Abstract
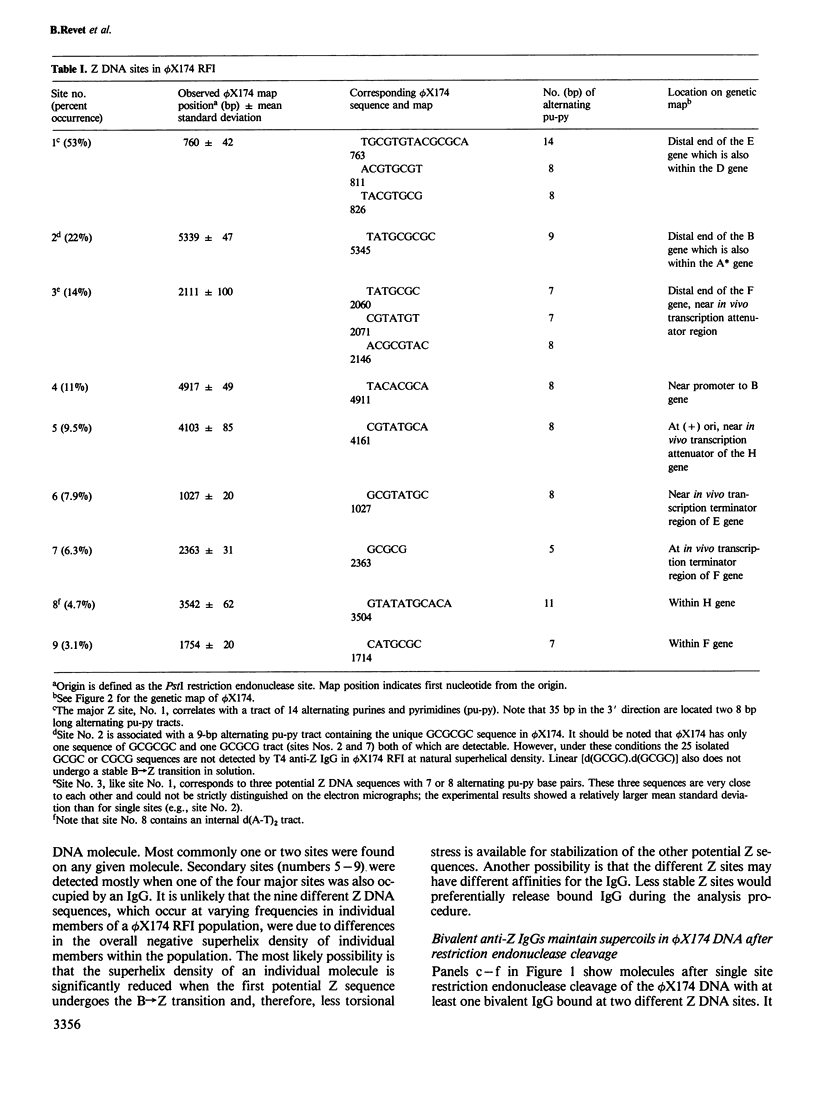
The specific interaction between left-handed Z DNA sequences in negatively supercoiled bacteriophage phi X174 replicative form I (RFI) DNA and anti-Z DNA immunoglobulin G (IgG) was investigated by high resolution darkfield immuno-electron microscopy. DNA-antibody complexes were formed and maintained under optimal binding conditions, purified by column chromatography, and visualized after uranyl acetate staining without using aldehyde fixation, shadowing, or second antibody. Bivalent anti-Z DNA IgGs bound to RFI molecules, thus forming intramolecular bridges. They could also oligomerize separate molecules by intermolecular linking of Z DNA sequences. At relatively low ionic strength and low temperature, high affinity anti-Z IgG was retained at certain loci even after restriction endonuclease cleavage of the DNA. In these cleaved molecules some superhelices could be visualized in the loops generated by the bivalent IgG. To our knowledge this is the first example of polypeptide stabilization of local superhelical strain in a cut molecule. Z DNA sequences in phi X174 RFI DNA were mapped. Alternating tracts of purines and pyrimidines starting at nucleotides 763, 1027, 1714, 2146, 2363, 3504, 4161, 4911 and 5345 occur within the nine different anti-Z IgG binding sites which were expressed with varying frequencies (53-3%) on the molecules. Usually, a limited number of sites (generally less than or equal to 2) exists on any one molecule. The formation of multiple Z sites (at the extracted superhelix density) in a given molecule is probably non-cooperative due to relaxation of torsional stress by the B----Z transition. Z sites occur in several different genes, including regions where transcription is attenuated and, in one case, in front of a promoter of transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Castleman H., Erlanger B. F. Electron microscopy of "Z-DNA". Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):133–142. doi: 10.1101/sqb.1983.047.01.018. [DOI] [PubMed] [Google Scholar]
- Di Capua E., Stasiak A., Koller T., Brahms S., Thomae R., Pohl F. M. Torsional stress induces left-handed helical stretches in DNA of natural base sequence: circular dichroism and antibody binding. EMBO J. 1983;2(9):1531–1535. doi: 10.1002/j.1460-2075.1983.tb01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. The in-vivo occurrence of Z DNA. J Biomol Struct Dyn. 1983 Dec;1(3):593–609. doi: 10.1080/07391102.1983.10507467. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Fujimura F. K., Hayashi M. Mapping of in vivo messenger RNAs for bacteriophage phiX-174. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3519–3523. doi: 10.1073/pnas.73.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Wei C. F., Gray H. B., Jr, Wells R. D. BAL 31 nuclease as a probe in concentrated salt for the B-Z DNA junction. Nucleic Acids Res. 1983 Jun 11;11(11):3811–3822. doi: 10.1093/nar/11.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevan L., Schumaker V. N. Stabilization of Z-DNA by polyarginine near physiological ionic strength. Nucleic Acids Res. 1982 Nov 11;10(21):6809–6817. doi: 10.1093/nar/10.21.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M. C., Malfoy B., Freund A. M., Daune M., Leng M. Visualization of Z sequences in form V of pBR322 by immuno-electron microscopy. EMBO J. 1982;1(10):1149–1153. doi: 10.1002/j.1460-2075.1982.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Jorgenson K. F., Winkfein R. J., van de Sande J. H., Zarling D. A., Stockton J., Rattner J. B. Natural occurrence of left-handed (Z) regions in PM2 DNA. J Biomol Struct Dyn. 1983 Dec;1(3):611–620. doi: 10.1080/07391102.1983.10507468. [DOI] [PubMed] [Google Scholar]
- Nahon-Merlin E., Delain E., Coulaud D., Lacour F. Electron microscopy of the reactions of anti-poly A. poly U and anti-poly I. poly C antibodies with synthetic polynucleotide complexes and natural nucleic acids. Nucleic Acids Res. 1980 Apr 25;8(8):1805–1822. doi: 10.1093/nar/8.8.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., DiCapua E. Antibodies to Z-DNA interact with form V DNA. Nature. 1982 Dec 9;300(5892):545–546. doi: 10.1038/300545a0. [DOI] [PubMed] [Google Scholar]
- Revet B., Delain E., Dante R., Niveleau A. Three dimensional association of double-stranded helices are produced in conditions for Z-DNA formation. J Biomol Struct Dyn. 1983 Dec;1(4):857–871. doi: 10.1080/07391102.1983.10507489. [DOI] [PubMed] [Google Scholar]
- Revet B., Delain E. The drosophila X virus contains a 1-microM double-stranded RNA circularized by a 67-kd terminal protein: high-resolution denaturation mapping of its genome. Virology. 1982 Nov;123(1):29–44. doi: 10.1016/0042-6822(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Wells R. D. Conformational flexibility of junctions between contiguous B- and Z-DNAs in supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 May;80(9):2447–2451. doi: 10.1073/pnas.80.9.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. H., Sinsheimer R. L. The in vitro transcription units of bacteriophage phiX174. III. Initiation with specific 5' end oligonucleotides of in vitro phiX174 RNA. J Mol Biol. 1976 Jun 5;103(4):711–735. doi: 10.1016/0022-2836(76)90205-9. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Kłysik J., Wells R. D. Energetic and structural inter-relationship between DNA supercoiling and the right- to left-handed Z helix transitions in recombinant plasmids. J Biol Chem. 1982 Sep 10;257(17):10159–10165. [PubMed] [Google Scholar]
- Stockton J. F., Miller F. D., Jorgenson K. F., Zarling D. A., Morgan A. R., Rattner J. B., van de Sande J. H. Left-handed Z-DNA regions are present in negatively supercoiled bacteriophage PM2 DNA. EMBO J. 1983;2(12):2123–2128. doi: 10.1002/j.1460-2075.1983.tb01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Avoort H. G., Teertstra R., Versteeg R., Weisbeek P. J. Genes and regulatory sequences of bacteriophage phi X174. Biochim Biophys Acta. 1983 Oct 13;741(1):94–102. doi: 10.1016/0167-4781(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Arndt-Jovin D. J., McIntosh L. P., Robert-Nicoud M., Jovin T. M. Interactions of anti-poly[d(G-br5C)] IgG with synthetic, viral and cellular Z DNAs. J Biomol Struct Dyn. 1984 Mar;1(5):1081–1107. doi: 10.1080/07391102.1984.10507506. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Arndt-Jovin D. J., Robert-Nicoud M., McIntosh L. P., Thomae R., Jovin T. M. Immunoglobulin recognition of synthetic and natural left-handed Z DNA conformations and sequences. J Mol Biol. 1984 Jul 5;176(3):369–415. doi: 10.1016/0022-2836(84)90495-9. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



