Abstract
Background
N-methyl-D-aspartate receptor antagonists, like ketamine, produce a rapid-acting and long-lasting antidepressant effect. Although the mechanism is not completely understood, ketamine is thought to preferentially target N-methyl-D-aspartate receptors on fast-spiking parvalbumin-containing interneurons. The function of parvalbumin-containing interneurons is dependent on perineuronal nets, a specialized form of extracellular matrix that surrounds these cells.
Methods
Chondroitinase was used to enzymatically degrade perineuronal nets surrounding parvalbumin-containing interneurons in the ventral hippocampus, a region that is involved in the antidepressant response to ketamine. Rats were tested on the forced swim test 30 minutes and 1 week after ketamine administration.
Results
Thirty minutes after ketamine injection, both chondroitinase-treated and control animals had a decrease in immobility. One week later, however, the antidepressant-like response observed with ketamine was completely abolished in the chondroitinase-treated animals.
Conclusion
This suggests that parvalbumin interneuron function in the ventral hippocampus is essential for the sustained antidepressant effect of ketamine.
Keywords: ketamine, perineuronal nets, ventral hippocampus
Introduction
Currently prescribed antidepressant drugs are not effective in all patients and often take weeks before the therapeutic benefit is realized (Williams et al., 2000). Recently, N-methyl-D-aspartate (NMDA) receptor antagonists, such as ketamine, have been examined for antidepressant efficacy. A single subanesthetic dose of ketamine has been shown to reduce symptoms of depression in rodents on the forced swim test (FST) (Autry et al., 2011; Carreno et al., 2016) and in humans suffering from treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006). Importantly, the therapeutic effect begins almost immediately and can persist in patients for up to 2 weeks (Murrough et al., 2013). Despite its promise, the mechanism of ketamine’s antidepressant effect has not been completely elucidated (Miller et al., 2016).
We recently demonstrated that activation of a ventral hippocampus (vHipp)–medial prefrontal cortex (mPFC) pathway is both necessary and sufficient for an antidepressant response to ketamine (Carreno et al., 2016). Indeed, this is consistent with the work of others demonstrating altered synaptic transmission in the mPFC is associated with the antidepressant action of ketamine (Li et al., 2010; Autry et al., 2011). To explain the seemingly contradictory finding that blocking the activity of an excitatory neurotransmitter would drive activity in the vHipp-mPFC pathway, it has been proposed that ketamine selectively antagonizes NMDA receptors on inhibitory interneurons, causing disinhibition of pyramidal cells (Miller et al., 2016). The reason why ketamine may preferentially target GABAergic interneurons relates to the physiological blockade of the NMDA receptor by Mg2+. Although NMDA receptors are ligand gated, they are also voltage dependent. Thus, at the resting potential of pyramidal neurons NMDA receptors are nearly completely blocked by Mg2+ (Nowak et al., 1984). In contrast, the fast-firing GABAergic interneurons are more depolarized and therefore less susceptible to Mg2+ blockade. Indeed, ketamine has been shown to selectively suppress the spiking activity of putative fast-spiking interneurons (Quirk et al., 2009). Thus, we posit that ketamine produces an antidepressant response by decreasing interneuron function in the hippocampus, leading to an augmented glutamatergic drive to the prefrontal cortex.
To target hippocampal interneurons selectively, we degraded the extracellular matrix surrounding fast-firing parvalbumin (PV)-containing interneurons known as a perineuronal net (PNN). PNNs are chondroitin sulfate proteoglycans that form an extracellular matrix around PV-containing interneurons (Fitch and Silver, 1997). The PNN is thought to play an important role in the function of PV-containing interneurons. For example, PNNs can act to stabilize synapses (Berardi et al., 2004), support synaptic plasticity (Bukalo et al., 2001; Brakebusch et al., 2002; Beurdeley et al., 2012), regulate motility of extracellular ions (Brückner et al., 1993; Härtig et al., 1999), and protect fast-spiking interneurons against oxidative stress (Cabungcal et al., 2013). Therefore, in the current experiments, we hypothesized that enzymatic degradation of PNNs surrounding PV-containing interneurons of the vHipp would attenuate the antidepressant effect of ketamine.
Methods
Animals
Adult male Sprague-Dawley rats (Harlan, 250–275 g at the time of arrival) were used for all experiments. Animals were maintained in a temperature-controlled environment on a 14:10-h light-dark cycle and had access to food and water ad libitum. All procedures were consistent with NIH guidelines (NIH publications no. 80-23, revised 1978) and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Chondroitinase Treatment
To degrade PNNs, animals were anesthetized (sodium pentobarbital, 60 mg/kg, i.p.) and placed into a stereotaxic apparatus. Bilateral cannula aimed at the vHipp (A/P -5.3, M/L ±5.2, DV -7.5 mm from bregma) were used to inject 0.75 μL of chondroitinase ABC from Proteus vulgaris (0.05 U μL-1). Penicillinase from Bacillus cereus (0.05 U μL-1), a nonrelevant enzyme, was used as a control, as published previously (Shah and Lodge, 2013). Rats were allowed at least 1 week to recover before testing.
FST
To measure the antidepressant effects of ketamine, the FST was used as previously reported (Carreno et al., 2016). Briefly, rats were placed into a plexiglass cylinder (21 x 46 cm) filled with water (25°C) for 5 min while behavior was digitally recorded for offline analysis. Rats were tested either 30 min or 1 week following a single administration of ketamine (Sigma, 10 mg/kg, i.p). To analyze the behavior, a time-sampling technique was used in which the most prominent behavior (immobility, swimming, climbing) observed was recorded in each 5-second bin. All videos were scored blind.
Histochemistry
To confirm the loss of PNNs, rats were transcardially perfused with saline, followed by formaldehyde, and brains were postfixed and cryopreserved for at least 24 hours. Coronal sections (50 μm) through the vHipp were blocked (2% normal goat serum), incubated with lectin from Wisteria floribunda (4 μg mL-1), then AlexaFluor594 conjugated to streptavidin. A subset of sections was also incubated with a rabbit anti-PV antibody (Abcam:AB11427), then anti-rabbit AlexaFluor488. Sections were mounted and coverslipped with Prolong Gold Antifade reagent (Molecular Probes). The representative images were acquired using an Olympus IX81 Motorized inverted confocal microscope and FV10-ASW software and enhanced using ImageJ.
Analysis
FST data were analyzed by 2-way ANOVA followed by the Holm-Sidak posthoc method. All tests were 2-tailed, and significance was determined at P<.05. In all figures, data are shown as mean ± SEM and n is indicated in the figure legend.
Results
FST
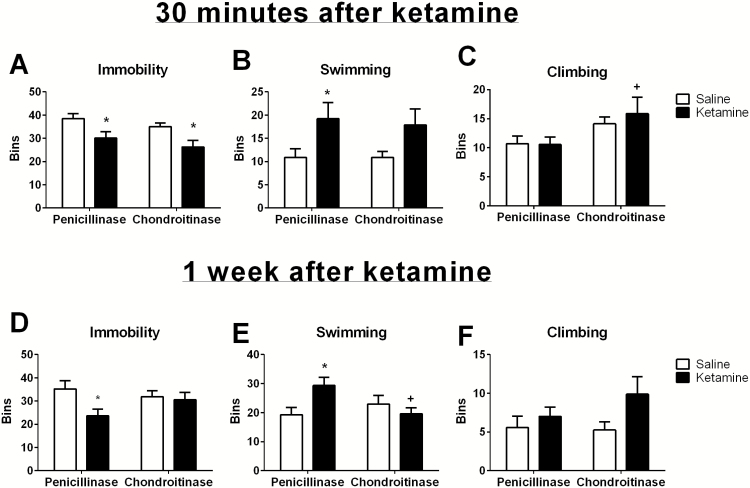
The FST was used to measure the acute and chronic antidepressant effect of ketamine 30 minutes or 1 week after drug administration, respectively. As we have shown previously, as soon as 30 minutes after administration, ketamine produces an antidepressant effect on the FST. Thirty minutes after ketamine administration, there was a decrease in immobility (Figure 1A; Saline/Pen=38.44±2.38, Ketamine/Pen 30.11±2.38; 2-way ANOVA: interaction F(1,33) = 0.007, drug F(1,33) = 12.18, enzyme F(1,33) = 2.22; Holm-Sidak: t=2.48, P=.02). The effect of ketamine on immobility was present even when PNNs were degraded (Saline/Chon = 35.00 ± 2.52, Ketamine/Chon = 26.25 ± 2.52, Holm-Sidak: t = 2.46, P=.02). This effect was driven primarily by an increase in swimming that was observed 30 minutes after ketamine administration in both penicillinase- and chondroitinase-treated animals (Figure 1B; Saline/Pen =10.89 ± 2.65, Ketamine/Pen=19.22±2.65, Saline/Chon=10.88±2.81, Ketamine/Chon=17.88±2.81; 2-way ANOVA: interaction F(1,33)=0.06, drug F(1,33)=7.87, enzyme F(1.33)=0.06; Holm-Sidak: t=2.22, P=.034). We also found that chondroitinase itself increased climbing behavior (Figure 1C; Saline/Pen=10.67±1.69, Ketamine/Pen=10.56±1.69, Saline/Chon=14.13 ± 1.80, Ketamine/Chon=15.88±1.80; 2-way ANOVA: interaction F(1,33) = 0.28, drug F(1,33) = 0.22, enzyme F(1.33)=6.32; Holm-Sidak: t=2.52, P=.02). Together, these results suggest that the acute antidepressant effect of ketamine is not dependent on PNN function.
Figure 1.
Perineuronal nets (PNNs) are required for the sustained but not acute antidepressant effect of ketamine. Thirty minutes after ketamine injection, both penicillinase- and chondroitinase-treated animals show a significant reduction in immobility (A) and an increase in swimming (B) compared with saline-treated control animals. Chondroitinase treatment itself produced an increase in climbing (C). One week after ketamine injection, penicillinase-treated animals continue to show reductions in immobility (D) and increases in swimming (E). This sustained antidepressant effect is abolished in the rats treated with chondroitinase in the vHipp. There was no significant effect on climbing when animals were tested one week after ketamine injection (F). *Significantly different than the corresponding saline control; +significantly different than penicillinase/ketamine. Chondroitinase n=8/group. Penicillinase n=9/group.
Interestingly, when animals were subjected to a second swim 1 week later, we found a different effect. Again, in penicillinase-treated animals, ketamine reduced immobility time (Figure 1D; Saline/Pen =35.22±2.98, Ketamine/Pen=23.67±2.98; 2-way ANOVA: interaction F(1,33) =2.748, drug F(1,33) =4.43, enzyme F(1,33)=0.32; Holm-Sidak: t=2.74, P=.01). However, in the rats that received chondroitinase to degrade PNNs in the vHipp, ketamine had no effect on immobility time (Saline/Chon = 31.88 ± 3.16, Ketamine/Chon = 30.50 ± 3.16; Holm-Sidak: t=0.31, P=.76). This effect was driven primarily by an increase in swimming time that was observed in the penicillinase-treated animals (Figure 1E; Saline/Pen =19.22±2.59, Ketamine/Pen = 29.33 ± 2.59; 2-way ANOVA: interaction F(1,33)=6.27, drug F(1,33)=1.65, enzyme F(1,33)=1.29; Holm-Sidak: t=2.76, P=.01), but not in the chondroitinase-treated rats (Saline/Chon = 22.88 ± 2.75, Ketamine/Chon = 19.63 ± 2.75; Holm-Sidak: t= 0.84, P=.41). One week after ketamine treatment, we did not observe significant changes in climbing behavior in response to any treatment (Figure 1F; Saline/Pen=5.56±1.52, Ketamine/Pen = 7.00 ± 1.52, Saline/Chon = 5.25 ± 1.61, Ketamine/Chon = 9.88 ± 1.61; 2-way ANOVA: interaction F(1,33) = 1.04, drug F(1,33) = 3.77, enzyme F(1,33)=0.68). Together, these results suggest that PNNs are required for the sustained antidepressant effect of ketamine.
Histochemistry
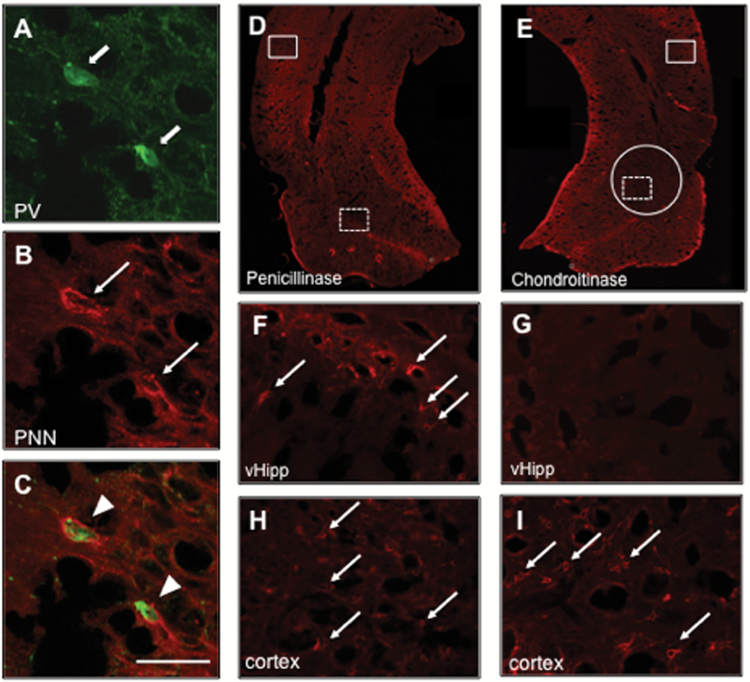
Dual florescence immunohistochemistry was used to confirm that PNNs surround PV-positive cells in the vHipp. Representative images are shown in Figure 2A-C. Further, immunohistochemistry was also used to confirm that chondroitinase injection produced a region specific reduction in PNNs (Figure 2D-I). In the penicillinase-treated rats, PNNs were observed throughout the vHipp (Figure 2D,F) and in cortical areas (Figure 2H). Chondroitinase injection in the vHipp reduced PNNs in this region (Figure 2E,G). PNNs were still observed in more dorsal cortical regions of chondroitinase-treated rats (Figure 2I), suggesting that the chondroitinase injection produced a region specific loss of PNNs.
Figure 2.
Chondroitinase degrades perineuronal nets (PNNs) surrounding parvalbumin (PV)-positive interneurons. A-C show high magnification PNNs surrounding PV cells. D and E depict a representative hippocampal section from a penicillinase- and chondroitinase-treated animal, respectively. Penicillinase-treated animals display PNNs in the ventral hippocampus (vHipp) (dashed box in D is magnified in F) as well as in other cortical areas (solid box in D is magnified in H). Chondroitinase injection caused a region-specific reduction of PNNs in the vHipp (dashed box in E is magnified in G) but not in more dorsal areas of the cortex (solid box in E is magnified in I). The diffusion area of the injection is shown in E by a solid circle. Block arrows indicate PV-positive cells, line arrows indicate PNNs, arrowheads indicate PV-positive cells surrounded by PNNs. Green, PV; red, PNNs. Scale bar = 50 μ.
Discussion
Here we demonstrate that PNNs in the vHipp are required for the sustained, but not the acute, antidepressant effect of ketamine. Ketamine is an antagonist at the NMDA family of glutamate receptors; however, low doses of ketamine enhance excitatory input to corticolimbic brain regions, which is thought to underlie its antidepressant effect (Cabungcal et al., 2013). Two primary hypotheses have been proposed to understand how blockade of excitatory glutamate receptors would produce an excitatory effect. First, it has been shown that at low doses, ketamine selectively antagonizes NMDA receptors on inhibitory interneurons, allowing disinhibition of pyramidal cells (Quirk et al., 2009). Ketamine has been shown to temporarily reduce expression of PV and the GABA-producing enzyme, GAD, further supporting the hypothesis that this drug reduces the function of inhibitory interneurons (Behrens et al., 2007; Zhou et al., 2015). Alternatively, it has been suggested that ketamine acts directly on pyramidal cells, inducing protein synthesis and causing a cell-autonomous form of synaptic plasticity such that the cells receive enhanced excitatory input (Miller et al., 2016). It should be noted that a recent publication suggested that the sustained antidepressant response of ketamine may be mediated by the ketamine metabolite, (2R, 6R)-HNK, which activates AMPA receptors and induces excitation in both pyramidal cells and interneurons of the hippocampus (Zanos et al., 2016). Nonetheless, our results demonstrate that the ketamine-induced increase in immobility on the FST is blocked by the degradation of perienuronal nets surrounding fast-spiking interneurons in the vHipp and supports the hypothesis that inhibitory interneurons play an important role in the sustained antidepressant activity of ketamine.
In the current experiments, we targeted PNNs specifically in the vHipp, a brain region that we have shown previously to be important for the sustained, but not acute, effect of ketamine (Carreno et al., 2016). Specifically, we have previously demonstrated that vHipp inactivation at the time of ketamine administration blocks the decrease in immobility in the FST seen at 1 week but not 30 minutes after ketamine (Carreno et al., 2016). Further, we demonstrated that activation of hippocampal projection neurons specifically to the mPFC were both necessary and sufficient for the sustained antidepressant effect of ketamine (Carreno et al., 2016). This finding is consistent with the work of others demonstrating that changes in synaptic function occur in the mPFC after ketamine administration (Li et al., 2010; Autry et al., 2011). In the current study, we blocked the sustained antidepressant effect of ketamine by enzymatically degrading PNNs in the vHipp. We hypothesize that this manipulation is disrupting the function of PV interneurons that regulate activity of pyramidal cells that project to the prefrontal cortex.
In addition to its connections to the mPFC, the vHipp has also been shown to regulate dopamine cells in the ventral tegmental area via a polysynaptic pathway beginning in the nucleus accumbens (NAc) (Lodge and Grace, 2011). Interestingly, activity in this vHipp-NAc projection is correlated with the antidepressant response to ketamine (Belujon and Grace, 2014). However, using designer receptors exclusively activated by designer drugs, we have shown that while activation of the vHipp-mPFC pathway produces an antidepressant-like response, activation of the vHipp-NAc pathway has no effect on the FST in unstressed rats (Carreno et al., 2016). Thus, it is possible that the vHipp-NAc-VTA pathway may be recruited in situations that involve stress, such as the learned helplessness paradigm, or may contribute to symptoms of depression not measured by the FST, such as anhedonia.
In the current experiments, we demonstrated that PNNs in the hippocampus are required for the sustained antidepressant effect of ketamine. Although PNNs have been shown to recover after chondroitinase treatment, we found that the nets were not present even at the time of the second swim test (Figure 2). It has been shown previously that PNNs can influence the function of PV cells through a variety of mechanisms. For example, removal of the PNNs in the hippocampus reduces long-term potentiation (Bukalo et al., 2001; Brakebusch et al., 2002), suggesting that this form of extracellular matrix plays an important role in regulating synaptic plasticity. As described above, we have shown previously that the sustained antidepressant effect of ketamine requires plasticity in the vHipp-mPFC pathway (Carreno et al., 2016). Therefore, we hypothesize that ablation of PNNs in the vHipp prevents this plasticity from occurring and ultimately abolishes the sustained but not acute antidepressant effect of ketamine. Further, PNNs have also been shown to attract maintenance factors like BDNF to PV cells (Harris et al., 2010). Interestingly, we and others have demonstrated that the sustained antidepressant response to ketamine is dependent on BDNF (Autry et al., 2011; Liu et al., 2012; Carreno et al., 2016). Specifically, ketamine increases phosphorylation of the BDNF receptor, TrkB, in the vHipp (Carreno et al., 2016). Furthermore, injection of K252a, a tyrosine kinase inhibitor, into the vHipp at the time of ketamine administration prevented the chronic antidepressant effect of ketamine (Carreno et al., 2016). These results suggest that ablation of PNNs may prevent the sustained antidepressant effect of ketamine by reducing neurotrophin signaling; again, this remains to be elucidated.
In conclusion, the current experiments demonstrate that PNNs, the specialized extracellular matrix that surround PV cells in the vHipp, are necessary for the sustained antidepressant response to ketamine. Disruptions in the extracellular matrix have been observed in patients with psychiatric disorders such as depression and schizophrenia (Lubbers et al., 2014). Therefore, understanding the role of PNNs in the antidepressant action of ketamine may have important implications for this novel and promising antidepressant drug.
Statement of Interest
None.
Acknowledgments
This work was supported by the Owens Foundation and by grant number R01 MH090067 from the National Institute of Mental Health at the National Institutes of Health.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P- F, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–1647. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. (2004) Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron 44:905–908. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. (2012) Otx2 Binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Böckers TM, Zhou X, Kreutz MR, Montag D, Gundelfinger ED, Fässler R. (2002) Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Bio 22:7417–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner G, Brauer K, Härtig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. (1993) Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8:183–200. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A. (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104:359–369. [DOI] [PubMed] [Google Scholar]
- Cabungcal J- H, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. (2013) Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad of Sci U S A 110:9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ. (2016) Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Fitch TM, Silver J. (1997) Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res 290:379–384. [DOI] [PubMed] [Google Scholar]
- Harris NG, Mironova YA, Hovda DA, Sutton RL. (2010) Pericontusion axon sprouting is spatially and temporally consistent with a growth-permissive environment after traumatic brain injury. J Neuropathol Exp Neurol 69:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W, Derouiche A, Welt K, Brauer K, Grosche J, Mäder M, Reichenbach A, Brückner G. (1999) Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res 842:15–29. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R- J, Banasr M, Dwyer JM, Iwata M, Li X- Y, Aghajanian G, Duman RS. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R- J, Lee FS, Li X- Y, Bambico F, Duman RS, Aghajanian GK. (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2011) Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers BR, Smit AB, Spijker S, van den Oever MC. (2014) Chapter 12 - Neural ECM in addiction, schizophrenia, and mood disorder. In: Prog Brain Res (Alexander Dityatev BW-H, Asla P, eds), pp 263–284. Elsevier. [DOI] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ. (2016) Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100:17–26. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307:462–465. [DOI] [PubMed] [Google Scholar]
- Quirk M, Sosulski D, Feierstein C, Uchida N, Mainen Z. (2009) A defined network of fast-spiking interneurons in orbitofrontal cortex: responses to behavioral contingencies and ketamine administration. Front Sys Neurosci 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Lodge DJ. (2013) A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 3:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. (2000) A systematic review of newer pharmacotherapies for depression in adults: evidence report summary: clinical guideline, part 2. Ann Intern Med 132:743–756. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, et al. (2006) A randomized trial of an n-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang G, Li X, Liu X, Wang N, Qiu L, Liu W, Zuo Z, Yang J. (2015) Loss of phenotype of parvalbumin interneurons in rat prefrontal cortex is involved in antidepressant- and propsychotic-like behaviors following acute and repeated ketamine administration. Mol Neurobiol 51:808–819. [DOI] [PubMed] [Google Scholar]