Abstract
Background
Atrial fibrillation (AF) is a common cause of stroke. Silent cerebral infarctions (SCIs) are known to occur in the presence and absence of AF, but the association between these disorders has not been well-defined.
Purpose
To estimate the association between AF and SCIs and the prevalence of SCIs in stroke-free patients with AF.
Data Sources
Searches of MEDLINE, PsycINFO, Cochrane Library, CINAHL, and EMBASE from inception to 8 May 2014 without language restrictions and manual screening of article references.
Study Selection
Observational studies involving adults with AF and no clinical history of stroke or prosthetic valves who reported SCIs.
Data Extraction
Study characteristics and study quality were assessed in duplicate.
Data Synthesis
Eleven studies including 5317 patients with mean ages from 50.0 to 83.6 years reported on the association between AF and SCIs. Autopsy studies were heterogeneous and low-quality; therefore, they were excluded from the meta-analysis of the risk estimates. When computed tomography (CT) and magnetic resonance imaging (MRI) studies were combined, AF was associated with SCIs in patients with no history of symptomatic stroke (odds ratio, 2.62 [95% CI, 1.81 to 3.80]; I2 = 32.12%; P for heterogeneity = 0.118). This association was independent of AF type (paroxysmal vs. persistent). The results were not altered significantly when the analysis was restricted to studies that met at least 70% of the maximum possible quality score (odds ratio, 3.06 [CI, 2.24 to 4.19]). Seventeen studies reported the prevalence of SCIs. The overall prevalence of SCI lesions on MRI and CT among patients with AF was 40% and 22%, respectively.
Limitation
Most studies were cross-sectional, and autopsy studies were heterogeneous and not sufficiently sensitive to detect small lesions.
Conclusion
Atrial fibrillation is associated with more than a 2-fold increase in the odds for SCI.
Primary Funding Source
Deane Institute for Integrative Research in Atrial Fibrillation and Stroke, Massachusetts General Hospital.
Atrial fibrillation (AF) is the most common arrhythmia in the elderly, currently affecting more than 2.7 million Americans (1), with a projected prevalence of 5.6 to 12.1 million by 2050 (2, 3). Patients with AF have not only a 4- to 5-fold increase in the risk for stroke (4) but also larger brain infarctions and worse poststroke outcomes than patients without AF (5–7). A recent meta-analysis demonstrated that AF is associated with a 40% increase in the risk for cognitive impairment (relative risk [RR], 1.40 [95% CI, 1.19 to 1.64]) (8). This association was independent of a history of symptomatic stroke (RR, 1.34 [CI, 1.13 to 1.58]) (8) and other comorbid conditions, such as advanced age, hypertension, heart failure, and diabetes (9, 10). These findings highlight the elusiveness of the mechanisms underlying this association and suggest pathways other than symptomatic stroke and shared risk factors listed previously as the underlying cause of this association. One such mechanism may be silent cerebral infarctions (SCIs). Several studies showed a significant association between AF and SCIs in patients without a history of clinically overt stroke (11, 12); however, the few studies that attempted to reproduce similar results did not demonstrate an association (13, 14).
There is a significant variability in the reported prevalence of SCIs in patients with AF (11, 12). One source of this heterogeneity may lie in the different methods of diagnosing SCIs. Whereas earlier studies used autopsy (15, 16) or computed tomography (CT) scans (17–19) to identify SCIs, recent studies have used magnetic resonance imaging (MRI) (12, 13), a more sensitive method, to detect such lesions. It is critical to understand the association between AF and SCIs, not only because it may be a mediating factor in the link between AF and cognitive impairment but also because SCIs may predict future symptomatic strokes and death (20–23).
No study has attempted to gather all of the available data in the literature on the association between AF and SCIs and on the prevalence of SCIs in patients with AF. Therefore, we performed a systematic review and metaanalysis of the literature to estimate the association between AF and SCIs and the prevalence of SCIs in patients with AF and no history of symptomatic stroke.
Methods
An internal study protocol was developed at the Cardiac Arrhythmia Service (Boston, Massachusetts) to perform this review; the protocol was not registered in any database.
Data Sources
Five leading bibliographic databases of biomedical science (MEDLINE [Ovid interface], PsycINFO, Cochrane Library [Ovid SP], CINAHL, and EMBASE) were searched electronically from their inception to 8 May 2014, using text and explosion of medical subject headings (Supplement, available at www.annals.org). One of the investigators designed the search strategies with the help of a trained librarian. In addition, bibliographies of pertinent articles as well as review papers were manually searched. No language restriction was applied. Authors of the included studies were contacted when required data were missing or ambiguous.
Study Selection
Prospective and nonprospective studies reporting the prevalence or incidence of SCIs in patients with AF, as well as prospective and nonprospective studies in which a risk estimate for the association between AF and SCIs was reported or could be calculated, were included in this metaanalysis. Magnetic resonance imaging studies were only included if they acknowledged the exclusion of leukoaraiosis from their analyses, used diffusion-weighted images, or used either hypodensity on fluid-attenuated inversion recovery images or prominent hypodensity on T1-weighted images as an additional criterion to separate leukoaraiosis from SCIs. Exclusion criteria included inappropriate study design (such as reviews, editorials, letters, case series, case reports, and conference proceedings); studies evaluating SCIs after a procedure, such as catheter ablation, coronary artery bypass graft surgery, or cardioversion, in patients with AF; studies with conflicting and inconsistent data in which discrepancies between text and tables could not be resolved by contacting authors or consensus; studies of patients with prosthetic heart valves; studies restricted to patients with mitral stenosis or rheumatic valve disease; studies using transcranial Doppler sonography or electroencephalography for diagnosis of SCIs; and studies of patients with acute stroke or a history of symptomatic stroke.
Data Extraction
Following MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (24), studies were initially screened on the basis of titles and abstracts. The full texts of potentially relevant papers were later reviewed. The reference lists of the review papers and studies that met the inclusion criteria were manually searched. Data from individual studies were extracted by 2 independent observers. Discrepancies were resolved by consensus or a third reviewer. The data extraction sheet included the first author; year; study design (prospective vs. nonprospective); study setting and comparison groups; follow-up period (when applicable); population characteristics (age; sex; country; comorbid conditions, particularly history of symptomatic stroke; and medication, particularly anticoagulants); inclusion and exclusion criteria; AF type (paroxysmal vs. persistent); and methods of AF ascertainment (electrocardiography vs. others). The data extraction sheet also included the method of outcome ascertainment (CT, MRI, or autopsy); CT, MRI, or autopsy specifications (such as section thickness or number and thickness of gaps between the slices); categories of SCI size; definition of SCI; total sample size; number of patients with and without AF; number of patients with SCIs within each group; type of RR estimate (odds ratio [OR], risk ratio, or hazard ratio); point estimate and 95% CI; list of variables in the multivariate model; and level of adjustment. We considered adjustment for confounding either at the study design stage (such as restriction and matching) or at the analyses stage (such as stratification or multivariate analyses) to be acceptable. Studies that used an acceptable method to control for at least 5 of the following 6 variables were considered to be at minimal risk of confounding bias: age, sex, hypertension, hyperlipidemia, diabetes mellitus, and significant carotid stenosis. Studies that did not control for 2 or more of these variables were considered at moderate risk of confounding bias, and those with no adjustment for any of the variables were considered at high risk of confounding bias and were excluded from analysis of pooled OR.
Quality Assessment
Because there are no standard criteria to assess the quality of observational studies, we used an adaptation of 2 published checklists (25, 26) and criteria that were applicable and relevant to the eligible studies. Risk for misclassification of AF and SCIs, as well as risk of confounding and selection bias, were assessed using 15 questions (Appendix Table 1, available at www.annals.org). Not all of the quality criteria were applicable to each study; therefore, to make valid comparisons among studies, we used the ratio of estimated quality score to maximum possible score (Appendix Table 2, available at www.annals.org). The total score was calculated and a sensitivity analysis was performed, including only studies that met at least 70% of the total possible quality score.
Data Synthesis and Analysis
Silent cerebral infarctions were broadly defined as evidence of brain infarctions on imaging or autopsy with no attributable clinical symptoms (such as neurologic deficits). When possible, SCIs were stratified according to their location as cortical versus noncortical. Cortical infarctions were defined as infarctions in the cerebral cortex with or without extension into the subcortical white matter. In the presence of heterogeneity, we used random-effects models and the profile likelihood method (27) to perform the meta-analysis of the association between AF and SCIs. In the absence of heterogeneity, results from fixed-effects models were reported. Forest plots were used to display and summarize the association between AF and SCIs according to SCI diagnosis methods. The Q statistic was used to test for heterogeneity. The degree of heterogeneity was quantified by I2 statistics with an I2 value of 30% to 60% representing a moderate level of heterogeneity (28). We estimated the prevalence of SCIs in patients with AF for each study using binomial distribution. The PROC GLIMMIX procedure in SAS, version 9.1, was used to estimate pooled prevalence of SCIs in patients with AF according to the method of SCI diagnosis. PROC GLIMMIX is able to fit generalized linear mixed models to correlated data with nonconstant variability where the response is not necessarily normally distributed (response distribution for the prevalence was specified as binomial). Stata/IC, version 12, was used to perform the remainder of the analyses. The significance level was defined as a P value less than 0.05 for all analyses.
Role of the Funding Source
This review was funded by the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke, Massachusetts General Hospital. The funding source had no role in the design or conduct of the study; collection or analysis of the data; or the decision to submit the manuscript for publication.
Results
Of 1723 titles and abstracts, 105 papers met the criteria for full-text screening. One German (29) and 2 Japanese (30, 31) articles were translated into English. Sixteen papers from the electronic search and 1 from the manual search met the inclusion criteria (Appendix Figure, available at www.annals.org). The prevalence of SCIs was extracted or calculated from 17 studies (9 using MRI, 6 using CT, and 2 using autopsy). However, only 11 studies (5 using MRI, 4 using CT, and 2 using autopsy) reported adjusted risk estimates. Table 1 lists the characteristics of included studies and patients’ demographic characteristics. Additional details about study design and setting, inclusion and exclusion criteria, AF type and method of ascertainment, and SCI method of ascertainment are presented in Appendix Table 3 (available at www.annals.org).
Table 1.
Demographic Characteristics and Comorbid Conditions of Patients
| Study, Year (Reference) | Diagnosis Method | Assessment | Country | Patients, n | Female | Patients, % |
|---|---|---|---|---|---|---|
| Shinkawa et al, 1995 (15) | Autopsy | Nonprospective | Japan | 543* | 45.3 | |
| Yamanouchi et al, 1997 (16) | Autopsy | Nonprospective | Japan | 367 | 50.7 | |
| Petersen et al, 1989 (19) | CT | Nonprospective | Denmark | 60 | – | |
| Petersen et al, 1989 (32) | CT | Nonprospective | Denmark | 58 | – | |
| Feinberg et al, 1990 (17) | CT | Nonprospective | United States | 141 | 20.6 | |
| Guidotti et al, 1990 (18) | CT | Nonprospective | Italy | 144 | 61.1 | |
| Ezekowitz et al, 1995 (33) | CT | Prospective and nonprospective | United States | 516 | 0 | |
| Zito et al, 1996 (34) | CT | Nonprospective | Italy | 78 | 61.5 | |
| Hara et al, 1995 (35) | MRI | Nonprospective | Japan | 72 | 29.2 | |
| Longstreth et al, 2002 (14) | MRI 1.5T or 0.35T†† | Prospective and nonprospective | United States | 1433 | – | |
| Vermeer et al, 2003 (23) | MRI 1.5T | Nonprospective | The Netherlands | 1015 | 51.8 | |
| Strach et al, 2005 (29) | MRI 1.5T | Prospective and nonprospective | Germany | 51 | 41.2 | |
| Das et al, 2008 (11) | MRI 1T | Nonprospective | United States | 2040 | 53.0 | |
| Kim et al, 2011 (13) | MRI 1.5T | Nonprospective | Republic of Korea | 406 | 61.6 | |
| Kobayashi et al, 2012 (36) | MRI 1.5T | Nonprospective | Japan | 142 | 32.4 | |
| Marfella et al, 2013 (12) | MRI 1.5T | Nonprospective | Italy | 464 | 48.7 | |
| Saito et al, 2014 (37) | MRI 1.5T | Nonprospective | Japan | 243 | 37 |
| Mean Age of Patients (SD), y | CAS, % | DM, % | Hypertension, % | HF, % | CAD, % | HLP, % |
|---|---|---|---|---|---|---|
| SCI: 78.3 (9.5) No SCI: 67.1 (17.9) |
– | – | – | – | – | – |
| AF: 83.5 (7.1) No AF: 83.6 (7.1) |
– | 14.2 | 59.4 | – | 20.7† | – |
| 64 (42–82)‡§ | – | – | – | – | 0 | – |
| 73 (57–87)‡§ | – | – | – | – | 0 | – |
| 68 (10) | – | 18.0 | 43.0 | 24.0 | 13† | – |
| AF: 68 (53–76)‡ No AF: 68 (54–77)‡ |
13.9 | 8.3 | 27.8 | 0 | 0 | 16.7 |
| SCI: 69.3 (6.8) No SCI: 67.0 (7.2) |
3.7‖ | 17.6 | 57.9 | 30.8 | 40.71¶ | – |
| AF: 80.6 (4.5) No AF: 80.4 (4.7) |
0** | 24.4 | 33.3 | 51.3 | – | – |
| SCI: 69 (6) No SCI: 68 (8) |
– | 9.7 | 29.2 | – | 0 | 19.4 |
| ≥65 (patients with no incident infarctions: 73.5; with incident infarctions: 74.4) | – | – | – | – | – | – |
| 72 (7) | – | 7.0 | 72.0 | – | – | – |
| Study group‡‡: 63 (10) Control group§§: 59 (12) |
– | – | – | – | – | – |
| 62 (9) | 15.0‖‖ | 8.7 | 37.0 | – | – | – |
| 64.4 (10.6) | – | 22.4 | 46.3 | – | 3.4 | 25.4 |
| AF: 74.4 (9.9) No AF: 73.7 (8.2) |
– | 27.5 | 60.6 | – | – | 38.7 |
| AF: 50 (5) No AF: 51 (6) |
– | 100 | – | – | – | – |
| AF: 69.4 (9.2) No AF: 69.0 (9.5) |
– | 21.4 | 47.7 | – | – | 25.1¶¶ |
AF = atrial fibrillation; CAD = coronary artery disease; CAS = carotid artery stenosis; CT = computed tomography; DM = diabetes mellitus; HF = heart failure; HLP = hyperlipidemia; MRI = magnetic resonance imaging; SCI = silent cerebral infarction.
Of 713 patients (125 in the SCI group and 588 in the group with no infarctions), only 543 (103 in SCI group and 440 in the group with no infarctions) were assessed for traditional risk factors for SCI, such as AF.
CAD was defined as myocardial infarction.
Median age (range).
Values are reported for patients with AF. Control patients did not have AF and were matched for age and sex with those with AF.
Stenosis ≥75% by ultrasonography.
CAD was defined as myocardial infarction and active angina.
Excluded irregular fibrocalcific ulcerative plaques or stenosis >50%.
From an original study of magnetic resonance abnormalities and cardiovascular disease in older adults (38).
The study group included patients with AF and echocardiographic evidence of left atrial thrombi.
The control group included patients with AF but no echocardiographic evidence of left atrial thrombi.
CAS ≥25%.
Statin therapy.
Definition of SCIs
Silent cerebral infarctions were identified by CT, MRI, or autopsy in 6, 9, and 2 studies, respectively (Appendix Table 4, available at www.annals.org). Autopsy studies were not sufficiently sensitive to detect small lesions because of thick slices and large interslice gaps. Studies that used CT for diagnosis consistently defined SCIs as hypodense foci. Three CT studies described additional criteria in their definition of SCIs (17, 33, 34). Feinberg (17) and Ezekowitz (33) and their colleagues required the presence of focal dilated sulci to be distinguished from SCIs that manifested as hypodense lesions in a vascular distribution with no mass effect or edema. Zito (34) and Feinberg (17) and their colleagues also separated SCIs from lesions suspicious for leukoaraiosis. However, MRI studies were more variable because of different MRI variables and criteria used for the evaluation of SCIs. With the exception of the study by Hara and colleagues (35), which did not report the exact criteria used, 2 criteria were common in the definition of SCIs on MRI images in all studies: focal, sharply demarcated hyperintense lesions on T2 and hypointense lesions on T1. A few studies applied additional criteria to distinguish SCIs from dilated Virchow-Robin spaces and leukoaraiosis. Criteria to distinguish SCIs from dilated Virchow-Robin spaces included a lesion size of 3 mm or greater (11–14, 23, 35, 36) and presence of a hyperintense rim around the hypointense lesion on fluid-attenuated inversion recovery images (13). Das and colleagues (11) required the lesion to be of cerebrospinal fluid intensity to distinguish SCIs from leukoaraiosis.
Association Between AF and SCIs in Patients Without a History of Symptomatic Stroke
Eleven studies (5 using MRI, 4 using CT, and 2 using autopsy) reported on the association between AF and SCIs. Autopsy studies were heterogeneous and low-quality; therefore, they were excluded from the meta-analysis of the risk estimates. When CT and MRI studies were combined, 4407 patients (505 with AF and 3902 without AF) were included in the analysis; 230 patients with AF (45.54%) and 610 patients without AF (15.63%) had SCIs. Atrial fibrillation was associated with SCIs in patients with no symptomatic stroke history (OR, 2.62 [CI, 1.81 to 3.80]; I2 = 32.12%; P for heterogeneity = 0.118) (Figure 1). The results were robust to further sensitivity analyses (Table 2). There was no significant heterogeneity between MRI and CT studies. Among MRI studies, Marfella and colleagues (12) reported a more significant association between AF and SCI. This could be because the study population had diabetes mellitus, which may have accentuated the vascular effect of AF on the brain. Removing this study did not alter the results significantly. All of the MRI studies that were included in this analysis defined SCI lesions as lesions greater than 3 mm.
Figure 1. Association between AF and SCIs according to the method of SCI diagnosis in 9 studies.
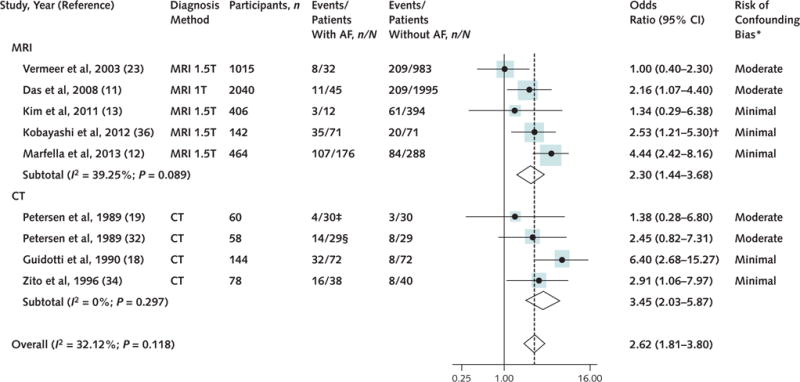
AF = atrial fibrillation; CT = computed tomography; MRI = magnetic resonance imaging; SCI = silent cerebral infarction.
*Studies that used an acceptable method to control for ≥5 of the following 6 variables were considered to be at minimal risk of bias: age, sex, hypertension, hyperlipidemia, diabetes mellitus, and presence of significant carotid stenosis. Studies that did not control for ≥2 variables were considered at moderate risk of bias.
†For the purpose of this meta-analysis, we used the reported odds ratio for the association between AF and SCI lesions >5 mm.
‡Paroxysmal AF.
§Persistent AF.
Table 2.
Sensitivity and Subgroup Analyses on the Association Between AF and SCIs
| Analysis Description | Studies, n | Odds Ratio (95% CI) |
Assessment of Heterogeneity | |
|---|---|---|---|---|
| I2, % | P Value | |||
| Original analysis | 9 | 2.62 (1.81–3.80) | 32.12 | 0.118 |
| Restricted to studies that met ≥70% of total quality score | 8 | 3.06 (2.24–4.19) | 2.35 | 0.37 |
| Restricted to studies with paroxysmal AF | 2 | 3.83 (2.17–6.75) | 0 | 0.180 |
| Restricted to studies with persistent AF | 3 | 3.87 (2.20–6.80) | 0 | 0.32 |
AF = atrial fibrillation; SCI = silent cerebral infarction.
Prevalence of SCIs in Patients With AF and No History of Symptomatic Stroke
Data on the prevalence of SCIs in patients with AF were available in 17 studies (9 MRI, 6 CT, and 2 autopsy). Because the prevalence of SCIs varies depending on the sensitivity of the diagnosis method, we reported SCI prevalence within each diagnosis category (CT, MRI, and autopsy). Autopsy studies were heterogeneous and of low sensitivity; therefore, we did not report a pooled prevalence estimate for these studies. The prevalence of SCIs was highest when lesions were detected by MRI, the most sensitive diagnostic method (prevalence, 0.40 [CI, 0.29 to 0.51]) (Figure 2). The prevalence of SCIs in CT scans was lower than the prevalence in MRI scans (prevalence in CT scans, 0.22 [CI, 0.13 to 0.32]) (Figure 2). One source of heterogeneity among MRI studies may be the variability in prevalence of vascular comorbid conditions (Table 1). For instance, the prevalence of diabetes varies from 7% to 100% among studies. The study in which all patients had diabetes reported a higher prevalence of SCI. The removal of this study from the analysis reduced the heterogeneity and the pooled prevalence to 0.31 (CI, 0.25 to 0.38). The mean age of the patients was similar across studies, ranging from 50.0 to 83.6 years. To minimize variability in the definition of SCIs among MRI studies, we also performed a sensitivity analysis limited to 7 MRI studies in which only lesions greater than 3 mm were included in the definition of SCIs (11–14, 23, 36). The prevalence of such lesions in MRI scans was estimated to be 0.44 (CI, 0.33 to 0.57).
Figure 2. Prevalence of SCIs in patients with AF according to the method of SCI diagnosis.
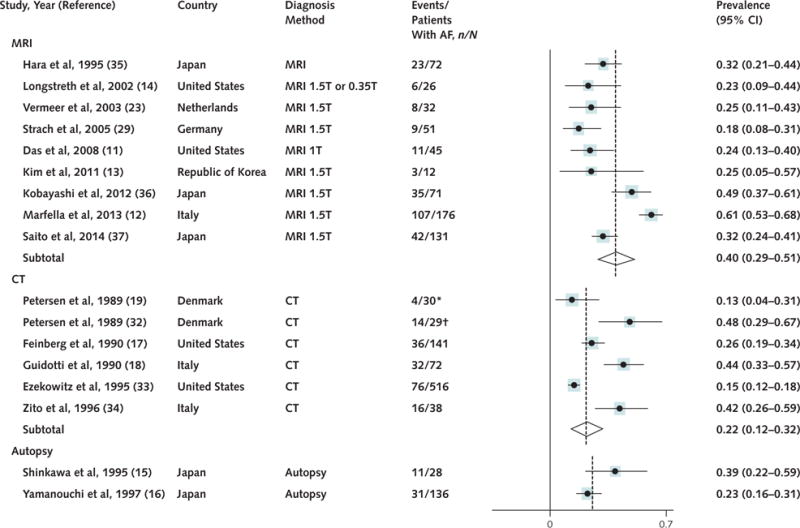
AF = atrial fibrillation; CT = computed tomography; MRI = magnetic resonance imaging; SCI = silent cerebral infarction.
Incidence of SCIs in Patients With AF and No History of Symptomatic Stroke
Data on the incidence of SCIs in patients with AF were scarce. Only 3 studies (14, 29, 33) reported longitudinal assessment of SCIs in patients with AF. Because of the significant variability among these studies in definition of SCIs, method of SCI diagnosis, and medications, we did not pool the results. Ezekowitz and colleagues (33) reported a mean incidence rate of 1.3 per 100 person-years on CT scans. In this study, the incidence of SCI lesions did not differ significantly between patients treated with warfarin or placebo. In the study by Longstreth and colleagues (14), 1433 patients aged 65 years or older with no baseline MRI lesions were followed for an average of 5 years for development of SCIs. During follow-up, 6 of 26 patients (23%) with AF developed SCIs. The anticoagulation status of these patients was unclear. Strach and colleagues (29) reported the occurrence of new MRI lesions in 3.9% of 51 patients during 12 months of follow-up despite therapeutic anticoagulation.
Variability in AF Type
Only a few studies explicitly differentiated paroxysmal from chronic or persistent AF by describing AF duration. The association between SCIs and paroxysmal AF and persistent AF was adequately described in 2 (12, 19) and 3 studies (18, 32, 34), respectively (Table 2). The association between AF and SCIs was statistically significant, regardless of the AF type. The pooled OR for the association between paroxysmal AF and SCIs (OR, 3.83 [CI, 2.17 to 6.75]; I2 = 0%; P for heterogeneity = 0.18) was similar to the pooled OR for the association between persistent AF and SCIs (OR, 3.87 [CI, 2.20 to 6.80]; I2 = 0%; P for heterogeneity = 0.32).
Variability in the Quality of Included Studies
The exclusion of studies that met less than 70% of total possible score did not change the results substantially (Table 2). With the exception of 3 studies (14, 29, 33) that reported both prevalence and incidence of SCIs in patients with AF, the remaining studies were crosssectional. Two autopsy (15, 16), 3 MRI (23, 35, 37), and 1 CT (17) study met less than 70% of the total quality score. No single criterion stood out as the primary problem. However, the potential for misclassification of SCIs and the history of symptomatic stroke, as well as unadjusted analysis, were mainly responsible for a lower quality score. Compared with the MRI studies, less sensitive CT and autopsy studies received lower quality scores because of the greater potential for misclassification of SCIs.
Discussion
This meta-analysis suggests that SCIs are very common in patients with AF. An SCI can be detected on MRI in 40% of patients with this arrhythmia. In addition, AF was associated with more than a 2-fold increase in the odds for any SCI. This association was independent of AF subtypes (paroxysmal vs. persistent). Restricting the analysis to studies with high-quality scores eliminated the heterogeneity among the studies without changing the results substantially.
The prevalence of MRI-diagnosed SCIs in the general population is between 8% and 28% (39). This prevalence is approximately one half of the estimated prevalence of SCI in the AF population. Although SCIs do not present with acute stroke symptoms, they have been reported to be associated with more than 3- and 2-fold increases in the risk for symptomatic stroke (23) and dementia (40), respectively. Few studies support a relationship between SCIs and death (21). Consequently, the higher prevalence of SCI in patients with AF may put this population at a greater risk for cognitive impairment, future stroke, and disability. Additional studies are warranted to identify appropriate preventive and treatment strategies to reduce the incidence of SCI in patients with AF.
Beyond rhythm-control strategies, anticoagulation has been the mainstay for the prevention of thromboembolic complications that may lead to SCI and symptomatic stroke in patients with AF. Although the overwhelming benefits of anticoagulant therapy for the prevention of symptomatic stroke in patients with AF have been extensively demonstrated, its effect on the prevention of SCI is unclear. Two studies included in this meta-analysis did not report any reduction in the incidence of SCI under anticoagulation (29, 33). In this meta-analysis, we could not report a separate estimate of SCI prevalence as a function of prophylactic anticoagulation or antiplatelet therapy because of a lack of comparable data. Studies that used anticoagulants were more recent and used more sensitive imaging methods, such as MRI; therefore, they reported a higher prevalence of SCI lesions. However, earlier studies investigating patients who were not treated with anticoagulants used less sensitive diagnostic methods and reported lower SCI prevalence; therefore, a valid comparison could not be made between the groups. Future studies will be needed to determine whether anticoagulation is associated with lower SCI incidence and whether such a change in SCI incidence will translate into a reduction in symptomatic stroke, dementia, and death.
Although the determinants of SCI versus symptomatic stroke are not well-defined, smaller lesions in deep white matter are more likely to remain silent, and larger cortical lesions are more likely to become symptomatic (15). The location of an infarction provides insight into the underlying mechanisms of its formation. Cortical and subcortical wedge-shaped infarctions indicate a thromboembolic mechanism (41, 42), whereas small infarctions in deep subcortical white matter indicate intrinsic small vessel disease, and junctional or watershed infarctions indicate hypoperfusion often caused by proximal arterial stenoses or cardiogenic shock (43). In contrast to embolic infarctions, nonembolic infarctions due to intrinsic small vessel disease typically occur in the subcortical white matter and can be confused with leukoaraiosis or chronic white matter hyperintensities that occur secondary to nonacute ischemic mechanisms. Data on the location of SCIs were limited, and the reported associations were unadjusted for potential confounders. Therefore, we could not report separate ORs by SCI location.
This meta-analysis has several strengths. First, we performed a thorough electronic and manual search of the literature, translated non-English studies, extracted data in duplicate, and contacted authors when required data were missing or ambiguous, all of which add assurance to the validity and thoroughness of data gathering in this study. Second, several sensitivity analyses were performed to identify potential sources of heterogeneity and evaluate the robustness of the results reported in this study. Third, we were able to report separate ORs according to AF type and methods of SCI diagnosis. Fourth, the geographic diversity of the studies included in this analysis adds to the generalizability of the results. Last, we performed a thorough quality assessment using 15 criteria, taking into account the potential risk for misclassification of exposure and outcome, as well as risk of confounding and selection bias.
This study also has several limitations. There was significant heterogeneity among autopsy studies. Included studies were mainly cross-sectional, and good-quality longitudinal data were lacking. The quality of included studies was variable; however, a sensitivity analysis restricted to studies that met more than 70% of total possible quality score showed similar results. There was inadequate information on the anticoagulation status of the patients in most studies. Last, the prevalence of MRI-defined SCIs varied depending on the MRI diagnostic criteria. Whereas studies that define SCIs as lesions of any size with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images may overestimate the prevalence of SCIs, those that only consider a fully cavitated lesion of greater than 3 mm with signal intensity similar to cerebrospinal fluid may underestimate their prevalence (44). Because the former definition does not distinguish among SCIs, dilated Virchow-Robin spaces, and leukoaraiosis, the latter definition is likely to be more specific and accurate, particularly because these 3 entities may have different causes and clinical significance. Although MRI variables (field strength of the magnet, matrix, slice thickness, and gap thickness) differed among studies, it is hard to assess the effect of these differences on the reported prevalence of SCIs. Thick slices with large interslice gaps may result in low detection of small lesions and underestimation of the prevalence of SCIs (44). This discussion highlights the need for a unified definition, similar to the one proposed in Appendix Table 5 (available at www.annals.org), to distinguish SCIs from leukoaraiosis and other lesions, such as dilated perivascular spaces in future studies.
In conclusion, this systematic review and meta-analysis provides strong evidence that AF is associated with more than a 2-fold increase in the odds of SCI. Future longitudinal studies with better adjustment for potential confounders and detailed information on the spatial distribution of lesions, as well as use of anticoagulant and antiplatelet therapy, are warranted to estimate the incidence of SCI lesions in patients with AF using unified SCI diagnostic criteria. Also, randomized clinical trials should be designed to investigate whether SCI diagnosis should be incorporated into the CHADS2 or CHA2DS2-VASc scoring system to identify patients who will benefit from anticoagulation.
Supplementary Material
Acknowledgments
The authors thank W.T. Longstreth Jr., MD, MPH, from the University of Washington, Seattle, Washington, and Tsukasa Saito, MD, PhD, from Asahikawa Medical University, Hokkaido, Japan, for providing additional data from their published studies.
Grant Support: By the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke, Massachusetts General Hospital, and with support from Harvard Catalyst and the Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers).
Appendix Figure. Summary of evidence search and selection.
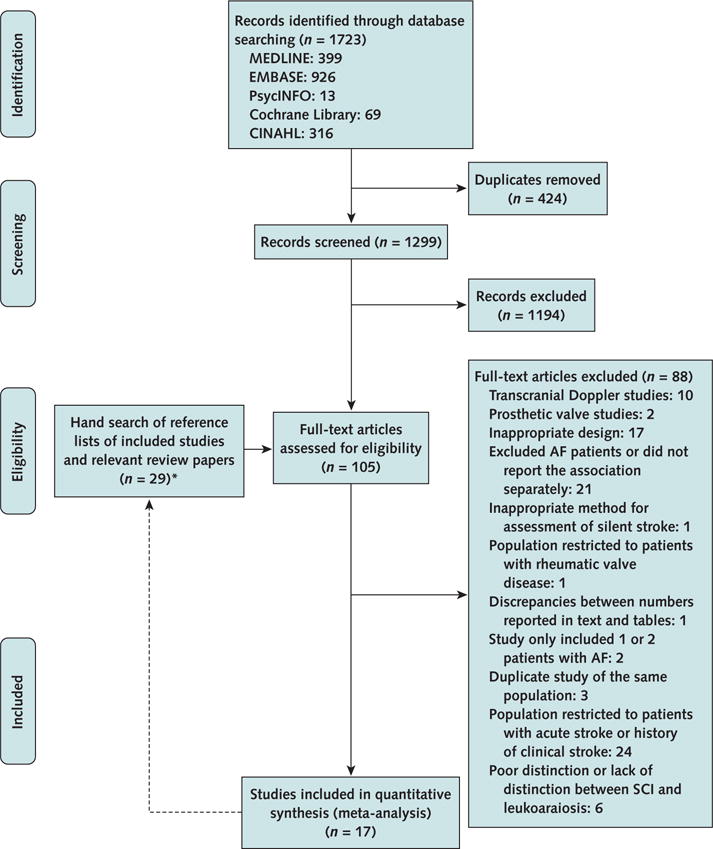
AF = atrial fibrillation; SCI = silent cerebral infarction.
* Once relevant papers were identified through electronic search, their reference lists were manually searched to identify any additional relevant papers. Twenty-nine full-text articles were reviewed from reference lists, and 1 was found to be relevant.
Appendix Table 1.
Fifteen Questions to Assess the Quality of Included Studies
| Question | Possible Answers (Score) | |
|---|---|---|
| 1. | Was AF the primary exposure of interest? | Yes (1) or no (0) |
| 2. | What was the reported temporal relationship between AF and SCI? | Prospective (1), not prospective (0), or both (1) |
| 3. | Were the inclusion and exclusion criteria adequately described? | Yes (1) or no (0) |
| 4. | Was the history of symptomatic stroke confirmed by detailed neurologic examination? | Yes (1) or no (0) |
| 5. | Were autopsies, MRI, and CT done for the purpose of research (as opposed to clinical care)? | Yes (1) or no (0) |
| 6. | What was the method of SCI diagnosis? | MRI (3), CT (2), or autopsy (1) |
| 7. | Were the investigators blinded to the clinical history of patients during the ascertainment of SCIs? | Yes (1) or no (0) |
| 8. | Were the SCIs ascertained by ≥1 investigator? | Yes (1) or no (0) |
| 9. | Did the investigators attempt to distinguish SCIs from dilated perivascular spaces? | Yes (1) or no (0) |
| 10. | Did the authors acknowledge whether they excluded leukoaraiosis from SCIs in their analysis? | Yes (1) or no (0) |
| 11. | Did the investigators use diffusion-weighted imaging to distinguish acute SCIs from leukoaraiosis? | Yes (1) or no (0) |
| 12. | Did the investigators distinguish chronic SCIs from leukoaraiosis by colocalizing the hyperintense lesions on T2-weighted images to nonterritorial FLAIR hyperdensity or prominent hypointense lesions (CSF intensity) on T1-weighted images? | Yes (1 or no (0) |
| 13. | Was AF documented on ≥1 electrocardiography? | Yes (1) or no (0) |
| 14. | Did the investigators assess or report carotid stenosis in study patients? | Yes (1) or no (0) |
| 15. | Risk of confounding bias? | Minimal (2) if studies controlled for ≥5 of following 6 potential confounders: age, sex, hypertension (or blood pressure measurements), DM, hyperlipidemia (or lipid profile measurements), and CAS (or carotid intima-media thickness) either by including these variables in the multivariate model or by ensuring that patients with and without AF were similar or matched on these variables; moderate (1) if studies did not control for ≥2 of these variables; or high (0) if studies did not control for any potential confounders. |
AF = atrial fibrillation; CAS = carotid artery stenosis; CT = computed tomography; CSF = cerebrospinal fluid; DM = diabetes mellitus; FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging; SCI = silent cerebral infarction.
Appendix Table 2.
Assessing the Quality of Included Studies Using 15 Criteria
| Study, Year (Reference) | AF Was the Primary Exposure of Interest (Score of 0/1) | Assessment Temporality (Score of 0/1) | Imaging/Autopsy for the Purpose of Research (Score of 0/1) | ECG Documentation of AF (Score of 0/1) | CAS Assessed/Reported (Score of 0/1) | Inclusion and Exclusion Criteria Clearly Stated (Score of 0/1) | Risk of Confounding Bias (Score of 0/1/2) | Diagnosis Method (Score of 1/2/3) | Attempted to Distinguish SCI From Leukoaraiosis in MRI Studies? (Score of 0/1/2/3) | Attempted to Distinguish SCI From EPVS (Score of 0/1) | Blinding to Clinical Data (Score of 0/1) | Assessment of Radiology or Autopsy Reports by >1 Person (Score of 0/1) | History of Stroke Confirmed by Neurologic Examination (Score of 0/1) | Total Quality Score Met, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Did Authors Acknowledge the Exclusion of Leukoaraiosis From the Final Analysis? (Score of 0/1) | Was DWI Used to Distinguish SCIs From Leukoaraiosis? (Score of 0/1) | Was Nonterritorial FLAIR Hyperdensity or Marked T1 Hypodensity Used as an Additional Criterion to Identify SCI? (Score of 0/1) | ||||||||||||||
| Shinkawa et al, 1995 (15) | No | Cross-sectional | Yes | Yes | No | Yes | Minimal | Autopsy | NA | NA | NA | Yes | – | – | – | 53.8 |
| Yamanouchi et al, 1997 (16) | Yes | Cross-sectional | Yes | Yes | No | Yes | Minimal | Autopsy | NA | NA | NA | Yes | – | Yes | – | 69.2 |
| Petersen et al, 1989 (19) | Yes | Cross-sectional | Yes | Yes | No | Yes | Moderate | CT | NA | NA | NA | No | Yes | Yes | Yes | 71.4 |
| Petersen et al, 1989 (32) | Yes | Cross-sectional | Yes | Yes | No | Yes | Moderate | CT | NA | NA | NA | No | Yes | Yes | Yes | 71.4 |
| Feinberg et al, 1990 (17) | Yes | Cross-sectional | Yes | Yes | No | Yes | N/A | CT | NA | NA | NA | No | Yes | Yes | – | 66.7 |
| Guidotti et al, 1990 (18) | Yes | Cross-sectional | No | Yes | Yes* | Yes | Minimal | CT | NA | NA | NA | No | Yes | No | Yes | 71.4 |
| Ezekowitz et al 1995 (33) | Yes | Both | Yes | Yes | Yes† | Yes | NA | CT | NA | NA | NA | No | Yes | Yes | Yes | 91.7 |
| Zito et al, 1996 (34) | Yes | Cross-sectional | Yes | Yes | Yes‡ | Yes | Minimal | CT | NA | NA | NA | No | Yes | Yes | – | 78.6 |
| Hara et al, 1995 (35) | Yes | Cross-sectional | Yes | Yes | No | Yes | NA | MRI | No | No | –§ | Yes | Yes | No | – | 56.3 |
| Longstreth et al, 2002 (14) | No | Both | Yes | Yes | Yes‖ | Yes | Unknown | MRI 1.5T or 0.35T | Yes | No | Yes | Yes | Yes | Yes¶ | Yes¶ | 77.8 |
| Vermeer et al, 2003 (23) | No | Cross-sectional | Yes | Yes | No | Yes | Moderate | MRI 1.5T | Yes | No | Yes | Yes | – | No | No | 55.6 |
| Strach et al, 2005 (29) | Yes | Both | Yes | Yes | No | Yes | NA | MRI 1.5T | No | Yes | Yes | Yes | Yes | Yes | Yes | 87.5 |
| Das et al, 2008 (11) | No | Cross-sectional | Yes | Yes | Yes** | Yes | Moderate | MRI 1T | Yes | No | No | Yes | Yes | Yes | Yes | 72.2 |
| Kim et al, 2011 (13) | No | Cross-sectional | Yes | Yes | No | Yes | Minimal | MRI 1.5T | Yes | No | No | Yes | Yes | Yes | Yes | 72.2 |
| Kobayashi et al, 2012 (36) | Yes | Cross-sectional | Yes | – | Yes†† | Yes | Minimal | MRI 1.5T | Yes | No | No | Yes | Yes | Yes | – | 72.2 |
| Marfella et al, 2013 (12) | Yes | Cross-sectional | Yes | Yes | Yes‡‡ | Yes | Minimal | MRI 1.5T | Yes | No | Yes | No | Yes | – | – | 72.2 |
| Saito et al, 2014 (37) | Yes | Cross-sectional | Yes | – | No | Yes | High | MRI 1.5T | No | No | Yes | Yes | Yes | – | – | 50.0 |
AF = atrial fibrillation; CAS = carotid artery stenosis; CT = computed tomography; DWI = diffusion-weighted imaging; ECG = electrocardiography; EPVS = enlarged perivascular space; FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging; NA= not applicable; SCI = silent cerebral infarction.
AF = 16.6%, and SR = 11.1% (no significant difference between groups).
Stenosis ≥75%; SCI = 5%; and no SCI = 4% (no significant difference between groups; P = 0.67).
Excluded stenosis >50%.
Only assessed cortical infarctions.
Maximum internal carotid artery stenosis was not a predictor of incidental infarction.
From reference 20.
15% had carotid artery stenosis ≥25%.
No significant difference in maximum intima–media thickness between nonvalvular atrial fibrillation and control patients.
Patients with carotid disease were excluded.
Appendix Table 3.
Study Design and Setting, Inclusion and Exclusion Criteria, AF and SCI Ascertainment Methods, and Other Patient Characteristics
| Study, Year (Reference) | Diagnosis Method | Design (Comparison Groups) | Inclusion Criteria | Exclusion Criteria | AF Ascertainment Method | Anticoagulation | SCI Ascertainment Method |
|---|---|---|---|---|---|---|---|
| Shinkawa et al, 1995 (15) | Autopsy | Nonprospective, population-based autopsy study reporting factors related to SCI and multivariate-adjusted risk estimate for the association between AF and SCI (original cohort followed for 26 y to elucidate CVA incidence in general population) | Patients aged ≥40 y from Hisayama, Japan, were included in the original cohort. After 13 and 22 y, new patients aged >40 y were selected from Hisayama for the new study cohort. Most of the patients who died had autopsies. | Patients with stroke diagnosed before the entry examination; patients with hemorrhagic stroke as the initial stroke episode; and patients with any clinical history, symptoms, or signs of stroke | ECG | – | Photographs of the brain slices in the autopsy documents were reviewed by authors |
| Yamanouchi et al, 1997 (16) | Autopsy | Nonprospective study assessing prevalence of SCI in patients with NRAF using autopsy specimens | Case patients were consecutive patients with chronic NRAF and no history of anticoagulation who were aged ≥70 y and evaluated between 1986 and 1993 in the Tokyo Metropolitan Geriatric Hospital. Control patients did not have NRAF and were matched for age, sex, history of hypertension, DM, pathologically confirmed MI, and severe atherosclerosis in the cerebral arteries. | Anticoagulant therapy during the patient’s lifetime, rheumatic valvular disease, and paroxysmal AF | Chronic NRAF diagnosed by ≥2 ECGs with no SR on subsequent ECGs | No | SCIs were diagnosed by 2 neurologists and a neuropathologist using autopsy specimens, clinical records, and macroscopic brain pathology |
| Petersen et al, 1989 (19) | CT | Nonprospective study with calculable prevalence and risk estimate (comparing patients with AF with age- and sex-matched control patients in SR) | Patients with paroxysmal AF documented on ≥1 ECG and ≥3 ECGs with SR obtained during the follow-up with an interval of ≥1 wk. The control group included age- and sex-matched patients in SR who previously participated in the Copenhagen City Heart Study and considered themselves healthy. | Paroxysmal AF linked to acute MI, intoxication, trauma or alcohol; history of anticoagulant, aspirin, or alcohol abuse in case patients; history of cerebrovascular disease without normal neurologic examination in both case and control patients; history of IHD or endocrinologic disease in control patients | Paroxysmal AF diagnosed by ECG | No | SCIs were diagnosed by 2 consultant neuroradiologists blindly |
| Petersen et al, 1989 (32) | CT | Nonprospective study with calculable prevalence and risk estimate (comparing patients with AF with age- and sex-matched control patients in SR) | Case patients had chronic AF of >l-y duration and were selected from patients referred to the outpatient clinic by their general practitioner for routine ECG. Control patients were age- and sex-matched in SR, considered themselves healthy, and previously participated in the Copenhagen City Heart Study. | History of cerebrovascular disease, abnormal neurologic examination, anticoagulants or aspirin therapy, history of alcohol abuse, and bad-quality CT; patients with cardiovascular events were excluded from the control group | Chronic AF of >1 y duration diagnosed by ECG | No | All CT results were evaluated blindly by 2 consultant neuroradiologists* |
| Feinberg et al, 1990 (17) | CT | Nonprospective study reporting the prevalence of SCIs in a large group of patients with NVAF participating in SPAF study | Patients with NVAF aged >21 y participating in the SPAF study from 15 centers in the United States | Cardiac exclusions: self-limited AF, mitral stenosis, NYHA-IV CHF, idiopathic cardiomyopathy in conjunction with CHF, prosthetic heart valve, MI, percutaneous transluminal angioplasty within 1 y, unstable angina, and CABG within 3 mo. Noncardiac exclusions: dementia, renal failure, any illness reducing life expectancy to <24 mo, any condition requiring antiplatelet or anticoagulant, and history of a stroke or TIA. | NVAF diagnosed by ECG † | No | 2 independent authors blinded to the clinical data read CT results using a standardized assessment form (disagreement in SCI diagnosis was seen in 8% of CT scans, and disagreement in SCI location was seen in 3%) |
| Guidotti et al, 1990 (18) | CT | Nonprospective study with calculable prevalence and risk estimate (comparing patients with AF with age- and sex-matched control patients without AF) | Case patients had ≥1 y ECG documented AF, without historical or instrumental evidence of rheumatic heart disease. Control patients were in SR without cardiopathy affected by muscle-tensive headache, diagnosed according to strict criteria. | Previous MI or thyroid disease, severe enlarged hearts, CHF, history of cerebrovascular events, abnormal neurologic examination, and antiaggregant or anticoagulant therapy | NRAF diagnosed by ECG | No | CT results were examined by a neuroradiologist who was blinded to the patients’ condition |
| Ezekowitz et al, 1995 (33) | CT | Prospective study of many patients with NRAF reporting prevalence and incidence of SCIs during 3 y of follow-up | Men of any age with AF documented by 2 ECGs ≥4 wk apart and no echocardiographic evidence of rheumatic heart disease; baseline prothrombin times had to be within the normal range; the neurologic examination taken at the entry had to be normal with no focal deficit | Previous stroke or a TIA within the 5 y before entry into the study | NRAF diagnosed by 2 ECGs ≥4 wk apart | No‡ | Scans were evaluated by 6 neurologists. Each scan was read by 2 neurologists blinded to physical condition or clinical course of the patient, and their consensus interpretation was recorded. Reading pairs were rotated after every 50 scans to minimize bias. When a consensus was not reached by the 2 primary readers, the scan in question was reviewed and classified by the entire 6-member committee. |
| Zito et al, 1996 (34) | CT | Nonprospective study with calculable prevalence and risk estimate (comparing patients with NVAF and their age- and sex-matched control patients in SR) | Patients with chronic AF >1 y, detected by ECG and defined as NVAF by ECG, all patients were admitted to the hospital because of symptoms related to AF and other minor diseases (such as benign disease of the upper and lower GI tract, arthrosis, urinary infection, etc.). The control group included elderly patients in SR matched for age and sex and educational level with the NVAF group. All control patients were randomly selected either among patients hospitalized for minor disease or among their relatives. | Previous stroke or TIA; lesions of large extracranial arteries; possible source of cerebral embolization (irregular fibrocalcific ulcerative plaques or stenosis >50%); significant abnormalities at neurologic examination; diseases leading to cognitive impairment or potentially interfering with cognitive functions (i.e., senile dementia [DSM III R], psychiatric diseases, or alcohol abuse); hypothyroidism; folate or vitamin B12 deficiency; advanced cardiac failure (NYHA class III and IV); and severe respiratory insufficiency renal failure (creatinine level >221 μmol/L [>2.5 mg/dL]); liver cirrhosis; anemia (hemoglobin level <10 g/dL); insulin-dependent DM; malignancy or other severe medical illness; and other conditions potentially interfering with physical abilities, such as visual impairments, deafness, and severe osteoarthritis | Chronic NVAF diagnosed by ECG | – | All CT scans were interpreted blindly by 2 consultant neuroradiologists with high levels of interobserver agreement. |
| Hara et al, 1995 (35) | MRI | Nonprospective study reporting the prevalence of SCIs in patients with AF at baseline (patients were followed for 2 y for incidence of symptomatic stroke) | Patients with chronic or paroxysmal AF who visited the department of internal medicine of the Health Insurance Nankai Hospital between November 1992 and January 1994 | Mitral stenosis and a history of MI or dilated cardiomyopathy and patients who had been treated with anticoagulants | Chronic or paroxysmal AF diagnosed by Holter electrocardiography and heart monitoring | No | Images were evaluated by a radiologist who was blinded to the study protocol. |
| Longstreth et al, 2002 (14) | MRI 1.5T, or 0.35T§ | Prospective cohort of CHS following patients with no stroke history for a mean of 5 y for development of clinical stroke or SCI | CHS patients with no infarctions on their initial MRI who had a second MRI during the 5-y follow-up. Patients had to be aged >65 y, able to give informed consent, and able to respond to questions without the aid of a surrogate respondent. | Patients were screened for a history of TIA or stroke before the initial MRI but were not excluded if they had such events; however, they had to be infarction-free on their initial MRI. | ECG | – | Imaging data were sent to a single reading center for interpretation by neuroradiologists trained in CHS protocol and without knowledge of the patients’ demographic or clinical profile‖ |
| Vermeer et al, 2003 (23) | MRI 1.5T | Prospective Rotterdam Scan Study following patients for an average of 4.2 y for incident stroke but with only cross-sectional assessment of AF and SCI, reporting prevalence and age- and sex-adjusted risk estimate | Patients without dementia aged 60–90 y from 32 large ongoing, population-based studies (the Rotterdam Elderly Study) | Symptomatic infarctions on MRI and patients with a previous stroke without infarctions on MRI | ECG (MEANS software) | – | Infarctions were rated by a single rater with good intrarater agreement (κ, 0.80). A history of stroke and TIA were determined by self-report, and by checking medical records. An experienced neurologist subsequently reviewed the medical history and scans and categorized the infarctions as silent or symptomatic. |
| Strach et al, 2005 (29) | MRI 1.5T | Prospective study of patients with AF reporting prevalence and incidence of SCIs with ≤52 wk of follow-up (comparing patients with AF and echocardiographic evidence of atrial thrombi with AF patients with no such evidence) | Consecutive patients with the diagnosis of AF that had either occurred for the first time or was diagnosed for the first time with subsequent initiation of therapeutic anticoagulation. The study group consisted of patients with echocardiographic evidence of a left atrial thrombus. Patients with AF and no echocardiographic evidence of left atrial thrombi served as control patients. | Acute neurologic event associated with the first-time diagnosis, presence of MRI-incompatible foreign bodies, biomedical implants, as well as the existence of the following potential cardiovascular sources for emboli: left ventricular thrombi, endocarditic vegetations on cardiac valves, aortic plaques >4 mm, floating aortic plaques, and stenosis of the internal carotid artery >70%, the presence of contraindications for TEE, and intravenous or oral anticoagulation within the last 12 mo | ECG | Yes | 2 experienced radiologists without knowledge of neurologic status or clinical therapy evaluated the averaged images (independent of direction) using the consensus method |
| Study, Year (Reference) | Diagnosis Method | Design (Comparison Groups) | Inclusion Criteria | Exclusion Criteria | AF Ascertainment Method | Anticoagulation | SCI Ascertainment Method |
| Das et al, 2008 (11) | MRI 1T | Prospective Framingham Offspring Study but with cross-sectional assessment of AF and SCIs reporting prevalence and age- and sex-adjusted risk estimate | Framingham Offspring patients who attended the sixth examination and had brain MRI at the seventh examination | Prevalent stroke or dementia or other neurologic conditions that may confound the diagnosis of SCI on MRI, claustrophobia, metal in the eyes or other body parts, valvular prosthesis, vascular clips, cardiac pacemakers, cochlear implants, or other implanted device sensitive to strong magnetic fields | Standard 12 lead ECG | – | 3 different raters blinded to patient demographic and stroke risk factor data processed and analyzed the scans (κ, 0.73–0.90). The final diagnosis about the presence and absence of stroke, type, and location was made at a stroke review by ≥2 neurologists with access to all Framingham and outside records, including brain imaging. |
| Kim et al, 2011 (13) | MRI 1.5T | Nonprospective study with reported risk estimate and prevalence (comparing patients with and without SCIs) | Retrospective analysis of the brain MRI findings of study patients who had visited the Neurology Clinic between March 2004 and August 2009 and had no neurologic disorder but requested a medical evaluation for symptoms, such as dizziness and headache, fear of stroke, a positive family history of stroke, or the presence of vascular risk factors | History of a neurologic disorder by medical interview and neurologic examination | Presence of a past history in medical records or routine ECG | – | 2 neurologists, unaware of the clinical information, evaluated the MRI, and SCI |
| Kobayashi et al, 2012 (36) | MRI 1.5T | Nonprospective study reporting multivariate risk estimate (comparing patients with NVAF with their age- and sex-matched control patients with SR) | Case patients had attended or were admitted to the department of neurology between 2003 and 2007 with chronic or paroxysmal AF. The control group was sex- and age-matched SR patients with subjective symptoms alone, such as headache, dizziness, tinnitus, and tingling of the hands or feet. | History of stroke or TIA, known neurologic disorders or CNS involvement, atrial fibrillation associated with valvular disease | Chronic or paroxysmal NVAF with no reported method of diagnosis | Yes | All images were shuffled before evaluation and interpreted by multiple neurologists and neuroradiologists who were blinded to the clinical data. The final diagnosis of each MRI lesion was made by consensus. |
| Marfella et al, 2013 (12) | MRI 1.5T | Perspective study with an average follow-up of 37 mo evaluating the association between subclinical episodes of AF with risk of SCI at baseline. Patients were followed for incident SCI and clinical stroke. | Patients were selected from a larger cohort of patients with type 2 diabetes from 4 participating institutions. The inclusion criteria were age <60 y, successful quarterly 48-h ECG, Holter monitoring, and assessment of the presence/absence of SCI by MRI of the brain. | Arrhythmia, documented persistent or permanent AF, documented stroke or TIA, anticoagulation therapy, coronary artery or valvular heart disease, cardiomyopathy, history of CHF, hypertension, carotid and peripheral vascular disease, hyperthyroidism, COPD, obstructive sleep apnea, and hepatic damage | 48-h ambulatory ECG recording at 3, 6, 9, and 12 mo during the screening for AF and then annually for another 3 y | No¶ | The MRI scans of the patients were randomly stored and interpreted without knowledge of the patients’ names and characteristics. |
| Saito et al, 2014 (37) | MRI 1.5T | Nonprospective study reporting the prevalence of SCI in patients with and without AF. Prospective assessment of predictors of new ACI in patients with AF. | AF patients: outpatients aged >45 y with AF who visited the cardiovascular, respiratory, and neurology divisions of Asahikawa Medical University Hospital. Control patients: patients without AF who had brain MRI scans for screening or diagnosing any neurologic disorders. | Patients with valvular AF or patients with any history of symptomatic cerebral infarction | NR | Yes | Trained observers who were blinded to the clinical information |
AF = atrial fibrillation; CABG = coronary artery bypass grafting; CHF = congestive heart failure; CHS = Cardiovascular Health Study; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CVA = cerebrovascular accident; DM = diabetes mellitus; DSM = Diagnostic and Statistical Manual of Mental Disorders; ECG = electrocardiography; GI = gastrointestinal; IHD = ischemic heart disease; MI = myocardial infarction; MRI = magnetic resonance imaging; NRAF = nonrheumatic atrial fibrillation; NVAF = nonvalvular atrial fibrillation; NYHA = New York Heart Association; SCI = silent cerebral infarction; SPAF = Stroke Prevention in Atrial Fibrillation; SR = sinus rhythm; TEE = transesophageal echocardiography; TIA = transient ischemic attack.
From reference 45.
From reference 46.
Patients were randomly assigned to warfarin and placebo at entry (they were not on any anticoagulants before entry).
From reference 38.
From reference 20.
All patients were treated with aspirin, 75 to 325 mg/day. If the CHADS2 score was 2, antiaggregant therapy was switched to oral anticoagulation therapy.
Appendix Table 4.
Variability in Definition of SCIs
| Study, Year (Reference) | Imaging Method | Section Thickness, mm | Section Gap, mm | Size Classification | SCI Characteristics |
|---|---|---|---|---|---|
| Shinkawa et al, 1995 (15) | Autopsy | 10–30 | NR | Small, <1 cm; medium, 1–3 cm; large, 3–5 cm; and massive, >5 cm* | Characteristics of SCIs were determined by pathologic examination |
| Yamanouchi et al, 1997 (16) | Autopsy | 10 cerebrum (coronal); 5 cerebellum (sagittal); 3–4 brain stem (axial) | NR | Massive, >2/3 of cerebral hemisphere involved; large, 1/3 to 2/3 of the hemisphere; medium, <1/3 of the hemisphere; small, <15 mm | Pathologic lesions to which no specific neurologic symptoms corresponded |
| Petersen et al, 1989 (19) | CT | 10 | NR | Lesion size was measured by ruler to the nearest half-millimeter in 2 dimensions and multiplied by the thickness of the slice | Low-density areas |
| Petersen et al, 1989 (32) | CT | 10 | NR | Lesion size was measured by ruler to the nearest half-millimeter in 2 dimensions and multiplied by the thickness of the slice | Low-density areas |
| Feinberg et al, 1990 (17) | CT | 4–5 from the base of the brain to the third ventricle; 8–10 mm sections from remainder of the brain | NR | Small cortical (largest diameter <2 cm) or large cortical (>2 cm); Small deep (subcortical) infarctions (<1 cm) or large subcortical infarctions (>1 cm) | Area of hypodensity with no mass effect or edema in vascular distribution excluding focal dilated sulci, and bilateral and symmetrical deep white matter hypodensity |
| Guidotti et al, 1990 (18) | CT | 10–13† | NR | NR | Focal hypodensity |
| Ezekowitz et al, 1995 (33) | CT | 10 | NR | The size of an infarction was estimated from the maximal lesion dimension in centimeters (x-axis) times the axis perpendicular to the x-axis (y-axis) times the number of 1-cm slices in which the infarction appeared. Lucencies of 4.2 cm3 (corresponding to the volume of a sphere of 2.0 cm in diameter) or greater were considered to be large infarctions. | A defect on the CT scan suggestive of SCI in a neurologically normal patient in anterior, posterior, or watershed vascular territories excluding enlarged cortical sulci |
| Zito et al, 1996 (34) | CT | 5 | NR | Maximum diameter <1 cm | Well-demarcated lesions in vascular distribution, usually involving internal capsule, basal ganglia, corona radiata, or thalamus, excluding ill-defined patchy or diffuse white matter hypointensities in the frontal and occipital horns and trigona of the lateral ventricles, medial cells of the lateral ventricles, and semioval center (i.e., leukoaraiosis) and unaccompanied small caps or pencil-thin linings around the frontal or occipital horns of the lateral ventricle |
| Hara et al, 1995 (35) | MRI | NR | NR | 3–18 mm | Lesions 3–18 mm in diameter distinguished from noninfarction lesions, such as état criblé |
| Longstreth et al, 2002 (14) | MRI 1.5T | 5‡ | 0‡ | ≥3 mm | An area of abnormal signal intensity ≥3 mm in a vascular distribution that lacked mass effect; in gray matter, hyperintense on both spin density and T2-weighted images; in white matter and brain stem, hypointense on T1-weighted images with intensities approaching that of CSF; lacunes were defined as subcortical infarctions 3–20 mm in size |
| Vermeer et al, 2003 (23) | MRI 1.5T | 5–6 | 20% | ≥3 mm | Hyperintense on T2-weighted images with corresponding prominent hypointensities on T1-weighted images to exclude cerebral white matter lesions and restricting to lesions ≥3 mm in size and using proton density scans to exclude dilated perivascular spaces |
| Strach et al, 2005 (29) | MRI 1.5T | 5 | NR | <5 mm, 5–10 mm, >10 mm | Focal hyperintensities on DWI that later correspond to lesion on T2-weighted and FLAIR sequences; wedge-shaped areas with the typical aspect of a territory infarction or the appearance of clearly newly occurred point-shaped or round lesions with a typical distribution in FLAIR and T2-weighted sequences without evidence of any diffusion abnormality. Diffuse plane hyperintensities in the diffusion imaging and diffusion abnormalities with typical configuration and localization of a vascular border-zone infarction were not regarded as indicative of thrombembolic events. |
| Das et al, 2008 (11) | MRI 1T | 4 | 0 | ≥3 mm | Lesions >3 mm with CSF density on subtraction images distinguished from the circle of Willis vessels for suspected basal ganglia infarctions |
| Kim et al, 2011 (13) | MRI 1.5T | 7 | 2 | ≥3–20 mm | Lesion 3–20 mm in diameter hypointense by T1-weighted images and hypointense surrounded by a hyperintense rim by FLAIR images (excluding lesions <3 mm to exclude dilated perivascular space) and excluded periventricular white matter hyperintensities diagnosed by FLAIR images |
| Kobayashi et al, 2012 (36) | MRI 1.5T | 7 | 0.5 | ≥3 mm | Lesions ≥3 mm hyperintense on T2-weighted images and hypointense on T1-weighted images. Lesions <3 mm were excluded because there was a high possibility to include dilatation of the perivascular space, demyelination, or gliosis. Separated SCIs from periventricular, and deep and subcortical white matter hyperintensity. |
| Marfella et al, 2013 (12) | MRI 1.5T | 7.8–8.0 | NR | 3–15 mm | Low signal intensity area (3–15 mm) on T1-weighted images that was also visible as a hyperintense lesion on T2-weighted images. All SCIs detected were lacunar infarctions <15 mm. |
| Saito et al, 2014 (37) | MRI 1.5T | 5.5 | 1 | NR | A cerebral infarction was defined as a focal hypointense lesion with a hyperintense rim on the FLAIR sequence images, with a corresponding hyperintensity on the T2-weighted images and corresponding hypointensity on the T1-weighted images. |
CSF = cerebrospinal fluid; CT = computed tomography; DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging; NR = not reported; SCI = silent cerebral infarction.
The infarction size in all cases could not be measured because autopsy materials were not available for all cases.
Obtained from searching the brochure of the EMI 1010 CT scan model.
From reference 38.
Appendix Table 5.
Criteria for Differentiating SCIs From Leukoaraiosis and Virchow-Robin Spaces
| MRI Lesion | Location | T1-Weighted Imaging | T2-Weighted Imaging | FLAIR | Shape/Appearance | Size |
|---|---|---|---|---|---|---|
| WMH or leukoaraiosis | Periventricular vs. deep vs. subcortical WMH | Isointense or mildly hypointense to brain parenchyma | Hyperintense | Hyperintense | Punctate, small foci; or cap, pencil-thin lining; or nodular band | Variable |
| SCIs | Along vascular territories: Anterior circulation (anterior cerebral artery and middle cerebral artery); posterior circulation (posterior cerebral artery, basilar artery, superior cerebellar artery, anterior inferior cerebellar artery, and posterior inferior cerebellar artery); watershed territories (anterior and posterior external watershed, internal watershed, and cerebellar watershed) | Markedly hypointense similar to CSF | Hyperintense (similar to CSF) | Hyperintense or hypointense with no mass effect* | Focal, sharply demarcated, regularly or irregularly shaped areas | ≥3 mm (variable) |
| Enlarged perivascular spaces or Virchow-Robin spaces or état criblé† | White matter (subregions of the frontal lobe, temporal lobe, parietal lobe, occipital lobe, and the centrum semiovale), basal ganglia region (including the caudate, lentiform nuclei, thalamus, internal capsule, substantia innominata, and insular area), brain stem, and hippocampal area (including the hippocampus, parahippocampal gyrus, and amygdalae) | Hypointense | Hyperintense | Hypointense (CSF-like content without abnormal surrounding signal intensity) | Small areas in the basal ganglia and centrum semiovale that follow the orientation of penetrating arterioles. They appear linear when parallel and dot-like when perpendicular to the imaging plane. | <3 mm‡ |
CSF = cerebrospinal fluid; FLAIR = fluid-attenuated inversion recovery; SCI = silent cerebral infarction; WMH = white matter hyperintensity.
Some lacunar infarctions may not progress to lacunes (i.e., they will not develop a cavity and remain hyperintense; therefore, they will be indistinguishable from nonspecific white matter lesions).
When there are several perivascular spaces, the brain can have a colander-like appearance referred to as état criblé.
Rarely huge perivascular spaces may be seen, which can have positive mass effect.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14-0538.
Author Contributions: Conception and design: S. Kalantarian, H. Ay, J.N. Ruskin.
Analysis and interpretation of the data: S. Kalantarian, H. Ay, R.L. Gollub, K. Retzepi, J.N. Ruskin.
Drafting of the article: S. Kalantarian, H. Ay, H. Lee, J.N. Ruskin. Critical revision of the article for important intellectual content: S. Kalantarian, H. Ay, H. Lee, M. Mansour, J.N. Ruskin.
Final approval of the article: S. Kalantarian, H. Ay, R.L. Gollub, H. Lee, M. Mansour, J.N. Ruskin.
Provision of study materials or patients: S. Kalantarian.
Statistical expertise: H. Lee.
Administrative, technical, or logistic support: R.L. Gollub, J.N. Ruskin. Collection and assembly of data: S. Kalantarian, K. Retzepi.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–7. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.de Falco FA, Mastroroberto G, Mazzei G, Montariello A, Zaccaria F, Sepe Visconti O. Atrial fibrillation and infarct area extent in ischemic stroke. A clinical and neuroradiological study in 104 patients. Acta Neurol (Napoli) 1991;13:249–54. [PubMed] [Google Scholar]
- 6.Harrison MJ, Marshall J. Atrial fibrillation, TIAs and completed strokes. Stroke. 1984;15:441–2. doi: 10.1161/01.str.15.3.441. [DOI] [PubMed] [Google Scholar]
- 7.Kaarisalo MM, Immonen-Räihä P, Marttila RJ, Lehtonen A, Salomaa V, Sarti C, et al. Atrial fibrillation in older stroke patients: association with recurrence and mortality after first ischemic stroke. J Am Geriatr Soc. 1997;45:1297–301. doi: 10.1111/j.1532-5415.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–46. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7:433–7. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59:1369–75. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–35. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marfella R, Sasso FC, Siniscalchi M, Cirillo M, Paolisso P, Sardu C, et al. Brief episodes of silent atrial fibrillation predict clinical vascular brain disease in type 2 diabetic patients. J Am Coll Cardiol. 2013;62:525–30. doi: 10.1016/j.jacc.2013.02.091. [DOI] [PubMed] [Google Scholar]
- 13.Kim MH, Moon JS, Pak SY, An SA, Kim OJ, Kim NK, et al. Different risk factor profiles between silent brain infarction and symptomatic lacunar infarction. Eur Neurol. 2011;65:250–6. doi: 10.1159/000324335. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–82. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 15.Shinkawa A, Ueda K, Kiyohara Y, Kato I, Sueishi K, Tsuneyoshi M, et al. Silent cerebral infarction in a community-based autopsy series in Japan. The Hisayama Study. Stroke. 1995;26:380–5. doi: 10.1161/01.str.26.3.380. [DOI] [PubMed] [Google Scholar]
- 16.Yamanouchi H, Nagura H, Mizutani T, Matsushita S, Esaki Y. Embolic brain infarction in nonrheumatic atrial fibrillation: a clinicopathologic study in the elderly. Neurology. 1997;48:1593–7. doi: 10.1212/wnl.48.6.1593. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg WM, Seeger JF, Carmody RF, Anderson DC, Hart RG, Pearce LA. Epidemiologic features of asymptomatic cerebral infarction in patients with nonvalvular atrial fibrillation. Arch Intern Med. 1990;150:2340–4. [PubMed] [Google Scholar]
- 18.Guidotti M, Tadeo G, Zanasi S, Pellegrini G. Silent cerebral ischemia in patients with chronic atrial fibrillation—a case-control study. Ir J Med Sci. 1990;159:96–7. doi: 10.1007/BF02937438. [DOI] [PubMed] [Google Scholar]
- 19.Petersen P, Pedersen F, Johnsen A, Madsen EB, Brun B, Boysen G, et al. Cerebral computed tomography in paroxysmal atrial fibrillation. Acta Neurol Scand. 1989;79:482–6. doi: 10.1111/j.1600-0404.1989.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Cardiovascular Health Study Collaborative Research Group Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–9. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 21.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Miwa K, Hoshi T, Hougaku H, Tanaka M, Furukado S, Abe Y, et al. Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness. Intern Med. 2010;49:817–22. doi: 10.2169/internalmedicine.49.3211. [DOI] [PubMed] [Google Scholar]
- 23.Vermeer SE, Hollander M, van Dijk EJ, Holman A, Koudstaal PJ, Breteler MM, Rotterdam Scan Study Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Thompson S, Ekelund U, Jebb S, Lindroos AK, Mander A, Sharp S, et al. A proposed method of bias adjustment for meta-analyses of published observational studies. Int J Epidemiol. 2011;40:765–77. doi: 10.1093/ije/dyq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol. 2005;161:280–8. doi: 10.1093/aje/kwi042. [DOI] [PubMed] [Google Scholar]
- 27.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160:267–70. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration. 2011 Accessed at www.cochrane-handbook.org on 10 September 2014.
- 29.Strach K, Meyer C, Hackenbroch M, Tiemann K, Haase J, Pizulli L, et al. [Long-term fate of left atrial thrombi and incidence of cerebral embolism under continuous anticoagulation therapy] Rofo. 2005;177:1706–12. doi: 10.1055/s-2005-858787. [DOI] [PubMed] [Google Scholar]
- 30.Sawai N, Yamano S, Minami S, Yamamoto Y, Akai M, Nomura K, et al. [Effects of atherogenic risk factors on asymptomatic brain infarct] Nihon Ronen Igakkai Zasshi. 1997;34:389–94. doi: 10.3143/geriatrics.34.389. [DOI] [PubMed] [Google Scholar]
- 31.Une F. [Nuclear magnetic resonance image and CT scan studies on silent strokes in non-valvular atrial fibrillation (NVAF)] Nihon Rinsho. 1993;51(Suppl):874–8. [PubMed] [Google Scholar]
- 32.Petersen P, Pedersen F, Madsen EB, Brun B, Gyldensted C, Boysen G. Echocardiography and cerebral computed tomography in chronic atrial fibrillation. Eur Heart J. 1989;10:1101–4. doi: 10.1093/oxfordjournals.eurheartj.a059432. [DOI] [PubMed] [Google Scholar]
- 33.Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, et al. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. Circulation. 1995;92:2178–82. doi: 10.1161/01.cir.92.8.2178. [DOI] [PubMed] [Google Scholar]
- 34.Zito M, Muscari A, Marini E, Di Iorio A, Puddu GM, Abate G. Silent lacunar infarcts in elderly patients with chronic non valvular atrial fibrillation. Aging (Milano) 1996;8:341–6. doi: 10.1007/BF03339591. [DOI] [PubMed] [Google Scholar]
- 35.Hara M, Ooie T, Yufu K, Tsunematsu Y, Kusakabe T, Ooga M, et al. Silent cortical strokes associated with atrial fibrillation. Clin Cardiol. 1995;18:573–4. doi: 10.1002/clc.4960181008. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi A, Iguchi M, Shimizu S, Uchiyama S. Silent cerebral infarcts and cerebral white matter lesions in patients with nonvalvular atrial fibrillation. J Stroke Cerebrovasc Dis. 2012;21:310–7. doi: 10.1016/j.jstrokecerebrovasdis.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Kawamura Y, Tanabe Y, Asanome A, Takahashi K, Sawada J, et al. Cerebral microbleeds and asymptomatic cerebral infarctions in patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2014;23:1616–22. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–27. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 39.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–9. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 40.Vermeer SE, Prins ND, den Heijer T, Holman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 41.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646–8. doi: 10.1161/01.str.29.12.2646. [DOI] [PubMed] [Google Scholar]
- 42.Bogousslavsky J. Topographic patterns of cerebral infarcts. Cerebrovasc Dis. 1991;1(Suppl 1):61–8. [Google Scholar]
- 43.Bogousslavsky J, Regli F. Unilateral watershed cerebral infarcts. Neurology. 1986;36:373–7. doi: 10.1212/wnl.36.3.373. [DOI] [PubMed] [Google Scholar]
- 44.Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42:1140–5. doi: 10.1161/STROKEAHA.110.600114. [DOI] [PubMed] [Google Scholar]
Web-Only References
- 45.Petersen P, Madsen EB, Brun B, Pedersen F, Gyldensted C, Boysen G. Silent cerebral infarction in chronic atrial fibrillation. Stroke. 1987;18:1098–100. doi: 10.1161/01.str.18.6.1098. [DOI] [PubMed] [Google Scholar]
- 46.Design of a multicenter randomized trial for the Stroke Prevention in Atrial Fibrillation Study. The Stroke Prevention in Atrial Fibrillation Investigators. Stroke. 1990;21:538–45. doi: 10.1161/01.str.21.4.538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


