Abstract
Objective
Metabolic syndrome (MetS) can lead to myocardial fibrosis, diastolic dysfunction and eventual heart failure. We evaluated alterations in myocardial microstructure in people with MetS using a novel algorithm to characterize ultrasonic signal intensity variation.
Methods
Among 254 participants without existing cardiovascular disease (mean age 42 ± 11 years, 75% women), there were 162 with MetS, 47 with obesity without MetS, and 45 non-obese controls. Standard echocardiography was performed, and a novel validated computational algorithm was used to investigate myocardial microstructure based on sonographic signal intensity and distribution. We examined the signal intensity coefficient (SIC, left ventricular microstructure).
Results
The SIC was significantly higher in people with MetS compared with people with (P<0.001) and without obesity (P=0.04), even after adjustment for age, sex, body mass index, hypertension, diabetes mellitus and triglyceride to HDL cholesterol (TG/HDL) ratio (P<0.05 for all). Clinical correlates of SIC included TG concentrations (r=0.21, P=0.0007) and the TG/HDL ratio (r=0.2, P=0.001).
Conclusions
Our findings suggest that preclinical MetS and dyslipidemia in particular, are associated with altered myocardial signal intensity variation. Future studies are needed to determine whether the SIC may help detect subclinical disease in people with metabolic disease, with the ultimate goal of targeting preventive efforts.
Keywords: obesity, echocardiography, metabolic syndrome
Introduction
One in four adults are living with obesity in the United States, a condition that is expect to affect more than half of the population by 2030 (1). Obesity predisposes to metabolic disease, with an increased risk of diabetes mellitus and metabolic syndrome (MetS) among those with higher body mass index(2, 3). Both obesity and metabolic disease have been linked to increased risk of cardiovascular disease, including heart failure (4). Further, subclinical changes in diastolic function and cardiac mechanics are apparent among individuals with obesity (5, 6), and may herald the future development of clinical heart failure. The underlying mechanisms driving cardiovascular disease in those with obesity are unclear, however many of the direct myocardial changes in metabolic disease are attributed to cardiac fibrosis (7), as well as increased myocardial lipid content, which appears correlated with the degree of adiposity (8). While experimental studies have demonstrated activation of myofibroblast phenotypes and myocardial macrophage infiltration leading to fibrosis in animal models of obesity and metabolic disease,(9) myocardial fibrosis has been more difficult to ascertain and examine in human investigations.
The quantitative aspects of reflected ultrasound beams have long been studied as potential noninvasive indicators of myocardial fibrosis (10). Specifically, scattering properties of the myocardium are directly correlated with histologic myocardial collagen content and extracellular matrix (11, 12). Previous clinical studies have demonstrated abnormalities in cyclic integrated signal intensity variation (historically termed ‘backscatter’) among patients with metabolic disease (13-15). Indeed, small human trials have demonstrated improvements in sonographic signal intensity variation with spironolactone therapy among patients with diabetes and obesity, both with abnormal diastolic function.(16, 17) However, cyclic integrated signal intensity variation has not gained widespread clinical use due to the need for prospective image acquisition with specialized software.
Our group recently validated a novel ultrasonic image analysis algorithm to ascertain myocardial signal intensity variation using a widely-available open access software, applied to routinely acquired echocardiographic images (18, 19). In initial studies, we demonstrated abnormalities in myocardial signal intensity variation in hypertensive heart disease, which correlated with experimental models simulating pressure overload states (18). Further, a recently published study demonstrated alterations in ultrasonic signal intensity variation and cardiac microstructure in hypertrophic cardiomyopathy, as well as sarcomere-mutation carriers without left ventricular hypertrophy (20). In this study, ultrasonic signal intensity variation correlated with extracellular volume by cardiac magnetic resonance imaging, as well as left ventricular diastolic function parameters. We sought to apply this novel methodology to a well-characterized sample of individuals with obesity with and without MetS, in order to investigate potential alterations in myocardial microstructure associated with metabolic dysfunction. Further, we hypothesized that myocardial signal intensity variation would be associated with measures of metabolic dysfunction, above and beyond its known association with hypertension.
Methods
Study sample
Participants were recruited from general cardiology and nutrition outpatient clinics at Boston Medical Center. Potential participants were screened for inclusion/exclusion criteria, and approached with permission of their primary physician. For the non-obese control group, research volunteers were recruited from the same clinics, as well as advertisements in the research community. Participants were grouped into three study groups: Participants with MetS met 3 or more of 5 accepted criteria:(21) (a) increased waist circumference (≥102 cm in men; ≥88 cm in women); (b) increased fasting triglyceride (≥150 mg/dL); (c) high blood pressure (≥130/85 mmHg) or treatment for hypertension; (d) decreased HDL-C (<40 mg/dL in men; <50 mg/dL in women); (e) impaired fasting glucose (≥100 mg/dL). Participants with obesity without MetS had a body mass index (BMI) ≥30 kg/m2, and met ≤1 MetS criterion other than increased waist circumference. Non-obese controls had a BMI <30 kg/m2 and no major medical comorbidities. Participants with a history of cardiovascular disease as ascertained by self-report and subsequent review of medical records (heart failure, coronary artery disease, valvular heart disease, or atrial fibrillation), severe hypertension (defined as taking >2 antihypertensive medications), or those with asymptomatic left ventricular (LV) systolic dysfunction (any previous echocardiogram with LV ejection fraction < 50%) were excluded from the study.
Clinical assessment
Participants underwent medical history, anthropometrics, assessment of resting blood pressure (average of three consecutive measurements), and fasting bloodwork. Diabetes mellitus was defined as a fasting serum glucose level ≥126 mg/dL and/or anti-diabetic therapy. All participants provided informed consent, and the study was approved by the Boston University Medical Center Institutional Review Board.
Image analysis
All participants underwent standard two-dimensional transthoracic echocardiograms using a 1 - 5 MHz transducer and a commercially available ultrasound machine (iE33, Phillips Medical Systems, Andover, Massachusetts, USA). Conventional echocardiographic measures, including cardiac dimensions, relative wall thickness (RWT), LV ejection fraction, and LV diastolic parameters using pulsed-wave and tissue Doppler assessment were measured according to published recommendations (22, 23). LV mass was ascertained by the cubed method and indexed to height to the power of 2.7 (LV mass/height2.7) (24). All echocardiographic measurements were averaged over three consecutive cardiac cycles (when available).
For advanced image analyses of myocardial ultrasonic signal intensity variation, a validated computational algorithm was used to measure signal intensity distributions within 2D ultrasound images using the ImageJ software platform v1.46 (NIH, Bethesda, MD)(18, 19). In brief, end-diastolic frames of the parasternal long axis echocardiographic views were obtained in .bmp format. A region of interest was defined as the pericardium adjacent to the mid-basal inferolateral LV segment, and the signal intensity distribution of the pixels within the region analyzed. The 25th percentile of pericardial signal intensity distribution (p) was used to calculate the Signal Intensity Coefficient (SIC) as follows: SIC = (1-p/256). The Myocardial Structural Index (MSI) was calculated using standardized values of SIC and RWT as follows: MSI = SIC/0.13 + RWT/0.05. As previously described, the MSI is calculated as sum of the relative increase in SIC (compared to a referent cohort standard deviation) plus the relative increase in RWT (compared to a referent cohort standard deviation). Thus, both the SIC and RWT were scaled, with 1 unit value on each scale equaling the referent cohort standard deviation. This method allowed for generating the sum of the scaled values to represent a single aggregate measure (with positive values) of both microstructural change and macrostructural cardiac wall-to-cavity remodeling that is not represented by either the SIC or RWT alone. Seven of 261 studies (2.7%) were deemed poor quality and were excluded from image analysis. All myocardial signal intensity variation images were analyzed by a single observer (YR) blinded to clinical status. We randomly selected 20 images from the study sample for analysis by a second observer in order to examine reproducibility. This demonstrated robust reproducibility with intra- and inter-observer coefficients of variation of 4.0% and 7.4%, respectively, and intra-class correlation coefficients of 0.92 and 0.94, respectively.
Statistical analysis
Baseline clinical and echocardiographic characteristics were compared between non-obese controls, participants with obesity with and without MetS using one-way analysis of variance (ANOVA) or chi-squared statistics as appropriate. Subsequent pairwise comparisons were performed using two sample t-tests (continuous) and chi-squared test (categorical), with Bonferroni correction for multiple testing. Between-group differences in myocardial microstructure (SIC and MSI) were assessed using analysis of covariance (ANCOVA) in order to account for potential clinical confounders. Due to a right-skewed distribution, the SIC was natural-log transformed in analyses. Analyses were first adjusted for age, sex, and BMI, and then additionally for systolic blood pressure, the use of anti-hypertensive medications, the presence of diabetes mellitus, and the triglyceride-to-HDL cholesterol ratio.
Correlations of myocardial microstructure measures and other echocardiographic parameters of cardiac structure and function, as well as clinical characteristics were assessed using pairwise Pearson correlation coefficients. We performed a sensitivity analysis after exclusion of diabetic individuals. In exploratory analyses, correlates of SIC among individuals with obesity with and without MetS were investigated using forward and backward stepwise selection models, forcing age and sex, with covariate retention at P<0.05. To account for the use of antihypertensive medications, correlations with systolic and diastolic blood pressure used a correction factor of 15 mmHg and 10 mmHg, as previously described in other studies (25). Lastly, we examined diastolic parameters among participants with SIC above and below the mean SIC and above and below the mean LV mass/ht2.7. All analyses were performed using STATA release 11 (StataCorp, College Station, TX), and results considered statistically significant using a two-sided P-value <0.05.
Results
A total of 162 participants with MetS (mean age 44 ± 11 years, 73% women), 47 individuals with obesity without MetS (38 ± 10 years, 89% women), and 45 healthy controls without obesity (44 ± 12 years, 51% women) were enrolled. Baseline clinical, laboratory, and echocardiographic characteristics are summarized in Table 1. Blood pressure was highest in the group with MetS, and the group with obesity had intermediate blood pressures compared with controls that were non-obese. Notably, BMI did not differ between groups with obesity and MetS (P=0.99), with 43% in those with obesity, and 48% in those with MetS classified as severe obesity (BMI ≥40 kg/m2). In contrast, the triglyceride-to-HDL cholesterol ratio was significantly higher among subjects with MetS, and was comparable among controls with and without obesity (4.1±3.4 in MetS; 1.8±1.0 in those with obesity; 1.5±0.9 in those without obesity, respectively).
Table 1. Clinical, laboratory, and echocardiographic characteristics of the sample.
| Controls n=45 | Obese n=47 | MetS n=162 | P ANOVA | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 44 (12) | 38 (10)* | 44 (11) | 0.008 |
| Women, n (%) | 33 (73) | 42 (89) | 118 (73) | 0.06 |
| White, n (%) | 23 (51) | 6 (13)* | 46 (28)*† | <0.001 |
|
| ||||
| Clinical | ||||
| Systolic blood pressure, mmHg | 111 (13) | 119 (11)* | 125 (15)*† | <0.0001 |
| Diastolic blood pressure, mmHg | 70 (8) | 75 (8)* | 78 (10)* | <0.0001 |
| Heart rate, bpm | 62 (10) | 67 (9) | 72 (13)*† | <0.0001 |
| Body mass index, kg/m2 | 24.3 (2.6) | 39.6 (10.7)* | 40.9 (9.2)* | <0.0001 |
| Waist circumference, cm | 82 (14) | 111 (19)* | 122 (19)*† | <0.0001 |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | 72 (44)*† | <0.001 |
| Anti-hypertensive treatment, n (%) | 0 (0) | 9 (19)* | 101 (62)*† | <0.001 |
| Current smoker, n (%) | 1 (2) | 4 (9) | 24 (15) | 0.05 |
|
| ||||
| Laboratory | ||||
| Total cholesterol, mg/dl | 192 (30) | 177 (32) | 184 (42) | 0.17 |
| HDL cholesterol, mg/dl | 59 (13) | 49 (10)* | 43 (10)*† | <0.0001 |
| Triglycerides, mg/dl | 78 (35) | 82 (39) | 164 (118)*† | <0.0001 |
| Fasting glucose, mg/dl | 86 (10) | 90 (10) | 116 (51)*† | <0.0001 |
| Creatinine, mg/dl | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.2) | 0.76 |
| TG/HDL ratio | 1.5 (0.9) | 1.8 (1.0) | 4.1 (3.4)*† | <0.0001 |
|
| ||||
| Echo characteristics | ||||
| Left atrial diameter, mm | 32 (4) | 38 (5)* | 38 (5)* | <0.0001 |
| LV end-diastolic dimension, mm | 46 (4) | 48 (5)* | 46 (5)† | 0.001 |
| LV end-systolic dimension, mm | 30 (4) | 32 (4) | 30 (5)† | 0.01 |
| Interventricular septal thickness, mm | 8 (1) | 9 (2)* | 10 (2)*† | <0.0001 |
| Posterior wall thickness, mm | 8 (1) | 10 (2)* | 10 (2)* | <0.0001 |
| Relative wall thickness | 0.36 (0.07) | 0.40 (0.09)* | 0.44 (0.08)*† | <0.0001 |
| LV mass, gm/ht2.7 | 28 (6) | 42 (12)* | 41 (10)* | <0.0001 |
| LV ejection fraction, % | 63 (6) | 61 (4) | 63 (6) | 0.31 |
| Mitral E wave, cm/s | 73 (14) | 83 (17)* | 79 (18) | 0.02 |
| Mitral A wave, cm/s | 51 (14) | 58 (13) | 68 (16)*† | <0.0001 |
| E/A ratio | 1.5 (0.5) | 1.5 (0.4) | 1.2 (0.4)*† | <0.0001 |
| Deceleration time, ms | 198 (33) | 190 (26) | 197 (35) | 0.36 |
| Isovolumic relaxation time, ms | 77 (12) | 78 (14) | 84 (15)*† | 0.002 |
| Tissue Doppler mitral e', cm/s | 11.2 (2.8) | 11.5 (2.2) | 9.4 (2.2)*† | <0.0001 |
| E/e' ratio | 6.8 (1.8) | 7.4 (2.0) | 8.7 (2.5)*† | <0.0001 |
P<0.05 compared with controls
P<0.05 compared with obese
Data are means and standard deviations for continuous variables, unless otherwise indicated.
Measures of myocardial microstructure are abnormal in metabolic syndrome
The SIC, a novel measure of myocardial microstructure, was significantly higher in individuals with MetS compared with those with and without obesity (P<0.001 vs those with obesity; P=0.04 vs controls without obesity, Table 2, Figure 1). This finding persisted after adjusting for age, sex, and BMI (P=0.004 vs those with obesity; P=0.0004 vs controls). The SIC also remained significantly higher in MetS after additionally adjusting for systolic blood pressure, anti-hypertensive treatment, diabetes mellitus, and the triglyceride-to-HDL cholesterol ratio (P=0.03 vs those with obesity, P=0.007 vs controls).
Table 2. Measures of myocardial microstructure by study group.
| Controls n=45 | Obese n=47 | MetS n=162 | P ANOVA | |
|---|---|---|---|---|
| Signal intensity coefficient | 0.15 (0.02) | 0.13 (0.02) | 0.22 (0.01)*† | 0.0001 |
| Age, sex, BMI-adjusted | 0.0004 | |||
| Multivariable-adjusted | 0.01 | |||
|
| ||||
| Myocardial structural index | 8.3 (0.3) | 9.0 (0.3) | 10.6 (0.2)*† | <0.0001 |
| Age, sex, BMI-adjusted | <0.0001 | |||
| Multivariable-adjusted | 0.0007 | |||
P<0.05 compared with controls,
P<0.05 compared with obese
Data are means and standard errors. Multivariable model adjusted for age, sex, body mass index, systolic blood pressure, hypertension treatment, diabetes mellitus, triglyceride-to-HDL ratio
Figure 1.
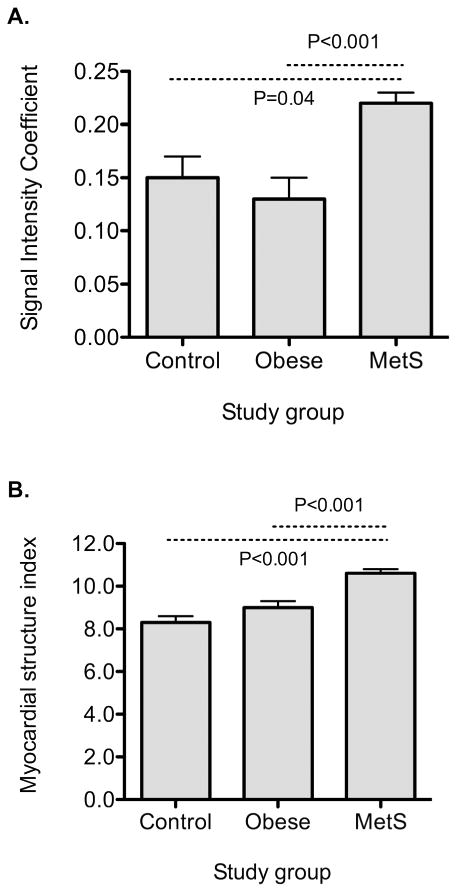
Measures of myocardial backscatter in individuals with MetS, obesity without MetS, and non-obese controls. Panel A displays the signal intensity coefficient, and panel B the myocardial structure index. Error bars represent standard errors.
Similarly, the MSI, an aggregate measure of myocardial microstructure and gross morphological changes, was highest in individuals with MetS (P<0.001 vs both controls with and without obesity, Table 2). The MSI remained significantly higher in MetS compared with other groups after multivariable adjustment (P=0.0003 vs those with obesity; P=0.02 vs controls without obesity after adjusting for age, sex, BMI, systolic blood pressure, anti-hypertensive treatment, diabetes mellitus, and the triglyceride-to-HDL cholesterol ratio).
Metabolic syndrome is associated with worse diastolic function
In addition to alterations in measures of myocardial microstructure, traditional echocardiographic measures of cardiac structure were also abnormal in MetS (Table 1). While there were no significant differences in LV ejection fraction between groups (P for ANOVA=0.31), those with obesity and/or MetS had greater LV mass and LA diameters. Further, those with MetS had worse diastolic function as assessed by the e' tissue Doppler mitral annular velocities, the mitral early wave to e' ratio, and the spectral Doppler mitral inflow pattern P<0.05 for each pairwise comparison of MetS vs those without obesity, and MetS vs those with obesity).
Measures of myocardial microstructure are associated with metabolic disease
The strongest clinical correlates of myocardial microstructure as assessed by the SIC were triglyceride concentrations (r=0.21, P=0.0007, Table 3, Figure 2) and the triglyceride-to-HDL cholesterol ratio (r=0.2, P=0.001), a measure associated with insulin resistance in previous studies.(26) An abnormal triglyceride-to-HDL ratio > 3.0 was associated with a higher SIC (0.22±0.02 versus 0.19±0.02, P=0.006, Figure 3). Similarly, the combination of hypertriglyceridemia and high waist circumference, also known as the hypertriglyceridemic-waist phenotype (27), was associated with significantly higher SIC (0.24±0.02 versus 0.19 ±0.01, P=0.02).
Table 3. Clinical and echocardiographic correlates of measures of myocardial microstructure.
| SIC | MSI | |||
|---|---|---|---|---|
|
| ||||
| r | P-value | r | P-value | |
| Clinical correlates | ||||
| Systolic blood pressure | 0.12 | 0.05 | 0.34 | <0.0001 |
| Diastolic blood pressure | 0.06 | 0.35 | 0.22 | 0.0005 |
| HDL cholesterol | −0.07 | 0.27 | −0.25 | <0.0001 |
| Triglycerides | 0.21 | 0.0007 | 0.24 | 0.0001 |
| TG/HDL ratio | 0.20 | 0.001 | 0.28 | <0.0001 |
| Total cholesterol | 0.04 | 0.52 | −0.004 | 0.95 |
| Fasting glucose | 0.08 | 0.18 | 0.22 | 0.0004 |
| Waist circumference | 0.10 | 0.10 | 0.30 | <0.0001 |
| Body mass index | −0.01 | 0.84 | 0.23 | 0.0002 |
|
| ||||
| Echocardiographic correlates | ||||
| Left atrial diameter | 0.12 | 0.06 | 0.20 | 0.001 |
| LV end-diastolic dimension | −0.06 | 0.36 | ||
| Relative wall thickness | 0.09 | 0.14 | ||
| LV mass | −0.01 | 0.82 | 0.32 | <0.0001 |
| E/A ratio | −0.14 | 0.02 | −0.30 | <0.0001 |
| mean e' | −0.08 | 0.16 | −0.32 | <0.0001 |
| E/e' ratio | 0.09 | 0.15 | 0.29 | <0.0001 |
Figure 2.
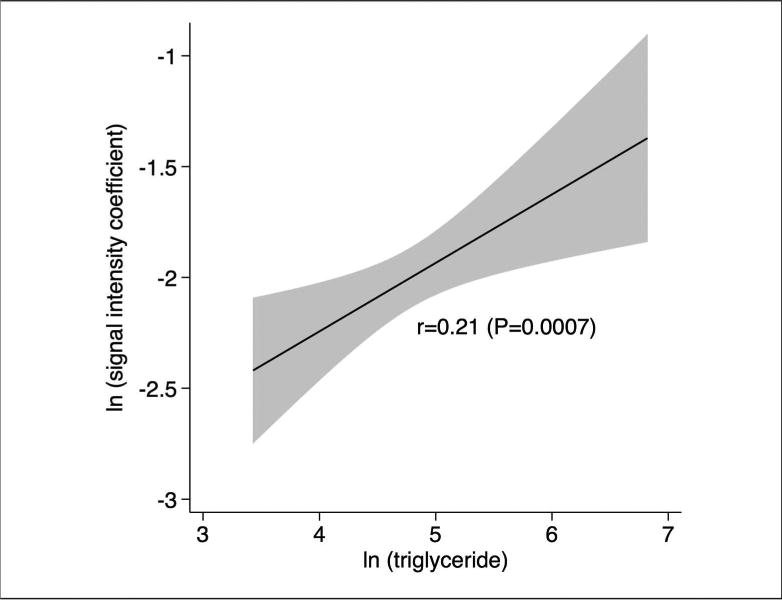
Correlation between myocardial microstructure (SIC) and triglyceride concentrations. Given skewed distributions, both SIC and triglyceride concentrations are log-transformed.
Figure 3.
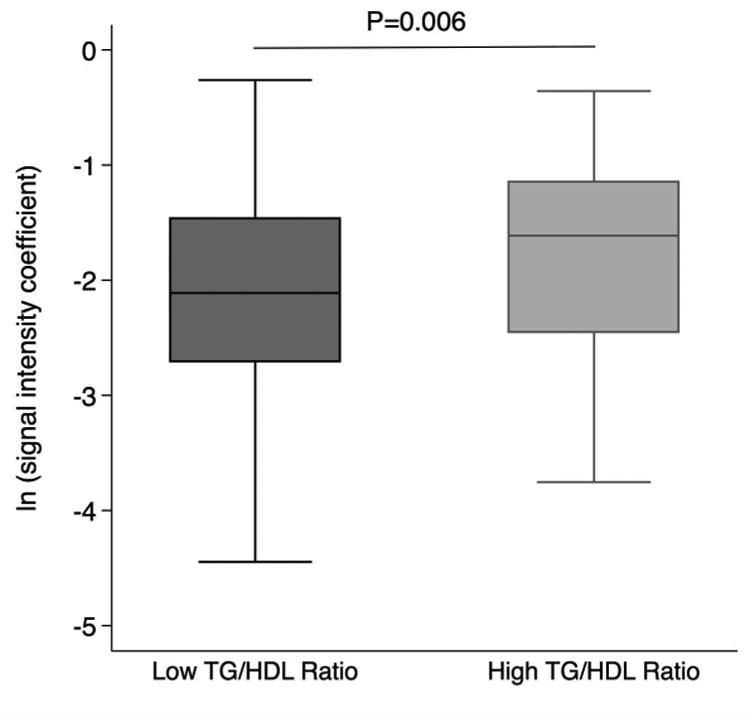
The SIC is higher among individuals with abnormal triglyceride-to-HDL cholesterol ratios greater than 3.
In exploratory analyses, triglyceride levels were the only clinical characteristic associated with the SIC in a stepwise model after adjusting for age and sex (P=0.049), and when considered a dichotomous variable, elevated triglycerides again represented the most significant clinical correlate of SIC in stepwise analyses (P=0.02).
Measures of myocardial microstructure are associated with diastolic dysfunction
The SIC was nominally correlated with echocardiographic parameters, including the mitral inflow E/A ratio (r=−0.14, P=0.02) and LA diameter (r=0.12, P=0.06). In contrast, the MSI was correlated with clinical phenotypes (blood pressure, HDL and triglyceride concentrations, fasting glucose, waist circumference, BMI) as well as cardiac structure and function (LA size, LV mass, E/A ratio, mean e', and E/e' ratio) as shown in Table 3. When examining diastolic parameters across high and low SIC and LV mass groups, we found minor but non-significant pairwise differences among those with high vs low SIC within a given LV mass stratum (P>0.05 for all, Table S1).
Sensitivity analysis excluding diabetic individuals
In a sensitivity analysis after exclusion of individuals with diabetes (leaving 90 participants with MetS), we found that both the SIC and MSI remained higher in those with MetS (SIC 0.21, s.e. 0.02, P<0.05 compared with those with obesity but non-significant compared with controls; MSI 10.2, s.e. 0.2, P<0.05 compared with both those with and without obesity). Further, the SIC remained significantly correlated with triglyceride concentrations among individuals without diabetes (r=0.21, P=0.005).
Discussion
We found that individuals with MetS without known cardiovascular disease had altered ultrasonic signal intensity variation, implying differences in myocardial microstructure compared with individuals with obesity that were metabolically healthy and controls without obesity. Notably, the most significant clinical correlates of myocardial microstructure were hypertriglyceridemia and the triglyceride-to-HDL cholesterol ratio. Our results show that myocardial signal intensity variation is associated with measures of metabolic dysfunction, above and beyond its known association with hypertension.
There are two notable aspects of our study: first, we used a novel ultrasonic algorithm to study changes in myocardial microstructure in individuals with metabolic dysfunction and obesity. Previous studies have focused on integrated ultrasonic signal intensity variation, historically termed ‘backscatter’, which examines sonographic properties through the cardiac cycle as a reflection of intrinsic scattering properties of the myocardium (28, 29). Specifically, integrated backscatter measures correlate directly with histologic myocardial collagen content and fibrosis (10, 11, 30). In clinical studies, integrated backscatter has been shown to be abnormal in a variety of conditions, including hypertension (31), and coronary artery disease (32). More relevant to our study population, integrated backscatter appears to be abnormal in diabetic cardiomyopathy (13, 14) where backscatter correlates with severity of insulin resistance and glycated hemoglobin (33), and also predicts worse exercise capacity (34). Abnormalities are also seen in individuals with obesity and MetS, where clinical correlates include abdominal adiposity (15). Further, antifibrotic therapy with spironolactone has been associated with improvements in integrated backscatter among people with diabetes (16) and obesity (17), both with impaired diastolic function. However, previous integrated techniques have been limited in utility due to the prospective application of complex imaging algorithms, variability due to poor quality images and random noise.(35)
We recently validated a novel image analysis algorithm, which can be applied to routinely acquired echocardiographic images using open access software (18, 19). This algorithm appears to be a sensitive measure of cardiac microstructure among those with hypertensive heart disease, where the SIC was more strongly associated with blood pressure elevation compared with traditional echocardiographic measures (18). In addition, alterations in microstructure were detected among patients with hypertrophic cardiomyopathy, as well as sarcomere gene mutation carriers in the absence of left ventricular hypetrophy. Importantly, the SIC was correlated with extracellular volume measures on cardiac magnetic resonance imaging, as well as other measures of LV diastolic function (20). We now extend these findings to cardiometabolic disease, and show that the SIC is abnormal in individuals with obesity and metabolic disease, even after accounting for hypertension. We and others have previously shown abnormalities in cardiac mechanics and diastolic function in individuals with obesity-related metabolic disease (5, 36). The current study suggests that along with functional abnormalities of the myocardium, structural changes are also detectable in a preclinical population.
The second notable finding of our study is that alterations in myocardial signal intensity variation are most strongly correlated with dyslipidemia among individuals with obesity, rather than elevated blood pressure. It is important to note that these results differ from a previous study showing a strong association with blood pressure (18). This may be due to differences in hypertension severity, with the current sample being younger with lower blood pressures compared with the prior study, allowing us to study individuals with obesity in the absence of severe hypertension. Cardiac structural changes in those with obesity and metabolic dysfunction include interstitial fat infiltration and myocyte steatosis, as well as fibrosis (7, 8, 37, 38). The underlying mechanisms of fibrosis remain poorly understood, with likely contributions from abnormal loading conditions and hypertension, systemic inflammation, and metabolic dysfunction (7). Our results highlight the importance of metabolic dysfunction, and in particular the association of dyslipidemia in altered ultrasonic signal intensity variation. The triglyceride-to-HDL ratio serves as a strong surrogate of insulin resistance (26), and it may be that hypertriglyceridemia in our sample is a marker of systemic metabolic dysfunction. It is also known that myocardial-specific lipid accumulation can directly lead to cardiac dysfunction and fibrosis. In experimental models of cardiomyocyte-specific overexpression of diacylglycerol acyl transference, myocardial triglyceride accumulation was associated with increased cardiac fibrosis and eventual cardiomyopathy (39). In human studies of diabetes, cardiac steatosis as assessed by magnetic resonance spectroscopy independently predicted concentric remodeling and cardiac mechanics, though interestingly was not associated with extracellular volume fraction (40).Whether the association of dyslipidemia and microstructural alterations in our study are due to systemic or cardiac-specific effects remains unknown.
Several limitations are worth mentioning. Our study design was cross-sectional and observational, thus causal inferences cannot be drawn, and the clinical implications of the described subclinical changes in myocardial microstructure remain to be elucidated. It is important to note that ultrasonic signal intensity variation can be influenced by many factors, including collagen content and fibrosis, myocyte size, sarcomere length, as well as fiber orientation (30). While a previous study showed correlation of the SIC with myocardial fibrosis ascertained by cardiac magnetic resonance imaging (20), such data are not available in our sample. Thus, abnormalities observed in our study may reflect changes in myocardial architecture due to a number of different processes that remain unclear, including possible myocyte steatosis and fat infiltration. Thus further studies are needed to elucidate potential clinical implications. We do not have measures of endothelial function or inflammation to further elucidate potential clinical correlates of ultrasonic signal intensity variation. Ours was a modest sample, and residual confounding could have influenced results. Lastly, our study sample was predominantly comprised of women, and the group with obesity without MetS in particular was comprised predominantly of premenopausal women, limiting generalizability to other populations. Hence, findings need to be validated in larger populations in future studies.
In summary, we used a novel ultrasonic image analysis algorithm to demonstrate that metabolic dysfunction was associated with altered myocardial signal intensity variation among obese individuals. Specifically, the SIC was more strongly correlated with hypertriglyceridemia compared with blood pressure. The SIC may represent a sensitive imaging modality to evaluate subclinical myocardial changes in cardiometabolic disease prior to progression to clinically overt cardiovascular disease. The ability to detect microstructural changes may help identify at-risk individuals, and future studies evaluating potential targeted preventive efforts in metabolic disease are warranted.
Supplementary Material
Table S1. Diastolic parameters by SIC and LV mass groups
What is known about this subject?
-
-
People with obesity and metabolic disease are at risk for the development of heart failure
-
-
Animal models support the development of cardiac fibrosis as a driver of cardiac remodeling in obesity
-
-
Ultrasound scattering properties are directly correlated with histologic myocardial collagen content
What does this study add?
-
-
We demonstrate alterations in myocardial microstructure in people with metabolic syndrome using a novel algorithm to characterize ultrasonic signal intensity variation
-
-
Dyslipidemia in particular is correlated with myocardial signal intensity variation
-
-
Future studies are needed to determine whether ultrasonic signal intensity variation may help detect subclinical disease in people with metabolic disease
Acknowledgments
None
Funding sources: This work was partially supported by National Heart, Lung and Blood Institute grants K23-HL116780 (Dr. Ho), a Massachusetts General Hospital Hassenfeld Scholar Award (Dr. Ho), NO1-00239 (Dr. Colucci), and R01HL131532 (Dr. Cheng).
Footnotes
Disclosures: None
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Wang YC, Liang CS, Gopal DM, et al. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6:142–152. doi: 10.1161/CIRCIMAGING.111.964627. [DOI] [PubMed] [Google Scholar]
- 7.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. American College of Physicians and the American Physiological Society.Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Kitamori K, Ichihara G, et al. Serial changes in adipocytokines and cardiac function in a rat model of the metabolic syndrome. Clin Exp Pharmacol Physiol. 2013;40:443–448. doi: 10.1111/1440-1681.12107. [DOI] [PubMed] [Google Scholar]
- 10.Hoyt RH, Collins SM, Skorton DJ, Ericksen EE, Conyers D. Assessment of fibrosis in infarcted human hearts by analysis of ultrasonic backscatter. Circulation. 1985;71:740–744. doi: 10.1161/01.cir.71.4.740. [DOI] [PubMed] [Google Scholar]
- 11.Picano E, Pelosi G, Marzilli M, et al. In vivo quantitative ultrasonic evaluation of myocardial fibrosis in humans. Circulation. 1990;81:58–64. doi: 10.1161/01.cir.81.1.58. [DOI] [PubMed] [Google Scholar]
- 12.Hall CS, Scott MJ, Lanza GM, Miller JG, Wickline SA. The extracellular matrix is an important source of ultrasound backscatter from myocardium. J Acoust Soc Am. 2000;107:612–619. doi: 10.1121/1.428327. [DOI] [PubMed] [Google Scholar]
- 13.Di Bello V, Talarico L, Picano E, et al. Increased echodensity of myocardial wall in the diabetic heart: an ultrasound tissue characterization study. J Am Coll Cardiol. 1995;25:1408–1415. doi: 10.1016/0735-1097(95)00026-Z. [DOI] [PubMed] [Google Scholar]
- 14.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41:611–617. doi: 10.1016/s0735-1097(02)02869-3. [DOI] [PubMed] [Google Scholar]
- 15.Kosmala W, Przewlocka-Kosmala M, Wojnalowicz A, Mysiak A, Marwick TH. Integrated backscatter as a fibrosis marker in the metabolic syndrome: association with biochemical evidence of fibrosis and left ventricular dysfunction. Eur Heart J Cardiovasc Imaging. 2012;13:459–467. doi: 10.1093/ejechocard/jer291. [DOI] [PubMed] [Google Scholar]
- 16.Jellis CL, Sacre JW, Wright J, et al. Biomarker and imaging responses to spironolactone in subclinical diabetic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2014;15:776–786. doi: 10.1093/ehjci/jeu013. [DOI] [PubMed] [Google Scholar]
- 17.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 18.Hiremath P, Bauer M, Aguirre AD, et al. Identifying early changes in myocardial microstructure in hypertensive heart disease. PLoS ONE. 2014;9:e97424. doi: 10.1371/journal.pone.0097424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiremath P, Bauer M, Cheng HW, Unno K, Liao R, Cheng S. Ultrasonic assessment of myocardial microstructure. J Vis Exp. 2014:e50850. doi: 10.3791/50850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiremath P, Lawler PR, Ho JE et al. Ultrasonic Assessment of Myocardial Microstructure in Hypertrophic Cardiomyopathy Sarcomere Mutation Carriers With and Without Left Ventricular Hypertrophy. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 24.De Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 25.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 27.Arsenault BJ, Lemieux I, Després JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182:1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olshansky B, Collins SM, Skorton DJ, Prasad NV. Variation of left ventricular myocardial gray level on two-dimensional echocardiograms as a result of cardiac contraction. Circulation. 1984;70:972–977. doi: 10.1161/01.cir.70.6.972. [DOI] [PubMed] [Google Scholar]
- 29.Holland MR, Gibson AA, Peterson LR, et al. Measurements of the cyclic variation of myocardial backscatter from two-dimensional echocardiographic images as an approach for characterizing diabetic cardiomyopathy. J Cardiometab Syndr. 2006;1:149–152. doi: 10.1111/j.1559-4564.2006.05493.x. [DOI] [PubMed] [Google Scholar]
- 30.Ciulla M, Paliotti R, Hess DB, et al. Echocardiographic patterns of myocardial fibrosis in hypertensive patients: endomyocardial biopsy versus ultrasonic tissue characterization. J Am Soc Echocardiogr. 1997;10:657–664. doi: 10.1016/s0894-7317(97)70028-2. [DOI] [PubMed] [Google Scholar]
- 31.Maceira AM, Barba J, Varo N, Beloqui O, Díez J. Ultrasonic backscatter and serum marker of cardiac fibrosis in hypertensives. Hypertension. 2002;39:923–928. doi: 10.1161/01.hyp.0000014616.48920.8f. [DOI] [PubMed] [Google Scholar]
- 32.Marini C, Picano E, Varga A, Marzullo P, Pingitore A, Paterni M. Cyclic variation in myocardial gray level as a marker of viability in man. A videodensitometric study. Eur Heart J. 1996;17:472–479. doi: 10.1093/oxfordjournals.eurheartj.a014882. [DOI] [PubMed] [Google Scholar]
- 33.Gibson AA, Schaffer JE, Peterson LR, et al. Quantitative analysis of the magnitude and time delay of cyclic variation of myocardial backscatter from asymptomatic type 2 diabetes mellitus subjects. Ultrasound Med Biol. 2009;35:1458–1467. doi: 10.1016/j.ultrasmedbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jellis CL, Stanton T, Leano R, Martin J, Marwick TH. Usefulness of at rest and exercise hemodynamics to detect subclinical myocardial disease in type 2 diabetes mellitus. Am J Cardiol. 2011;107:615–621. doi: 10.1016/j.amjcard.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno R, Fujimoto S, Saito Y, Nakamura S. Non-invasive quantitation of myocardial fibrosis using combined tissue harmonic imaging and integrated backscatter analysis in dilated cardiomyopathy. Cardiology. 2007;108:11–17. doi: 10.1159/000095595. [DOI] [PubMed] [Google Scholar]
- 36.Crendal E, Walther G, Vinet A, et al. Myocardial deformation and twist mechanics in adults with metabolic syndrome: Impact of cumulative metabolic burden. Obesity (Silver Spring) 2013;21:E679–E686. doi: 10.1002/oby.20537. [DOI] [PubMed] [Google Scholar]
- 37.De Simone G, Izzo R, De Luca N, Gerdts E. Left ventricular geometry in obesity: Is it what we expect? Nutr Metab Cardiovasc Dis. 2013;23:905–912. doi: 10.1016/j.numecd.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Calligaris SD, Lecanda M, Solis F, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS ONE. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glenn DJ, Wang F, Nishimoto M, et al. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–222. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levelt E, Mahmod M, Piechnik SK, et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes. 2016;65:44–52. doi: 10.2337/db15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diastolic parameters by SIC and LV mass groups


