Abstract
The dermal glands of many amphibian species secrete toxins or other noxious substances as a defense strategy against natural enemies. Newts in particular possess the potent neurotoxin tetrodotoxin (TTX), for which the highest concentrations are found in species within the genus Taricha. Adult Taricha are hypothesized to use TTX as a chemical defense against vertebrate predators such as garter snakes (Thamnophis spp.). However, less is known about how TTX functions to defend aquatic-developing newt larvae against natural enemies, including trematode parasites and aquatic macroinvertebrates. Here we experimentally investigated the effects of exogenous TTX exposure on survivorship of the infectious stages (cercariae) of five species of trematode parasites that infect larval amphibians. Specifically, we used dose-response curves to test the sensitivity of trematode cercariae to progressively increasing concentrations of TTX (0.0 [control], 0.63, 3.13, 6.26, 31.32, and 62.64 nmol L−1) and how this differed among parasite species. We further compared these results to the effects of TTX exposure (0 and 1,000 nmolL−1) over 24 hr on seven macroinvertebrate taxa commonly found in aquatic habitats with newt larvae. TTX significantly reduced the survivorship of trematode cercariae for all species, but the magnitude of such effects varied among species. Ribeiroia ondatrae – which causes mortality and limb malformations in amphibians – was the least sensitive to TTX, whereas the kidney-encysting Echinostoma trivolvis was the most sensitive. Among the macroinvertebrate taxa, only mayflies (Ephemeroptera) showed a significant increase in mortality following exogenous TTX exposure, despite the use of a concentration 16x higher than the maximum used for trematodes. Our results suggest that maternal investment of TTX into larval newts may provide protection against certain trematode infections and highlight the importance of future work assessing the effects of newt toxicity on both parasite infection success and the palatability of larval newts to invertebrate predators.
Keywords: tetrodotoxin, animal toxicity, natural enemy ecology, infectious disease, amphibian decline
1. Introduction
Tetrodotoxin (TTX) is a naturally occurring neurotoxin found in at least 140 animal species spanning a broad range of taxa (Lorentz et al., 2016). Flatworms (genus Planocera), the eggs of horseshoe crabs (genus Limulus), pufferfish (genus Fugu), xanthid crabs (genus Xantho), blue-ringed octopi (genus Hapalochlaena), and newts (family Salamandridae) are all known to possess TTX (Brodie III and Brodie Jr, 1990; Geffeney et al., 2005; Hanifin, 2010; Lorentz et al., 2016; Miyazawa et al., 1986; Noguchi et al., 1984; Ritson-Williams et al., 2006), which is recognized as an exceptionally deadly poison to many vertebrates (Guzmán et al., 2007). Among the 12 species of newts in the family Salamandridae known to possess TTX (Hanifin, 2010), the highest average dermal concentrations (25 µg cm2 −1) occur in members of the genus Taricha (Wakely et al., 1966). Concentrations in other genera such as Notophthalmus and Cynops often range from 1 –4 µg TTX g−1, while only trace amounts have been detected in Triturus (Wakely et al., 1966; Yotsu et al., 1990). Adult Taricha are hypothesized to use TTX as a chemical defense against predators, including birds, fishes, and garter snakes (Brodie III et al., 2005; Brodie Jr, 1968; Farner and Kezer, 1953; Storm, 1948). Predation on newts has often been studied as part of a coevolutionary framework positing that adult Taricha have evolved greater toxicity in response to predation by garter snakes, which can develop genetic resistance to the toxin (Brodie III et al., 2005; Brodie III and Brodie Jr, 1999, 1990; Toledo et al., 2016; Williams et al., 2003).
Considerably less is known about the role of TTX in defending larval newts against natural enemies, including macroinvertebrate predators, competitors, and infectious parasites. Although all newts appear to possess TTX, it is not clear how they produce the neurotoxin. Specifically, it may be endogenously controlled through a biosynthetic pathway or the result of bacterial symbionts. As a result, it is unclear how newts evolved TTX, leaving open many questions about its evolutionary origins and potential ecological roles in ecosystems. While TTX concentrations tend to be highest in adult newts (Wakely et al., 1966), maternal investment of the toxin into eggs is hypothesized to help defend embryos and larvae against enemy attack during aquatic development (Gall et al., 2011; Hanifin, 2010). In experimental studies, Taricha embroys and larvae were relatively unpalatable to dragonfly nymphs (Gall et al., 2010) and experimental expose to TTX from adult newts impaired their feeding behavior (Bucciarelli and Kats, 2015). However, other aquatic invertebrate predators such as caddisfly larvae show remarkably high tolerance to TTX (Gall et al., 2012).
Whether TTX in the skin of larval newts affects their susceptibility to water-borne parasites, such as trematodes, remains an unexplored question. Adult newts are known to support a diversity of parasites, including nematodes (Rhabdias tarichae, Cosmocercoides variabilis, Megalobatrachonemea moraveci, Hedruris siredonis), protozoans (Eimeria tarichae, Tritrichomonas sp., T. augusta, Hexamita ovatus, Karotomorpha swezi, Trypanosoma ambystomae, and T. granulosa), trematodes (Ribeiroia ondatrae, Clinostomum sp., Megalodiscus microphagus, M. americanus, Brachycoelium salamandrae, and Glypthelmins sp.), and an acanthocephalan (Neoechinorhynchus sp.) (Bolek, 1997; Goldberg et al., 1998; Johnson et al., 2013, 2017; Johnson and Hoverman, 2012; Kuzmin et al., 2003; Lehmann, 1954; Macy, 1960; Parkinson, 2010; Richardson and Adamson, 1988; Vanderburgh and Anderson, 1987). In a recent study of macro- and microparasites in adult T. granulosa and T. torosa, Johnson et al. (personal communication) reported a negative correlation between host TTX concentration and both the presence of several microparasites and the total load of macroparasites. Encysted trematodes in the skin were notably rare, despite the fact that larval newts are exposed to many different trematodes (e.g., Caffara et al., 2014; Etges, 1961; Miller et al., 2004; Owen, 1946). Because water-borne trematode larvae (cercariae) often penetrate the skin of larval amphibians and encyst within host tissues, they may be exposed to higher concentrations of TTX relative to parasites within the gastrointestinal tract, where toxicity levels are relatively lower. However, little is known about the direct sensitivity of parasites exposed to exogenous TTX.
In this study, we experimentally tested how exposure to waterborne-TTX affected the survivorship of larval trematodes and aquatic macroinvertebrates. Specifically, we compared the sensitivity of cercariae representing five trematode species known to infect larval amphibians to progressively increasing concentrations of TTX. We then compared these results with the effects of TTX exposure on seven common macroinvertebrate taxa found in aquatic ecosystems. This work has implications for understanding the degree to which TTX provides protection from parasites generally and especially virulent trematodes, such as the trematode R. ondatrae, which causes limb malformations and elevated mortality in many amphibians (Johnson et al., 2012). Our results also underscore the importance of future work to assess the effects of TTX on parasite infection success in vitro and on the palatability of larval newts to invertebrate predators.
2. Materials and Methods
2.1 Trematode bioassays
Between May and August of 2015, we collected freshwater snails (Helisoma trivolvis and Physa spp.) using dip-nets (45.7 cm D-frame with 1.2 mm mesh), seines (1.2 × 1.8 m), or by hand from pond ecosystems in California and Oregon (California: Alameda, Contra Costa, and Santa Clara counties; Oregon: Multnomah and Washington counties). These snails function as the first intermediate hosts for many trematode parasites known to infect pond-breeding amphibians. Snails were screened for infection using methods described in Calhoun et al. (2015) and Paull et al. (2012) and identified using morphological features (Bray et al., 2008; Gibson et al., 2005; Johnson et al., 2004; Jones et al., 2005; Schell, 1985, 1970). Once identified, we housed snails in 720 ml plastic containers with dechlorinated, UV-sterilized, and carbon-filtered tapwater (hereafter referred to as ‘treated’ water). We replaced water every other day and fed snails ad libitum a mixture of Tetramin™, agar, and calcium. Air temperature within the environmental chambers ranged between 21 –23 °C.
To test the sensitivity of different trematode species to TTX, we collected cercariae by placing infected snails in 50 ml conical tubes filled with 40 ml of treated water (A. marcianae, E. trivolvis, M. syntomentera, R. ondatrae, and Cephalogonimus sp.). Cercariae for each species were collected within 4 hrs of release from snails to ensure viability. We purchased commercially pure TTX (Fisher Scientific, Acros Organics) and prepared following manufacturer’s instructions to rehydrate to 1 mg ml−1. To begin, we made a stock solution of TTX at 10 µg ml−1, which was used to complete five serial dilutions (0.0, 0.63, 3.13, 6.26, 31.2, and 62.64 TTX nmol L−1). Finally, we prepared individual wells on sterile, 96-well plates for trials by adding 0.1 µg ml−1 of TTX prepared at various concentrations to 0.4 ml of treated water achieving our final doses. One µl of treated water was used as the control solution. We pipetted individual cercariae into each well with a minimal transfer of water (<0.1 µl) using an automatic 0.1 µl micropipette (Fisher Scientific, Eppendorf™) and no pipette contact to the well solution. Cercariae were collected from a single snail when possible, otherwise pooled from no more than three snails (Alaria marcianae [n = 140 cercariae], Echinostoma trivolvis [n =168 cercariae], Manodistomum syntomentera [n =144 cercariae], R. ondatrae [n = 474 cercariae], and Cephalogonimus sp. [n = 168 cercariae]). Each plate included replicates of all treatments to control for plate effects and contained only a single parasite species. Finally, we used alternating rows to limit the risk of cross-contamination of parasites or doses. Each treatment was replicated at least 24 times per parasite taxon. Once all cercariae were added, plates were covered to prevent evaporation and maintained at 22°C for 24 hrs or until all cercariae had died. We examined each well every 2 hrs using an Olympus SZX10 and recorded the status of the cercaria (alive and swimming, alive but not swimming, or dead). The typically lifespan of trematode cercariae is <24 hrs (Singh, 2015), such that our experiment sought to evaluate how different concentrations of TTX altered the survivorship of cercariae relative to the control treatment. Cercariae were considered dead when they failed to respond to a probe or when the tail detached from the body, as described previously (Calhoun et al., 2015; Paull et al., 2012; Reddy et al., 2004; Rohr et al., 2008).
2.2 Invertebrate bioassays
We collected macroinvertebrates from three adjacent streams in the Santa Monica Mountains (California: Los Angeles County) using either D-frame nets (30 cm wide) or by hand from the underside of rocks (Peck et al., 2006). We separated macroinvertebrates by order into Nalgene™ containers filled with 750 mL of stream water, transported them in a cooler to the laboratory, and housed them in acrylic containers (30 × 15 × 12 cm, l × w × d) until bioassays commenced (∼24 hrs). Specific invertebrate taxa included caddisflies (order Trichoptera, Lepidostoma sp.), dragonflies (order Odonata, Anax sp.), damselflies (order Odonata, Enallagma sp.), hellgrammites (order Megaloptera, Corydalus sp.), mayflies (order Ephemeroptera, Paraleptophlebia sp.), stoneflies (order Plecoptera, Malenka sp.), and toe biters (order Hemiptera, Abetus sp.). Although we have high confidence that all macroinvertebrates within a genus were the same species, we are limited by the taxonomic resolution in some species that require adult sex organs to identify the species. Because some orders of macroinvertebrates in our experimental bioassays undergo hemimetabolism, we measured the sclerotized head capsule of each specimen in the orders of Ephemeroptera, Hemiptera, Odonata, and Plecoptera. We evaluated if measurements were all within one standard deviation of the mean for each order (genera for Odonata) to determine if development differed between specimens. Any individuals that exceeded one standard deviation were excluded. In our statistical analysis, we subdivided order Odonata into the suborders Zygoptera and Anisoptera. We fabricated individual chambers from translucent acrylic tubing and sheet material to house individual insect larva for the 24 hrs bioassay period. Chamber diameters ranged from 2–6 cm and were scaled to accommodate various-sized larvae. We rinsed all chambers with HCl and then molecular grade water prior to use. The TTX solution of 1,000 nmol TTX was mixed using 1 mg TTX standards (sodium citrate, Fisher Scientific) and 5-µm filtered, dechlorinated, tapwater. Filtered water was used as the control solution. We used an upper concentration of 1,000 nmol L−1, rather than the dose-response curve used for the cercariae assays, both because this is lethal to many invertebrates and vertebrates (Bucciarelli et al., 2016) and because we hypothesized that the invertebrates might have a higher tolerance of TTX given that they live a large part of their life cycle exposed to TTX in the water.
We performed bioassays in a climate-controlled, walk-in room where chambers were placed on a numbered matrix affixed to a workbench. Trichoptera (n = 65), Zygoptera (n = 38, Anisoptera (n = 38), Megaloptera (n =15), Ephemeroptera (n = 59), Plecoptera (n = 22), and Hemiptera (n = 38) were collected from their containers, assigned a random number, and placed in the corresponding pre-filled chamber. All chambers were filled with TTX solution or treated tapwater (control). We monitored invertebrates hourly for 24 hrs for movement of limbs or gills or signs of mortality following protocols outlined in Brown (1980), whereby we used a paintbrush tip to touch the water surface or the animal itself. If the macroinvertebrate was not moving or unresponsive to the stimulation, we tested whether it had died or was sublethally poisoned using a Doppler flow probe (Conforma DP-NSP1, Cook Medical, Vandergrift, PA) that monitored whether hemolymph was moving within the circulatory system. If alive, we returned the macroinvertebrate to the experiment and continued monitoring.
2.3 Statistical analyses
For the parasite data, we used Cox proportional hazards regression models to assess the effects of TTX concentration (a continuous variable), trematode species identity (a factor with five levels), and their interaction on survivorship (hours to death, measured in 2 hr intervals) (Fox and Weisberg, 2011). Parasites that survived the entire observational period (typically 24 hrs) were classified as ‘censored’ (although the majority of parasites died within this time frame, as expected for short-lived infectious stages (Paull et al., 2012). Because we detected a significant interaction between parasite species and TTX concentration, we subsequently subset the dataset by trematode species and evaluated the effects of TTX on each individually. The estimated coefficients and their standard errors from these analyses were used to compare sensitivity to TTX among parasites. Because cercariae typically die within 24 hr, using coefficients was preferable to metrics such as LC50s because we sought to quantify the effect of TTX on survival relative to the control treatment. For the macroinvertebrate data, we used a similar approach to analyze survivorship with the only difference being that individuals experienced only two dosages (0 and 1,000 nmol TTX L−1) and their status was assessed hourly. All analyses were conducted using the R statistical program (2012) with the function coxph in the package survival (Therneau and Lomley, 2017). For analyses of invertebrate survivorship, which sometimes exhibited the problem of monotone likelihood because no individuals died within the 24 hr period, we used coxphf to implement Cox regression with Firth’s penalized likelihood (Heinze and Ploner, 2016).
3. Results
In total, 974 cercariae representing five species of trematode were tested for their sensitivity to TTX exposure. Consistent with the short lifespan of these free-living stages 98.9% of all cercariae died over the course of the observation period, with censored cases (living more than 24 hrs) manifesting only for M. syntomentera (which exhibited high survivorship in many treatments, including 11 of 145 cercariae). Based on the overall analysis, we detected significant effects of TTX concentration, parasite identity, and their interaction on cercariae time-to-death (Log rank test = 562.2, df = 9, n = 974, P < 0.00001). TTX strongly reduced the survivorship of cercariae overall (TTX coefficient: 0.022156 ± 0.004152 SE, z = 5.336, P < 0.000001; Figure 1). The model including the interaction effect had a much lower Akaike Information Criterion (AIC) value, which is a measure of the relative quality of a model for which lower values are better (▲AIC: 66.54).
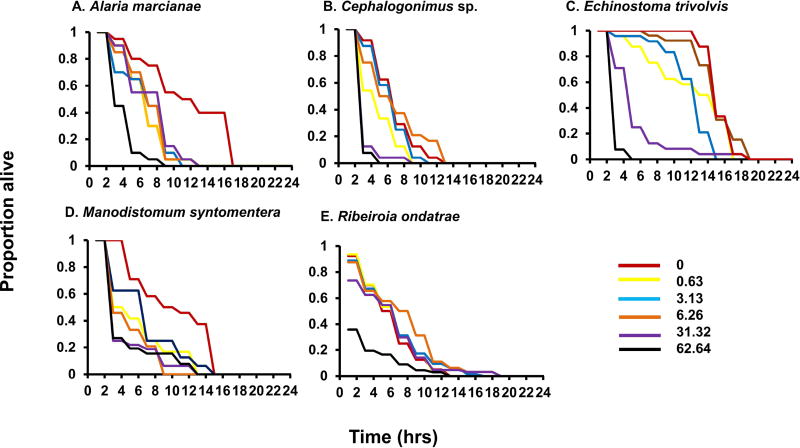
Figure 1.
Survivorship curves for cercariae of five trematode species exposed to increasing concentrations of tetrodotoxin (0.0, 0.63, 3.13, 6.26, 31.2, and 62.64 TTX nmol L−1) over 24 hrs. Presented is the proportion of surviving cercariae every 2 hrs throughout the exposure period. For sample sizes and statistical results, see Table 1.
After subsetting the dataset, each trematode species exhibited significantly elevated mortality in response to progressively higher TTX concentrations (range of R2: 0.086 [R. ondatrae] to 0.558 [E. trivolvis]; Table 1). At TTX concentrations of 31.32 nmol L−1, for instance, only 47% of cercariae survived longer than 2 hrs, relative to 82% in the control treatment. However, sensitivity varied considerably among trematode species (Figure 1). Echinostoma trivolvis exhibited the greatest increase in mortality associated with TTX exposure (coefficient: 0.066164 ± 0.006058 SE, z = 10.92, P < 0.0001), such that each 1.0 nmol L−1 increase of the toxin enhanced the risk of death by 6.6%. This was followed by Cephalogonimus sp. and A. marcianae, which had coefficients of 0.034850 ± 0.004483 SE (z = 7.773, P < 0.00001) and 0.022845 ± 0.004525 SE (z = 5.049, P < 0.00001), respectively. Manodistomum syntomentera and R. ondatrae exhibited the weakest responses to TTX (M. syntomentera TTX coefficient: 0.017673 ± 0.003664 SE, z = 4.823, P < 0.00001; R. ondatrae TTX coefficient: 0.015223 ± 0.002314 SE, z = 6.579, P < 0.00001) (Figure 2). It is important to note, however, that while cercariae mortality generally increased with TTX concentration, this effect was not always linear (Figure 1). For example, E. trivolvis average mortality at dose 3.13 nmol L−1 was 11 hrs whereas at 6.26 nmol L−1 it was 13.37 hrs. Such non-monotonic dose-response patterns are not uncommon in toxicological studies (Birnbaum, 2012; Conolly and Lutz, 2004; Vandenberg, 2014), particularly because of reactions in a complex biological system (Conolly and Lutz, 2004).
Table 1.
Survivorship results for freshwater trematode cercariae exposed exogenously to different concentrations of tetrodotoxin (TTX). For each trematode species, we present the number of cercariae included in trials, the encystment location of the next life stage within the amphibian hosts, and model statistics for the best-fitting Cox proportional hazards model, including R2 value and coefficient of TTX (value ± 1 SE).
| Trematode taxon | N (cercariae tested) |
Encystment location | R2 value | Coefficient of TTX ± 1 SE |
|---|---|---|---|---|
| Alaria marcianae | 120 | subcutaneous, mandible, tongue, and muscle | 0.17 | 0.022845 ± 0.004525 |
| Cephalogonimus sp. | 144 | subcutaneous and muscle | 0.312 | 0.034850 ± 0.004483 |
| Echinostoma trivolvis | 144 | kidneys | 0.558 | 0.066164 ± 0.006058 |
| Manodistomum syntomentera | 144 | subcutaneous | 0.134 | 0.017673 ± 0.003664 |
| Ribeiroia ondatrae | 422 | subcutaneous | 0.086 | 0.015223 ± 0.002314 |
Figure 2.
Results of Cox proportional hazard regression models to analyze the experimental effects of tetrodoxtoxin exposure on (A) five trematode species (n = 974) known to infect larval newts at increasing concentrations (0.0, 0.63, 3.13, 6.26, 31.2, and 62.64 TTX nmol L−1) over 24 hrs, and (B) seven macroinvertebrates taxa (n = 296) known inhabit similar habitats as larval aquatic newts at two concentrations (0 vs. 1000 TTX nmol L−1). Presented is the coefficient estimate ± 1 SE that characterizes the proportional increase in mortality associated with each 1.0-unit increase in TTX nmol L−1
Among the six orders of macroinvertebrates tested (n = 296 total individuals), we observed very little mortality over the 24 hr trials, regardless of TTX exposure. Only 21 Ephemeroptera (36%) and 2 Anisoptera (5 %) died over 24 hrs. Specifically, 1 mayfly died in the first hour, 11 died over the next 15 hrs, and 7 died in final 8 hours (for a total of 21). All invertebrates that died during testing were in the TTX exposure treatment, except one control Anisoptera. Ninety-two percent of tested invertebrates survived for 24 hrs. Based on the analysis, we detected a significant, negative effect of TTX (coefficient: 0.0026 ± 0.00087 SE, P = 0.000065) on survivorship with a main effect of invertebrate taxon (Table 2). More specifically, the effects of TTX were significant only in trials involving Ephemeroptera (coefficient: 0.0038 ± 0.0015 SE, P = 0.000014, n = 59) (Figure 2).
Table 2.
Survivorship results for freshwater macroinvertebrates exposed exogenously to tetrodotoxin (TTX). For each taxonomic order or suborder, we present the number of individuals included in trials, and the model statistics for the best-fitting model, including the coefficient of TTX (value ± 1 SE). Because several invertebrate groups experienced no mortality, we used Cox regression with Firth’s penalized likelihood.
| Macroinvertebrate taxon |
Common name | N (individuals tested) |
Coefficient of TTX ± 1 SE |
|---|---|---|---|
| Trichoptera | caddisflies | 64 | 0 ± 0 |
| Zygoptera | damselflies | 38 | 0 ± 0 |
| Megaloptera | hellgrammites | 15 | 0 ± 0 |
| Ephemeroptera | mayflies | 59 | 0.0038 ± 0.0015 |
| Plecoptera | stoneflies | 22 | 0 ± 0 |
| Hemiptera | toe biters | 38 | 0 ± 0 |
| Anisoptera | dragonflies | 38 | −0.00041 ± 0.0014 |
4. Discussion
Chemical forms of defense conventionally studied as anti-predator strategies have increasingly been recognized for their potential to limit or prevent infections by parasites ranging from tachinid flies to protozoans (Bos et al., 2015; de Roode et al., 2011; Huffman, 2003; Singer et al., 2009). For example, de Roode and colleagues (2008) found that consumption of milkweed-containing cardenolides by monarch caterpillars reduced the probability of subsequent infection by the apicomplexan parasite, Ophryocystis elektroscirrha. Infected monarchs have a shorter life span than uninfected individuals, suggesting that foraging on milkweed provides a positive fitness influence. Among amphibians, newts in the genus Taricha contain high concentrations of tetrodotoxin, which helps deter predation by vertebrates such as snakes, birds, and mammals (Brodie III and Brodie Jr, 1990; Brodie Jr, 1968). However, the effects of this toxin on infection by parasites remains relatively unexplored. Results of the current study indicate that exogenous exposure to TTX caused significant, dose-dependent mortality in trematode free-living stages (cercariae). This effect was true for all five species tested, each of which is known to infect or encounter larval newts in aquatic environments (Johnson et al., 2013, 2017). By comparison, TTX had no detectable effects on 6 of the 7 tested macroinvertebrate taxa, even at a substantially greater concentration. The only group that showed a significant response was mayflies, which experienced 27% mortality within 24 hrs, relative to zero mortality in the control treatment. This result is consistent with the overall sensitivity of mayfly larvae to chemicals and low oxygen availability previously established in the literature (Courtney and Clements, 2000; Hickey and Vickers, 1992; Johnson et al., 2010).
Among the trematode species included in this study, the magnitude of TTX-mediated reductions in survivorship varied by roughly 5-fold. All species exhibited a significant mortality response as TTX dosage increased. This effect was greatest in the kidney-infecting species E. trivolvis, for which a 1.0 increase in TTX nmol L−1 was associated with an 6.8% increase in mortality risk, and weakest for R. ondatrae, for which the equivalent increase in TTX led to a 1.5% increase. Cephalogonimus sp. exhibited the second strongest response, while A. marcianae and M. syntomentera had intermediate sensitivity values and were not appreciably different from each other (Figure 2). This variation in response could relate to differences among the parasites with respect to the type of infectious stages utilized or the encystment location within the host. For instance, four of the five parasites (R. ondatrae, M. syntomentera, E. trivolvis, Cephalogonimus sp.) form metacercariae within amphibian hosts, whereas A. marcianae form mesocercariae (Schell, 1985). Metacercariae are protected by a cyst wall that varies in thickness and is the product of both parasite and host tissue (Fried, 1993; Sukhdeo and Sukhdeo, 1994), which may help reduce exposure to toxins or host immune defenses (Bryan-Walker et al., 2007; Morley et al., 2003; Siddall, 1992). In contrast, mesocercariae lack a protective coating and are more mobile within intermediate hosts, making it somewhat surprising that A. marcianae was not more sensitive. A second salient factor is the location of infection, which could affect the amount of TTX experienced by a parasite and whether they have developed any tolerance to the toxin. Echinostoma trivolvis cercariae travel up the cloaca of larval amphibians to encyst within the developing kidneys, which have low concentrations of TTX relative to the skin (Wakely et al., 1966). Skin-encysting trematodes such as R. ondatrae likely experience higher TTX exposure (Cardall et al., 2004; Mosher et al., 1964; Tsuruda et al., 2001) and may therefore have greater tolerance to the toxin. An important next step will be to couple these in vitro experimental results with in vivo infection trials using newt hosts that vary in TTX concentration, which will also offer insights into natural patterns of infection by different trematodes observed in larval newt populations.
Based on the current results, larval trematodes were more sensitive to TTX relative to common aquatic invertebrates that co-occur with newt larvae in freshwater ecosystems. Among the 7 invertebrate taxa, we detected reductions in survivorship only within mayfly larvae, for which exposure to 1,000 nmol L−1 was associated with a 36% decrease in survivorship. Because the lifespan of trematode cercariae is short, the most relevant comparison is of the coefficients from the Cox proportional hazards models on survivorship. While the 1.0 unit (nmol L−1) effect of TTX on mayfly mortality was 0.0038 ± 0.0015, the overall effect on trematode mortality was 0.0236 ± 0.00153 – a 6-fold increase (Figure 2). This value accounts for the inherently shorter lifespan of cercariae. The lesser sensitivity of macroinvertebrates is consistent with the ability of some insect taxa to buffer the effects of poisons by investing energetic resources into cuticle deposition, thereby decreasing the need to upregulate transcription of more enzymes to catalyze metabolism (Wood et al., 2010). Correspondingly, several macroinvertebrate taxa have been shown to consume larval or embryonic Taricha without notably ill effects, including larvae of Trichoptera, Zygoptera, and Anisoptera (Gall et al., 2012, 2011; Toledo et al., 2016). For instance, caddisfly larvae consume the eggs of T. granulosa (maximum of 1.53 µg TTX/egg) (Gall et al., 2012; Mebs et al., 2016) while dragonfly nymphs will eat larvae of both T. granulosa and T. torosa (0.296 ± 0.103 µg TTX/larva) (Bucciarelli and Kats, 2015; Mebs et al., 2016). Further consistent with our results, mayfly larvae – which are not predators of newt larvae – are often cited as bioindicator taxa sensitive to adverse environmental conditions, including hypoxia and contaminants (Gerhardt, 1996; Hodkinson and Jackson, 2005; Menetrey et al., 2008; O’Halloran et al., 2008).
Of relevance to conservation, trematode parasites can be harmful to amphibian development, fitness, and survival (Blaustein and Johnson, 2003; Koprivnikar et al., 2012, 2008). Previous research has found that exposure to R. ondatrae increases amphibian mortality as well as the risk of developing severe hindlimb malformations, which help to increase parasite transmission to bird definitive hosts via predation (Johnson and Lunde, 2005). In lab-based studies, parasite-induced malformations are associated with reduced jumping ability, lower prey intake, and reduced recapture rates (Goodman and Johnson, 2011a, 2011b). Similarly, infection by echinostome trematodes can inhibit osmoregulatory function in amphibians and reduce growth or survival (Holland et al., 2007; Johnson and McKenzie, 2009; Schotthoefer et al., 2003). With respect to newt hosts specifically, Johnson et. al (2012) found that R. ondatrae exposure reduced length and mass-at-metamorphosis of T. torosa larvae in a dose-dependent manner, although this species showed only a low frequency of parasite-induced malformations. The current results indicated that R. ondatrae was the least sensitive trematode to exogenous TTX exposure, consistent with the frequent detection of this parasite in larval newts in natural populations (e.g., (Johnson et al., 2013, 2002). Understanding whether TTX provides defense against such pathogenic trematodes and how it limits pathology is an important future research arena, including an examination of how infection susceptibility and TTX concentrations covary in populations (Johnson personal communication).
The effects of toxic pollutants on the functional biology of aquatic parasites has been a subject of increasing interest in recent years (Koprivnikar and Walker, 2011; Marcogliese, 2005). Notably, studies of contaminants have found a reduction in the production of trematode cercariae from molluscan hosts as well as their capacity to infect downstream hosts (Cross et al., 2001; Morley et al., 2003). For example, Rohr et al. (2008) reported an increase in cercariae mortality when E. trivolvis cercariae were exposed to atrazine at ecologically relevant concentrations. However, the effect of herbicide on cercariae mortality was smaller than the pesticide-induced increase in tadpole susceptibility to infections, leading to a net increase in total amphibian infection (Rohr et al., 2008). Alongside pesticides, heavy metals such as cadmium and zinc can decrease locomotion and survival of cercariae for multiple trematode species (Cross et al., 2001; Morley et al., 2003, 2001). Several researchers have suggested that, owing to their heightened sensitivity, parasites may function as effective bioindicators in the study of pollution in aquatic ecosystems (e.g., Bongers and Ferris, 1999; Huspeni et al., 2005; Marcogliese, 2005).
Overall, our study indicates that TTX exposure has a substantial effect on the mortality of trematode cercariae, which suggests that host toxicity has the potential to decrease infection in naturally occurring animals. An important subsequent step will involve in vivo parasite exposures of amphibian hosts that vary naturally in TTX concentration to investigate whether host toxicity can limit either initial infection, the temporal trajectory of parasite dynamics within the host, or overall pathology. Interestingly, our results indicate that macroinvertebrates exogenously exposed to TTX showed little to no changes in survivorship, at least over a period of 24 hrs. Beyond the implications of these findings for the infection or consumption of larval newts in aquatic ecosystems, they also raise interesting questions about the amount of waterborne-TTX to which aquatic organisms are exposed; particularly in systems or seasons with large breeding aggregations of adult Taricha, it is possible that concentrations of TTX within aquatic habitat could become non-trivial and thus have consequences for co-occurring vertebrate or invertebrate organisms.
Highlights.
Trematode cercariae exposed to tetrodotoxin all exhibited reduced survivorship.
Trematodes survivorship varied, Ribeiroia ondatrae was the least sensitive to TTX.
Host toxicity may decrease infection in naturally occurring populations.
Of macroinvertebrates, only mayflies showed a decrease in survivorship to TTX.
Maternal investment of TTX into newts may provide protection against trematodes.
Acknowledgments
We thank T. McDevitt-Galles, J. Bowerman, and L. Guderyahn for collecting the snails used in this study. We also thank S. Shaw, J. Walker, A. Kimball, C. Garcia, I. Newton, and R. Van Hove for assisting with animal husbandry and checking survivorship of cercariae. Finally, we thank two reviewers. This research was supported by funding from the National Science Foundation (DEB 1149308, IOS 08-29643, and OCE 08-52361), the National Institutes of Health (R01 GM109499), and the David and Lucile Packard Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: DMC and PTJJ conceived, designed, and performed the parasite experiment; PTJJ analyzed the data; GMB, LBK, and RKZ conceived, designed, and performed the invertebrate experiment; DMC, GMB, and PTJJ wrote the paper and all authors provided editorial input and agreed approved final version of manuscript.
Conflicts of Interest: The authors declare no conflict of interest. The funding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Author Agreement/Declaration is a statement to certify that all authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere.
References
- Birnbaum LS. Environmental chemicals: evaluating low-dose effects. Environ. Health Perspect. 2012;120:A143–A144. doi: 10.1289/ehp.1205179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Johnson PTJ. Explaining frog deformities. Sci. Am. 2003;288:60–65. doi: 10.1038/scientificamerican0203-60. [DOI] [PubMed] [Google Scholar]
- Bolek MG. Seasonal occurrence of Cosmocercoides dukae and prey analysis in the blue-spotted salamander, Ambystoma laterale, in southeastern Wisconsin. J.-Helminthol. Soc. Wash. 1997;64:292–295. [Google Scholar]
- Bongers T, Ferris H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999;14:224–228. doi: 10.1016/s0169-5347(98)01583-3. [DOI] [PubMed] [Google Scholar]
- Bos N, Sundström L, Fuchs S, Freitak D. Ants medicate to fight disease. Evolution. 2015;69:2979–2984. doi: 10.1111/evo.12752. [DOI] [PubMed] [Google Scholar]
- Bray RA, Gibson DI, Jones A, et al. Keys to the Trematoda. 2008;3 CABI. [Google Scholar]
- Brodie III ED, Feldman CR, Hanifin CT, Motychak JE, Mulcahy DG, Williams BL, Brodie ED., Jr Parallel arms races between garter snakes and newts involving tetrodotoxin as the phenotypic interface of coevolution. J. Chem. Ecol. 2005;31:343–356. doi: 10.1007/s10886-005-1345-x. [DOI] [PubMed] [Google Scholar]
- Brodie III ED, Brodie ED., Jr Costs of exploiting poisonous prey: evolutionary tradeoffs in a predator-prey arms race. Evolution. 1999;53:626–631. doi: 10.1111/j.1558-5646.1999.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Brodie ED, III, Brodie ED., Jr Tetrodotoxin resistance in garter snakes: an evolutionary response of predators to dangerous prey. Evolution. 1990;43:651–659. doi: 10.1111/j.1558-5646.1990.tb05945.x. [DOI] [PubMed] [Google Scholar]
- Brodie ED., Jr Investigations on the skin toxin of the adult rough-skinned newt, Taricha granulosa. Copeia. 1968:307–313. [Google Scholar]
- Brown L. The use of Hydrobia jenkinsi to detect intermittent toxic discharges to a river. Water Res. 1980;14:941–947. [Google Scholar]
- Bryan-Walker K, Leung TL, Poulin R. Local adaptation of immunity against a trematode parasite in marine amphipod populations. Mar. Biol. 2007;152:687–695. [Google Scholar]
- Bucciarelli GM, Green DB, Shaffer HB, Kats LB. Individual fluctuations in toxin levels affect breeding site fidelity in a chemically defended amphibian. Proc R Soc B. 2016;283:20160468. doi: 10.1098/rspb.2016.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciarelli GM, Kats LB. Effects of newt chemical cues on the distribution and foraging behavior of stream macroinvertebrates. Hydrobiologia. 2015;749:69–81. [Google Scholar]
- Caffara M, Bruni G, Paoletti C, Gustinelli A, Fioravanti ML. Metacercariae of Clinostomum complanatum (Trematoda: Digenea) in European newts Triturus carnifex and Lissotriton vulgaris (Caudata: Salamandridae) J. Helminthol. 2014;88:278–285. doi: 10.1017/S0022149X13000151. [DOI] [PubMed] [Google Scholar]
- Calhoun DM, Schaffer PA, Gregory JR, Hardy KM, Johnson PTJ. Experimental infections of bluegill with the trematode Ribeiroia ondatrae (Digenea: Cathaemasiidae): histopathology and hematological Response. J. Aquat. Anim. Health. 2015;27:185–191. doi: 10.1080/08997659.2015.1084068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardall BL, Brodie ED, Jr, Hanifin CT. Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa) Toxicon. 2004;44:933–938. doi: 10.1016/j.toxicon.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol. Sci. 2004;77:151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- Courtney LA, Clements WH. Sensitivity to acidic pH in benthic invertebrate assemblages with different histories of exposure to metals. J. North Am. Benthol. Soc. 2000;19:112–127. [Google Scholar]
- Cross MA, Irwin SWB, Fitzpatrick SM. Effects of heavy metal pollution on swimming and longevity in cercariae of Cryptocotyle lingua (Digenea: Heterophyidae) Parasitology. 2001;123:499–507. doi: 10.1017/s0031182001008708. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Pedersen AB, Hunter MD, Altizer S. Host plant species affects virulence in monarch butterfly parasites. J. Anim. Ecol. 2008;77:120–126. doi: 10.1111/j.1365-2656.2007.01305.x. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Rarick RM, Mongue AJ, Gerardo NM, Hunter MD. Aphids indirectly increase virulence and transmission potential of a monarch butterfly parasite by reducing defensive chemistry of a shared food plant. Ecol. Lett. 2011;14:453–461. doi: 10.1111/j.1461-0248.2011.01604.x. [DOI] [PubMed] [Google Scholar]
- Etges FJ. Contributions to the life history of the brain fluke of newts and fish, Diplostomulum scheuringi Hughes, 1929 (Trematoda: Diplostomatidae) J. Parasitol. 1961;47:453–458. [PubMed] [Google Scholar]
- Farner DS, Kezer J. Notes on the amphibians and reptiles of Crater Lake National Park. Am. Midl. Nat. 1953;50:448–462. [Google Scholar]
- Fox J, Weisberg S. Cox proportional-hazards regression for survival data in R. An appendix to an R companion to applied regression. Thousand Oaks: SAGE Publications; 2011. [Google Scholar]
- Fried B. Metacercarial excystment of trematodes. Adv. Parasitol. 1993;33:92–92. doi: 10.1016/s0065-308x(08)60412-1. [DOI] [PubMed] [Google Scholar]
- Gall BG, Brodie ED, III, Brodie ED., Jr Survival and growth of the caddisfly Limnephilus flavastellus after predation on toxic eggs of the rough-skinned newt (Taricha granulosa) Can. J. Zool. 2011;89:483–489. [Google Scholar]
- Gall BG, Stokes AN, French SS, Brodie III ED. Predatory caddisfly larvae sequester tetrodotoxin from their prey, eggs of the rough-skinned newt (Taricha granulosa) Can. J. Ecol. 2012;38:1351–1357. doi: 10.1007/s10886-012-0213-8. [DOI] [PubMed] [Google Scholar]
- Geffeney SL, Fujimoto E, Brodie III ED, Brodie ED, Jr, Ruben PC. Evolutionary diversification of TTX-resistant sodium channels in a predator-prey interaction. Nature. 2005;434:759–763. doi: 10.1038/nature03444. [DOI] [PubMed] [Google Scholar]
- Gerhardt A. Behavioural early warning responses to polluted water. Environ. Sci. Pollut. Res. 1996;3:63–70. doi: 10.1007/BF02985490. [DOI] [PubMed] [Google Scholar]
- Gibson DI, Jones A, Bray RA. Keys to the Trematoda. 2005 CABI. [Google Scholar]
- Goldberg SR, Bursey CR, Cheam H. Composition and structure of helminth communities of the salamanders, Aneides lugubris, Batrachoseps nigriventris, Ensatina eschscholtzii (Plethodontidae), and Taricha torosa (Salamandridae) from California. J. Parasitol. 1998;84:248–251. [PubMed] [Google Scholar]
- Goodman BA, Johnson PTJ. Disease and the extended phenotype: parasites control host performance and survival through induced changes in body plan. PLoS One. 2011a;6:e20193. doi: 10.1371/journal.pone.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman BA, Johnson PTJ. Ecomorphology and disease: cryptic effects of parasitism on host habitat use, thermoregulation, and predator avoidance. Ecology. 2011b;92:542–548. doi: 10.1890/10-0516.1. [DOI] [PubMed] [Google Scholar]
- Guzmán A, de Henestrosa ARF, Marín A-P, Ho A, Borroto JIG, Carasa I, Pritchard L. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat. Res. Toxicol. Environ. Mutagen. 2007;634:14–24. doi: 10.1016/j.mrgentox.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Hanifin CT. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs. 2010;8:577–593. doi: 10.3390/md8030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G, Ploner M. [accessed 4.1.17];Package “coxphf” - R [WWW Document] 2016 URL http://cemsiis.meduiwien.ac.at/kb/wf/software/statistische-software/fccoxphf.
- Hickey CW, Vickers ML. Comparison of the sensitivity to heavy metals and pentachlorophenol of the mayflies Deleatidium spp. and the cladoceran Daphnia magna. N. Z. J. Mar. Freshw. Res. 1992;26:87–93. [Google Scholar]
- Hodkinson ID, Jackson JK. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ. Manage. 2005;35:649–666. doi: 10.1007/s00267-004-0211-x. [DOI] [PubMed] [Google Scholar]
- Holland MP, Skelly DK, Kashgarian M, Bolden SR, Harrison LM, Cappello M. Echinostome infection in green frogs (Rana clamitans) is stage and age dependent. J. Zool. 2007;271:455–462. [Google Scholar]
- Huffman MA. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003;62:371–381. doi: 10.1079/pns2003257. [DOI] [PubMed] [Google Scholar]
- Huspeni TC, Hechinger RF, Lafferty KD. Estuar. Indic. CRC Press; Boca Raton: 2005. Trematode parasites as estuarine indicators: opportunities, applications and comparisons with conventional community approaches; pp. 297–314. [Google Scholar]
- Johnson PTJ, Hoverman JT. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc. Natl. Acad. Sci. 2012;109:9006–9011. doi: 10.1073/pnas.1201790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Lunde KB, Thurman EM, Ritchie EG, Wray SN, Sutherland DR, Kapfer JM, Frest TJ, Bowerman J, Blaustein AR. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol. Monogr. 2002;72:151–168. [Google Scholar]
- Johnson PTJ, McKenzie VJ. The Biology of Echinostomes. Springer; 2009. Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America; pp. 249–280. [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl. Acad. Sci. 2013;110:16916–16921. doi: 10.1073/pnas.1310557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB. Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history and pathogenesis with special emphasis on the amphibian malformation problem. Adv. Parasitol. 2004;57:191–253. doi: 10.1016/S0065-308X(04)57003-3. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Townsend AR, Cleveland CC, Glibert PM, Howarth RW, McKenzie VJ, Rejmankova E, Ward MH. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol. Appl. 2010;20:16–29. doi: 10.1890/08-0633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Lunde KB. Amphib. Declines Conserv. Status U. S. Species. Univ. Calif. Press; Berkeley: 2005. Parasite infection and limb malformations: a growing problem in amphibian conservation; pp. 124–138. [Google Scholar]
- Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Jones A, Bray RA, Gibson DI. Keys to the Trematoda. CABI Publishing and The Natural History Museum; Cambridge: 2005. [Google Scholar]
- Koprivnikar J, Forbes MR, Baker RL. Larval amphibian growth and development under varying density: are parasitized individuals poor competitors? Oecologia. 2008;155:641–649. doi: 10.1007/s00442-007-0937-2. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Marcogliese DJ, Rohr JR, Orlofske SA, Raffel TR, Johnson PTJ. Macroparasite infections of amphibians: what can they tell us? EcoHealth. 2012;9:342–360. doi: 10.1007/s10393-012-0785-3. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Walker PA. Effects of the herbicide atrazine’s metabolites on host snail mortality and production of trematode cercariae. J. Parasitol. 2011;97:822–827. doi: 10.1645/GE-2814.1. [DOI] [PubMed] [Google Scholar]
- Kuzmin Y, Tkach VV, Snyder SD. The nematode genus Rhabdias (Nematoda: Rhabdiasidae) from amphibians and reptiles of the Nearctic. Comp. Parasitol. 2003;70:101–114. [Google Scholar]
- Lehmann DL. Some helminths of west coast urodeles. J. Parasitol. 1954;40:1–11. [Google Scholar]
- Lorentz MN, Stokes AN, Rössler DC, Lötters S. Tetrodotoxin. Curr. Biol. 2016;26:R870–R872. doi: 10.1016/j.cub.2016.05.067. [DOI] [PubMed] [Google Scholar]
- Macy RW. On the life cycle of Megalodiscus microphagus Ingles (Trematoda: Paramphistomatidae) J. Parasitol. 1960;46:662–675. [Google Scholar]
- Marcogliese DJ. Parasites of the superorganism: Are they indicators of ecosystem health? Int. J. Parasitol. 2005;35:705–716. doi: 10.1016/j.ijpara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Mebs D, Yotsu-Yamashita M, Arakawa O. The praying mantis (Mantodea) as predator of the poisonous red-spotted newt Notophthalmus viridescens (Amphibia: Urodela: Salamandridae) Chemoecology. 2016;26:121–126. [Google Scholar]
- Menetrey N, Oertli B, Sartori M, Wagner A, Lachavanne JB. Eutrophication: are mayflies (Ephemeroptera) good bioindicators for ponds? Hydrobiologia. 2008;597:125–135. [Google Scholar]
- Miller DL, Bursey CR, Gray MJ, Smith LM. Metacercariae of Clinostomum attenuatum in Ambystoma tigrinum mavortium, Bufo cognatus and Spea multiplicata from west Texas. J. Helminthol. 2004;78:373–376. doi: 10.1079/joh2004248. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Jeon JK, Maruyama J, Noguchi T, Ito K, Hashimoto K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon. 1986;24:645–650. doi: 10.1016/0041-0101(86)90027-9. [DOI] [PubMed] [Google Scholar]
- Morley NJ, Crane M, Lewis JW. Toxicity of cadmium and zinc to miracidia of Schistosoma mansoni. Parasitology. 2001;122:81–85. doi: 10.1017/s0031182000007083. [DOI] [PubMed] [Google Scholar]
- Morley NJ, Leung KMY, Morritt D, Crane M. Toxicity of anti-fouling biocides to Parorchis acanthus (Digenea: Philophthalmidae) cercarial encystment. Dis. Aquat. Organ. 2003;54:55–60. doi: 10.3354/dao054055. [DOI] [PubMed] [Google Scholar]
- Mosher HS, Fuhrman FA, Buchwald HD, Fischer HG. Tarichatoxin-tetrodotoxin: a potent neurotoxin. Science. 1964;144:1100–1110. doi: 10.1126/science.144.3622.1100. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Maruyama J, Narita H, Kanehisa H. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell) Toxicon. 1984;22:219–226. doi: 10.1016/0041-0101(84)90022-9. [DOI] [PubMed] [Google Scholar]
- O’Halloran K, Cavanagh J-A, Harding JS. Response of a New Zealand mayfly (Deleatidium spp.) to acid mine drainage: implications for mine remediation. Environ. Toxicol. Chem. 2008;27:1135–1140. doi: 10.1897/07-199.1. [DOI] [PubMed] [Google Scholar]
- Owen HM. The life history of Plagitura salamandra Holl, 1928 (Trematoda: Plagiorchiidae) J. Parasitol. 1946;32:553–562. [PubMed] [Google Scholar]
- Parkinson L. Helminth parasite infracommunities and dietary analysis of the rough-skinned newt, Taricha granulosa from south-eastern Vancouver Island, BC. Malaspina University-College; Nanaimo, British Columbia: 2010. [Google Scholar]
- Paull SH, LaFonte BE, Johnson PTJ. Temperature-driven shifts in a host-parasite interaction drive nonlinear changes in disease risk. Glob. Change Biol. 2012;18:3558–3567. [Google Scholar]
- Peck LS, Clarke A, Chapman AL. Metabolism and development of pelagic larvae of Antarctic gastropods with mixed reproductive strategies. Mar. Ecol. Prog. Ser. 2006;318:213–220. [Google Scholar]
- Reddy A, Ponder EL, Fried B. Effects of copper sulfate toxicity on cercariae and metacercariae of Echinostoma caproni and Echinostoma trivolvis and on the survival of Biomphalaria glabrata snails. J. Parasitol. 2004;90:1332–1337. doi: 10.1645/GE-321R. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Adamson ML. A new Kathlaniidae (Cosmocercoidea; Nematoda), Megalobatrachonema (Chabaudgolvania) moraveci sp. n. from the intestine of the rough-skinned newt, Taricha granulosa. Proc. Helminthol. Soc. Wash. 1988;55:155–159. [Google Scholar]
- Ritson-Williams R, Yotsu-Yamashita M, Paul VJ. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3176–3179. doi: 10.1073/pnas.0506093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 2008;18:1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- Schell SC. Handbook of trematodes of North America north of Mexico. University Press of Idaho; Moscow, Indiana: 1985. [Google Scholar]
- Schell SC. How to know the trematodes. MW. C. Brown; Dubuque, Iowa: 1970. [Google Scholar]
- Schotthoefer AM, Cole RA, Beasley VR. Relationship of tadpole stage to location of echinostome cercariae encystment and the consequences for tadpole survival. J. Parasitol. 2003;89:475–482. doi: 10.1645/0022-3395(2003)089[0475:ROTSTL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Siddall R. Parasites as biological indicators of sewage sludge disposal. University of Aberdeen; United Kingdom: 1992. [Google Scholar]
- Singer MS, Mace KC, Bernays EA. Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars. Plos One. 2009;4:e4796. doi: 10.1371/journal.pone.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK. Human emerging and re-emerging infections Set. John Wiley & Sons; 2015. [Google Scholar]
- Storm RM. The herpetology of Benton County. Oregon: 1948. [Google Scholar]
- Sukhdeo MVK, Sukhdeo SC. Optimal habitat selection by helminths within the host environment. Parasitology. 1994;109:S41–S55. doi: 10.1017/s0031182000085073. [DOI] [PubMed] [Google Scholar]
- Team R. Development core. R Lang. Environ. Stat. Comput 2012 [Google Scholar]
- Therneau T, Lomley T. [accessed 4.28.17];Package “survival” [WWW Document] 2017 URL https://github.com/therneau/survival.
- Toledo G, Hanifin C, Geffeney S, Brodie ED., Jr Chapter four-convergent evolution of tetrodotoxin-resistant sodium channels in predators and prey. Curr. Top. Membr. 2016;78:87–113. doi: 10.1016/bs.ctm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Tsuruda K, Arakawa O, Noguchi T. Toxicity and toxin profiles of the newt, Cynops pyrrhogaster from western Japan. J. Nat. Toxins. 2001;10:79–89. [PubMed] [Google Scholar]
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study. Dose-Response. 2014;12:259–276. doi: 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderburgh DJ, Anderson RC. The relationship between nematodes of the genus Cosmocercoides Wilkie, 1930 (Nematoda: Cosmocercoidea) in toads (Bufo americanus) and slugs (Deroceras laeve) Can. J. Zool. 1987;65:1650–1661. [Google Scholar]
- Wakely JF, Fuhrman GJ, Fuhrman FA, Fischer HG, Mosher HS. The occurrence of tetrodotoxin (tarichatoxin) in Amphibia and the distribution of the toxin in the organs of newts (Taricha) Toxicon. 1966;3:195–203. doi: 10.1016/0041-0101(66)90021-3. [DOI] [PubMed] [Google Scholar]
- Williams BL, Brodie ED, Jr, Brodie ED., III Coevolution of deadly toxins and predator resistance: Self-assessment of resistance by garter snakes leads to behavioral rejection of toxic newt prey. Herpetologica. 2003;59:155–163. [Google Scholar]
- Wood PJ, Boulton AJ, Little S, Stubbington R. Is the hyporheic zone a refugium for aquatic macroinvertebrates during severe low flow conditions? Fundam. Appl. Limnol. Für Hydrobiol. 2010;176:377–390. [Google Scholar]
- Yotsu M, Iorizzi M, Yasumoto T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon. 1990;28:238–241. doi: 10.1016/0041-0101(90)90419-8. [DOI] [PubMed] [Google Scholar]