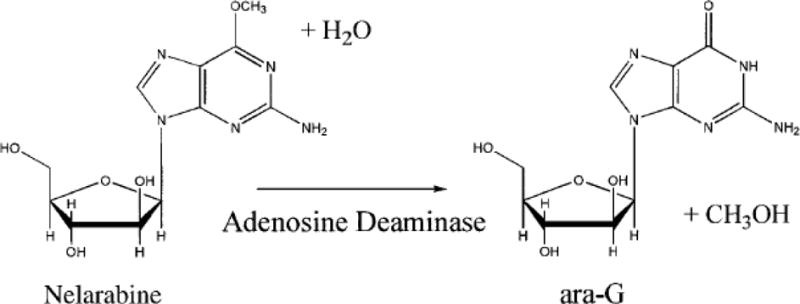
Conversion of nelarabine to 9-β-D-Arabinofuranosylguanine (ara-G) by adenosine deaminase (ADA). [Adapted from Kisor DF, Plunkett W, et. al. J Clin Oncol 2000.]
The current paper focuses on the clinical development of nelarabine in the treatment of adults and children with T-cell leukemias and lymphomas, from early phase I dose-finding and safety studies, to phase II efficacy studies and more recent combination approaches (Table 1). In preparing this review, we queried the PubMed database with the search term “nelarabine” and reviewed the clinical studies associated with this term, with a particular focus on clinical trials in leukemia. Abstract proceedings of national meetings (American Society of Hematology and American Society of Clinical Oncology) were also searched for similar terms. Nelarabine has also been known as Compound 506, 506U78, GW506U78, and 6-methoxy-ara-G. Preclinical studies, early development, and mechanism of action of this compound have been reviewed previously.17–23
Table 1.
Studies of Nelarabine in Patients with Relapsed and/or Refractory Leukemias.(T-ALL: T Acute Lymphoblastic Leukemia; T-LBL: T Lymphoblastic Lymphoma; D or d: day; EM: extramedullary; CNS: central nervous system)
| Reference (Ref. #) | Phase | N | Agent(s) | Dose(s) and Schedule | N | Overall response rate | Any Neurotoxicity | ≥ Grade 3 Neurotoxicity |
|---|---|---|---|---|---|---|---|---|
| Kurtzberg, et. al. (25) | I | 93 | Nelarabine | Escalating 5 mg/kg/d - 75 mg/kg/d on D1-5; (MTD 1200 mg/m2/d on D1-5) | 93 | 31% overall | 72% | |
| 39 | 54% in T-ALL | |||||||
| Gandhi, et. al. (33) | I | 13 | Nelarabine | 1200 mg/m2/d on D1, 3, 5 | 13 | 54% | 54% | |
| Fludarabine | 30 mg/m2/d on D3 and 5 | |||||||
| Berg, et. al. (34) | II | 121 | Nelarabine | started 1200 mg/m2/d on D1-5, but needed 2 de-escalations to 650 mg/m2/d on D1-5 and 400 mg/m2/d on D1-5 | 106 | Overall - 33% | 18% | |
| 33 | First relapse - 55% | |||||||
| 30 | Beyond first relapse -27% | |||||||
| 21 | With CNS disease - 33% | |||||||
| 22 | With isolated EM disease - 14% | |||||||
| DeAngelo, et. al. (35) | II | 39 | Nelarabine | 1500 mg/m2/d on D1, 3, 5 |
39 (13 T-LBL) |
Overall - 41% | 37% peripheral sensory neuropathy | 18% |
| 11 | First relapse - 55% | 21% peripheral motor neuropathy | ||||||
| 28 | Beyond first relapse - 36% | |||||||
| Gokbuget, et. al. (38) | II | 126 | Nelarabine | 1500 mg/m2/d on D1, 3, 5 |
126 (19 T-LBL) |
46% | 16% | 7% |
| Commander, et. al. (46) | I | 7 | Nelarabine | 650 mg/m2/d on D1 - 5 | 7 | 100% | 86% | 57% |
| Etoposide | 100 mg/m2/d on D6 - 10 | |||||||
| Cyclophosphamide | 440 mg/m2/d on D6 - 10 | |||||||
| Kadia, et. al. (51) | I | 23 | Nelarabine | Escalating doses from 100 - 700 mg/m2/d x 5 days by 24hr continuous infusion | 23 | Overall - 30% | 22% peripheral neuropathy | 4% |
| 10 | First relapse - 30% | 9% muscle weakness | ||||||
| 7 | Second relapse - 29% | |||||||
| 6 | Beyond second relpase - 33% | |||||||
| 6 | Thymic/Mature histology - 50% |
2.0 Preclinical and Early Phase Clinical Studies
Preclinical studies confirmed the efficient conversion of nelarabine to ara-G in the plasma of non-human primates, yielding peak ara-G levels at the end of nelarabine infusion.16 The conversion of nelarabine to ara-G is by adenosine deaminase (enzyme) which is present in high specific activity in several large body organs as well as in erythrocytes. The ara-G is then phosphorylated to mono-, di-, and triphosphate selectively in circulating leukemic T-cells. Accumulation of ara-GTP in malignant T-cells was relevant, as higher peak intracellular ara-GTP levels correlated with response.24
Based on promising preclinical results, nelarabine was first studied in phase I dose-escalation trial in patients with relapsed and refractory (R/R) hematologic malignancies.25 At total of 93 patients were treated, including 59 adults with a median age of 48 years, and 34 children with a median age of 10 years. Patients were treated with escalating doses ranging from 5 mg/kg/d to 75 mg/kg/d given as a 1 hour infusion on days 1 to 5. The maximum tolerated dose was found to be 60 mg/kg/d in children and 40 mg/kg/d in adults. The dose-limiting toxicity was neurologic. Neurologic events were seen in 72% of patients, including 50% of children and 85% of adults, most being reversible. Most of the neurologic toxicity started within 12 days of infusion and some was noted to be cumulative with successive cycles. The most common grade 3/4 neurologic adverse events reported were malaise, somnolence, confusion, ataxia, muscle weakness, and peripheral neuropathies. Nausea, vomiting, fever, and anorexia were observed, but acceptable. Notably, in patients without bone marrow disease (i.e. lymphoblastic lymphoma only) and normal marrow function, significant hematologic toxicity was not seen with this nucleoside analogue. The overall response rate in this mixed population of hematologic malignancies was 31%, including 54% of patients with T-ALL achieving a complete or partial response. Myeloid leukemia did not show any benefit with this drug. Most patients that responded had T-cell malignancy; one patient with CML in T-lymphoid blast crisis26 and one patient with chronic lymphocytic leukemia (CLL) achieved PR. Based on cumulative safety and efficacy data, the recommended phase 2 dose (RP2D) was 1200 mg/m2/d, which was equivalent to approximately 30 mg/kg/d in adults and 40 mg/kg/d in children. This Phase I study clearly defined, that similar to preclinical observations, the drug was most effective in T-cell malignancies.
Extensive in vivo plasma pharmacokinetic analysis of nelarabine and its metabolite ara-G, was performed as part of this phase I study.27 Of the 93 patients treated, 78 patients were characterized for plasma pharmacology. The analysis confirmed that nelarabine was an excellent prodrug of ara-G, achieving a Cmax at or near the end of the 1-hour infusion and demonstrating linear increase in plasma concentration with increasing nelarabine dose. The plasma T1/2 of ara-G was relatively long, approximately 2.1 hours in children and 3 hours in adults; and the clearance had a linear relationship with the patient’s creatinine clearance. At the RP2D of approximately 1200mg/m2, the plasma ara-G concentration remained > 10 micromolar (the concentration required for linear accumulation of intracellular ara-GTP) for more than 8 hours.
From the initial studies, it was clear that the intracellular accumulation of ara-GTP was directly related to the clinical success of nelarabine.24,27 Modulating cellular biochemistry to increase intracellular ara-GTP accumulation28 as had previously been demonstrated with nucleoside analogues could potentially enhance clinical efficacy. Fludarabine is a potent inhibitor of ribonucleotide reductase (RNR) and had been used previously in combination with cytarabine to increase intracellular levels of ara-CTP in the treatment of acute myeloid leukemia.29–31 Inhibition of RNR by fludarabine could (1) deplete intracellular deoxynucleotides and (2) lead to induction of deoxycytidine kinase and deoxyguanosine kinase through reduced feedback inhibition. These 2 enzymes are responsible for phosphorylating ara-G to ara-GTP32, increasing its intracellular concentration, and facilitating its incorporation into DNA. The combination of fludarabine with nelarabine, therefore, could potentiate intracellular ara-GTP accumulation, increase cytotoxicity, and translate into clinical benefit. This concept was studied in a phase I study of a sequential combination of fludarabine and nelarabine. Because fludarabine is used as a standard of care for patients with CLL and nelarabine showed some activity in this disease and in T-cell leukemias, this combination was tested in patients with R/R CLL and T-ALL.33 Thirteen patients with a median age of 62 years were treated with nelarabine at a dose of 1200 mg/m2 on days 1, 3, and 5 combined with the standard dose of fludarabine (30 mg/m2) on days 3 and 5. This protocol design facilitated pharmacokinetic evaluation of ara-GTP accumulation in circulating leukemia cells before and after fludarabine infusion. The regimen was tolerated with grade 2 sensory neuropathy reported in 7 (54%) patients, and grade 3/4 hematologic toxicity in 44% of patients. The overall response rate was 54%, including 6 responses among 9 patients (67%) with indolent leukemias and 1 of 2 patients with R/R T-ALL achieving a complete remission. The addition of fludarabine did not affect the plasma pharmacokinetic profiles of nelarabine or ara-G. However, intracellular accumulation of ara-GTP was significantly higher on day 2 compared to day 1, suggesting some biochemical modulation with the combination. Similar to preclinical studies, cells from responding patients demonstrated significantly higher ara-GTP levels compared to those who were nonresponders.33 The combination showed promising clinical synergy even in patients who were previously fludarabine-refractory and merits further study.
3.0 Phase II Studies
After determining the safety profile of nelarabine and estimating an RP2D, several phase II trials were conducted to establish its efficacy in patients with R/R T-ALL and T-lymphoblastic lymphoma (LBL). Berg and colleagues first reported on phase II study in children with R/R T-ALL and LBL.34 A total of 121 patients with a median age of 11.5 years were enrolled in 4 predefined strata: (1) patients with ≥ 25% bone marrow blasts in first relapse, (2) those with ≥ 25% bone marrow blasts beyond first relapse, (3) those with CNS disease document by positive CSF pathology, and (4) patients with isolated extramedullary relapse (i.e. with no bone marrow disease). Patients were treated initially at a dose of 1200 mg/m2/day on days 1 to 5 of a 21 days cycle. However, two de-escalations in the dose were necessary due to excessive toxicity. Patients in strata 1 and 2 received a dose of 650 mg/m2 for 5 days and those in strata 3 and 4 received a dose of 400 mg/m2. Among 106 evaluable patients at the final dose levels, the overall response rate (CR + PR) was 33%. When examined within each strata, the ORR were 55%, 27%, 33%, and 14% for strata 1, 2, 3, and 4, respectively. Interestingly, 8 of 22 patients who had positive CSF cytology prior to study entry converted to negative CSF cytology by day 7, prior to their scheduled intrathecal chemotherapy, suggesting a role for nelarabine in treatment and/or prophylaxis of CNS leukemia. Treatment with nelarabine was tolerated, but did require dose reductions for management of clinically significant neurotoxicity. Overall 18% of patients had ≥ grade 3 neurotoxicity which was dose-dependent. At doses ≥ 900 mg/m2, the rate of neurologic adverse events was 28%, compared to 17% at doses ≤ 650 mg/m2. The most common neurologic adverse events were peripheral neuropathy and reversible somnolence. There were 13 episodes of ≥ grade 3 peripheral neurologic adverse events and 18 episodes of ≥ grade 3 central neurologic adverse events. As expected, patients with bone marrow involvement had higher rates of hematologic toxicity compared to those in strata 4, which had only extramedullary disease. The study confirmed the high single-agent activity of nelarabine in R/R T-ALL in children, particular for those in the first salvage setting. Additionally, the trial further refined the treatment dose to 650 mg/m2/day for 5 days in children.
A second phase II study was conducted to confirm the efficacy in adult patients with R/R T-ALL or T-LBL.35 Thirty-nine patients, with a median age of 34 years and a median of 2 prior therapies were treated on the study. Two-thirds of the patients had T-ALL and a third had T-LBL. The dosing regimen, borrowed from a previous phase I study in an effort to reduce neurotoxicity, was 1500 mg/m2/d on days 1, 3, and 5 every 3 to 4 weeks. Additional rationale for an alternate day schedule was the observation that levels of ara-GTP in the circulating leukemia cells were maintained for more than 24 hours. In a heavily pretreated population, the overall response rate (CR+CRi+PR) was 41%, including 31% CR+CRi. The ORR was 55% for patients in first salvage and 36% for those in ≥ 2nd salvage. The median disease-free survival (DFS) was 20 weeks. The median overall survival (OS) was 20 weeks and the 1-year OS probability was 28%. The most prominent clinical toxicity was grade 3 and 4 neutropenia and thrombocytopenia. The most frequent ≥ grade 3 non-hematologic adverse events were fatigue in 18% and muscle weakness in 11%. Grade 3 or 4 neurologic adverse events occurred in 18% of patients. Grade 1 to 2 neurologic adverse events were common, with 37% of patients having peripheral sensory neuropathy and 21% having peripheral motor neuropathy.
Based on these studies and in the context of a critical unmet need, nelarabine was given accelerated approval by the US Food and Drug Administration in October of 2005 for the treatment of patients with T-ALL/LBL whose disease has not responded to treatment or has relapsed following treatment with at least two chemotherapy regimens.17,36,37
Several studies of nelarabine following its approval have confirmed and broadened its applicability. The large German Multicenter Study Group for Adult ALL (GMALL) published their large prospective phase II study in patients with R/R T-ALL/LBL.38 A cohort of 126 patients with a median age of 33 years were treated with single-agent nelarabine at a dose of 1500 mg/m2 over 2 hours on days 1, 3, and 5 of a 3 week cycle. The overall response rate was 46%, with 36% CR and 10% PR. Two-thirds of the patients were in the first salvage setting while 10% were in second salvage; 38% of patients in the second salvage setting achieved a CR. The 1-year OS was 25% and the median OS was 6 months. Eighty percent of patients achieving a CR went on to allogeneic SCT. For those receiving a SCT the 3-year OS was 31% and the RFS was 37%. Neurotoxicity was seen in 16% of patients, reaching grade 3 or 4 in severity in only 7%. Grades 3 and 4 neutropenia and thrombocytopenia was seen in 37% and 17%, respectively. This is the largest prospective trial of nelarabine to date in patients with R/R T-ALL and confirms its safety and single-agent efficacy. These data also demonstrate that nelarabine can facilitate adequate disease control to make SCT feasible.
In order to expand from experience in T-cell leukemia, a small pilot phase II study was conducted in patients with cutaneous- and/or systemic peripheral T-cell lymphoma.39 Nineteen patients were treated with nelarabine at a dose of 1500 mg/m2 on days 1, 3, and 5 of a 21 day cycle, for a minimum of 2 cycles. Grade 3 or 4 neurotoxicity was observed in 33% of the patients, including ataxia, vertigo, peripheral neuropathy, and/or altered consciousness. The overall response rate was 10.5%, including 2 PR’s of short duration. The investigators concluded that nelarabine was not recommended as monotherapy for these diseases based on its efficacy and toxicity profile.39
4.0 Nelarabine Combination Studies
These investigations with nelarabine highlight particular characteristics of the drug that could steer further development. First, it was clear that less-heavily treated patients had higher remission rates with nelarabine; and second, nelarabine was not profoundly myelosuppressive in the absence of marrow disease and could potentially be combined with other leukemia drugs with non-overlapping toxicities. It is important to note, however, that in each of the following studies the nelarabine is combined as part of regimen but not administered simultaneously with other chemotherapy drugs.
The Children’s Oncology Group (COG) conducted a pilot study adding nelarabine to the Berlin-Frankfurt-Munster (BFM)-86 multiagent chemotherapy regimen in 88 children (median age 10 years) with newly diagnosed T-ALL/LBL (Study AALL00P2).40 Poor early response to prednisone (PPR) and detectable minimal residual disease (MRD) at the end of induction are strong predictors of inferior outcome in children with newly diagnosed T-ALL treated on a BFM regimen. Efforts are made, therefore, to identify these patients with ‘slow early response’ (SER) and offer more intensification of their therapy and allogeneic stem cell transplant to improve outcomes.
This pilot trial aimed to intensify chemotherapy in patients with SER with the addition of nelarabine to the BFM-86 regimen. The trial had 2 stages to first evaluate for tolerability. Nelarabine was administered according to the pediatric schedule of either 400 mg/m2/d or 650 mg/m2/d for 5 days during induction, reinduction, and the first 4 courses of maintenance. In the first stage, patients with SER by PPR (N=8) and MRD (N=4) received chemotherapy plus nelarabine while those with rapid early response (RER, N=16) received chemotherapy alone. In stage 1, there was no difference in 5-year EFS between those with SER or RER (73% vs. 69%, respectively). During stage 2, once tolerability was confirmed, all patients (SER or RER) received chemotherapy plus nelarabine. In stage 2, once again, there was no difference in 5-year EFS between patients with SER or RER (67% vs. 74%, respectively). This lack of difference in long-term outcome in two prognostically distinct groups suggested that the addition of nelarabine was able to overcome the negative impact of SER. The addition of nelarabine to multiagent chemotherapy was safe and tolerable. Myelosuppression was universal, but somewhat paradoxically, there were significantly less neutropenic infections (42% vs. 81%, p = 0.005) in patients who received nelarabine. However, there were a significantly higher percentage of neurologic adverse events (25% vs. 4%, p = 0.02) in patients who received nelarabine. Grade 3 or 4 peripheral neuropathy occurred in 15% of patients receiving nelarabine compared to 0% who did not receive the drug.
This pilot study (AALL00P2) was followed by a larger, randomized phase III study by COG (AALL0434) investigating the safety and efficacy of adding nelarabine to an augmented-BFM backbone in children with higher risk T-ALL.41 Since the augmented-BFM backbone includes more intensive intrathecal therapy and more frequent vincristine administration, a lead-in phase of 94 patients was required establish safety. Results from this lead-in phase were recently reported.41 All patients received the same induction therapy and high risk patients underwent 2 randomizations at the time of consolidation. Patients were randomized to high-dose (HD) methotrexate (MTX) vs. Capizzi MTX + asparaginase and also randomized between nelarabine (650 mg/m2/d for 5 days) vs. no nelarabine – yielding 4 cohorts of patients. In the nelarabine arms, the nelarabine was incorporated as 6 total cycles intercalated during consolidation, delayed intensification, and maintenance, but not simultaneously with other chemotherapeutic agents. In this safety analysis, the authors found no difference in the rate of sensory, motor, or central neurotoxicities between patients who did and did not receive nelarabine. Longer follow-up to document efficacy in longer term outcomes continues.
An additional study reported the MD Anderson experience of adding nelarabine to multiagent chemotherapy in adults with previously untreated T-ALL/LBL.42 The study used the hyper-CVAD backbone which is composed of 8 alternating cycles of multiagent chemotherapy and 8 doses of prophylactic intrathecal chemotherapy followed by 30 months of maintenance.43–45 In this prospective phase II study in 40 patients with a median age of 38 years, the nelarabine cycles were intercalated among the 8 cycles of intensive chemotherapy and during 2 of the months of maintenance. For this study, nelarabine was administered at a dose of 650 mg/m2/d x 5 days on a 3 to 4-week cycle. Two cycles of nelarabine were given between cycles 4 and 5 of hyper-CVAD and 2 more cycles were given during months 6 and 7 of maintenance. The overall response rate with the combination was 97%, with 91% of patients achieving a CR; 47% of the patients achieving a CR were MRD negative. The 3-year disease-free survival was 65% and the 3-year OS probability was 65%. The addition of nelarabine to hyper-CVAD was feasible. Myelosuppression was universal and expected and ≥ grade 3 infections occurred in 87% of patients. Neurological adverse events were observed, but there were no grade 3 or 4 neurologic adverse events attributed to the nelarabine. Grade 1 or 2 peripheral neuropathy occurred in 55% of patients and other grade 1 or 2 neurologic adverse events (including muscle weakness, altered consciousness, tremors, memory impairment) occurred in 20 % of patients. The study determined that the regimen was safe and effective for adults with newly diagnosed T-ALL/LBL. However, given the timing of the nelarabine later in the course of therapy, its contribution to CR rate is not clear, although it may contribute to more durable responses. The optimal timing and schedule with multiagent chemotherapy remains to be determined. Earlier or concomitant introduction of nelarabine as well as treatment of MRD is being tested.
Nelarabine is also being tested in combination in the salvage setting, harnessing potential synergy with other active agents in ALL. In one pilot study, investigators combined nelarabine with etoposide and cyclophosphamide (EC) in patients with R/R T-ALL.46 Seven patients, ranging from 2 to 19 years of age, were treated on a sequential schedule of nelarabine and EC. One patient was treated with concomitant nelarabine and EC, but had unacceptable toxicity. Two patient were treated with nelarabine followed by EC and 2 others were treated with EC followed by nelarabine. Nelarabine was administered at the dose of 650 mg/m2/d for 5 days, etoposide was given at 100 mg/m2/d x 5 combined with cyclophosphamide 440 mg/m2/d x 5. The overall response rate in this heavily pretreated population was 100%, with 5 of 7 (71%) patients achieving a CR. Grade 3 or higher hematologic toxicity was universal and 4 patients had grade 3 febrile neutropenia. Six of the 7 patients (86%) had some neurotoxicity, including grade 2 to 3 sensory/motor neuropathy in 4 of the 7 (57%) patients.
5.0 Nelarabine Continuous Infusion
While there is significant potential for nelarabine-based combinations for patients with R/R T-ALL, overall significant neurotoxicity limits its robust development. Modifying the dose and schedule of nelarabine may affect the toxicity profile and could expand the therapeutic window. Clinical experience with the nucleoside analogue such as cytarabine may provide some leads. High-dose cytarabine given as a short or bolus infusion has been associated with significant neurotoxicity, including seizures, somnolence, and coma.47 When the infusion time was prolonged to continuous infusion over 24 hours, there was a significant decrease in neurotoxicity, with a change in toxicity profile. The new infusional schedule was associated with more myelosuppression and gastrointestinal toxicity.48–50 A similar approach has been taken with nelarabine for patients with R/R T-ALL/LBL. A phase I dose-escalation trial studied the safety and efficacy of administering nelarabine over a 24-hour continuous infusion for 5 days, rather than the standard, short-infusion approach.51 Twenty-three patients, including 21 with R/R T-ALL were treated with escalating doses of nelarabine from 100 to 700 mg/m2/d x 5 days by continuous infusion. The median age of the patients was 38 years (range, 14 to 77 years) and they had received a median of 2 prior therapies, some having previously received nelarabine. Given their disease and prior treatment, all patients had myelosuppression. Peripheral neuropathy was observed in 22% of patients, including one ≥ grade 3 event related to nelarabine. Muscle weakness was noted in 9% of patients with one ≥ grade 3 event attributed to nelarabine. Notably, there was no central neurotoxicity observed on study, including seizures, somnolence, or coma. The MTD was not reached. The overall response rate was 30%, including 22% CR/CRi (CR with incomplete blood count recovery). The overall response rates by salvage status were 30%, 29%, and 33% for patients in 1st salvage, 2nd salvage, and > 2nd salvage, respectively. The ORR by immunophenotype were 20%, 50%, and 50% for patients with early T-precursor, thymic, and mature histologies, respectively. This study is ongoing, however, these early data suggest that this promising approach could broaden the use of nelarabine and mitigate some of its toxicities.
6.0 Conclusions
In a disease like T-ALL, where new drug development has been limited, the discovery and approval of nelarabine has been an important and fundamental step to improve patient outcomes. Nelarabine has exhibited T-cell specificity, is highly active in a relapsed and refractory population, and has a toxicity profile that makes it amenable to combination therapy with other agents. Two schedules, including the 5-day pediatric dosing or the thrice weekly adult dosing have been developed and widely used with success. Neurotoxicity is dose-limiting and had initially curbed the enthusiasm of developing robust combination strategies. However, newer schedules such as continuous infusion and newer, rational combinations have demonstrated better tolerability and significant clinical activity.
7.0 Expert Commentary
A better understanding of the purine metabolism pathway and its effect on T-cells in patients with PNP deficiency prompted the development of a nucleoside analogue to naturally occurring guanosine. 9-β-D-Arabinofuranosylguanine (ara-G) is resistant to cleavage by cellular PNP and thereby mimics the toxic intracellular accumulation of the dGTP in T-cells. Metabolically ara-G is phosphorylated by intracellular nucleoside kinases preferentially in T-cells24 or in indolent CLL cells52 and mechanistically, the triphosphate of ara-G is incorporated into DNA, leading to apoptosis in rapidly cycling cells.15,53 The creation of a water soluble prodrug of ara-G, nelarabine, allowed its clinical development through phase I and II trials, each with carefully conducted correlative studies to better define its metabolism and kinetics. These studies demonstrated the rapid conversion of prodrug nelarabine to ara-G within the serum, the relationship between intracellular ara-GTP accumulation and cytotoxicity, and hints on how to optimize drug combinations. These foundational studies led to the approval of nelarabine for relapsed and refractory T-ALL and T-LBL, but much work needs to be done. Optimizing dose and schedule to widen the therapeutic window will be important. Moving the drug earlier into the T-ALL treatment paradigm to treat higher risk patients or those with resistant disease may produce higher response rates and more durable remissions. Similarly, the use of nelarabine for salvage therapy in the post-transplant setting may also have promise and should be studied further.54 Rational combination strategies using agents with non-overlapping toxicities and increased synergy will be key to improving salvage options for T-ALL and extending the use of nelarabine beyond just a single-agent option. The use of nelarabine combinations in other T-cell malignancies such as T-cell lymphoma and mature T-cell lymphoproliferative disorders (e.g. T-prolymphocytic leukemia, T-large granular lymphocyte leukemia) should be explored, along with novel combinations such as asparaginase, NOTCH inhibitors, and monoclonal antibodies.
8.0 Five-Year View
While nelarabine prodrug and its metabolite ara-G are nucleoside analogues, the selectivity of these drugs to leukemia and lymphoma subtypes make them more akin with targeted agents rather than cytotoxic chemotherapies. The primary limitation for the development and broad use of nelarabine in T-cell and indolent leukemias is neurotoxicity. Identification of causal events for this untoward toxicity is a primary requirement for sustainability in the use of this agent. Additional studies with continuous infusion may define optimal usage of the drug for maximal mitigation of neurological events. Finally, toxicity with other agents will continue to be explored in the next few years.
Key Issues.
Nelarabine is a rationally designed nucleoside analogue that is targeted to specific subtypes of hematological malignancies.
Consequential decline in normal T-cells in pediatric patients with PNP deficiency, accumulation of plasma dGuo and cellular dGTP selectively in T-cells were the key observations for genesis of ara-G and nelarabine.
Nelarabine serves as an optimal prodrug and gets converted effectively and efficiently to ara-G. Cellular ara-GTP accumulation was specific to selective subtypes of leukemia.
Phase I and phase II studies in adult patients with T-cell ALL clearly demonstrated activity of nelarabine. Similar observations were mimicked in pediatric population with this subtype of leukemia. Nelarabine was approved for both populations with relapsed/refractory T-cell leukemia and lymphoma.
In addition to T-cell diseases, nelarabine had activity in indolent leukemia, such as CLL.
Neurological toxicities have dampened a rigorous development of this agent, however, newer infusion strategies along with combination approaches are being evaluated.
Nelarabine is also being incorporated into frontline combination regimens to improve outcomes in high-risk subtypes of T-ALL.
Acknowledgments
Funding
This paper has been supported in part by the National Institutes of Health/National Cancer Institute under award number P30 CA016672.
TM Kadia and V Gandhi have received financial support as a Sponsored Research Agreement from GlaxoSmithKline.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
*Article of interest
**Article of considerable interest
- 1.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016 doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maury S, Chevret S, Thomas X, et al. Addition of Rituximab Improves the Outcome of Adult Patients with CD20-Positive, Ph-Negative, B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL): Results of the Randomized Graall-R 2005 Study. Blood. 2015;126(23):1–1. [Google Scholar]
- 3.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 4.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Giblett ER, Ammann AJ, Wara DW, Sandman R, Diamond LK. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. Initial observation in pediatric patients that deficiency of PNP enzyme leads to loss of T and B cells. [DOI] [PubMed] [Google Scholar]
- 8.Markert ML. Purine nucleoside phosphorylase deficiency. Immunodefic Rev. 1991;3(1):45–81. [PubMed] [Google Scholar]
- 9.Taddeo A, Fairbanks LD, Simmonds HA, Duley JA, Morris GS. Deoxy GTP accumulates in thymocytes, but not in T or B lymphocytes in simulated PNP deficiency. Adv Exp Med Biol. 1989;253B:275–280. doi: 10.1007/978-1-4684-5676-9_40. [DOI] [PubMed] [Google Scholar]
- 10*.Mitchell BS, Mejias E, Daddona PE, Kelley WN. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. Selective accumulation of deoxyguanosine triphosphate in T-cells specified its activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson DA, Wasson DB, Lakow E, Kamatani N. Possible metabolic basis for the different immunodeficient states associated with genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase. Proc Natl Acad Sci U S A. 1982;79(12):3848–3852. doi: 10.1073/pnas.79.12.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shewach DS, Mitchell BS. Differential metabolism of 9-beta-D-arabinofuranosylguanine in human leukemic cells. Cancer Res. 1989;49(23):6498–6502. [PubMed] [Google Scholar]
- 13.Ullman B, Martin DW., Jr Specific cytotoxicity of arabinosylguanine toward cultured T lymphoblasts. J Clin Invest. 1984;74(3):951–955. doi: 10.1172/JCI111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen A, Lee JW, Gelfand EW. Selective toxicity of deoxyguanosine and arabinosyl guanine for T-leukemic cells. Blood. 1983;61(4):660–666. [PubMed] [Google Scholar]
- 15**.Rodriguez CO, Jr, Stellrecht CM, Gandhi V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood. 2003;102(5):1842–1848. doi: 10.1182/blood-2003-01-0317. A systematic evaluation of ara-G in T-, B-, and myeloid cell lines to demosntrate selectivity of ara-G in T-cell lines. [DOI] [PubMed] [Google Scholar]
- 16**.Lambe CU, Averett DR, Paff MT, Reardon JE, Wilson JG, Krenitsky TA. 2-Amino-6-methoxypurine arabinoside: an agent for T-cell malignancies. Cancer Res. 1995;55(15):3352–3356. Synthesis of 2-amino-6-methoxypurine arabinoside (nelarabine) to serve as a prodrug for ara-G. This was water soluble and was eventually used in clinic. [PubMed] [Google Scholar]
- 17.Gandhi V, Keating MJ, Bate G, Kirkpatrick P. Nelarabine. Nat Rev Drug Discov. 2006;5(1):17–18. doi: 10.1038/nrd1933. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi V, Plunkett W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr Opin Oncol. 2006;18(6):584–590. doi: 10.1097/01.cco.0000245326.65152.af. [DOI] [PubMed] [Google Scholar]
- 19.Ravandi F, Gandhi V. Novel purine nucleoside analogues for T-cell-lineage acute lymphoblastic leukaemia and lymphoma. Expert Opin Investig Drugs. 2006;15(12):1601–1613. doi: 10.1517/13543784.15.12.1601. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez CO, Gandhi V. Nelarabine: A new drug for hematological malignancies. Haematologica. 2001;86(Suppl 1–11):43–47. [Google Scholar]
- 21.Buie LW, Epstein SS, Lindley CM. Nelarabine: a novel purine antimetabolite antineoplastic agent. Clin Ther. 2007;29(9):1887–1899. doi: 10.1016/j.clinthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Reilly KM, Kisor DF. Profile of nelarabine: use in the treatment of T-cell acute lymphoblastic leukemia. Onco Targets Ther. 2009;2:219–228. doi: 10.2147/ott.s4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanford M, Lyseng-Williamson KA. Nelarabine. Drugs. 2008;68(4):439–447. doi: 10.2165/00003495-200868040-00004. [DOI] [PubMed] [Google Scholar]
- 24**.Gandhi V, Plunkett W, Rodriguez CO, Jr, et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacokinetics and clinical response. J Clin Oncol. 1998;16(11):3607–3615. doi: 10.1200/JCO.1998.16.11.3607. First publication of plasma and cellualr pharmacokinetics showing a strong relationship between cellular acculumation of triphosphate and clinical responses. [DOI] [PubMed] [Google Scholar]
- 25**.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23(15):3396–3403. doi: 10.1200/JCO.2005.03.199. Clinical responses, toxicity, and other data from the first phase I study of nelarabine in both pediatric and adult patietns with hematological malignancies. [DOI] [PubMed] [Google Scholar]
- 26.Aguayo A, Cortes JE, Kantarjian HM, et al. Complete hematologic and cytogenetic response to 2-amino-9-beta-D-arabinosyl-6-methoxy-9H-guanine in a patient with chronic myelogenous leukemia in T-cell blastic phase: a case report and review of the literature. Cancer. 1999;85(1):58–64. [PubMed] [Google Scholar]
- 27*.Kisor DF, Plunkett W, Kurtzberg J, et al. Pharmacokinetics of nelarabine and 9-beta-D-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18(5):995–1003. doi: 10.1200/JCO.2000.18.5.995. Detailed evaluation of nelarabine and ara-G plasma pharmacokinetics. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez CO, Jr, Legha JK, Estey E, Keating MJ, Gandhi V. Pharmacological and biochemical strategies to increase the accumulation of arabinofuranosylguanine triphosphatein primary human leukemia cells. Clin Cancer Res. 1997;3(11):2107–2113. [PubMed] [Google Scholar]
- 29.Estey E, Thall P, Andreeff M, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994;12(4):671–678. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11(1):116–124. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 31.Seymour JF, Huang P, Plunkett W, Gandhi V. Influence of fludarabine on pharmacokinetics and pharmacodynamics of cytarabine: implications for a continuous infusion schedule. Clin Cancer Res. 1996;2(4):653–658. [PubMed] [Google Scholar]
- 32.Rodriguez CO, Jr, Mitchell BS, Ayres M, Eriksson S, Gandhi V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002;62(11):3100–3105. [PubMed] [Google Scholar]
- 33*.Gandhi V, Plunkett W, Weller S, et al. Evaluation of the combination of nelarabine and fludarabine in leukemias: clinical response, pharmacokinetics, and pharmacodynamics in leukemia cells. J Clin Oncol. 2001;19(8):2142–2152. doi: 10.1200/JCO.2001.19.8.2142. Clinical data and plasma and cellular pharmacokinetics of combination of nelarabine and fludarabine. [DOI] [PubMed] [Google Scholar]
- 34**.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol. 2005;23(15):3376–3382. doi: 10.1200/JCO.2005.03.426. Clinical responses, toxicity, and other data from the phase II study of nelarabine in pediatric patients with T-cell malignancies. These data were included in the FDA approval package. [DOI] [PubMed] [Google Scholar]
- 35**.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109(12):5136–5142. doi: 10.1182/blood-2006-11-056754. Clinical responses, toxicity, and other data from the phase II study of nelarabine in adult patients with T-cell malignancies. These data were included in the FDA approval package. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Cohen MH, Johnson JR, Justice R, Pazdur R. FDA drug approval summary: nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008;13(6):709–714. doi: 10.1634/theoncologist.2006-0017. This paper summarizes all data and describes FDA approval of nelarabine. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MH, Johnson JR, Massie T, et al. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/lymphoma. Clin Cancer Res. 2006;12(18):5329–5335. doi: 10.1158/1078-0432.CCR-06-0606. [DOI] [PubMed] [Google Scholar]
- 38**.Gokbuget N, Basara N, Baurmann H, et al. High single-drug activity of nelarabine in relapsed T-lymphoblastic leukemia/lymphoma offers curative option with subsequent stem cell transplantation. Blood. 2011;118(13):3504–3511. doi: 10.1182/blood-2011-01-329441. Major clinical study outside of the United States that describes clinical responses and toxicity. [DOI] [PubMed] [Google Scholar]
- 39.Czuczman MS, Porcu P, Johnson J, et al. Results of a phase II study of 506U78 in cutaneous T-cell lymphoma and peripheral T-cell lymphoma: CALGB 59901. Leuk Lymphoma. 2007;48(1):97–103. doi: 10.1080/10428190600961058. [DOI] [PubMed] [Google Scholar]
- 40.Dunsmore KP, Devidas M, Linda SB, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(22):2753–2759. doi: 10.1200/JCO.2011.40.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62(7):1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain P, Kantarjian H, Ravandi F, et al. The combination of hyper-CVAD plus nelarabine as frontline therapy in adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma: MD Anderson Cancer Center experience. Leukemia. 2014;28(4):973–975. doi: 10.1038/leu.2013.312. [DOI] [PubMed] [Google Scholar]
- 43.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 44.Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104(6):1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 45.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Commander LA, Seif AE, Insogna IG, Rheingold SR. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol. 2010;150(3):345–351. doi: 10.1111/j.1365-2141.2010.08236.x. [DOI] [PubMed] [Google Scholar]
- 47.Baker WJ, Royer GL, Jr, Weiss RB. Cytarabine and neurologic toxicity. J Clin Oncol. 1991;9(4):679–693. doi: 10.1200/JCO.1991.9.4.679. [DOI] [PubMed] [Google Scholar]
- 48.Capizzi RL. Curative chemotherapy for acute myeloid leukemia: the development of high-dose ara-C from the laboratory to bedside. Invest New Drugs. 1996;14(3):249–256. doi: 10.1007/BF00194527. [DOI] [PubMed] [Google Scholar]
- 49.Donehower RC, Karp JE, Burke PJ. Pharmacology and toxicity of high-dose cytarabine by 72-hour continuous infusion. Cancer Treat Rep. 1986;70(9):1059–1065. [PubMed] [Google Scholar]
- 50.Ochs J, Sinkule JA, Danks MK, Look AT, Bowman WP, Rivera G. Continuous infusion high-dose cytosine arabinoside in refractory childhood leukemia. J Clin Oncol. 1984;2(10):1092–1097. doi: 10.1200/JCO.1984.2.10.1092. [DOI] [PubMed] [Google Scholar]
- 51*.Kadia T, Gandhi V, Thomas DA, et al. Phase I Study of Continuous-Infusion Nelarabine in Patients with Advanced Lymphoid Malignancies. Blood. 2011;118(21):4239–4239. Data from first clinical evaluation of continuous dose of nelarabine. [Google Scholar]
- 52.Gandhi V, Tam C, O’Brien S, et al. Phase I trial of nelarabine in indolent leukemias. J Clin Oncol. 2008;26(7):1098–1105. doi: 10.1200/JCO.2007.14.1986. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez CO, Jr, Gandhi V. Arabinosylguanine-induced apoptosis of T-lymphoblastic cells: incorporation into DNA is a necessary step. Cancer Res. 1999;59(19):4937–4943. [PubMed] [Google Scholar]
- 54.Forcade E, Leguay T, Vey N, et al. Nelarabine for T cell acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation: an opportunity to improve survival. Biol Blood Marrow Transplant. 2013;19(7):1124–1126. doi: 10.1016/j.bbmt.2013.04.010. [DOI] [PubMed] [Google Scholar]


