Abstract
Background
At high medicinal doses perchlorate is known to decrease the production of thyroid hormone, a critical factor for fetal development. In a large and uniquely exposed cohort of pregnant women, we recently identified associations between environmental perchlorate exposures and decreased maternal thyroid hormone during pregnancy. Here, we investigate whether perchlorate might be associated with birthweight or preterm birth in the offspring of these women.
Methods
Maternal urinary perchlorate, serum thyroid hormone concentrations, birthweight, gestational age, and urinary nitrate, thiocyanate, and iodide were collected in 1,957 mother-infant pairs from San Diego County during 2000–2003, a period when the county’s water supply was contaminated with perchlorate. Associations between perchlorate exposure and birth outcomes were examined using linear and logistic regression analyses adjusted for maternal age, weight, race/ethnicity, and other factors.
Results
Perchlorate was not associated with birth outcomes in the overall population. However, in analyses confined to male infants, log10 maternal perchlorate concentrations were associated with increasing birthweight (β=143.1 gm, p=0.01), especially among preterm births (β=829.1 g, p<0.001). Perchlorate was associated with male preterm births >2500 g (odds ratio=3.03, 95% confidence interval=1.09–8.40, p-trend=0.03). Similar associations were not seen in females.
Conclusions
This is the first study to identify associations between perchlorate and increasing birthweight. Further research is needed to explore the differences we identified related to infant sex, preterm birth, and other factors. Given that perchlorate exposure is ubiquitous, and that long-term impacts can follow altered birth outcomes, future research on perchlorate could have widespread public health importance.
Keywords: perchlorate, thyroid, birthweight, pregnancy, preterm
INTRODUCTION
Perchlorate (ClO4−) is a highly stable oxidizing chemical component of missile fuel, road flares, and other products. Perchlorate exposure in the US is ubiquitous, typically occurring through contaminated food or water. In a recent nationwide US survey, perchlorate was detected in the urine of every person tested (1).
At medicinal levels (e.g. 800 mg), perchlorate competitively inhibits uptake of iodide by the sodium iodide symporter (NIS) in the thyroid gland (2). Iodide is the predominant form of iodine found in diet. Iodide anion accounts of >90% of total iodine in urine and is the biologically available form that is transported into the thyroid to produce thyroid hormones. Since iodide is a key component of thyroid hormone, inhibiting its uptake into the thyroid can decrease thyroid hormone production. In the developing fetus and child, thyroid hormone is vital for proper development, and decreases in this hormone have been linked to significant decreases in IQ and other adverse neurologic outcomes (3–6). Pregnancy is a time of thyroid stress and increased iodine demands, and this could lead to pregnancy being a period of particular susceptibility to perchlorate (7).
Thyroxine (T4) is the major circulating form of thyroid hormone, and free thyroxine (fT4) is its non-protein bound form. Thyroid stimulating hormone (TSH) is produced by the pituitary in response to low thyroid hormone concentrations in plasma, and stimulates the thyroid to produce more T4. Elevated TSH acts as a sensitive marker for thyroid stress since TSH is commonly effective at maintaining adequate serum concentrations of T4. In the largest study to date of perchlorate in pregnant women, we identified statistically significant associations between increasing urinary perchlorate concentrations and decreasing serum T4 and fT4 and increasing TSH concentrations (8). This study involved samples and data collected from pregnant women who gave birth in San Diego County between November 2000 and March 2003. This was a time when a large groundwater plume of perchlorate from a former perchlorate manufacturing plant contaminated the Las Vegas Wash, a tributary of the Colorado River (9). This contamination led to widespread perchlorate exposure, since the Colorado River supplies drinking water for 15–20 million people in Nevada, Arizona, and California, including much of San Diego County. The contamination resulted in perchlorate concentrations approaching the current California regulatory standard of 6 µg/L in the county’s major drinking water supply. Other water sources in the county had much lower perchlorate concentrations. This exposure situation, combined with likely variations in intake of perchlorate from food, provided a wide range of perchlorate exposure among county residents.
Although several studies, including ours, have linked perchlorate to impacts on thyroid function, few studies have examined perchlorate in relation to overt adverse health effects. Based on the associations we identified in our previous study, and the well-known role thyroid hormone plays in fetal development, we investigated whether maternal perchlorate may have impacted birthweight or gestational age in our San Diego cohort. We also evaluated whether any associations we identified might be greater in certain potentially susceptible subpopulations. In previous research, perchlorate-thyroid hormone associations were greatest in subjects with elevated intakes of thiocyanate and nitrate, or with very high or low urinary levels of iodide (1, 8, 10). Thiocyanate (from foods or tobacco smoke) and nitrate (from foods or contaminated water) also competitively inhibit thyroid iodide uptake, and may have additive impacts with perchlorate (2). Previous research has also shown that birth outcomes or the adverse effects of environmental chemicals may be related to gender, ethnicity or race, or migration status (11, 12), and we assessed potential susceptibility related to these factors as well.
METHODS
Study design and data collection
Subjects were a convenience sample of pregnant women and their newborns from San Diego County, delivered from November 2000 to March 2003, and already recruited as part of Project Baby’s Breath (PBB), a study of tobacco and other environmental exposures during pregnancy. PBB involved 14 hospitals and 41 community clinics and obstetrical care providers widely spread throughout the county, and the collection and frozen storage of urine, blood, and cord blood samples originally obtained for non-study purposes. Urine samples assayed for the present analysis were left over from spot urine samples collected from PBB subjects for pregnancy tests at a median of seven weeks gestation. After pregnancy testing, clinic staff transferred the remaining urine into 5ml Corning cryovials that were refrigerated and transferred within one day to a central laboratory for storage at −20°C. Perchlorate is stable for many months in water at room temperature (13), and for years in frozen urine (14). Serum samples assayed for the present analysis were left over from samples collected by obstetrical care providers at approximately 15–20 weeks gestation from PBB subjects who participated in the California Prenatal Screening (PNS) Program (15). After collection in 4ml serum separator tubes, specimens were spun down and tested for chromosomal abnormalities and neural tube defects, with a median time between collection and PNS testing of three days. After PNS testing and 1–2 days of refrigeration, remaining serum from PBB study participants was transferred to 4ml Corning cryovials and stored at −20°C. Samples not assayed within seven days of collection were excluded from the PNS and not available for PBB. Mannisto et al. reported that fT4 or TSH were relatively stable for 6 days at 4°C and up to 23 years at −25°C (16). Information on mother’s age, highest education, prenatal weight, payment method (e.g., private insurance vs. MediCal), and race/ethnicity was collected from birth records. Information on prenatal weight was derived from the PNS program. Gestational age was calculated using the date of conception estimated from ultrasound and information on last menstrual period collected during prenatal screening, a method that produces more accurate gestational age estimates than relying on last menstrual period dates from birth records (17, 18). For subjects not participating in prenatal screening (approximately 10%), gestational age was based on the date of last menstrual period recorded on birth records. Urine and serum samples, PNS program data, and birth records were linked using probabilistic matching software. PNS to birth match rates using this method are generally 93% (19). Informed consent was obtained for collection of leftover urine and blood specimens for the PBB study and for future testing of stored specimens for environmental contaminants. PNS program participants signed a consent/refusal form and received a privacy notification regarding research use of their specimen. The PBB study and the study presented here were approved by the State of California Committee for the Protection of Human Subjects.
Laboratory measurements
Urinary perchlorate is the most common biomarker for evaluating perchlorate exposure, since most ingested perchlorate is excreted unchanged in the urine (20). Urine samples were shipped overnight to the Centers for Disease Control and Prevention (CDC) on dry ice and analyzed by the CDC’s Perchlorate Biomonitoring Laboratory for perchlorate (detection limit, 0.05 µg/L), thiocyanate (20 µg/L), nitrate (700 µg/L), and iodide (0.5 µg/L) using ion chromatography tandem mass spectrometry (21). Urinary cotinine levels were measured in 856 subjects by the CDC as part of a previous study. Results met the division’s quality control criteria for accuracy and precision similar to those outlined in Caudill et al. (22). After overnight shipping on dry ice, serum samples were measured for total T4, free thyroxine (fT4), thyroid stimulating hormone (TSH)), and thyroperoxidase (TPO) and thyroglobulin (Tg) antibody concentrations at the University of Washington, Seattle, using a Beckman automated immunoassay chemiluminescence platform and microparticle enzyme immunoassay (Beckman Coulter). Quality control measures included 2-level quantitative controls for each assay on every reagent run, monitoring run integrity using Levey-Jennings charts, and participation in proficiency surveys by the College of American Pathologists. Manufacturers’ values for TPO and Tg antibody positivity are >9 and >4 IU/mL, respectively.
Statistical analysis
This study initially included all pregnant women (n=2,184) who participated in PBB and had a urine and/or blood sample with sufficient remaining specimen for laboratory analyses. Additional exclusion criteria for the analyses presented here included missing data on urinary perchlorate, thiocyanate, nitrate, iodide, or creatinine concentrations (n=139); missing data on birthweight or gestational age (n=32); multiple births (e.g. twins)(n=19); birth or pregnancy complications (e.g., hypertension, breech birth, diabetes)(n=30); or abnormally high maternal T4 (>20 µg/dL) or TSH (>10 µIU/mL) values (n=7).
Most statistical analyses were done using SAS version 9.1 (SAS Institute Inc.) and all reported p-values are two-sided. Univariate analyses of urinary analytes, serum thyroid hormones, birth outcomes, and sociodemographic variables were examined for distributions and outliers. Unadjusted analyses of perchlorate concentrations and birth outcomes by various sociodemographic characteristics were conducted using Wilcoxon rank sum tests, Spearman correlation coefficients, and Chi-square tests. Mean concentrations of perchlorate and the other urinary analytes in this study were compared to those calculated for reproductive-aged women (ages 15–45 years) from the 2001–2002 National Health and Nutrition Examination Survey using methods described elsewhere (10).
Associations between perchlorate and birthweight were examined using linear regression analyses in models both unadjusted and adjusted for urinary creatinine (log10), maternal age (year), maternal weight (lbs), race (indicator variables for White, Black, Asian, Other), infant sex, maternal education (<high school, some high school, high school, beyond high school), Caesarean birth, medical insurance (private vs. MediCal), timing of urine sample collection (weeks gestation), ethnicity (Hispanic vs. non-Hispanic), and mother’s birthplace (Mexico vs. US vs. other). When assessing subgroups based on race and ethnicity, we classified Hispanics as all subjects identifying as Hispanic regardless of race, and our other categories of race excluded Hispanics. Independent variables, including perchlorate, thiocyanate, nitrate, iodide, and creatinine were log10 transformed to stabilize variance and reduce outlier influence. Variables that changed regression coefficients by more than 10% were retained in the final models and included creatinine, maternal age and weight, race, and sex. In addition to linear regression analyses, perchlorate-birthweight associations were evaluated by calculating mean birthweights by quartiles of perchlorate concentrations using proc GLM. Potential effect modification by factors such as infant sex, co-exposure to other NIS-related agents (nitrate, iodide, thiocyanate), and other factors (e.g. Cesarean birth, cotinine) was assessed by stratifying the linear regression analyses based on these factors or by entering interaction terms (e.g. perchlorate*thiocyanate) into the regression models. For nitrate, thiocyanate, and cotinine category cutoff points were based on tertiles. For urinary iodide we used categories of <100, 100–300, and >300 µg/L which correspond to median values used by the World Health Organization (WHO) to define iodine deficiency, normal values, and elevated iodine levels, respectively, in non-pregnant populations ≥6 years old (23). A lower category cutoff of 150 µg/L, the WHO recommended population level for pregnant women, was assessed but produced similar results. Because previous studies have reported differences in birth outcomes by ethnicity or mother’s migration status, stratified analyses were also done based on these factors (24).
Because the known toxic mechanism of perchlorate involves thyroid hormone and altered thyroid hormone levels have been linked to adverse birth outcomes (25), mediation by this pathway was assessed by comparing perchlorate-birthweight linear regression coefficients with and without adjustment for T4, fT4, or log10 TSH levels and using the Sobel test and the SAS mediation macro provided by Valeri and VanderWeele (26).
Logistic regression was used to calculate odds ratios for preterm birth (<37 weeks gestation) across quartiles of urinary perchlorate concentration using the lower quartile as the reference. These models also included log10 creatinine, maternal age and weight, race, and infant sex. The impact of adjusting for other factors such as iodide, Cesarean birth, mother’s education, or sample collection timing was also assessed. Because the health implications of preterm birth can vary by birthweight (27), and to explore possible impacts of perchlorate on birthweight and gestational age combined, we also calculated ORs for various birthweight-gestational age strata (preterm birth below 2500 g, term birth below 2500 g, preterm birth 2500 grams or above, and term birth 2500 grams or above) using the categories similar to those proposed by Yerushalmy (27). Here, all births not in the selected category were used as the reference group. The adjusted proportions of births in each category were assessed using the adjprop command in StataSE 14.0.
RESULTS
Characteristics of the study population
Overall, 1,957 mother-infant pairs met the inclusion criteria for this study. Distributions of perchlorate, thyroid hormones and other factors in the study population as a whole are shown in Table 1. Urine perchlorate concentrations ranged from 0.23 to 177 µg/L, with a mean of 8.57 µg/L, which is higher than that seen in reproductive-aged US women from the 2001–2002 US National Health and Nutrition Examination Survey (mean of 4.73 µg/L) (Table A1). The median urinary iodide concentration was 153 µg/L, which is similar to the recommended population level for pregnant women of 150 µg/L (23).
Table 1.
Distributions of perchlorate, other urinary analytes, serum thyroid hormones, and other factors in pregnant women from San Diego County 2000–2003
| Percentiles
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SD | Min | 25th | 50th | 75th | Max |
| Urine perchlorate (µg/L)2 | 1957 | 8.57 | 9.99 | 0.23 | 3.94 | 6.50 | 10.00 | 177.00 |
| Urine thiocyanate (µg/L)2 | 1957 | 1328 | 1519 | 20 | 446 | 881 | 1580 | 16200 |
| Urine nitrate (mg/L)2 | 1957 | 66.14 | 57.71 | 0.70 | 32.50 | 54.80 | 83.70 | 796.00 |
| Urine iodide (µg/L)2 | 1957 | 216.61 | 256.81 | 0.81 | 77.00 | 153.00 | 269.00 | 3330.00 |
| Urine creatinine (g/L) | 1957 | 130.27 | 75.04 | 7.90 | 72.91 | 119.37 | 171.83 | 472.46 |
| Birthweight (gm) | 1957 | 3418 | 521 | 610 | 3130 | 3430 | 3742 | 5970 |
| Gestational age (weeks) | 1957 | 39.32 | 1.87 | 23.00 | 38.57 | 39.57 | 40.43 | 46.14 |
| Maternal age (years) | 1957 | 25.57 | 5.74 | 14.00 | 21.00 | 25.00 | 30.00 | 43.00 |
| T4 (µg/dl)1 | 1766 | 12.31 | 1.87 | 0.56 | 11.21 | 12.26 | 13.39 | 19.99 |
| TSH (µIU/mL)1 | 1765 | 1.36 | 0.86 | 0.00 | 0.81 | 1.20 | 1.69 | 8.39 |
| fT4 (ng/dL)a | 1766 | 0.86 | 0.19 | 0.11 | 0.77 | 0.85 | 0.93 | 5.44 |
Abbreviations: fT4, free thyroxine; Max, maximum; Min, minimum; N, number of participants; SD, standard deviation; T4, total thyroxine; TSH, thyroid stimulating hormone
Reference ranges from the University of Washington for all ages and genders are: 4.8–10.8 µg/dL for total T4; 0.6–1.2 ng/dL for fT4 and 0.4–5.0 µIU/mL for TSH. The American Thyroid Association upper reference range for TSH in second trimester pregnancy is 4.0 µIU/mL (53).
Urinary concentrations are not corrected for urinary creatinine concentration
The numbers of subjects, as well as mean perchlorate concentrations and birthweights, and the proportions of preterm births, in categories of various sociodemographic characteristics are shown in Table 2. Overall, the study population was mostly Hispanic (69.1%), mostly used public medical insurance (69.9%), and mothers were mostly born in the US (45.1%) or Mexico (47.7%). Sociodemographic characteristics were similar in included and excluded subjects (Table A2). Mean perchlorate concentrations were higher in Blacks than Hispanics (11.85 vs. 8.42 µg/L, p<0.001), and lower in mothers born in Mexico and elsewhere compared to those born in the US (means of 8.40 (p=0.02), 7.63 (p=0.02), and 8.90 µg/L, respectively). Lower birthweights were associated with female sex (3341.4 vs. 3498.2 g, p<0.001), decreasing maternal age (Spearman R=0.08, <0.001), decreasing maternal weight (Spearman R=0.26, p<0.001), and normal vaginal delivery (3400.8 vs. 3480.5 g, p<0.001). Preterm births were somewhat higher in those with increasing maternal age (p-trend=0.06), and somewhat lower in mothers born in Mexico than in the US (7.5 vs. 8.8%, OR=0.84, 95% CI: 0.60–1.17).
Table 2.
Mean maternal urinary perchlorate concentrations (µg/L) and birthweights (grams), and percentage preterm births by categories of various sociodemographic characteristics in the study population of pregnant women from San Diego County 2000–2003
| Variable | Category | Category N (%)1 |
Perchlorate2 Mean (SD) |
Birthweight Mean (SD) |
Preterm N (%)3 |
|---|---|---|---|---|---|
| All subjects | 1957 (100%) | 8.57 (9.99) | 3418.3 (520.7) | 158 (8.1%) | |
| Sex | Female | 998 (51.0%) | 8.49 (10.48) | 3341.4 (519.5) | 73 (7.3%) |
| Male | 959 (49.0%) | 8.66 (9.47) | 3498.2 (510.0) | 85 (8.9%) | |
| Race/ethnicity | Hispanic | 1352 (69.1%) | 8.42 (10.36) | 3426.8 (519.3) | 104 (7.7%) |
| Asian | 25 (1.3%) | 9.65 (14.12) | 3477.8 (549.7) | 3 (12.0%) | |
| Black | 81 (4.1%) | 11.85 (15.28) | 3278.8 (503.1) | 9 (11.1%) | |
| Other | 90 (4.6%) | 6.81 (4.75) | 3240.1 (527.6) | 9 (10.0%) | |
| White | 409 (20.9%) | 8.73 (7.63) | 3453.2 (515.8) | 33 (8.1%) | |
| Mother’s birthplace | US | 882 (45.1%) | 8.90 (10.35) | 3424.9 (533.4) | 78 (8.8%) |
| Mexico | 933 (47.7%) | 8.40 (10.03) | 3422.9 (508.2) | 70 (7.5%) | |
| Other | 142 (7.3%) | 7.63 (6.98) | 3347.2 (520.3) | 10 (7.0%) | |
| Maternal age (years) | <21 | 446 (22.8%) | 7.68 (7.51) | 3348.7 (491.5) | 32 (7.2%) |
| 21–25 | 575 (29.4%) | 8.99 (11.18) | 3423.6 (487.6) | 36 (6.3%) | |
| 26–30 | 435 (22.2%) | 9.12 (12.96) | 3442.1 (534.3) | 36 (8.3%) | |
| >30 | 501 (25.6%) | 8.39 (7.07) | 3453.4 (564.6) | 54 (10.8%) | |
| Maternal education (highest grade) | <9 | 537 (28.0%) | 8.52 (9.24) | 3440.5 (493.6) | 34 (6.3%) |
| 9–11 | 319 (16.6%) | 8.70 (11.59) | 3375.8 (532.0) | 29 (9.1%) | |
| 12 | 580 (30.2%) | 8.69 (11.02) | 3400.2 (532.8) | 54 (9.3%) | |
| >12 | 484 (25.2%) | 8.43 (8.50) | 3441.9 (534.3) | 39 (8.1%) | |
| Maternal weight (lbs) | <130 | 441 (25.0%) | 7.78 (8.12) | 3243.4 (459.1) | 31 (7.0%) |
| 131–147 | 438 (24.8%) | 8.10 (10.01) | 3358.0 (463.4) | 33 (7.5%) | |
| 148–160 | 449 (25.4%) | 9.41 (13.15) | 3478.2 (497.9) | 33 (7.3%) | |
| >160 | 438 (24.8%) | 8.86 (8.10) | 3584.7 (591.0) | 38 (8.7%) | |
| Delivery | NVD | 1523 (77.8%) | 8.66 (10.55) | 3400.5 (487.6) | 119 (7.8%) |
| Caesarean | 434 (22.2%) | 8.25 (7.73) | 3480.5 (619.8) | 39 (9.0%) | |
| Insurance | Private | 589 (30.1%) | 8.58 (10.31) | 3429.2 (507.5) | 41 (7.0%) |
| Public | 1368 (69.9%) | 8.57 (9.86) | 3413.6 (526.4) | 117 (8.6%) |
Abbreviations: N, number of participants; NVD, normal vaginal delivery; SD, standard deviation
Percentage of all births in this category
Urinary perchlorate concentrations are not corrected for urinary creatinine concentration
Percentage of all births in this category that were preterm
Perchlorate-birthweight
In linear regression analyses, clear associations between log10 perchlorate and birthweight were not seen when both sexes were combined (Table 3). However, in analyses stratified by sex, an association was seen for male births (β=143.1 gram increase for each 10-fold increase in perchlorate, p=0.01), but not for female births (β=−38.3 grams, p=0.49). Given a median birthweight of 3498 grams in male infants, the regression coefficient of 143.1 grams identified here represents a 4.1 percent increase in birthweight for each 10-fold increase in urinary perchlorate concentration. In male infants, perchlorate-birthweight associations were greater in preterm births (β=829.1, p<0.001) than in births ≥37 weeks gestation (β=97.3, p=0.06). Associations in male infants were also greater for those with US born mothers (β=297.2, p<0.001) (Figure 1) than those with Mexico-born mothers (β=29.9, p=0.68). In male births of US born mothers similar results were seen in categorical analyses where mean birthweights increased from the lowest to highest perchlorate quartile (3402.5, 3461.6, 3563.6, 3566.8 grams, respectively; p-trend=0.009) (Table A3). In US born mothers, results were similar in strata of Hispanics and Whites (data not shown). Adjusting for thiocyanate, iodide, nitrate, cotinine, education, Caesarean birth, Tg or TPO positivity, race, or insurance type had little impact on these birthweight results.
Table 3.
Regression coefficients between maternal log10 urine perchlorate concentrations (µg/L) and birthweight (grams) in the study population of pregnant women from San Diego County 2000–2003
| Both sexes
|
Males
|
Females
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | β1 | SE | p | N | β1 | SE | p | N | β1 | SE | p |
| All | 1957 | 51.3 | 39.7 | 0.20 | 959 | 143.1 | 56.3 | 0.01 | 998 | −38.3 | 56.0 | 0.49 |
| Urinary iodide2 | ||||||||||||
| <100 µg/L | 629 | 87.2 | 68.2 | 0.20 | 313 | 177.3 | 91.7 | 0.05 | 316 | −14.8 | 102.1 | 0.88 |
| 100–300 µg/L | 920 | −34.7 | 57.8 | 0.55 | 444 | 25.4 | 84.6 | 0.76 | 476 | −84.6 | 79.6 | 0.29 |
| >300 µg/L | 408 | 185.2 | 113.3 | 0.10 | 202 | 301.1 | 161.5 | 0.06 | 206 | −1.1 | 160.1 | 0.99 |
| Gestational age | ||||||||||||
| <37 weeks | 158 | 547.3 | 202.6 | 0.01 | 85 | 829.1 | 263.1 | <0.001 | 73 | 230.4 | 319.1 | 0.47 |
| ≥37 weeks | 1799 | 28.8 | 36.0 | 0.42 | 874 | 97.3 | 51.5 | 0.06 | 925 | −39.5 | 50.2 | 0.43 |
| Mothers | birthplace | |||||||||||
| Mexico | 933 | 27.1 | 53.1 | 0.61 | 451 | 29.9 | 72.6 | 0.68 | 482 | 26.0 | 77.8 | 0.74 |
| US born | ||||||||||||
| All | 882 | 95.3 | 63.0 | 0.13 | 445 | 297.2 | 91.9 | <0.001 | 437 | −95.1 | 86.4 | 0.27 |
| White | 381 | 166.7 | 104.9 | 0.11 | 204 | 325.6 | 151.4 | 0.03 | 177 | −34.8 | 144.7 | 0.81 |
| Hispanic | 394 | 40.4 | 94.7 | 0.67 | 186 | 305.4 | 136.9 | 0.03 | 208 | −186.0 | 129.3 | 0.15 |
| Other | 107 | 137.3 | 144.4 | 0.34 | 55 | 408.3 | 202.5 | 0.05 | 52 | −43.2 | 184.2 | 0.82 |
| Other birthplace | 142 | −39.0 | 167.9 | 0.82 | 63 | 23.8 | 255.6 | 0.93 | 79 | −115.8 | 237.1 | 0.63 |
Abbreviations: β, regression coefficient; N, sample size; SE, standard error
Adjusted for urinary creatinine (log10), maternal age, maternal weight, race, and sex
Urinary iodide concentrations were not corrected for urinary creatinine concentrations
Figure 1.
Log10 maternal urinary perchlorate concentrations and birthweight in male infants of US born mothers (regression coefficient between birthweight and log10 perchlorate = 297.2 g, p <0.001) in the study population of pregnant women from San Diego County 2000–2003
The dotted line represents the trend calculated using linear regression.
Analyses using interaction terms or stratified analyses showed no clear evidence of interaction with thiocyanate or nitrate on perchlorate-birthweight associations in either sex (not shown). The perchlorate-birthweight association was greater for male births with maternal iodide concentrations below 100 µg/L (β=177.3, p=0.05) and above 300 µg/L (β=301.1, p=0.06) compared to those with iodide concentrations between these levels (β=25.4, p=0.76) (Table 3).
Perchlorate-preterm birth
In both sexes combined, and in males and females separately, clear associations were not seen between increasing perchlorate quartiles and ORs for all preterm births combined (Table 4). However, the OR for having a preterm birth ≥2500 grams comparing the upper to lower perchlorate quartiles was elevated in male (OR=3.03; 95% CI: 1.09–8.40; p-trend=0.03) but not female infants (OR=1.07; 95% CI: 0.39–2.89). For male infants, this OR was greater in infants of Mexico (OR=4.33; 95% CI: 1.05–17.87) vs. US born mothers (OR=1.46; 95% CI: 0.33–6.55) (Figure A1). Adjustments for thiocyanate, nitrate, iodide, cotinine, maternal weight or education, Tg or TPO positivity, or public vs. private insurance use, and removing the 191 subjects for whom gestational age was estimated based solely on birth certificate data, had little impact on perchlorate-preterm ORs.
Table 4.
Odds ratios for preterm birth by quartile of maternal urinary perchlorate concentration in the study population of pregnant women from San Diego County 2000–2003
| Both sexes
|
Males
|
Females
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gro up |
Per c qua rt. |
Cas es |
Co nt |
O R1 |
95 % CI L |
95 % CI U |
p- tre nd |
Cas es |
Co nt |
O R1 |
95 % CI L |
95 % CI U |
p- tre nd |
Cas es |
Co nt |
O R1 |
95 % CI L |
95 % CI U |
p- tre nd |
| All preterm births: | |||||||||||||||||||
| 1 | 36 | 452 | 1.00 | Ref | 21 | 218 | 1.00 | Ref | 15 | 234 | 1.00 | Ref | |||||||
| 2 | 33 | 458 | 0.86 | 0.51 | 1.45 | 17 | 220 | 0.77 | 0.38 | 1.56 | 16 | 238 | 0.94 | 0.43 | 2.04 | ||||
| 3 | 43 | 447 | 1.09 | 0.64 | 1.85 | 21 | 219 | 0.86 | 0.41 | 1.82 | 22 | 228 | 1.30 | 0.60 | 2.82 | ||||
| 4 | 46 | 442 | 1.11 | 0.64 | 1.92 | 0.72 | 26 | 217 | 1.14 | 0.53 | 2.45 | 0.73 | 20 | 225 | 1.06 | 0.47 | 2.38 | 0.90 | |
| Preterm ≥2500 g: | |||||||||||||||||||
| 1 | 19 | 469 | 1.00 | Ref | 9 | 230 | 1.00 | Ref | 10 | 239 | 1.00 | Ref | |||||||
| 2 | 22 | 469 | 1.09 | 0.56 | 2.11 | 12 | 225 | 1.39 | 0.54 | 3.57 | 10 | 244 | 0.86 | 0.33 | 2.22 | ||||
| 3 | 33 | 457 | 1.53 | 0.79 | 2.99 | 19 | 221 | 1.82 | 0.69 | 4.80 | 14 | 236 | 1.22 | 0.47 | 3.13 | ||||
| 4 | 37 | 451 | 1.84 | 0.91 | 3.72 | 0.09 | 24 | 219 | 3.03 | 1.09 | 8.40 | 0.03 | 13 | 232 | 1.07 | 0.39 | 2.89 | 0.90 | |
| Preterm <2500 g: | |||||||||||||||||||
| 1 | 17 | 471 | 1.00 | Ref | 12 | 227 | 1.00 | Ref | 5 | 244 | 1.00 | Ref | |||||||
| 2 | 11 | 480 | 0.62 | 0.27 | 1.39 | 5 | 232 | 0.37 | 0.12 | 1.12 | 6 | 248 | 1.11 | 0.30 | 4.08 | ||||
| 3 | 10 | 480 | 0.57 | 0.23 | 1.39 | 2 | 238 | 0.16 | 0.03 | 0.79 | 8 | 242 | 1.44 | 0.40 | 5.21 | ||||
| 4 | 9 | 479 | 0.42 | 0.16 | 1.06 | 0.07 | 2 | 241 | 0.12 | 0.02 | 0.61 | 0.01 | 7 | 238 | 1.06 | 0.28 | 3.97 | 0.94 | |
Abbreviations: CIL, lower confidence interval; CIU, upper confidence interval; Cont, controls; OR, odds ratio; Perc quart, maternal urinary perchlorate concentration quartile unadjusted for urinary creatinine concentrations (cut-off points are 3.94, 6.50, and 10.0 µg/L); Ref, Reference category
Adjusted for creatinine (log10), maternal age, maternal weight, race, (and sex, for analyses of both sexes)
For preterm births <2500 grams, the OR in male infants comparing the upper to lower perchlorate quartiles was below 1.0 (OR=0.12, 95% CI: 0.02–0.61) although the number of births in this category was small (n=2 cases in the upper perchlorate quartile). This OR was close to 1.0 in females infants (OR=1.06; 95% CI: 0.28–3.97). Analyses stratified by mother’s birthplace suggest the low OR in male infants for preterm birth <2500 grams primarily occurs in infants of US born mothers although sample sizes were very small (e.g. a total of 3 preterm births <2500g in the upper three perchlorate quartiles) (Figure A1).
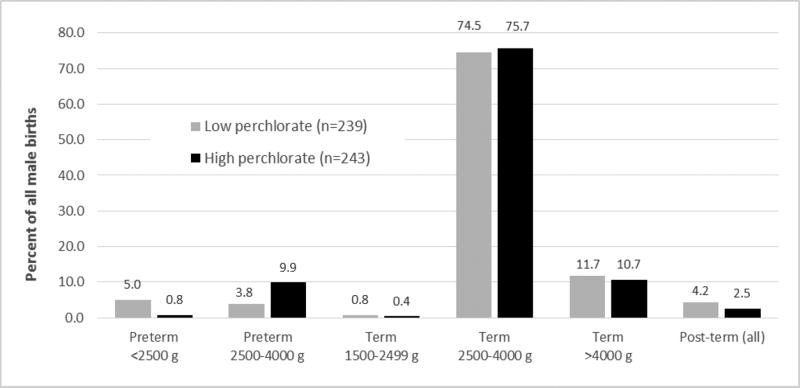
Figure 2 displays the unadjusted proportions of all male births in several additional birthweight-gestational age categories, comparing subjects in the upper and lower perchlorate quartiles. As shown, there was a greater percentage of births in the preterm 2500–4000 g (9.9 vs. 3.8%) group and a lower percentage in the preterm <2500 g (5.0% vs. 0.8%) group among subjects in the higher vs. lower perchlorate quartiles. Only small perchlorate-related differences were seen in the other birthweight-gestational age categories, including term >4000 g babies. Adjustments for creatinine, maternal age and weight, and race had little impact on these results but led to non-robust results in categories with small numbers (not shown).
Figure 2.
Proportions of male births in various birthweight-gestational age categories (27), comparing infants of mothers with high and low urinary perchlorate concentrations in the study population of pregnant women from San Diego County 2000–2003
The high and low perchlorate groups are the upper (range: 10.0–177.0 µg/L, unadjusted for urinary creatinine concentration) and lower (range: 0.23–3.94 µg/L, unadjusted for urinary creatinine concentration) quartiles in all subjects, respectively. Some groups are not displayed because of small numbers.
Thyroid hormone mediation
For the analyses of possible mediation of the perchlorate-birthweight association by thyroid hormone, 862 male births had data on maternal serum T4, fT4, and TSH concentrations. In these subjects, increasing log10 perchlorate was associated with decreasing T4 (β=−0.73, p<0.001) (Table A4), decreasing fT4 (β=−0.043, p=0.05), and increasing log10 TSH (β=0.069, p=0.03). Serum T4 was inversely associated with birthweight, when adjusting for log10 perchlorate (β=−22.1 g, p=0.02) (Table A4). The association between T4 and birthweight unadjusted for perchlorate and creatinine was −0.24 g (p=0.009). The log10 perchlorate-birthweight linear regression coefficients unadjusted and adjusted for T4 were 149.8 (p=0.01) and 133.6 (p=0.03), respectively (Table A4), giving a Sobel value (“indirect effect”) of 16.2 (p=0.05) and suggesting a possible weak mediation effect. Further adjustments for maternal age, maternal weight, race, sex, or other factors had little impact on mediation results. The Sobel values for fT4 and log10 TSH were near 4.0 and not statistically significant (not shown). No evidence of mediation by T4, fT4 or TSH was seen for the elevated odds ratios in male births for perchlorate and preterm births >2500 g.
DISCUSSION
We identified an association between increasing maternal urinary perchlorate concentrations and increasing birthweight in male infants. Increasing perchlorate was associated with increases in the proportion of preterm births >2500 g in males and a corresponding decrease in the proportion of preterm births <2500 g in males. These latter findings are consistent with our birthweight analysis in which the largest perchlorate-increased male birthweight associations were seen in the analyses confined to pre-term births. Overall, these results suggest that perchlorate may increase male infant birthweight, particularly in infants born preterm.
An important strength of our study was the large size. With 1,957 subjects this is the largest study of perchlorate in pregnant women to date. Another important feature was the unique contamination situation in the study area which is likely at least partially responsible for the wide range of perchlorate exposure seen in our study subjects. Wide contrasts in exposure like this increase the likelihood that true associations can be identified with good statistical power. Another strength was our access to data on a large number of variables including race and ethnicity; birth and pregnancy complications; socioeconomic factors like maternal education and insurance type; anti-thyroid antibodies; and related chemicals including cotinine, thiocyanate, nitrate, and iodine. These data allowed us to evaluate the potential confounding or modifying effects of a large number of factors that can potentially influence birth outcomes.
Only a few previous perchlorate studies have examined birth outcomes, and clear associations have not been seen (28–30). For example, in northern Chile, birthweights were similar in infants from cities with drinking water perchlorate concentrations of 113.9 µg/L (n=55 subjects), 5.82 µg/L (n=48), and below detection (n=55) although a large fraction of the subjects from the higher exposure city moved to and gave birth in the larger lower exposure city (30). In a recent study in New Jersey, maternal urinary perchlorate concentrations were not related to birthweight, although the sample size was small (n=107) and all subjects were recruited from a high risk pregnancy clinic (29). The study presented here is the first report of perchlorate exposure potentially altering birthweight, and the first to examine perchlorate and preterm birth.
The mechanisms that underlie our results are unknown. However, given the well-established mechanism linking high doses (e.g. 500–800 mg/day) of perchlorate to decreased thyroid iodide uptake and decreased thyroid hormone production, and the well-known role of thyroid hormone in fetal development, pathways involving this hormone seem most likely (31). Several studies have linked overt or subclinical hypothyroidism to increases in preterm birth, although findings are not consistent across all studies (25). Data linking thyroid function to changes in birthweight have also been inconsistent (25), although several results support the possibility that thyroid hormone could mediate our findings. For example, in the FASTER Trial of 10,990 births from throughout the US, first trimester hypothyroxinemia was associated with birthweights >4000 g (OR=1.97, 95% CI: 1.37–2.83) (32). In the Northern Finland Birth Cohort, clinical hypothyroidism (TSH >95th percentile) was associated with a greater ponderal index (28.4 vs. 27.6 kg/m3, p<0.05) and greater mean birthweight (3676 vs. 3568 g) although the later was not statistically significant (33). In a study of all singleton births in Denmark from 1976–2006 (n=1,638,338) maternal hypothyroidism was associated with a 20 g (95% CI: 10–30 g) increase in birthweight and an odds ratio of 1.24 (95% CI: 1.17–1.31) for large-for-gestational age children (34). Other studies have not found clear associations between maternal hypothyroidism and birthweight (35, 36). Alterations in thyroid function have also been linked to gender-related effects on gonadal development, sex hormone and sex hormone binding globulin levels, and other outcomes (25), and it’s possible one or more of these effects are related to the different results we identified in male versus female infants. As reported earlier, we found evidence that perchlorate decreases serum T4 and fT4 and increases serum TSH in the pregnant women in this cohort (8). In our analysis of mediation by T4 we also found evidence that decreasing serum T4 was associated with increasing birthweight (Table A3), highlighting the possibility that thyroid hormones could be mediating the perchlorate-birthweight associations we identified. Overall however, our mediation analyses suggested that T4 had only a weak mediating effect. Importantly though, this analysis was based on only a single assessment of thyroid hormone, levels of which change over the course of pregnancy and which have some day to day and within-day variability (25). In addition, pregnancy-related changes in serum proteins can impact the immunoassays we used to measure fT4 (37). As a whole, these factors may have limited our ability to identify the true extent by which our results are mediated by thyroid hormones.
The magnitude of the perchlorate-birthweight association we identified for male infants was greater when mothers had lower (<100 µg/L) or higher (>300 µg/L) urinary iodide concentrations (Table 3). For those with lower urinary iodide levels, this finding is consistent with the known mechanism of perchlorate (iodide uptake inhibition in the thyroid) and the potential impacts of thyroid hormone on birthweight discussed above. In most individuals, very high iodine intakes can transiently and paradoxically inhibit thyroid hormone production, termed the acute Wolff-Chaikoff effect (38). Typically, this is only temporary and thyroid function returns to normal after a few days, even if high iodine exposure continues (39). However, several studies have reported increased rates of thyroid autoimmunity and hypothyroidism in areas where people have chronically high iodine intakes (40–44). This would suggest that chronic excessive high iodine intakes might lead to long-term hypothyroidism in some susceptible individuals. Although further research is needed on this issue, it may be that high iodine and perchlorate combine to reduce thyroid hormone production, and this combined exposure is responsible for the greater perchlorate-birthweight regression coefficient we saw in those with high urinary iodide levels.
The results of this study were also based on single assessments of urinary concentrations of perchlorate, iodide, thiocyanate, nitrate, and creatinine. All of these can also vary throughout the day and from day to day. The direction of any bias caused by this variability is unpredictable, however these variations would most likely reduce statistical power or bias any true associations to the null (45). The half-life of perchlorate excretion is fairly short (e.g., 48–72 hours) (46–48). However, a study in New York City children following urinary perchlorate levels over a six-month period has shown that a single measurement can be used to accurately classify participants into low, medium, and high long-term exposure groups (49). Overall, although our metric of perchlorate exposure likely involved some misclassification, this was unlikely to have caused the positive results we identified.
Our analyses incorporated several potential confounding factors known to influence birth outcomes, including maternal age, race and ethnicity, adverse pregnancy or birth conditions, or indicators of low socioeconomic status (SES) like maternal education or insurance payment method. None of these appeared to cause major confounding in our study. We did not have information on some of the other factors that may influence birth outcomes, including certain genetic conditions, diet (including consumption of foods like dairy products that can be high in perchlorate), some medications, or the presence of treated thyroid disease. Some of these factors, including the use of most medications during pregnancy or genetic abnormalities are likely too rare to cause major confounding. Overall, although we did not see evidence of major confounding, residual confounding or unknown confounding by factors we did not assess cannot be completely excluded.
Several of our findings varied by mother’s birthplace. The exact reasons for this are unknown, although some of this may be related to some of the same issues influencing the Hispanic paradox, the phenomenon that despite lower socioeconomic status, Hispanic mothers born in Mexico who migrate to the US may have better pregnancy outcomes than US-born mothers (50). Although the exact causes of this have not been clearly delineated possible explanations could be greater family or community support, diet, lower rates of smoking or alcohol intake, or fewer birth or delivery complications. In our study, US- and Mexico-born mothers differed on several factors including urinary thiocyanate levels and public insurance use (data not shown), however none of these factors was found to be an important effect modifier in the perchlorate-birthweight associations we identified. Overall, given the small sample sizes in some analyses and the multiple comparisons we performed, chance cannot be ruled out as an explanation for these birthplace differences. As such any conclusions based on these particular findings are speculative and these analyses should be replicated in larger detailed studies before any firm conclusions regarding perchlorate and mother’s birthplace can be made.
In conclusion, we found evidence of association between perchlorate and male infant birthweight, particularly in preterm births. The long-term consequences of these associations are unknown. A number of previous studies have identified associations between increased birthweight overall and adult obesity, a well-known risk factor for several chronic adult diseases (51), as well as overall increased morbidity and mortality (52). Whether or not these findings are directly relevant to our results is currently unknown. Regardless these previous studies highlight the importance and potentially adverse impacts of any factor that may alter birthweight or any other birth outcome. Given the novelty of our findings, the fact that some sample sizes are small in some analyses, and that confounding or other bias cannot be ruled out, further research is needed in other populations, and these findings should be considered preliminary. However, because perchlorate exposure is ubiquitous, and because it can potentially impact thyroid function and fetal development, further research on this agent could have widespread public health importance.
Highlights.
Perchlorate was associated with increased birth weight in male infants.
Perchlorate-birth weight associations were greater for US- vs. Mexico-born mothers.
Perchlorate was associated with increased preterm births ≥2500g in male infants.
Acknowledgments
This project was funded by grant R01ES020365 from the National Institute of Environmental Health Sciences (National Institutes of Health)
APPENDIX
Table A1.
Distributions of perchlorate and other urinary analytes in pregnant women from San Diego County 2000–2003 and in reproductive-age women aged 15–45 years in the National Health and Nutrition Examination Survey (NHANES) 2001–2002
| San Diego study
|
NHANES
|
|||||
|---|---|---|---|---|---|---|
| Variable1 | N | Mean | IQR | N | Mean | IQR |
| Urine perchlorate (µg/L) | 1957 | 8.57 | 3.94–10.00 | 618 | 4.73 | 1.8–5.6 |
| Urine thiocyanate (µg/L) | 1957 | 1328 | 446–1580 | 617 | 2286 | 640–2600 |
| Urine nitrate (mg/L) | 1957 | 66.14 | 32.50–83.70 | 618 | 57.84 | 30.00–74.00 |
| Urine iodide (µg/L) | 1957 | 216.61 | 77.00–269.00 | 618 | 210.83 | 75.00–257.00 |
| Urine creatinine (g/L) | 1957 | 130.27 | 72.91–171.83 | 618 | 129.53 | 64.00–177.00 |
Abbreviations: N, number of participants; IQR, interquartile range
Urinary concentrations are not corrected for urinary creatinine concentration
Table A2.
Sociodemographic variables of the included and excluded subjects in the study population of pregnant women from San Diego County 2000–2003
| Variable | Category | Included N (%) |
Excluded N (%) |
|---|---|---|---|
| All subjects | 1957 (100%) | 227 (100%) | |
| Sex | Female | 998 (51.0%) | 112 (49.3%) |
| Male | 959 (49.0%) | 115 (50.7%) | |
| Race/ethnicity | Hispanic | 1352 (69.1%) | 171 (75.3%) |
| Asian | 25 (1.3%) | 3 (1.3%) | |
| Black | 81 (4.1%) | 6 (2.6%) | |
| Other | 90 (4.6%) | 8 (3.5%) | |
| White | 409 (20.9%) | 39 (17.2%) | |
| Mother’s birthplace | US | 882 (45.1%) | 98 (43.2%) |
| Mexico | 933 (47.7%) | 115 (50.7%) | |
| Other | 142 (7.3%) | 14 (6.2%) | |
| Maternal age (years) | <21 | 446 (22.8%) | 54 (23.8%) |
| 21–25 | 575 (29.4%) | 59 (26.0%) | |
| 26–30 | 435 (22.2%) | 61 (26.9%) | |
| >30 | 501 (25.6%) | 53 (23.3%) | |
| Maternal education (highest grade) | <9 | 537 (28.0%) | 63 (28.3%) |
| 9–11 | 319 (16.6%) | 38 (17.0%) | |
| 12 | 580 (30.2%) | 74 (33.2%) | |
| >12 | 484 (25.2%) | 48 (21.5%) | |
| Maternal weight (lbs) | <130 | 441 (25.0%) | 55 (24.2%) |
| 131–147 | 438 (24.8%) | 37 (16.3%) | |
| 148–160 | 449 (25.4%) | 78 (34.4%) | |
| >160 | 438 (24.8%) | 57 (25.1%) | |
| Delivery | NVD | 1523 (77.8%) | 161 (70.9%) |
| Caesarean | 434 (22.2%) | 66 (29.1%) | |
| Insurance | Private | 589 (30.1%) | 68 (30.1%) |
| Public | 1368 (69.9%) | 158 (69.9%) |
Abbreviations: N, number of participants; NVD, normal vaginal delivery
Table A3.
Mean birthweight (grams) by quartiles of perchlorate concentrations in male births from US born mothers in the study population of pregnant women from San Diego County 2000–2003 (p-trend in mean birthweights across quartiles=0.009)
| Perchlorate (µg/L)1 | N | Birthweight2 | 95% CI | p-value3 |
|---|---|---|---|---|
| 0.82–4.71 | 111 | 3402.5 | 3303.1–3502.0 | Reference |
| 4.72–6.89 | 111 | 3461.6 | 3362.7–3560.4 | 0.41 |
| 6.90–10.15 | 112 | 3563.6 | 3465.4–3661.8 | 0.02 |
| 10.25–86.83 | 111 | 3566.8 | 3467.8–3665.8 | 0.02 |
Abbreviations: CI, confidence interval; N, sample size
Unadjusted for urinary creatinine concentration
Adjusted means, adjusted for maternal age, maternal weight, urinary creatinine (log10), and race
p-value compared to the lowest quartile
Table A4.
Results of the mediation analysis of maternal serum total thyroxine (T4) on the association between maternal urine perchlorate and birthweight in male infants (n=862) in the study population of pregnant women from San Diego County 2000–20031
| Model | Dependent variable |
Independent variables |
β | SE | p-value |
|---|---|---|---|---|---|
| MODEL 1 | T4 | Log10 perchlorate | −0.73 | 0.22 | 0.0008 |
| MODEL 22 | Birthweight | Log10 perchlorate | 133.6 | 60.4 | 0.03 |
| T4 | −22.1 | 9.4 | 0.02 | ||
| MODEL 3 | Birthweight | Log10 perchlorate | 149.8 | 60.2 | 0.01 |
All models adjusted for urinary creatinine (log10). All models include the same 862 male subjects with information on perchlorate, creatinine, and maternal T4
This model includes both log10 perchlorate and serum T4
Figure A1.
Odds ratio of preterm birth by urinary maternal perchlorate concentration quartiles (Q1-Q4) in male infants of Mexico and US born mothers in the study population of pregnant women from San Diego County 2000–20031
1. Lowest quartile of unadjusted maternal urinary perchlorate concentration (Q1) is used as the reference group. Odds ratios are for preterm birth compared to all other births (i.e. all term and post-term births). ORs are adjusted for creatinine (log10), maternal age, maternal weight, and race
2. p-trend <0.05
For US born mothers there were no cases of preterm births <2500 g in third perchlorate quartile
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention, the Office of Environmental Health Hazard Assessment, the California Environmental Protection Agency, the California Department of Public Health, or the State of California.
The authors declare they have no competing financial interests.
References
- 1.Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, et al. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006;114(12):1865–71. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonacchera M, Pinchera A, Dimida A, Ferrarini E, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14(12):1012–9. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 3.Haddow JE, Palomaki GE, Allan WC, Williams JR, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 4.Pop VJ, Brouwers EP, Vader HL, Vulsma T, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59(3):282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 5.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50(2):149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 6.Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79(2):152–62. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- 7.Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117(7):1033–41. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinmaus C, Pearl M, Kharrazi M, Blount BC, et al. Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in southern California. Environ Health Perspect. 2015;124:861–7. doi: 10.1289/ehp.1409614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. EPA. Perchlorate monitoring results. Henderson, Nevada to the lower Colorado River. 2005 Available from: http://www.epa.gov/fedfac/pdf/perrpt12_05.pdf.
- 10.Steinmaus C, Miller MD, Howd R. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001-2002 National Health and Nutrition Examination Survey. Environ Health Perspect. 2007;115(9):1333–8. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heck JE, Park AS, Contreras ZA, Davidson TB, et al. Risk of childhood cancer by maternal birthplace: a test of the Hispanic paradox. JAMA Pediatr. 2016;170(6):585–92. doi: 10.1001/jamapediatrics.2016.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United Nations Development Programme. UNDP Environment and Energy Group. Energy and Environment Practice Gender Mainstreaming Guidance Series Chemicals Management. Chemicals and Gender. 2011 Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjx6tWTlMjNAhVJWD4KHaidAcYQFggmMAA&url=http%3A%2F%2Fwww.undp.org%2Fcontent%2Fdam%2Faplaws%2Fpublication%2Fen%2Fpublications%2Fenvironment-energy%2Fwww-ee-library%2Fchemicals-management%2Fchemicals-andgender%2F2011%2520Chemical%26Gender.pdf&usg=AFQjCNFM_7jcDJUs9l5394VLd2mbjvdApg&sig2=wbbaguIj0qNHr0tZb6UKGQ.
- 13.Stetson SJ, Wanty RB, Helsel DR, Kalkhoff SJ, et al. Stability of low levels of perchlorate in drinking water and natural water samples. Anal Chim Acta. 2006;567(1):108–13. doi: 10.1016/j.aca.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Blount BC, Valentin-Blasini L, Osterloh JD, Mauldin JP, et al. Perchlorate exposure of the US population, 2001-2002. J Expo Sci Environ Epidemiol. 2007;17(4):400–7. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- 15.California Department of Public Health. California Newborn Screening Program. 2010 Available from: http://www.cdph.ca.gov/programs/NBS/Pages/default.aspx.
- 16.Mannisto T, Surcel HM, Bloigu A, Ruokonen A, et al. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53(11):1986–7. doi: 10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- 17.Dietz PM, England LJ, Callaghan WM, Pearl M, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 18.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21(Suppl 2):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 19.Kharrazi M, Pearl M, Yang J, DeLorenze GN, et al. California Very Preterm Birth Study: design and characteristics of the population- and biospecimen bank-based nested case-control study. Paediatr Perinat Epidemiol. 2012;26(3):250–63. doi: 10.1111/j.1365-3016.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blount BC, Valentin-Blasini L. Biomonitoring as a method for assessing exposure to perchlorate. Thyroid. 2007;17(9):837–41. doi: 10.1089/thy.2007.0106. [DOI] [PubMed] [Google Scholar]
- 21.Valentin-Blasini L, Blount BC, Delinsky A. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J Chromatogr A. 2007;1155(1):40–6. doi: 10.1016/j.chroma.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Assessment of iodine deficieny disorders and monitoring their elimination: a guide for Programme managers. 3. World Health Organization; Geneva: 2007. [Google Scholar]
- 24.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101(3):253–65. [PMC free article] [PubMed] [Google Scholar]
- 25.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–55. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 26.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yerushalmy J. The classification of newborn infants by birth weight and gestational age. J Pediatr. 1967;71(2):164–72. doi: 10.1016/s0022-3476(67)80067-2. [DOI] [PubMed] [Google Scholar]
- 28.Blount BC, Rich DQ, Valentin-Blasini L, Lashley S, et al. Perinatal exposure to perchlorate, thiocyanate, and nitrate in New Jersey mothers and newborns. Environ Sci Technol. 2009;43(19):7543–9. doi: 10.1021/es9008486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans KA, Rich DQ, Weinberger B, Vetrano AM, et al. Association of prenatal perchlorate, thiocyanate, and nitrate exposure with neonatal size and gestational age. Reprod Toxicol. 2015;57:183–9. doi: 10.1016/j.reprotox.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tellez Tellez R, Michaud Chacon P, Reyes Abarca C, Blount BC, et al. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15(9):963–75. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- 31.Leung AM, Pearce EN, Braverman LE. Perchlorate, iodine and the thyroid. Best Pract Res Clin Endocrinol Metab. 2010;24(1):133–41. doi: 10.1016/j.beem.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112(1):85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannisto T, Vaarasmaki M, Pouta A, Hartikainen AL, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94(3):772–9. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- 34.Andersen SL, Olsen J, Wu CS, Laurberg P. Low Birth Weight in Children Born to Mothers with Hyperthyroidism and High Birth Weight in Hypothyroidism, whereas Preterm Birth Is Common in Both Conditions: A Danish National Hospital Register Study. Eur Thyroid J. 2013;2(2):135–44. doi: 10.1159/000350513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey BM, Dashe JS, Wells CE, McIntire DD, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239–45. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 36.Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, et al. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab. 2013;98(4):1417–26. doi: 10.1210/jc.2012-3389. [DOI] [PubMed] [Google Scholar]
- 37.Lee RH, Spencer CA, Mestman JH, Miller EA, et al. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200(3):260, e1–6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Wolff J, Chaikoff I. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biological Chem. 1948;174:555–564. [PubMed] [Google Scholar]
- 39.Eng PH, Cardona GR, Fang SL, Previti M, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140(8):3404–10. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- 40.Konno N, Makita H, Yuri K, Iizuka N, et al. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab. 1994;78(2):393–7. doi: 10.1210/jcem.78.2.8106628. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen IB, Laurberg P, Knudsen N, Jorgensen T, et al. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007;92(8):3122–7. doi: 10.1210/jc.2007-0732. [DOI] [PubMed] [Google Scholar]
- 42.Tajiri J, Higashi K, Morita M, Umeda T, et al. Studies of hypothyroidism in patients with high iodine intake. J Clin Endocrinol Metab. 1986;63(2):412–7. doi: 10.1210/jcem-63-2-412. [DOI] [PubMed] [Google Scholar]
- 43.Teng W, Shan Z, Teng X, Guan H, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783–93. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Liu DR, Qu CY, Zhang PY, et al. Endemic goitre in central China caused by excessive iodine intake. Lancet. 1987;2(8553):257–9. [PubMed] [Google Scholar]
- 45.Roy T. Fitting a straight line when both variables are subject to error: pharmaceutical applications. J Pharm Biomed Anal. 1994;12(10):1265–9. doi: 10.1016/0731-7085(94)00057-3. [DOI] [PubMed] [Google Scholar]
- 46.Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect. 2002;110(9):927–37. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamm SH, Braverman LE, Li FX, Richman K, et al. Thyroid health status of ammonium perchlorate workers: a cross-sectional occupational health study. J Occup Environ Med. 1999;41(4):248–60. doi: 10.1097/00043764-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Selivanova L, Arefaeva Z. The dynamics behind the absorption and elimination of perchloric acid salts in laboratory animals and agricultural livestock. Chemistry P.S.X. 1986;24:43–45. [Google Scholar]
- 49.Mervish N, Blount B, Valentin-Blasini L, Brenner B, et al. Temporal variability in urinary concentrations of perchlorate, nitrate, thiocyanate and iodide among children. J Exp Sci Environ Epidemiol. 2011;22(2):212–8. doi: 10.1038/jes.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeCamp LR, Choi H, Fuentes-Afflick E, Sastry N. Immigrant Latino neighborhoods and mortality among infants born to Mexican-origin Latina women. Matern Child Health J. 2015;19(6):1354–63. doi: 10.1007/s10995-014-1640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7(10):e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Berg BJ, Yerushalmy J. The relationship of the rate of intrauterine growth of infants of low birth weight to mortality, morbidity, and congenital anomalies. J Pediatr. 1966;69(4):531–45. doi: 10.1016/s0022-3476(66)80038-0. [DOI] [PubMed] [Google Scholar]
- 53.Alexander EK, Pearce EN, Brent GA, Brown RS, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]