Abstract
Despite a high rate of early revascularization and use of intraaortic balloon pump counterpulsation (IABP) therapy, the prognosis of patients with cardiogenic shock (CS) has remained poor. In the hopes of improving outcomes, clinicians are increasing turning to percutaneous left- and right- mechanical circulatory support devices. Until recently, the evidence base for these devices had consisted only of observational data, meta-analyses, and small feasibility trials. In this manuscript, we describe the contemporary outcomes of patients with CS, the hemodynamics of CS, and hemodynamic effects of percutaneous mechanical circulatory support devices. We then use this discussion to provide clinicians with a useful framework for understanding when selecting between or while managing patients with a percutaneous mechanical circulatory support devices. We critically review the recently published data for and against the use of commercially-available devices – the IABP, the Impella system, the TandemHeart, and V-A ECMO - and highlight gaps in our understanding. Given such gaps, a consensus multidisciplinary approach which combines expertise from interventional cardiologists, heart failure specialists, cardiac surgeons, and cardiac anesthesiologists may help pair the right patient with the right device at the right time.
Introduction
The incidence of cardiogenic shock (CS) is increasing. Data from the Nationwide Inpatient Sample (NIS), the largest publicly available all-payor inpatient care database in the United States, shows a more than two-fold rise in the number of discharges complicated by CS, from 55,123 in 2004 to 126, 555 in 2014 (ptrend < 0.05; Figure 1). Despite a high rate of early revascularization and use of intraaortic balloon pump counterpulsation (IABP) therapy, the prognosis of patients with CS has remained poor, with 48% of patients in 2014 not surviving to discharge (Figure 1). Rapid innovation in percutaneous left- and right-ventricular mechanical circulatory support (MCS) devices is fundamentally altering the management of CS, requiring not only technical proficiency with these devices but also novel models of team-based care among Cardiologists, Cardiac Surgeons, Heart failure specialists, and Cardiac Anesthesiologists. The purpose of this manuscript is to review the hemodynamics and outcomes associated with CS, to describe the hemodynamic effects of contemporary MCS devices, and to highlight and review the evidence regarding left-and right- ventricular support devices.
Figure 1.
We queried the 2004–2014 Nationwide Inpatient Sample databases to identify all patients with CS (ICD-9 CM 785.51). The Mantel-Haenszel X2 test to analyze trends. (A) The overall incidence of CS increased from 55,123 discharges in 2004 to 126, 555 discharges in 2014 (ptrend = 0.002). (B) In-hospital mortality decreased from 62% in 2004 to 48% in 2014 (ptrend = 0.02).
Cardiogenic shock (CS)
CS is a state of end-organ dysfunction often attributed to insufficient cardiac output due to LV, RV, or biventricular dysfunction. However, CS is not simply a decrease in cardiac contractile function, but also a multi-organ dysfunction syndrome (MODS) involving the entire circulatory system, often complicated by a systemic inflammatory response syndrome (SIRS). 1 Interestingly, SIRS scoring systems (e.g. APACHE II or SAPS II) and biomarkers of SIRS (IL-6 and receptor of advanced glycation end-products [RAGE]) can more accurately predict mortality in CS than hemodynamic indices or biomarkers of heart failure. 2
Clinically, CS is defined by both hemodynamic parameters (persistent hypotension [systolic blood pressure <80–90 mm Hg or mean arterial pressure 30 mm Hg lower than baseline], a cardiac index <1.8 L/min/m2 without support or <2.0 to 2.2 L/min/m2 with support, and elevated filling pressures [LV end-diastolic pressure >18 mm Hg or RV end-diastolic pressure >10–15 mm Hg]) as well as clinical signs/symptoms of hypoperfusion (cool extremities, decreased urine output, and/or altered mental status). 1
Several cohorts with CS may benefit from the use of percutaneous MCS devices (Table 1). In the United States, acute myocardial infarction (AMI) remains the most common precipitant of CS. From 2003–2010, while the incidence of CS complicating AMI rose from 6.5% to 10.1% (p<0.001), in-hospital mortality decreased significantly from 44.6% to 33.8% (p<0.001). 3 Among patients with congestive heart failure not precipitated by AMI, the incidence of CS rose from 0.5% to 1.0% (p<0.001), while in-hospital mortality decreased significantly from 44.2% to 26.1%. 4
Table 1.
Cohorts that may benefit from percutaneous left- and right-ventricular MCS devices.
| AMI without mechanical complications complicated by CS |
| Acute or acute-on-chronic LV or biventricular failure complicated by CS (bridge-to-recovery, bridge-to-bridge, or bridge-to-transplantation) |
| Peri-partum cardiomyopathy |
| Tatosubo/stress-induced cardiomyopathy |
| Cardiac allograft failure/rejection |
| Acute myocarditis |
| Post-cardiotomy |
| Hypertrophic cardiomyopathy with severe outflow tract obstruction |
| Acute RV failure complicated by CS |
| Post-LVAD implantation |
| Post-cardiotomy |
| Post-transplantation |
| Pulmonary embolism |
| Refractory arrhythmias |
| (Extracorporeal Cardiopulmonary resuscitation) eCPR |
Hemodynamic Effects of MCS Devices
Current modes of percutaneous MCS can be characterized by one of four circuit configurations: 1) LV to aorta assist devices (IABP and Impella); 2) LA to systemic artery assist devices (TandemHeart); 3) RA to systemic artery assist devices (V-A ECMO); and 4) RA to PA assist devices (Impella RP and adapted TandemHeart). To varying degrees, all available devices improve cardiac output and blood pressure, but their specific features result in distinct hemodynamic profiles. 5 The implications of these differences on clinical outcomes have not yet been studied in adequately powered randomized trials. Contemporary, commercially available percutaneous left- and right- ventricular MCS devices may be seen in Figure 2.
Figure 2.
Contemporary, commercially available percutaneous left- (Panel A) and right- (Panel B) ventricular mechanical support devices.
Several key concepts exist that may provide clinicians with a useful framework for understanding when choosing a percutaneous MCS device for a patient or while managing a patient with a percutaneous MCS device. First, derangements in RV systolic and diastolic function and pulmonary vascular resistance (PVR) have a major impact on the performance characteristics of LV to aorta (IABP therapy and Impella LP) and LA to systemic artery (TandemHeart) assist devices. In these cases, percutaneous V-A ECMO or the combination of an LV assist device with an RV assist device (Impella RP and adapted TandemHeart) may be necessary. Second, IABP therapy and the Impella are associated with significant decreases in total peripheral resistance (TPR) via the lowering of both peak LV systolic and diastolic pressures as well as improved coronary perfusion, although Impella likely has a greater effect than IABP. 6 In contrast, significant flow-mediated increases in TPR are seen with percutaneous V-A ECMO. If TPR and LV contractility are fixed, the only way for the LV to overcome increased TPR is via increases in the LVEDP, LA pressure, and PCWP. These changes markedly increase myocardial oxygen consumption (MVO2) and may inhibit LV recovery. Thus, except when acute LV recovery is anticipated following initiation of MCS (e.g. extracorporeal cardiopulmonary resuscitation [eCPR]), V-A ECMO is most often used as a bridge-to-bridge or bridge-to-transplant. While increases in TPR are also seen with the TandemHeart, given that blood is withdrawn directly from the LA, large decreases in the LVEDP, LA pressure, and PCWP are typically seen. Third, continuous pumping of blood from the LV (Impella) results in proportionally greater degrees of LV unloading than pumping from the LA or RA (Tandem Heart).
Left ventricle to aorta assist devices (LV volume and/or pressure unloading)
Intraaortic balloon pump (IABP) counterpulsation therapy
Introduced nearly five decades ago, IABP therapy is commonly used in patients with CS on the assumption that it is associated with hemodynamic improvements including decreased myocardial oxygen consumption, increased coronary artery perfusion, decreased afterload, and modestly enhanced cardiac output (0.8–1.0 L/min). 5 IABP therapy typically requires an 8 Fr sheath in the femoral or axillary artery.7 (Table 2).
Table 2.
Comparison of left- and right-ventricular percutaneous mechanical circulatory support devices. Adapted with permission from reference 7.
| IABP (Ascending Ao) | Impella 2.5 (LV/LVOT) | Impella 5.0 (LV/LVOT) | TandemHeart (LA to systemic artery) | ECMO (Femoral artery and vein) | TandemHeart (RA to Ao) | Impella RP (RA to PA) | |
|---|---|---|---|---|---|---|---|
| Primary hemodynamic effect(s) | LV volume and/or pressure unloading | LV volume and/or pressure unloading | LV volume and/or pressure unloading | LV volume unloading | Biventricular pressure and volume unloading | RV pressure unloading | LV volume and/or pressure unloading |
| TPR | Decreased | Decreased | Decreased | Mildly increased | Highly increased | None | None |
| Bridge-to-recovery | Yes | Yes | Yes | Yes | No* | Yes | Yes |
| Hemodynamic support | Low | Moderate | High | High | High | High | Moderate |
| Pump mechanism | Pneumatic | Axial flow | Axial flow | Centrifugal | Centrifugal | Centrifugal | Axial flow |
| Cannula size | 7.9 Fr | 13 Fr | 22 Fr | 21 Fr inflow; 15–17 Fr outflow | 18–21 Fr inflow;15–22 Fr outflow | 21 Fr inflow; 15–17 Fr outflow | 13 Fr |
| Implantation time | Very low | Low | Moderate | High | Moderate | High | Low |
| Risk of limb ischemia | Very low | Low | Low | High | High | High | Low |
| Anticoagulation | Very low | Very low | Very low | High | High | High | Very low |
| Hemolysis | Very low | Low | Low | Low | Low | Low | Low |
| Post-implantation management complexity | Very low | Moderate | Moderate | High | Very high | High | Moderate |
Except in extracorporeal cardiopulmonary resuscitation (eCPR)
IABP therapy in AMI patients with CS
Until recently, the evidence base for IABP therapy in AMI patients with CS had consisted only of observational data 8–21 and meta-analyses 22–25, perhaps because few alternatives had been available to support patients with severely compromised hemodynamics.
Prospective randomized controlled trials have failed to demonstrate conclusive a proof of benefit in patients with CS due to AMI.
In 2005, the TACTICS trial randomized 57 AMI patients with CS status post fibrinolytic therapy to 48 hours of IABP therapy or optimal medical therapy. The primary endpoint was all-cause mortality at 6 months. Secondary endpoints included in-hospital death, re-infarction, and safety events. At 6 months, 43% of the fibrinolysis-only group had died versus 34% of the fibrinolysis-IABP group (p=0.23). Among those patients with Killip class>II, 6-month mortality was significantly lower in the fibrinolysis-IABP group when compared with fibrinolysis only (39% versus 80%, respectively; p=0.05). Similar rates of in-hospital death, re-infarction, and safety events were seen.
Prondzinsky et al randomized 45 AMI patients with CS status post primary PCI to IABP therapy or optimal medical therapy. 26 The primary endpoint was change in Acute Physiology and Chronic Health Evaluation (APACHE) II scores over 4 days. Secondary endpoints included inflammatory markers, BNP levels, hemodynamic values, and in-hospital mortality. APACHE II scores, IL-6 levels, and CI were not significantly different between the cohorts at 4 days while BNP levels were lower in patients receiving IABP therapy (suggesting an element of LV unloading). In-hospital mortality was not significantly different between the two groups (38.6% versus 28.6% for IABP therapy and optimal medical therapy, respectively; p=ns).
The IABP-SHOCK II trial was a multi-center, open label study that randomized 600 AMI patients with CS of less than 12 hrs duration not due to mechanical causes (eg. ventricular septal defect or papillary muscle rupture) and not requiring CPR for more than 30 minutes or clinically comatose to IABP therapy or optimal medical therapy. 27 Interestingly, no significant differences were seen in the study groups with respect to 30-day mortality (primary outcome; 39.7% in the IABP group vs. 41.3% in the optimal medical therapy group, RR 0.96, 95% CI 0.79–1.17, p=0.69) nor time to hemodynamic stabilization, the length of stay in the intensive care unit, serum lactate levels, the dose and duration of catecholamine therapy, renal function, and adverse events (eg. bleeding, stroke, peripheral ischemic complications requiring intervention, or infection). In pre-specified subgroup analyses, a significant benefit of IABP therapy was seen only in patients under the age of 50 and those with a first myocardial infarction. Limitations of this study included its relatively small sample size (>900 patients would have been required to detect the specified treatment effect); the preponderance of more patients with mild to moderate CS (average SBP 89 mm Hg and average HR 92) compared with other trials and registries, leading to questions about its applicability to more severe CS; a low rate (13.4%) of IABP insertion before PCI (when the risk of hemodynamic compromise is the greatest); the number of crossovers from the optimal medical therapy group to the IABP group (10%), which may have influenced the analysis that was done on an intention-to-treat principle; and lack of longer-term outcomes data, especially given that the overall event rate was lower than expected. It remains an important trial and following its publication, the 2012 ESC Guidelines downgraded the use of IABP in STEMI patients with CS from a 1C recommendation to 2B. 28 The 2013 ACC/AHA guidelines, which do not incorporate the results of the IABP-SHOCK II trial, maintain IABP therapy as a 2A recommendation in STEMI patients with CS. 29
Recent meta-analyses of IABP therapy in AMI patients with CS (incorporating the results of the IABP-SHOCK II trial) have further called into question the utility of IABP therapy in these patients. Analyzing data from 17 studies, Romeo et al reported no overall differences in short- or long-term mortality in patients receiving IABP therapy. 30 Interestingly, when stratified by initial treatment, IABP therapy significantly reduced mortality (RR 0.77, 95% CI 0.68–0.87) in patients receiving thrombolytic therapy but significant increased mortality (RR 1.18, 95% CI 1.04–1.34) in patients receiving primary PCI. More recently, using data from 12 RCTs and 15 observational studies, Ahmed et al reported no benefit of IABP therapy in AMI on 30-day mortality, regardless of the presence (OR 0.94, 95% CI 0.69–1.28) or absence (OR 0.98, 95% CI 0.57–1.69) of CS. 31
IABP therapy in advanced heart failure patients with CS
While limited to small, single-center studies, 32–35 IABP therapy in advanced heart failure patients with CS appears to be a reasonable bridge to more advanced therapies.
Gjesdal et al compared the outcomes of 32 patients with advanced heart failure patients treated with IABP therapy as a bridge to transplantation with 135 electively transplanted patients. 32 The mean duration of IABP therapy was 21±16 days and 80% of patients survived to transplant without additional mechanical circulatory support. 3 patients developed vascular complications, 2 patients developed sepsis, and 1 patient developed ileus, corresponding to 0.05 complications per patient-week of support. Most notably, mortality was similar in the IABP and electively transplanted cohorts at 1-year (9.4% versus 11.1%, p=0.80).
More recently, axillary IABPs have generated considerable interest as they permit ambulation and limit debility during the waiting period for advanced therapies. Among 50 patients with end-stage heart failure awaiting transplantation, 92% survived to transplantation on left axillary-subclavian artery IABP support for a median of 18 days. 90-day post-transplant survival was 90%. 34 Prior to transplant, patients received a median of 3 (2 to 15) nursing-guided ambulation sessions. No significant bleeding or arterial ischemic complications were seen at the time of IABP placement. Prior to transplantation, 2 patients required surgical evaluation for acquired left-hand ischemia and 3 patients underwent removal of the IABP secondary to axillary IABP complications. IABP removal was followed by percutaneous closure with an 8-Fr Angioseal device in 58% of patients. Similarly, Tanaka et al reported outcomes in 88 patients with end-stage heart failure awaiting transplantation who received axillary-subclavian IABP therapy. 36 80% of patients their IABPs surgically implanted in the right subclavian artery. The median duration of IABP support was 21 days (maximum 135 days). 93.2% of patients survived to recovery, transplantation, or durable mechanical support. 95.5% of patients ambulated extensively and demonstrated a significant increase in their aerobic capacity, as assessed by the 2-minute step-in-place test. Access site complications (hematomas, infection, asymptomatic bleeding) occurred in 8 (9.1%) patients.
Impella
The Impella (AbioMed, Danvers, MA, USA) is a continuous, non-pulsatile, axial flow Archimedes-screw pump that provides active support by expelling aspirated blood from the LV into the ascending aorta. (Figure 2) Importantly, unlike IABP therapy, the Impella does not require EKG or arterial waveform triggering, facilitating stability even in the setting of tachyarrhythmias or electromechanical disassociation. However, careful patient selection and meticulous attention to good technique are critical given reported complications, which include device migration, device malfunction due to thrombosis, hemolysis, bleeding requiring transfusion, arrhythmias, limb ischemia, tamponade, aortic or mitral valve injury, and stroke. 37, 38
Three versions for LV support are currently available: the Impella LP 2.5 that can deliver 2.5 L/min of CO, the Impella CP that can deliver 3.7 L/min of CO, and the Impella LP 5.0 that can deliver 5.0 L/min of CO. While the Impella LP 2.5 and Impella CP can be delivered percutaneously via a 12–14 Fr sheath, insertion of the Impella LP 5.0 requires surgical cutdown of the femoral or axillary artery prior to insertion of a 22 Fr sheath.
Impella therapy in AMI patients with CS
The ISAR-SHOCK trial was a 2-center, randomized controlled pilot study that randomized 26 AMI patients with CS to hemodynamic support with the Impella LP 2.5 or IABP therapy. 39 The primary endpoint was change in cardiac index (CI) from baseline to 30 min after implantation. Secondary endpoints included lactic acidosis, hemolysis, and mortality after 30 days. While the CI after 30 min of support was significantly increased in patients with the Impella LP 2.5 compared with patients with IABP therapy (0.49±0.46 versus 0.11±0.31 l/min/m2 respectively; p=0.02), no significant differences were seen by 4 hrs in the CI, modified cardiac power index (CPI), or serum lactate. By 24 hrs, no significant differences were seen in urine output, vasopressor dose, median vasopressor support time, or mechanical ventilation support time. Hemolysis and packed red blood cell or fresh frozen plasma administration were significantly higher in patients with the Impella LP 2.5 and 1 case of acute limb ischemia requiring surgery after device explanation was seen. Overall 30-day mortality was 46% in both groups. The most obvious limitation of this pilot study was its small number of patients, which precluded a meaningful evaluation of potential mortality differences.
More recently, Ouweneel et al reported results from the IMPRESS trial, which randomized 48 AMI patients with CS to hemodynamic support with the Impella CP or IABP therapy. 37 Device placement occurred at the discretion of the treating physician, either prior to PCI, during PCI, or immediately after PCI. The primary and secondary endpoints were 30-day and 6-month mortality, respectively. Notably, 92% of study population had a history of recent cardiac arrest requiring resuscitation. At 30 days, mortality was similar (50% versus 46% for patients receiving support with the Impella CP or IABP therapy, respectively; p=0.92). 6-month mortality was 50% in both groups. More bleeding events (8 versus 2) occurred in patients receiving support with the Impella CP than in the IABP group, respectively, and significant hemolysis requiring the cessation of Impella CP therapy occurred in 2 patients.
Impella therapy in post-cardiotomy patients with CS
The RECOVER I feasibility study evaluated the safety and efficacy of the Impella LD 5.0 in post-cardiotomy CS patients. 40 In 16 patients, the Impella LP 5.0 significantly improved hemodynamic indices following insertion and was associated with 94%, 81%, and 75% survival at 30 days, 6 months, and 1 year, respectively. The primary safety endpoint (a composite of death and stroke) occurred in 2 patients. Other complications included bleeding requiring re-operation (7 patients), sepsis (6 patients), renal failure (3 patients), hepatic failure (1 patient), and major vascular injury (1 patient).
IABP therapy in advanced heart failure patients with CS
In a single case series, the Impella LP 5.0 has been shown to be effective as a bridge-to-transplantation, a bridge-to-bridge, and a bridge-to-recovery. 41
Left atrium to systemic artery assist devices (Left ventricular volume unloading)
TandemHeart
The TandemHeart (TandemLife, Pittsburgh, PA, USA) provides mechanical circulatory support of up to 4 L/min via a continuous flow centrifugal pump. Oxygenated blood is withdrawn from the left atrium via a 21 Fr inflow cannula placed via trans-septal puncture and then re-injected into the lower abdominal aorta or iliac arteries via a 15–17 Fr outflow cannula. (Figure 2) The TandemHeart is inserted through the femoral vein and is advanced across the inter-atrial septum into the LA. The need for trans-septal puncture is a potential limitation for operators not facile with this technique.
TandemHeart therapy in AMI patients with CS
In 2005, Thiele et al reported their early experience with TandemHeart therapy in AMI patients with CS. 42 41 AMI patients with CS were randomized to hemodynamic support with either IABP therapy or the TandemHeart. The primary endpoint was hemodynamic improvement (as measured by the cardiac power index [CPI]). Secondary endpoints included 30-day mortality and safety endpoints. While greater improvements in CPI, CI, PAP, and PCWP were seen in patients receiving the TandemHeart, 30-day mortality was similar (43% versus 45%, p=0.86) in both groups. In the TandemHeart cohort, 7 patients (33%) developed limb ischemia requiring intervention (IABP n=0; p=0.009) 19 patients (90%) required blood transfusions (median 8.0 units, IQR 3.8–16.5 units; IABP n=8; p=0.002), and 13 patients (62%) had signs of DIC (IABP n=3; p=s).
The TandemHeart Investigators Group randomized 33 AMI patients with CS to treatment with IABP therapy or the TandemHeart. 43 Major exclusion criteria included coagulopathy, sepsis, stroke within 6 months, severe peripheral vascular disease, isolated right heart failure, ≥2+ aortic regurgitation, and ventricular septal rupture. The primary endpoint was hemodynamic changes. Secondary endpoints included safety and 30-day mortality. Compared with IABP therapy, patients receiving the TandemHeart had significantly greater increases in CI (0.6±0.6 versus 1.2±0.8 L/min, respectively) and significantly greater decreases in PCWP over the first 16 hours. The percentage of patients with at least 1 adverse event was not significantly different between patients receiving IABP therapy and the TandemHeart (p=0.12), with bleeding being the most common adverse event in both groups. 30-day mortality was 36% in the IABP group compared with 47% in the TandemHeart group (p=ns).
In 2011, Kar et al reported outcomes following TandemHeart therapy in 80 AMI patients with CS. 44 Notably, almost half of these patients had undergone CPR immediately before or at the time of implantation. TandemHeart therapy was associated with significant improvements in hemodynamic indices. The 30-day and 6-month mortality rates were 40.2% and 45.3%, respectively. Notably, 1 patient died following wire-mediated perforation of the left atrium. Other complications included the need for blood transfusions (71%), sepsis/SIRS (29.9%), bleeding around the cannula (29.1%), gastrointestinal bleeding (19.7%), coagulopathy (11%), stroke (6.8%), and device-related limb ischemia (3.4%).
TandemHeart therapy in advanced heart failure patients with CS
Kar et al also reported outcomes following TandemHeart therapy in 37 NICM patients with CS. 44 In the NICM group, the 30-day and 6-month mortality rates were 32% and 35%, respectively (when compared with ICM, p=ns). Fewer blood transfusions were needed (35.1% versus 71%) in patients with NICM when compared with patients ICM. The TandemHeart has been validated in smaller case series as a bridge-to-transplantation, bridge-to-bridge, bridge-to-decision, and bridge-to-recovery. 45–47
RA to systemic artery assist devices (Biventricular pressure and volume unloading)
Venous-Arterial extracorporeal membrane oxygenation (V-A ECMO)
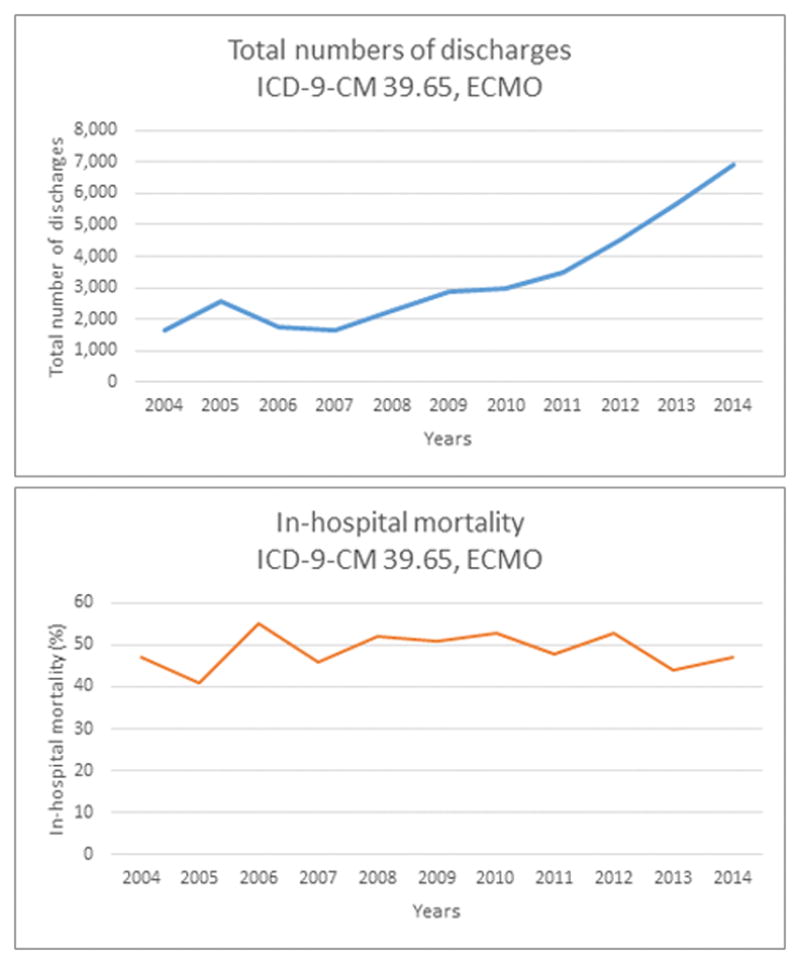
While the use of ECMO has increased from 2004–2014 (ptrend < 0.05), outcomes remain poor with an in-hospital mortality of 47% in 2014 (Figure 3).
Figure 3.

We queried the 2004–2014 Nationwide Inpatient Sample databases to identify all patients undergoing ECMO (ICD-9 CM 39.65). The Mantel-Haenszel X2 test was used to analyze trends. (A) ECMO was used in 1,653 patients in 2004 and 6,890 patients in 2014 (ptrend = 0.005). (B) In-hospital mortality was stable between 2004 and 2014 at 47% (ptrend = 0.80).
In peripheral V-A ECMO, blood is aspirated via a 18–21 Fr venous inflow cannula in the femoral or internal jugular vein, directed into a membrane oxygenator, and returned to the arterial system via a 15–22 Fr outflow cannula in the femoral or axillary artery, thereby bypassing the heart and lungs and providing mechanical circulatory support of >4.5 L/min. (Figure 2)
There are two important caveats to its use. First, despite adequate peripheral unloading, venous blood return to the left heart (primarily from the bronchial circulation) in the setting of peripheral flow-mediated elevated TPR can result in blood pooling in the LV and thereby elevated LV pressures. 6, 48 The clinical consequences of this may include LV thrombus as well as pulmonary edema. While concomitant IABP therapy to “vent” the LV does not improve outcomes in patients receiving V-A ECMO, 49 IABP therapy does effectively lower PA artery pressures and decrease LV dimensions. 48 Cheng et al have reported the use of the Impella 2.5 as an LV vent in patients on V-A ECMO with evidence of LV distension, resulting in a decreased LVEDD as measured by echocardiography (7.8±1.4 cm vs 6.2±0.8 cm, p=0.001). 50 Other approaches include percutaneous atrial septostomy (to allow left-to-right shunting) or a surgically placed LV vent.
Second, if femoral artery cannulation is chosen, steps must be taken to ensure the adequate retrograde flow of extracorporeally oxygenated blood into the arch and proximal ascending aorta to ensure delivery to the cerebral vessels and coronary arteries 51 This is especially important as native cardiac function recovers and the mixing of anterograde deoxygenated (in patients with respiratory failure) blood and retrograde (extracorporeally) oxygenated blood occurs. The serial monitoring of right upper extremity oxygen saturations and EKGs (for ST-T changes) may provide information on cerebral and cardiac oxygenation, respectively.
V-A ECMO in AMI patients with CS
The evidence base for V-A ECMO in AMI patients with CS not requiring CPR consists largely of small, single-center case series. In the largest of these, Muller et al assessed clinical and quality of life outcomes in 138 AMI patients with CS not requiring CPR receiving V-A ECMO. 52 47%, 41%, and 38% of patients were alive at discharge, 6 months, and 1 year, respectively. ECMO was a bridge-to-bridge in 18 patients and a bridge-to-transplant in 13 patients. ECMO-associated complications (leg ischemia, femoral hemorrhage due to arterial laceration, cannula insertion-site infection, pulmonary edema, and circuit-related hemolysis) occurred in 39% of patients.
V-A ECMO can also be considered in AMI patients with CS experiencing a cardiac arrest who remain refractory to initial resuscitative efforts, so called “eCPR.” 53–57 In 2008, Chen et al reported outcomes for 178 in-hospital cardiac arrest patients (117 patients diagnosed with AMI) receiving conventional CPR or eCPR. 56 Following propensity matching, there was still a significant difference in survival to discharge (HR 0.51, 95% CI 0.35–0.74), 30-day survival (HR 0.47, 95% CI 0.28–0·77), and 1-year survival (HR 0.53, 95% CI 0.33–0.83, p=0·006) favoring eCPR over conventional CPR. Thiagarajan et al analyzed 295 patients (75% with AMI) in the ELSO registry receiving eCPR and reported a survival of 27% in patients otherwise facing imminent mortality. 57 Similarly, Kagawa et al reported single-center outcomes in 86 patients with AMI who were unresponsive to conventional CPR. 54 Emergency coronary angiography was performed in 81 patients (94%) and intra-arrest PCI was performed in 61 patients (71%). The rates of ROSC, 30-day survival, and favorable neurologic outcomes were 88%, 29%, and 24%, respectively.
V-A ECMO in post-cardiotomy/advanced heart failure patients with CS
V-A ECMO has been used in patients with CS due to acute myocarditis, primary graft dysfunction, rejection, and as a bridge-to-bridge or bridge-to-transplant. 58, 59
Combes et al analyzed 65 patients receiving V-A ECMO (72% of patients were cannulated percutaneously) for CS secondary to dilated cardiomyopathy (n=18), fulminant myocarditis (n=16), post-cardiotomy (n=16), post-transplantation (n=10), and miscellaneous (n=5). 58 Peripheral ECMO was switched to central ECMO in 3 patients (6%) because of leg ischemia and/or inadequate support. In-hospital mortality was 58%. The majority of patients (57%) experienced more than 1 major ECMO-related complication including major bleeding (32%), femoral vein thrombosis (10%), arterial ischemia (19%), vena cava thrombosis (7.4%), surgical wound infection (17%), pulmonary edema (12%), and stroke (8.6%). Rastan et al reported outcomes for 517 patients requiring V-A ECMO following cardiac surgery. 59 203 (29.2%) patients were cannulated percutaneously. The mean duration of ECMO support was 3.28±2.85 days. Weaning from ECMO was successful in 63.3% of patients and 24.8% were discharged. Cumulative survival after 6 months, 1 year, and 5 years were 17.6%, 16.5%, and 13.7%, respectively. Risk factors for hospital mortality were age order than 70 years old (OR 1.6, 95% CI 1.01–2.69, p=0.049), diabetes (OR 2.47, 95% CI 1.48–4.13, p=0.001), and renal insufficiency (OR 2.11, 95% CI 1.04–4.29, p=0.038). No differences in mortality were seen between patients undergoing central or peripheral cannulation. Complication rates were similar to those described by Combes et al.
Right atrium (RA) to pulmonary artery (PA) assist devices
Acute RV failure may occur in multiple settings, including AMI, fulminant myocarditis, acute decompensated heart failure, acute pulmonary embolism, decompensated pulmonary hypertension, post-cardiotomy, OHT, and increasingly following LVAD implantation. When acute RV failure occurs, the mainstays of therapy include inotropic and pulmonary vasodilator support and the optimization of volume status.
Temporary mechanical support devices for RV failure are an attractive option because RV function often improves sufficiency (albeit over days to weeks) to allow for device removal.
RV pressure unloading
The RECOVER RIGHT study evaluated the safety and efficacy of the Impella RP (4.0 L/min of cardiac support) in 30 patients with RV failure refractory to medical therapy. 60 For the purposes of analysis, the cohort was divided into patients with RV failure following LVAD implantation and patients with RV failure following AMI or cardiotomy. At baseline, patients were on an average of 3.2 inotropes/pressors. Major exclusion criteria included patients with INTERMACS Profile 1 (“crash and burn”), anoxic brain injury, right heart thrombus, right heart prosthetic valves, severe pulmonary hypertension, mechanical complications after AMI, and patients supported with a surgical RVAD or ECMO. The primary endpoint was survival to 30 days or hospital discharge (whichever was longer). Secondary endpoints included safety parameters. The primary endpoint was achieved in 73.3% of the overall study population, with 83.3% of patients with RV failure following LVAD implantation and 58.3% of patients with RV failure following AMI or cardiotomy alive at 30 days or discharge. All discharged patients were alive at 180 days. Bleeding occurred in 60% of patients and hemolysis occurred in 13% patients, with no differences seen between the cohorts. These results are particularly compelling when considering that prior studies of RVAD devices in patients with CS reported survival rates of 42–57% to discharge. 61, 62 In 2015, based on the data above, the Impella RP received humanitarian device exemption (HDE) approval from the FDA for adult or pediatric patients with a BSA≥1.5 m2 who develop acute RV failure following LVAD implantation, AMI, heart transplantation, or cardiotomy.
RV volume unloading
In 2013, Kapur et al retrospectively reviewed outcomes in 46 patients with RV failure who received an TandemHeart adapted to provide RV support. 62 22 patients were cannulated percutaneously. TandemHeart implantation was associated with a decrease in RA pressure (21±8 to 16±7, p<0.01) and increase in CI (1.7±0.7 to 2.2±0.6, p=0.01). In-hospital mortality was similar between the percutaneous and surgical groups (50% versus 62%, p=0.4) and highest among patients with RV failure with acute myocarditis (100%), following valve surgery (87%), or following CABG (67%) and lowest among patients with RV failure following OHT (40%), AMI (33%), chronic LV failure (33%), and LVAD implantation (20%). TIMI-major bleeding was seen in 41% of patients cannulated percutaneously with one patient developing a retroperitoneal bleed.
Conclusions
While the pace of innovation in percutaneous left- and right- MCS devices has accelerated recently, the available evidence for or against their use in CS is sparse, often comprised of observational studies with surrogate endpoints, and outcomes remain poor.
Any attempt to improve outcomes in CS should begin with its early identification. Models of care including a multi-disciplinary CS team 63 hold potential in speeding the early identification and treatment of CS.
Among the many challenges that remain include how best to pair the right patient with the right device at the right time. AMI patients with CS appear to have the worst prognosis, while patients with CS status post cardiac surgery with acute RV failure appear to fare the best. Data from the above-mentioned studies with a focus on the timecourse of CS indicates that percutaneous MCS has a limited ability to change outcome if initiated when overt multi-organ dysfunction has already occurred. Accordingly, perhaps MCS should not be considered the treatment of last resort in AMI patients with CS but should probably be initiated early in the disease course (pre-PCI). Supporting data for this, however, beyond a single study from the U.S. Pella investigators initiation where outcomes were improved when MCS was initiated prior to reperfusion, 38 is currently lacking. The ongoing “Door to Unloading (DTU) with Impella CP System in Acute Myocardial Infarction to Reduce Infarct Size” prospective, multi-center trial may shed further light on this issue.
While randomization in CS poses logistical and ethical challenges, randomized controlled trials of percutaneous MCS devices with clinical not surrogate endpoints and long-term follow-up are urgently needed, especially given that their use has risen just as quickly as IABP therapy use has fallen. Significant research opportunities remain.
Acknowledgments
Sources of Funding
This research was supported by NIH training grant # 5T32HL069749-13 (A. Mandawat).
Footnotes
Disclosures
None
References
- 1.Reynolds HR, Hochman JS. Cardiogenic Shock. Current Concepts and Improving Outcomes. 2008;117:686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 2.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156–67. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 3.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N, Aggarwal S, Gaglianello N, Bangalore S, Cinquegrani MP. Abstract 13061: Trends in Incidence, Management and Outcomes of Heart Failure Patients with Cardiogenic Shock. Circulation. 2014;130:A13061–A13061. [Google Scholar]
- 5.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular CareEndorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention*. Journal of the American College of Cardiology. 2015;65:e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of Mechanical Circulatory Support. Journal of the American College of Cardiology. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Ouweneel DM, Henriques JPS. Percutaneous cardiac support devices for cardiogenic shock: current indications and recommendations. Heart. 2012;98:1246–1254. doi: 10.1136/heartjnl-2012-301963. [DOI] [PubMed] [Google Scholar]
- 8.Moulopoulos S, Stamatelopoulos S, Petrou P. INTRAAORTIC BALLOON ASSISTANCE IN INTRACTABLE CARDIOGENIC-SHOCK. European Heart Journal. 1986;7:396–403. doi: 10.1093/oxfordjournals.eurheartj.a062080. [DOI] [PubMed] [Google Scholar]
- 9.Bengtson JR, Kaplan AJ, Pieper KS, Wildermann NM, Mark DB, Pryor DB, Phillips HR, Califf RM. PROGNOSIS IN CARDIOGENIC-SHOCK AFTER ACUTE MYOCARDIAL-INFARCTION IN THE INTERVENTIONAL ERA. Journal of the American College of Cardiology. 1992;20:1482–1489. doi: 10.1016/0735-1097(92)90440-x. [DOI] [PubMed] [Google Scholar]
- 10.Waksman R, Weiss AT, Gotsman MS, Hasin Y. INTRAAORTIC BALLOON COUNTERPULSATION IMPROVES SURVIVAL IN CARDIOGENIC-SHOCK COMPLICATING ACUTE MYOCARDIAL-INFARCTION. European Heart Journal. 1993;14:71–74. doi: 10.1093/eurheartj/14.1.71. [DOI] [PubMed] [Google Scholar]
- 11.Stomel RJ, Rasak M, Bates ER. TREATMENT STRATEGIES FOR ACUTE MYOCARDIAL-INFARCTION COMPLICATED BY CARDIOGENIC-SHOCK IN A COMMUNITY-HOSPITAL. Chest. 1994;105:997–1002. doi: 10.1378/chest.105.4.997. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RD, Ohman EM, Holmes DR, Col J, Stebbins AL, Bates ER, Stomel RJ, Granger CB, Topol EJ, Califf RM. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: Observations from the GUSTO-I study. Journal of the American College of Cardiology. 1997;30:708–715. doi: 10.1016/s0735-1097(97)00227-1. [DOI] [PubMed] [Google Scholar]
- 13.Kovack PJ, Rasak MA, Bates ER, Ohman EM, Stomel RJ. Thrombolysis plus aortic counterpulsation: Improved survival in patients who present to community hospitals with cardiogenic shock. Journal of the American College of Cardiology. 1997;29:1454–1458. doi: 10.1016/s0735-1097(97)82537-5. [DOI] [PubMed] [Google Scholar]
- 14.Sanborn TA, Sleeper LA, Bates ER, Jacobs AK, Boland J, French JK, Dens J, Dzavik V, Palmeri ST, Webb JG, Goldberger M, Hochman JS, Investigators S. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: A report from the SHOCK Trial Registry. Journal of the American College of Cardiology. 2000;36:1123–1129. doi: 10.1016/s0735-1097(00)00875-5. [DOI] [PubMed] [Google Scholar]
- 15.Barron HV, Every NR, Parsons LS, Angeja B, Goldberg RJ, Gore JM, Chou TM Investigators Natl Registry M. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the National Registry of Myocardial Infarction 2. American heart journal. 2001;141:933–939. doi: 10.1067/mhj.2001.115295. [DOI] [PubMed] [Google Scholar]
- 16.French JK, Feldman HA, Assmann SF, Sanborn T, Palmeri ST, Miller D, Boland J, Buller CE, Steingart R, Sleeper LA, Hochman JS, Investigators S. Influence of thrombolytic therapy, with or without intra-aortic balloon counterpulsation, on 12-month survival in the SHOCK trial. American heart journal. 2003;146:804–810. doi: 10.1016/S0002-8703(03)00392-2. [DOI] [PubMed] [Google Scholar]
- 17.Vis MM, Sjauw KD, van der Schaaf RJ, Baan J, Koch KT, DeVries JH, Tijissen JGP, de Winter RJ, Piek JJ, Henriques JPS. In patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of diabetes. American heart journal. 2007;154:1184–1190. doi: 10.1016/j.ahj.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Vis MM, Sjauw KD, van der Schaaf RJ, Koch KT, Baan J, Tijssen JGP, Piek JJ, de Winter RJ, Henriques JPS. Prognostic value of admission hemoglobin levels in ST-segment elevation myocardial infarction patients presenting with cardiogenic shock. American Journal of Cardiology. 2007;99:1201–1202. doi: 10.1016/j.amjcard.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-Year Trends (1975 to 2005) in the Magnitude of, Management of, and Hospital Death Rates Associated With Cardiogenic Shock in Patients With Acute Myocardial Infarction A Population-Based Perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Hu W, Xiao HB, Feng XD, Chen YG, Zhang DD. Intra-Aortic Balloon Pump Improves Clinical Prognosis and Attenuates C-Reactive Protein Level in Acute STEMI Complicated by Cardiogenic Shock. Cardiology. 2010;117:75–80. doi: 10.1159/000319618. [DOI] [PubMed] [Google Scholar]
- 21.Zeymer U, Bauer T, Hamm C, Zahn R, Weidinger F, Seabra-Gomes R, Hochadel M, Marco J, Gitt A Euro Heart Survey PCIS. Use and impact of intra-aortic balloon pump on mortality in patients with acute myocardial infarction complicated by cardiogenic shock: results of the Euro Heart Survey on PCI. Eurointervention. 2011;7:437–441. doi: 10.4244/EIJV7I4A72. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LSD, van Domburg RT, Serruys PW. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. European Heart Journal. 2009;30:2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 23.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Koch KT, de Winter RJ, Piek JJ, Tijssen JGP, Henriques JPS. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? European Heart Journal. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 24.Bahekar A, Singh M, Singh S, Bhuriya R, Ahmad K, Khosla S, Arora R. Cardiovascular Outcomes Using Intra-Aortic Balloon Pump in High-Risk Acute Myocardial Infarction With or Without Cardiogenic Shock: A Meta-Analysis. Journal of Cardiovascular Pharmacology and Therapeutics. 2012;17:44–56. doi: 10.1177/1074248410395019. [DOI] [PubMed] [Google Scholar]
- 25.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr, Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30:459–68. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 26.Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, Schmidt H, Winkler M, Thiery J, Werdan K, Buerke M. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152–160. doi: 10.1097/CCM.0b013e3181b78671. [DOI] [PubMed] [Google Scholar]
- 27.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. New England Journal of Medicine. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 28.Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van’t Hof A, Widimsky P, Zahger D, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Hasdai D, Astin F, Åström-Olsson K, Budaj A, Clemmensen P, Collet J-P, Fox KA, Fuat A, Gustiene O, Hamm CW, Kala P, Lancellotti P, Maggioni AP, Merkely B, Neumann F-J, Piepoli MF, Van de Werf F, Verheugt F, Wallentin L. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 29.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX Writing Comm M. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:E362. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 30.Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, Gensini GF, Chiarotti F, Caretta Q. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. American heart journal. 2013;165:679–92. doi: 10.1016/j.ahj.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad Y, Sen S, Shun-Shin MJ, Ouyang J, Finegold JA, Al-Lamee RK, Davies JE, Cole GD, Francis DP. Intra-aortic balloon pump therapy for acute myocardial infarction: A meta-analysis. JAMA Internal Medicine. 2015;175:931–939. doi: 10.1001/jamainternmed.2015.0569. [DOI] [PubMed] [Google Scholar]
- 32.Gjesdal O, Gude E, Arora S, Leivestad T, Andreassen AK, Gullestad L, Aaberge L, Brunvand H, Edvardsen T, Geiran OR, Simonsen S. Intra-aortic balloon counterpulsation as a bridge to heart transplantation does not impair long-term survival. European journal of heart failure. 2009;11:709–14. doi: 10.1093/eurjhf/hfp078. [DOI] [PubMed] [Google Scholar]
- 33.Russo MJ, Jeevanandam V, Stepney J, Merlo A, Johnson EM, Malyala R, Raman J. Intra-aortic balloon pump inserted through the subclavian artery: A minimally invasive approach to mechanical support in the ambulatory end-stage heart failure patient. J Thorac Cardiovasc Surg. 2012;144:951–5. doi: 10.1016/j.jtcvs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Estep JD, Cordero-Reyes AM, Bhimaraj A, Trachtenberg B, Khalil N, Loebe M, Bruckner B, Orrego CM, Bismuth J, Kleiman NS, Torre-Amione G. Percutaneous Placement of an Intra-Aortic Balloon Pump in the Left Axillary/Subclavian Position Provides Safe, Ambulatory Long-Term Support as Bridge to Heart Transplantation. JACC: Heart Failure. 2013;1:382–388. doi: 10.1016/j.jchf.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Bezerra CG, Adam EL, Baptista ML, Ciambelli GS, Bernoche LKC, Lopes LNGD, Macatrão-Costa MF, Falcão BdAA, Lage SG. Aortic Counterpulsation Therapy in Patients with Advanced Heart Failure: Analysis of the TBRIDGE Registry. Arquivos Brasileiros de Cardiologia. 2016;106:26–32. doi: 10.5935/abc.20150147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka A, Tuladhar SM, Onsager D, Asfaw Z, Ota T, Juricek C, Lahart M, Lonchyna VA, Kim G, Fedson S, Sayer G, Uriel N, Jeevanandam V. The Subclavian Intraaortic Balloon Pump: A Compelling Bridge Device for Advanced Heart Failure. The Annals of Thoracic Surgery. 2015;100:2151–2158. doi: 10.1016/j.athoracsur.2015.05.087. [DOI] [PubMed] [Google Scholar]
- 37.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill WW, Schreiber T, Wohns DH, Rihal C, Naidu SS, Civitello AB, Dixon SR, Massaro JM, Maini B, Ohman EM. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. Journal of interventional cardiology. 2014;27:1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. Journal of the American College of Cardiology. 2008;52:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 40.Griffith BP, Anderson MB, Samuels LE, Pae WE, Jr, Naka Y, Frazier OH. The RECOVER I: a multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg. 2013;145:548–54. doi: 10.1016/j.jtcvs.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 41.Lima B, Kale P, Gonzalez-Stawinski GV, Kuiper JJ, Carey S, Hall SA. Effectiveness and Safety of the Impella 5.0 as a Bridge to Cardiac Transplantation or Durable Left Ventricular Assist Device. The American Journal of Cardiology. 2016;117:1622–1628. doi: 10.1016/j.amjcard.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 42.Thiele H, Sick P, Boudriot E, Diederich K-W, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. European Heart Journal. 2005;26:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 43.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. American heart journal. 2006;152:469.e1–469.e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The Percutaneous Ventricular Assist Device in Severe Refractory Cardiogenic Shock. Journal of the American College of Cardiology. 2011;57:688–696. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 45.Bruckner BA, Jacob LP, Gregoric ID, Loyalka P, Kar B, Cohn WE, La Francesca S, Radovancevic B, Frazier OH. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Tex Heart Inst J. 2008;35:447–50. [PMC free article] [PubMed] [Google Scholar]
- 46.Gregoric ID, Jacob LP, La Francesca S, Bruckner BA, Cohn WE, Loyalka P, Kar B, Frazier OH. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge) Tex Heart Inst J. 2008;35:125–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Velez-Martinez M, Rao K, Warner J, Dimaio J, Ewing G, Mishkin JD, Mammen PP, Drazner MH, Markham DW, Patel PC. Successful use of the TandemHeart percutaneous ventricular assist device as a bridge to recovery for acute cellular rejection in a cardiac transplant patient. Transplantation proceedings. 2011;43:3882–4. doi: 10.1016/j.transproceed.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 48.Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Duranteau J, Combes A. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 2014;42:2075–82. doi: 10.1097/CCM.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 49.Lin L-Y, Liao C-W, Wang C-H, Chi N-H, Yu H-Y, Chou N-K, Hwang J-J, Lin J-L, Chiang F-T, Chen Y-S. Effects of Additional Intra-aortic Balloon Counter-Pulsation Therapy to Cardiogenic Shock Patients Supported by Extra-corporeal Membranous Oxygenation. Scientific Reports. 2016;6:23838. doi: 10.1038/srep23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng A, Swartz MF, Massey HT. Impella to unload the left ventricle during peripheral extracorporeal membrane oxygenation. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2013;59:533–6. doi: 10.1097/MAT.0b013e31829f0e52. [DOI] [PubMed] [Google Scholar]
- 51.Lawler PR, Silver DA, Scirica BM, Couper GS, Weinhouse GL, Camp PC. Extracorporeal Membrane Oxygenation in Adults With Cardiogenic Shock. Circulation. 2015;131:676–680. doi: 10.1161/CIRCULATIONAHA.114.006647. [DOI] [PubMed] [Google Scholar]
- 52.Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, Anselmi A, Combes A. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive care medicine. 2016;42:370–8. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg. 2012;94:1–7. doi: 10.1016/j.athoracsur.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 54.Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–13. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 55.Kim H, Lim SH, Hong J, Hong YS, Lee CJ, Jung JH, Yu S. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation. 2012;83:971–5. doi: 10.1016/j.resuscitation.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 56.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet (London, England) 2008;372:554–61. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 57.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87:778–85. doi: 10.1016/j.athoracsur.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 58.Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, Pavie A, Chastre J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–11. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 59.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, Mohr FW. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. The Journal of Thoracic and Cardiovascular Surgery. 139:302–311.e1. doi: 10.1016/j.jtcvs.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 60.Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, Kapur NK, Bansal A, Garcia J, Baker JN, Silvestry S, Holman WL, Douglas PS, O’Neill W. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2015;34:1549–60. doi: 10.1016/j.healun.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 61.John R, Long JW, Massey HT, Griffith BP, Sun BC, Tector AJ, Frazier OH, Joyce LD. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. The Journal of Thoracic and Cardiovascular Surgery. 141:932–939. doi: 10.1016/j.jtcvs.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 62.Kapur NK, Paruchuri V, Jagannathan A, Steinberg D, Chakrabarti AK, Pinto D, Aghili N, Najjar S, Finley J, Orr NM, Tempelhof M, Mudd JO, Kiernan MS, Pham DT, DeNofrio D. Mechanical Circulatory Support for Right Ventricular Failure. JACC: Heart Failure. 2013;1:127–134. doi: 10.1016/j.jchf.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Doll JA, Ohman EM, Patel MR, Milano CA, Rogers JG, Wohns DH, Kapur NK, Rao SV. A team-based approach to patients in cardiogenic shock. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2016;88:424–33. doi: 10.1002/ccd.26297. [DOI] [PubMed] [Google Scholar]




