Abstract
Background
QUALIFY was a 28-week, randomized, open-label, head-to-head trial that assessed improvements across multiple measures in stable patients with schizophrenia with aripiprazole once-monthly 400 mg vs paliperidone palmitate.
Methods
Secondary effectiveness assessments included physician-rated readiness for work using the Work Readiness Questionnaire, the Clinical Global Impression–Severity and Clinical Global Impression–Improvement scales, and quality of life with the rater-blinded Heinrichs-Carpenter Quality of Life Scale. Patients assessed their treatment satisfaction and quality of life with Subjective Well-Being under Neuroleptic Treatment−short version and Tolerability and Quality of Life questionnaires.
Results
Odds of being ready for work at week 28 were significantly higher with aripiprazole once-monthly 400 mg vs paliperidone palmitate (adjusted odds ratio, 2.67; 95% CI, 1.39−5.14; P=.003). Aripiprazole once-monthly 400 mg produced numerically or significantly greater improvements from baseline vs paliperidone palmitate in all Quality of Life Scale items. With aripiprazole once-monthly 400 mg vs paliperidone palmitate at week 28, there were significantly more Clinical Global Impression–Severity and Clinical Global Impression–Improvement responders (adjusted odds ratio, 2.26; P=.010, and 2.51; P=.0032) and significantly better Clinical Global Impression–Improvement scores (least squares mean treatment difference, −0.326; 95% CI, −0.60 to −0.05; P=.020). Numerically larger improvements with aripiprazole once-monthly 400 mg vs paliperidone palmitate were observed for patient-rated scales Subjective Well-Being under Neuroleptic Treatment−short version and Tolerability and Quality of Life. Partial correlations were strongest among clinician-rated and among patient-rated scales but poorest between clinician and patient-rated scales.
Conclusions
Consistently greater improvements were observed with aripiprazole once-monthly 400 mg vs paliperidone palmitate across all measures. Partial correlations between scales demonstrate the multidimensionality of various measures of improvement. More patients on aripiprazole once-monthly 400 mg were deemed ready to work by the study end.
Trial registry
National Institutes of Health registry, NCT01795547, https://clinicaltrials.gov/ct2/results?id=NCT01795547)
Keywords: aripiprazole once-monthly, paliperidone palmitate, quality of life, functioning, schizophrenia
Significance Statement
The QUALIFY study (QUAlity of LIfe with AbiliFY Maintena) compared aripiprazole once-monthly 400 mg with paliperidone palmitate across multifaceted clinical and functional scales addressing changes in symptoms, quality of life, and functional capacity. Overall, our findings show consistently greater improvements with aripiprazole once-monthly 400 mg vs paliperidone palmitate across scales reflecting a broad range of symptoms and functioning, regardless of whether the raters were blinded or not and whether the scale was rated by the patient or clinician. These results across multiple domains of health-related quality of life and functioning and in enhanced work readiness help to differentiate aripiprazole once-monthly 400 mg and paliperidone palmitate, 2 long-acting injectable antipsychotics with different pharmacologic profiles. Aripiprazole is a partial agonist at dopamine D2 and serotonin 5HT1A receptors and an antagonist at 5HT2A receptors while paliperidone is an antagonist at D2 and 5HT2A receptors. Our results may help provide guidance to clinicians when choosing between antipsychotic treatments and psychosocial options for their patients with schizophrenia.
Introduction
The chronic nature of schizophrenia often requires continuous treatment to prevent relapse and maintain or improve functioning and quality of life over the long term (Kreyenbuhl et al., 2010; Hasan et al., 2013). Longitudinal studies demonstrate that early symptomatic improvement may have an enduring benefit regarding quality of life and that quality-of-life improvement affects long-term outcomes, such as symptomatic remission and functional recovery (Boden et al., 2009; Lambert et al., 2009, 2010; Karow et al., 2014). Patients who do not achieve symptomatic remission are more likely to report poor quality of life, to receive low scores on physician-rated scales of functioning (Boden et al., 2009), and to remain unemployed (Haynes et al., 2012).
Atypical (second-generation) antipsychotics offer the opportunity to individualize and optimize treatment, and this is reflected in more ambitious treatment goals that encompass quality of life and other relevant patient outcomes. Given the low degree of overlap between quality-of-life measures and clinician-rated assessments of psychopathologic symptoms, multifactorial assessment should be considered essential in clinical trials of antipsychotic drugs (Naber et al., 2005; Chen et al., 2011). Moreover, measurement of quality of life and functioning is of great importance given the role these can play in treatment adherence and satisfaction as well as clinical outcomes and prognosis in patients with schizophrenia (Boyer et al., 2013). Assessing patients’ quality of life, functioning, and key milestones such as returning to work or school or maintaining employment (Schennach et al., 2012) can differentiate the benefits of different atypical antipsychotics, which have distinct pharmacologic and tolerability profiles (Fervaha et al., 2014a, 2014b, 2015, 2016) as well as identify areas for psychosocial intervention.
Quality of Life with Abilify Maintena (QUALIFY; NCT01795547) is the first head-to-head randomized study of 2 atypical long-acting injectable antipsychotics (LAIs) with different pharmacologic profiles, aripiprazole once-monthly 400 mg (AOM 400) and paliperidone palmitate once-monthly (PP), and the first to directly compare treatment effectiveness on a measure of health-related quality of life and functioning as the primary outcome (Naber et al., 2015). These 2 LAIs have different pharmacologic activities: aripiprazole is a partial agonist at dopamine D2 and serotonin 5HT1A receptors and antagonist at 5HT2A receptors (Abilify Maintena Prescribing Information, 2016), whereas paliperidone is an antagonist at D2 and 5HT2A receptors (Invega Sustenna Prescribing Information, 2015). Patients in QUALIFY were stable at baseline but, in their physician’s judgment, needed a change from their current oral antipsychotic and might benefit from LAI treatment. Compared with PP, AOM 400 showed noninferiority and met prespecified superiority criteria vs PP on the primary endpoint, which was changed from baseline to week 28 on the rater-blinded Heinrichs-Carpenter Quality of Life Scale (QLS). The clinician-rated QLS assesses function and health-related quality of life in patients with schizophrenia. It comprises a semistructured interview assessing 21 items in 4 domains: interpersonal relations (e.g., capacity for intimacy, avoidance, and withdrawal tendencies), instrumental role (e.g., satisfaction from work/school/parenting, level of accomplishment), intrapsychic foundations (e.g., sense of purpose, motivation, empathy), and commonplace objects and activities (e.g., engagement in regular/everyday activities) (Heinrichs et al., 1984). The QLS was originally designed in part to measure deficit symptoms of schizophrenia not adequately captured by symptom scales like the Positive and Negative Syndrome Scale. In QUALIFY, significantly greater improvements with AOM 400 compared with PP were also observed for the rater-blinded Investigator’s Assessment Questionnaire (IAQ) (Naber et al., 2015), which measures medication effectiveness relative to prior antipsychotic medication (Tandon et al., 2005), and the clinician-rated Clinical Global Impression—Severity of Illness scale (CGI-S), which assesses the clinician’s impression of current disease severity (Guy, 1976).
QUALIFY included multifaceted clinical and functional scales addressing changes in symptoms, quality of life, and functional capacity. Here, we report the effects of AOM 400 and PP on validated measures of clinical effectiveness, quality of life, functioning, and tolerability and their relationship to readiness to work, as measured by the Work Readiness Questionnaire (WoRQ), a newly developed clinician-administered assessment of the functional capacity to work in patients with schizophrenia, independent of their current employment status or opportunities (Potkin et al., 2016).
Methods
Study Design
Details regarding the study design were previously described (Naber et al., 2015). Briefly, this was a multicenter, open-label, rater-blinded, 28-week, randomized study (NCT01795547). The study took place from February 2013 through September 2014 (last patient enrolled February 2014; last visit occurred September 2014) and adhered to the principles of Good Clinical Practice and the Declaration of Helsinki; protocol approval was obtained from the relevant ethics committees by the national coordinating investigator for each country (EU) or the principal investigator at each site. Patients provided written informed consent after receiving a complete description of the study.
Study investigators enrolled patients at 71 study sites in 10 countries (Canada, Czech Republic, Estonia, France, Germany, Italy, Spain, Sweden, United Kingdom, and United States). Patients were randomized 1:1 to AOM 400 (option to reduce to 300 mg if patients experienced tolerability issues) or PP (flexible dosing, per label, with 50–150 mg/mo as paliperidone [EU and Canada], equivalent to 78–234 mg/mo as paliperidone palmitate [US]). Following randomization, patients’ treatment was first converted to oral aripiprazole (5–30 mg/d) or paliperidone (3–12 mg/d) during 3 weeks while discontinuing their prior antipsychotic (oral conversion phase), followed by administration of the respective LAI formulation (LAI continuation phase) (Naber et al., 2015).
Randomization and Masking
Randomization was stratified by age (≤35 years or >35 years) and was achieved using an interactive voice response system using block randomization to ensure that equal numbers of patients entered each treatment group within each stratum. Treatment was not masked, because the objective was to design a study closely reflective of clinical practice, and AOM 400 and PP differed in appearance, available dose strengths, dose initiation regimens, and administration site (at the time of the QUALIFY study, Abilify Maintena (aripiprazole once-monthly) was approved for gluteal administration only). Nevertheless, raters for 2 outcome measures (QLS and IAQ) were unaware of (blinded to) the patient’s treatment assignment.
Patients
Patient inclusion and exclusion criteria were previously described (Naber et al., 2015). Briefly, eligible patients included men and women, aged 18 to 60 years, with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision diagnosis of schizophrenia. Eligible patients were stable, were treated with an oral antipsychotic for at least 3 months before screening, and, in the opinion of the investigator, required a treatment change from their oral antipsychotic because of inadequate response, poor tolerability, or lack of adherence, and might benefit from LAI therapy. At screening and baseline visits, CGI-S scores ≥3 (mildly ill) and ≤5 (markedly ill) were required. Key exclusion criteria were an Axis I disorder or psychiatric disorder other than schizophrenia, a substance use disorder that might confound assessment (other than nicotine use), or treatment with an atypical LAI within 6 months before study screening (Naber et al., 2015).
Assessments
The QLS and IAQ were administered by trained raters who were blind to treatment assignment (Naber et al., 2015). Clinicians rating the additional secondary effectiveness measures (CGI-S, CGI-I, and WoRQ) were not blinded. The CGI-S scale quantifies the rater’s impression of the patient’s current illness severity on a scale ranging from 1 (normal, not at all ill) to 7 (among the most extremely ill patients) (Guy, 1976). CGI-S response was defined as a decrease of ≥1 point from baseline to week 28. The CGI-I scale quantifies the clinician’s impression of patient improvement or worsening relative to baseline on a 7-point scale ranging from 1 (very much improved) to 7 (much worse) (Naber et al., 2015). CGI-I response was defined as a score of 1 or 2 at week 28. WoRQ comprises 7 statements rated by the clinician on a 4-point scale (strongly agree to strongly disagree) based on the clinician’s perception of the patient’s ability or capacity to engage in daily activities; interact with others including co-workers, customers, and supervisors; maintain adherence to treatment; and of the patient’s appearance, behavior, and impulse control with others (Potkin et al., 2016). Following rating of the 7 statements, a global yes/no question based on the clinician’s judgment of the patient’s readiness to work is answered; individual ratings for the 7 statements are used to aid the clinician in determining whether or not the patient is ready for work. Combined scores for the 7 statements constitute the WoRQ total score (Potkin et al., 2016). The judgment on readiness to work or not is made separately from the total score. Work is defined as an independent effort that merits pay and does not include supported work or job coach.
Patient-rated measures included the Subjective Well-Being Under Neuroleptic Treatment−short version (SWN-S) (Naber et al., 2001) and Tolerability and Quality of Life questionnaire (TooL) (Lindström et al., 2009). The SWN-S provides patients with a scale to rate the subjective effects of their neuroleptic drugs on psychopathology, quality of life, and adherence over the past 7 days. The scale contains 10 positive and 10 negative statements that comprise 5 subscales (mental functioning, self-control, physical functioning, emotional regulation, and social integration; 4 items per subscale). Items are rated on a 6-point Likert scale ranging from 1 (not at all) to 6 (very much). When calculating the total score, negative statements are reverse scored (e.g., 6=1, 5=2). Possible total scores range from 20 to 120 (4 to 24 for each subscale), with higher scores indicating better well-being (Naber et al., 2001). The TooL asks patients to rate the impact of antipsychotic side effects on their quality of life on 8 domains (mood [worry/upset], function/capabilities, fatigue/weakness, weight gain, stiffness/tremor, physical restlessness, sexual dysfunction, dizziness/nausea) using a 4-point scale ranging from 1 (no impact) to 4 (maximum impact); possible total scores range from 8 to 32, with lower scores indicating less impact of side effects on quality of life.
Safety and tolerability were assessed throughout the study, with a safety follow-up visit 4 weeks after completion of, or withdrawal from, treatment. The safety and tolerability of AOM 400 and PP in QUALIFY were previously reported (Naber et al., 2015).
Statistical Analyses
Effectiveness analyses were performed using the full analysis set (FAS), which included all randomized patients who took at least 1 dose of oral antipsychotic medication and had a valid baseline and post-baseline assessment of the QLS total score. All statistical hypothesis tests of endpoints were 2-sided tests performed on a 5% significance level, and no adjustment for multiplicity was made.
Preplanned secondary analyses included mean total CGI-I and IAQ scores at week 28 and changes from baseline in SWN-S and TooL at week 28. In addition, posthoc analyses of the change from baseline in QLS items at week 28, the proportion of CGI-S responders at week 28, and the proportion of CGI-I responders were performed. Mean changes from baseline in SWN-S, TooL, and QLS items at week 28 were calculated using a mixed model for repeated measures. To compare AOM 400 with PP, region, baseline score-by-visit interaction, geographical region, age group, visit, and treatment-by-visit interaction were included as fixed effects, using an unstructured covariance matrix and Kenward-Rogers method for handling degrees of freedom. The proportions of CGI-S and CGI-I responders at week 28 were compared in AOM 400 and PP treatment groups using logistic regressions adjusting for baseline score, region, and age group. Change from baseline at week 28 in WoRQ total score was analyzed using an ANCOVA model adjusting for baseline score, region, and age group. In addition, a shift analysis of the WoRQ readiness-to-work question (shifts from not ready to work to ready to work) was performed, and the odds of being ready for work at week 28 were estimated with logistic regression adjusted for baseline status of work readiness.
To examine the relationships between assessments, mixed model for repeated measures was used to derive baseline adjusted partial correlations of changes from baseline to week 28 for QLS, CGI-S, WoRQ, TooL, and SWN-S, and between scores at week 28 for IAQ, and CGI-I. Partial correlation analyses were performed for each treatment group.
Safety was assessed in all treated patients (AOM 400, n=144; PP, n=137); adverse events were summarized using descriptive statistics and were previously reported in detail (Naber et al., 2015).
Results
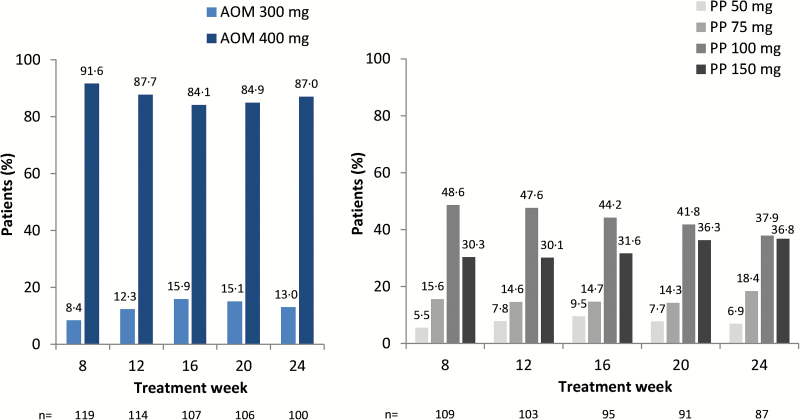
There were 295 patients randomly assigned to AOM 400 (n=148) or PP (n=147). Of those randomized, 67.6% (100/148) of patients in the AOM 400 group and 56.5% (83/147) of patients in the PP group completed 28 weeks of treatment (Naber et al., 2015). The FAS included 136/144 (94.4%) treated patients in the AOM 400 group and 132/137 (96.4%) treated patients in the PP group (Naber et al., 2015). Baseline demographics and disease severity characteristics were similar between treatment groups (Table 1). Across treatment weeks, most patients in the AOM 400 treatment group (i.e., >84%) were treated with the 400-mg dose, and most patients in the PP treatment group (i.e., >74%) were treated with the 100-mg or 150-mg dose (as paliperidone, equivalent to 156 mg or 234 mg paliperidone palmitate) (Figure 1). In addition to study drug, 39 (27.1%) and 33 (24.1%) patients in the AOM 400 and PP treatment groups, respectively, received concomitant benzodiazepines (most frequently lorazepam, diazepam, and alprazolam), while 20 (13.9%) and 14 (10.2%) received concomitant non-benzodiazepine hypnotics (zolpidem or zopiclone). Concomitant anticholinergic medications, most often benztropine, were taken by 18 (12.5%) and 12 (8.8%) patients in the AOM 400 and PP treatment groups, respectively.
Table 1.
Patient Baseline Demographics and Disease Severity
| AOM 400 | PP | |
|---|---|---|
| Randomized patients | n=148 | n=147 |
| All-patients-treated set | n=144 | n=137 |
| Full analysis set | n=136 | n=132 |
| Baseline demographicsa | ||
| Mean (SD) age, y | 42.6 (10.9) | 41.2 (10.7) |
| Mean (SD) age at schizophrenia onset, y | 28.5 (10.7) | 26.8 (9.5) |
| Mean (SD) schizophrenia duration, y | 14.1 (10.5) | 14.4 (10.7) |
| Male, n (%) | 86 (59.7) | 82 (59.9) |
| Baseline scale scoresb | ||
| QLS total score, possible range 0–126 (higher = better) | ||
| Mean (SD) | 66.0 (21.7) | 62.9 (21.5) |
| CGI-S score, possible range 1–7 (lower = better) | ||
| Mean (SD) | 4.0 (0.65) | 4.0 (0.65) |
| WoRQ total score, possible range 7–28 (lower = better) | ||
| Mean (SD) | 14.5 (3.45) | 15.3 (3.25) |
| SWN-S total score, possible range 20–120 (higher = better) | ||
| Mean (SD) | 83.6 (15.9) | 85.2 (14.6) |
| TooL total score, possible range 8–32 (lower = better) | ||
| Mean (SD) | 14.4 (4.4) | 14.3 (3.9) |
Abbreviations: AOM 400, aripiprazole once-monthly 400 mg; CGI-S, Clinical Global Impression–Severity of Illness; QLS, Heinrichs-Carpenter Quality-of-Life Scale; PP, paliperidone palmitate once monthly; SWN-S, Subjective Well-Being Under Neuroleptics–short version; TooL, Tolerability and Quality of Life Questionnaire; WoRQ, The Work Readiness Questionnaire.
aBaseline and demographic data are from the all-patients-treated set.
bBaseline scale scores are from the full analysis set.
Figure 1.
Distribution of aripiprazole once-monthly 400 mg (AOM 400) and paliperidone palmitate once-monthly (PP) doses by treatment week in the all-patients-treated set (i.e., all patients randomized who took ≥1 dose of study medication). Percentages of patients receiving each dose is given above each bar, numbers below the axes refer to number of treated patients in the given treatment week. PP doses refer to paliperidone (50, 75, 100, and 150 mg [EU]) equivalent to paliperidone palmitate (78, 117, 156, and 234 mg [US]).
Clinician-Rated Outcomes
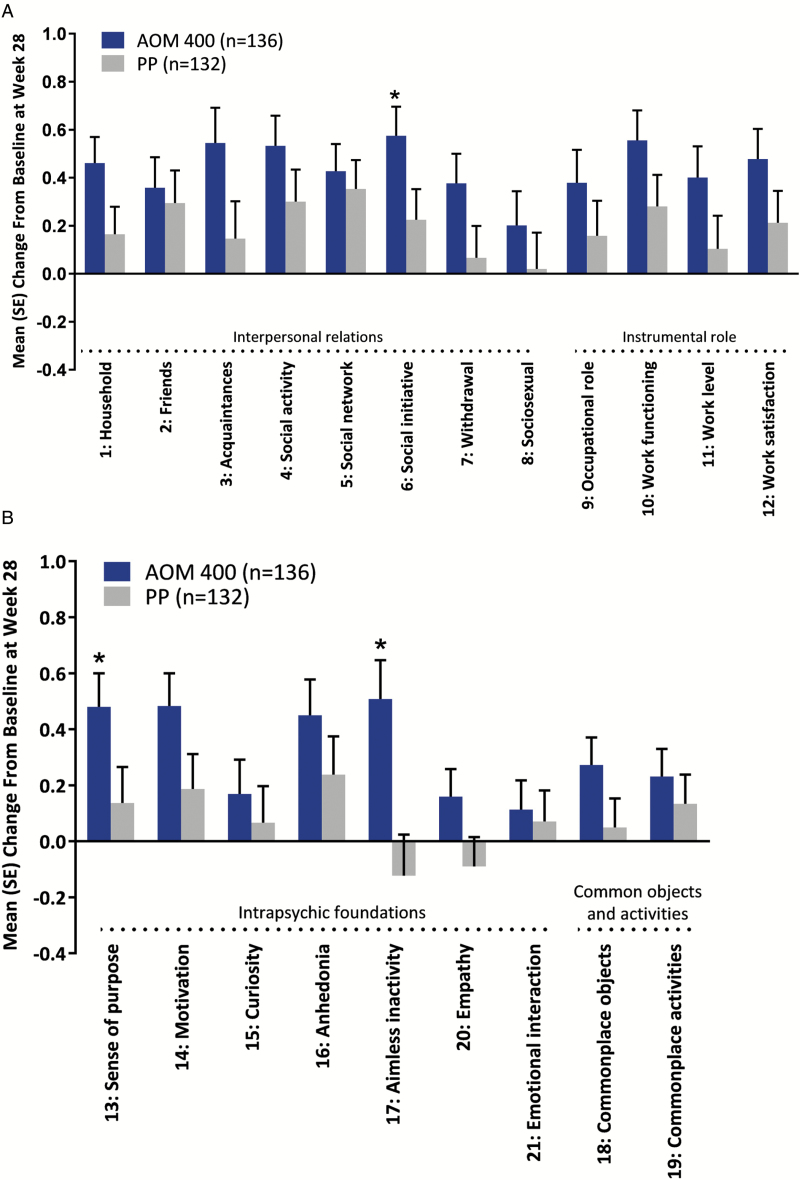
Among QLS items, numerically greater improvements from baseline were observed with AOM 400 treatment compared with PP treatment on all items, and sense of purpose, aimless inactivity (both items of the intrapsychic foundations domain), and social initiative improved significantly more with AOM 400 than with PP (P<.05 for both comparisons) (Figure 2).
Figure 2.
Change from baseline at week 28 on Heinrichs-Carpenter Quality of Life Scale (QLS) items. Full analysis set. Results are shown for (a) the first 12 items on the scale and (b) the last 9 items on the scale. AOM 400, aripiprazole once-monthly 400 mg; PP, paliperidone palmitate once-monthly. *P<.05.
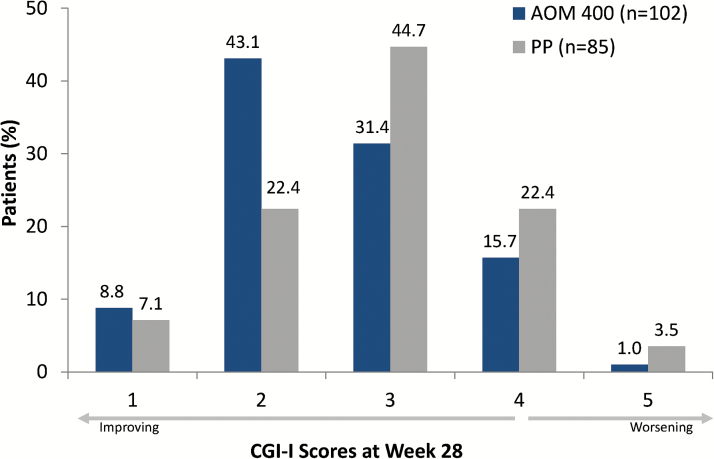
At week 28, a greater proportion of patients in the AOM 400 treatment group compared with the PP treatment group were classified as responders on the clinician-rated CGI-S (62.7% vs 43.5%, respectively; adjusted odds ratio (OR), 2.26; P=.010) and on the clinician-rated CGI-I (52.0% vs 29.4%; adjusted OR, 2.51; P=.0032) (Figure 3). Patients treated with AOM 400 also demonstrated significantly lower (better) CGI-I scores compared with patients treated with PP, indicating greater clinical improvement with AOM 400 (least squares mean treatment difference at week 28, −0.326 [95% CI, −0.60 to −0.05]; P=.020).
Figure 3.
Distribution of Clinical Global Impressions–Improvement (CGI-I) scores at week 28. Full analysis set. 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse. Patients with CGI-I scores of 1 or 2 at week 28 were considered to be responders. AOM 400, aripiprazole once-monthly 400 mg; PP, paliperidone palmitate once-monthly.
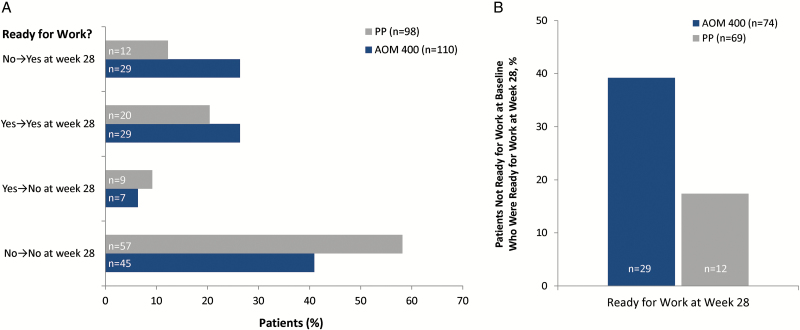
Change from baseline at week 28 on the WoRQ total score was significantly greater with AOM 400 compared with PP treatment (least squares mean treatment difference [standard error of the mean] at week 28, –1.162 [0.399] 95% CI, –1.96 to –0.37; P=.004). At baseline, similar percentages of patients in the AOM 400 and PP treatment groups (32.7% and 29.6%, respectively) were perceived by clinicians as being ready for work. At week 28, a numerically larger percentage of patients in the AOM 400 group (52.7%) was rated as being ready for work compared with the PP treatment group (32.7%) (Figure 4a). Furthermore, a greater percentage of patients receiving AOM 400 shifted from No (not ready to work) at baseline to Yes (ready to work) at week 28 (26.4%) compared with those receiving PP (12.2%) in the FAS population. Among patients who were not ready to work at baseline (n=74 patients in the AOM 400 treatment group and 69 patients in the PP treatment group), 39.2% of patients who received AOM 400 and 17.4% of patients who received PP were rated by clinicians as being ready for work at week 28 (Figure 4b). The adjusted odds of being ready for work at week 28 were significantly higher for patients treated with AOM 400 compared with patients treated with PP (adjusted OR at week 28, 2.67 [95% CI, 1.39−5.14]; P=.003).
Figure 4.
Shift from baseline in readiness for work status among (a) the full analysis set and (b) patients not ready for work at baseline. Shift analysis was based on the WoRQ readiness to work question (item 8) at baseline and week 28: Based on your clinical judgment, is this patient ready for work?
AOM 400, aripiprazole once-monthly 400 mg; FAS, full analysis set; PP, paliperidone palmitate once-monthly; WoRQ, Work Readiness Questionnaire.
Patient-Rated Outcomes
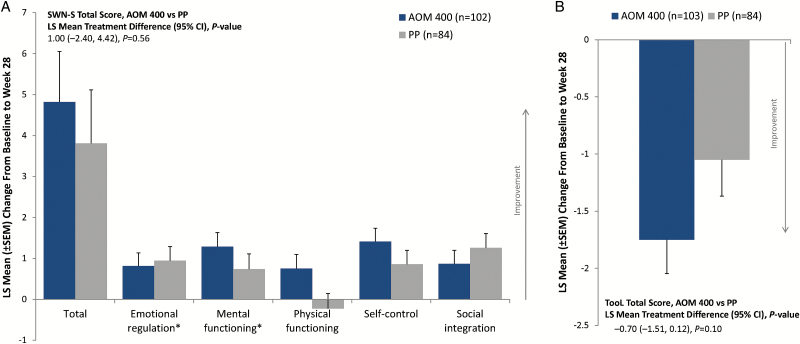
At baseline, mean SWN-S total scores indicated that patients in both treatment groups had a relatively positive perception of their well-being on their current medication (Table 1). Improvements from baseline to week 28 on SWN-S total score were reported in both treatment groups, and the difference between treatment arms did not reach statistical significance (Figure 5a). Improvements were noted on all SWN-S subscales in the AOM 400 treatment group at week 28 and 4 of 5 subscales in the PP group (Figure 5a). Improvements numerically favored AOM 400 over PP on the subscales of mental functioning, physical functioning, and self-control.
Figure 5.
Change from baseline to week 28 in patient-rated (a) Subjective Well-Being Under Neuroleptics–short version (SWN-S) total and subscale scores and (b) Tolerability and Quality of Life (TooL) total score. Full analysis set. AOM 400, aripiprazole once-monthly 400 mg; FAS, full analysis set; LS, least squares. *n=83 for the PP treatment group.
Likewise, mean TooL scores were relatively low (i.e., minimal impact of side effects on quality of life) at baseline (Table 1). The impact of antipsychotic side effects on quality of life improved at week 28 in both treatment groups, with numerically greater improvement in mean changes from baseline in TooL total scores for AOM 400 vs PP (P=.10) (Figure 5b).
Partial Correlations between Assessments
Posthoc analysis of responses to treatment based on clinician- and patient-rated scales indicated that, in general, the clinician-rated assessments (i.e., CGI-S, IAQ, CGI-I) were modestly correlated with change from baseline in the patient-rated TooL and SWN-S scales (Table 2). Change from baseline on the WoRQ total score was correlated with the clinician-rated scales of symptom severity (i.e., CGI-S, IAQ, CGI-I, and QLS) and patient-rated TooL or SWN-S scales (Table 2). Likewise, change from baseline on the clinician-rated QLS total score was modestly correlated with change from baseline on the WoRQ total score, and improvements in QLS were correlated with clinician-rated measures of symptom severity (CGI-S, CGI-I, IAQ) and modestly correlated with patient ratings of well-being and quality of life (Tool, SWN-S) (Table 2).
Table 2.
Correlations between Change from Baseline to Week 28 (QLS, CGI-S, WoRQ, TooL, SWN-S) or Scores at Week 28 (IAQ, CGI-I) by Treatment
| Correlation Estimates | QLS Total | CGI-S | IAQ Total | CGI-I | WoRQ Total | TooL Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOM 400 | PP | AOM 400 | PP | AOM 400 | PP | AOM 400 | PP | AOM 400 | PP | AOM 400 | PP | |
| CGI-S | -0.598 | -0.381 | ||||||||||
| IAQ-total | -0.587 | -0.510 | 0.392 | 0.473 | ||||||||
| CGI-I | -0.459 | -0.400 | 0.765 | 0.715 | 0.459 | 0.578 | ||||||
| WoRQ-total | -0.322 | -0.396 | 0.468 | 0.236 | 0.260 | 0.181 | 0.380 | 0.333 | ||||
| TooL total | -0.374 | -0.365 | 0.323 | 0.213 | 0.355 | 0.058 | 0.491 | 0.168 | 0.117 | -0.158 | ||
| SWN-S total | 0.375 | 0.376 | -0.390 | -0.355 | -0.330 | -0.259 | -0.260 | -0.348 | -0.113 | -0.064 | -0.689 | -0.557 |
Analyzed posthoc at patient level with MMRM to assess partial correlations between scales in the full analysis set. Abbreviations: CGI-I, Clinical Global Impression–Improvement; CGI-S, CGI–Severity; IAQ, Investigators’ Assessment Questionnaire; MMRM, mixed model for repeated measures; QLS, Heinrichs-Carpenter Quality of Life Scale; SWN-S, Subjective Well-Being Under Neuroleptic Treatment−short version; Tool, Tolerability and Quality of Life Questionnaire; WoRQ, Work Readiness Questionnaire.
Higher QLS and SWN-S total scores indicate clinical improvements, whereas lower CGI-S, IAQ, CGI-I, WoRQ, and TooL scores indicate better clinical condition.
Safety and Tolerability
Safety and tolerability results were previously reported (Naber et al., 2015). In both treatment groups, there were no new safety signals detected, and no deaths occurred during the study.
Discussion
Here we report endpoints from the QUALIFY study in patients with schizophrenia, demonstrating consistent and multidimensional improvements in patients on AOM 400, particularly in functional measures including readiness for work. Patients with schizophrenia are often unemployed, and those with chronic schizophrenia are more likely to be unemployed than patients with first-episode schizophrenia (Marwaha et al., 2007). In QUALIFY, there were significantly greater improvements in the WoRQ total score as well as significantly higher odds of being rated as ready for work at week 28 in patients treated with AOM 400 compared with those treated with PP. The improvement on the WoRQ shows that this questionnaire is sensitive to drug effects and may be appropriate as a scale of functioning in future clinical trials. In particular, the simplicity of administering the WoRQ questionnaire makes it potentially useful in clinical practice as a good indicator of functioning.
As previously reported, patients treated with AOM 400 had significantly greater mean improvements from baseline to week 28 in the QLS total score compared with patients in the PP treatment group, and the mean change from baseline at week 28 was numerically greater with AOM 400 for all 4 QLS domains, reaching statistical significance for the intrapsychic foundations domain (Naber et al., 2015). The current analysis demonstrated numeric improvements with AOM 400 vs PP on all items, indicating that improvements in the QLS total score and domain scores resulted from improvements across QLS items. Specific items across domains, including sense of purpose and aimless inactivity (both items of the intrapsychic foundations domain) as well as social initiative, improved significantly more with AOM 400 than with PP. Improvement on these items and in the intrapsychic foundations domain of the QLS indicates improved motivation and goal-oriented thoughts, thus addressing key negative symptoms that underlie impaired functioning in patients with schizophrenia (Milev et al., 2005; Gard et al., 2009; Konstantakopoulos et al., 2011; Fervaha et al., 2014c;Robertson et al., 2014).
Greater improvement with AOM 400 compared with PP on clinician-rated scales was consistent across measures assessed by blinded (i.e., QLS, IAQ) and nonblinded raters (e.g., CGI-S and CGI-I). Effectiveness measures, including significantly better CGI-I scores at week 28 and significantly greater proportions of CGI-S and CGI-I responders, also demonstrated superiority of AOM 400 vs PP. Numeric improvements with AOM 400 vs PP were observed on the patient-rated SWN-S scale. On average, patients rated their experience on their current neuroleptic drugs as relatively good at baseline, leaving less room for improvement after switching to the LAI test drug. Scores remained stable or improved modestly, indicating improved overall satisfaction with general functioning and with 28 weeks of treatment with either LAI.
Using the TooL, patients noted minimal impact of medication side effects on quality of life at baseline and modest improvements in medication side effects at week 28 that trended in favor of AOM 400 vs PP. For the IAQ, physicians rated efficacy and tolerability of AOM 400 or PP as improved compared with prior antipsychotic therapy; greater improvements were noted with AOM 400 vs PP (Naber et al., 2015).
Correlations between scales indicated that different scales measure separate facets of schizophrenia. When comparing various assessment scales, the weakest correlations were observed between the clinician- and patient-rated scales, suggesting that patient and clinician perspectives provide distinct information and therefore, paying additional attention to the patient’s perspective may be useful as it might not always be captured by the clinician-rated scales. The clinicians’ rating of tolerability (i.e., IAQ) had a higher correlation with the patients’ assessments (i.e., Tool and SWN-S) for AOM than for PP. However, the divergence of clinician and patient ratings could be best explored in a larger dataset with blinded ratings. WoRQ was modestly correlated with other clinician-rated scales but poorly correlated with patient-rated scales, suggesting that work readiness was not related to patient subjective assessments. As a whole, correlations between scales were modest, which suggests that the assessments in QUALIFY measured different dimensions of schizophrenia. By virtue of this fact, this study can be considered to offer broader information than most randomized controlled trials that more narrowly focus on the assessment of psychopathologic symptoms using measures such as the Positive and Negative Syndrome Scale and CGI, which strongly correlate with one another (Levine et al., 2008) but less so with subjective measures of well-being and quality of life (Naber et al., 2005; Chen et al., 2011). Across the broad range of scales assessed in QUALIFY, AOM 400 produced numerical or statistically significantly greater improvements compared with PP.
The QUALIFY study is one of few randomized head-to-head studies of LAIs and is the first to compare 2 atypical LAIs with different pharmacologic profiles on health-related quality of life and functioning in patients with schizophrenia. Patients were stable at enrollment, and thus our findings may not be applicable to patients experiencing an acute episode of schizophrenia (Naber et al., 2015). Study limitations were previously reported in detail—key limitations include the open-label study design in which raters of only the QLS and IAQ were blinded to study treatment (Naber et al., 2015). Also, the study was powered based on a 5-point noninferiority margin for the primary endpoint (Naber et al., 2015), which may have limited the ability to detect smaller statistically significant differences on some secondary and other endpoints, including QLS items. Finally, although the study findings were consistent across rater-blinded, rater unblinded, and patient-rated scales, the open-label design intended to more closely parallel clinical practice may have introduced some unmeasured bias.
Overall, our findings indicate consistently greater improvements with AOM 400 vs PP across all rater-blinded (i.e., QLS, IAQ) and unblinded (e.g., WoRQ, CGI-S and CGI-I) clinician- as well as patient-rated scales, reflecting a broad range of symptoms and functioning. These included significantly better CGI-I scores at week 28, significantly greater proportions of CGI-S and CGI-I responders, and significantly higher odds of being judged as ready for work. These multidimensional results extend our primary finding of superior improvement on the QLS total score with AOM 400 vs PP (Naber et al., 2015), demonstrating improvements across multiple domains of health-related quality of life and functioning and an additional important potential benefit of clinical and functional improvement in enhanced work readiness using the newly developed WoRQ. These results coupled with results from other studies may help to differentiate AOM 400 and PP, 2 LAI antipsychotics with different pharmacologic profiles: aripiprazole is a partial agonist at dopamine D2 and serotonin 5HT1A receptors and an antagonist at 5HT2A receptors while paliperidone is an antagonist at D2 and 5HT2A receptors.. Our results may help provide guidance to clinicians when choosing between antipsychotic treatments and psychosocial options for their patients with schizophrenia.
Statement of Interest
Steven G. Potkin has been a consultant or participated in advisory boards for Otsuka, Sunovion, Roche, Lundbeck, FORUM, Acadia, and Alkermes; has received grants or research support from Eli Lilly, Toyama, Otsuka, FORUM, Alkermes, Eisai, and Lundbeck; and has been a member of the speakers bureau or received speaker honoraria for Otsuka, Sunovion, Novartis, Acadia, and Forest. Jean-Yves Loze is an employee of Otsuka Pharmaceutical Europe. Carlos Forray and Anna Eramo are employees of Lundbeck LLC. Ross A. Baker and Timothy Peters-Strickland are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Christophe Sapin, Maud Beillat, and Karina Hansen are employees of Lundbeck SAS. Anna-Greta Nylander, Peter Hertel, and Simon Nitschky Schmidt are employees of H. Lundbeck A/S. Dieter Naber has participated in advisory boards for Janssen/Cilag, Eli Lilly, Lundbeck, Otsuka, and Servier; and has received speaker honoraria from Astra Zeneca, Janssen/Cilag, Eli Lilly, Lundbeck, and Otsuka; neither he nor his wife has stock in or other financial relationship to any company.
Acknowledgments
Editorial support for development of this manuscript was provided by Amy Roth Shaberman, PhD, at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company.
This work was supported by H. Lundbeck A/S and Otsuka Pharmaceutical Development & Commercialization, Inc.
References
- Abilify Maintena US (Aripiprazole) (2016) Full prescribing information. Tokyo: Otsuka Pharmaceutical Co., Ltd. [Google Scholar]
- Boden R, Sundstrom J, Lindstrom E, Lindstrom L. (2009) Association between symptomatic remission and functional outcome in first-episode schizophrenia. Schizophr Res 107:232–237. [DOI] [PubMed] [Google Scholar]
- Boyer L, Baumstarck K, Boucekine M, Blanc J, Lancon C, Auquier P. (2013) Measuring quality of life in patients with schizophrenia: an overview. Expert Rev Pharmacoecon Outcomes Res 13:343–349. [DOI] [PubMed] [Google Scholar]
- Chen L, Phillips G, Johnston J, Kinon BJ, Ascher-Svanum H, Kollack-Walker S, Succop P, Naber D. (2011) The relationship, structure and profiles of schizophrenia measurements: a post-hoc analysis of the baseline measures from a randomized clinical trial. BMC Psychiatry 11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Agid O, Foussias G, Remington G. (2014. a) Toward a more parsimonious assessment of neurocognition in schizophrenia: a 10-minute assessment tool. J Psychiatr Res 52:50–56. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Agid O, Takeuchi H, Foussias G, Remington G. (2014. b) Effect of antipsychotic medication on overall life satisfaction among individuals with chronic schizophrenia: findings from the NIMH CATIE study. Eur Neuropsychopharmacol 24:1078–1085. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. (2014. c) Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry 29:449–455. [DOI] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. (2009) Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr Res 115:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976) Clinical Global Impression Scale (CGI) ECDEU assessment manual for psychopharmacology, pp217–222. Rockville, MD: US Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs. [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ, Wfsbp Task Force on Treatment Guidelines for Schizophrenia (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 14:2–44. [DOI] [PubMed] [Google Scholar]
- Haynes VS, Zhu B, Stauffer VL, Kinon BJ, Stensland MD, Xu L, Ascher-Svanum H. (2012) Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry 12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT., Jr (1984) The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 10:388–398. [DOI] [PubMed] [Google Scholar]
- Invega Sustenna (paliperidone palmitate) (2015) Full prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc. [Google Scholar]
- Karow A, Wittmann L, Schottle D, Schafer I, Lambert M. (2014) The assessment of quality of life in clinical practice in patients with schizophrenia. Dialogues Clin Neurosci 16:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantakopoulos G, Ploumpidis D, Oulis P, Patrikelis P, Soumani A, Papadimitriou GN, Politis AM. (2011) Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr Res 133:193–198. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Schizophrenia Patient Outcomes Research Team (2010) The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull 36:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Schimmelmann BG, Naber D, Eich FX, Schulz H, Huber CG, Karow A. (2009) Early- and delayed antipsychotic response and prediction of outcome in 528 severely impaired patients with schizophrenia treated with amisulpride. Pharmacopsychiatry 42:277–283. [DOI] [PubMed] [Google Scholar]
- Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. (2010) Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci 12:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SZ, Rabinowitz J, Engel R, Etschel E, Leucht S. (2008) Extrapolation between measures of symptom severity and change: an examination of the PANSS and CGI. Schizophr Res 98:318–322. [DOI] [PubMed] [Google Scholar]
- Lindström E, Jönsson L, Berntsson A. A patient perspective on side effects of antipsychotic therapy: the TooL instrument. Poster presented at: International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 12th Annual European Congress; October 24–27, 2009; Paris, France. [Google Scholar]
- Marwaha S, Johnson S, Bebbington P, Stafford M, Angermeyer MC, Brugha T, Azorin JM, Kilian R, Hansen K, Toumi M. (2007) Rates and correlates of employment in people with schizophrenia in the UK, France and Germany. Br J Psychiatry 191:30–37. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. (2005) Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry 162:495–506. [DOI] [PubMed] [Google Scholar]
- Naber D, Moritz S, Lambert M, Pajonk FG, Holzbach R, Mass R, Andresen B. (2001) Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res 50:79–88. [DOI] [PubMed] [Google Scholar]
- Naber D, Riedel M, Klimke A, Vorbach EU, Lambert M, Kuhn KU, Bender S, Bandelow B, Lemmer W, Moritz S, Dittmann RW. (2005) Randomized double blind comparison of olanzapine vs. clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta Psychiatr Scand 111:106–115. [DOI] [PubMed] [Google Scholar]
- Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, Peters-Strickland T, Nylander AG, Hertel P, Andersen HS, Eramo A, Loze JY, Potkin SG. (2015) Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res 168:498–504. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Bugarski-Kirola D, Edgar CJ, Soliman S, Le Scouiller S, Kunovac J, Miguel Velasco E, Garibaldi GM. (2016) Psychometric evaluation of the Work Readiness Questionnaire in schizophrenia. CNS Spectr 21:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, Harvey PD. (2014) Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr Res 160:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schennach R, Musil R, Moller HJ, Riedel M. (2012) Functional outcomes in schizophrenia: employment status as a metric of treatment outcome. Curr Psychiatry Rep 14:229–236. [DOI] [PubMed] [Google Scholar]
- Tandon R, Devellis RF, Han J, Li H, Frangou S, Dursun S, Beuzen JN, Carson W, Corey-Lisle PK, Falissard B, Jody DN, Kujawa MJ, L’Italien G, Marcus RN, McQuade RD, Ray S, Van Peborgh P, Group IAQVS (2005) Validation of the Investigator’s Assessment Questionnaire, a new clinical tool for relative assessment of response to antipsychotics in patients with schizophrenia and schizoaffective disorder. Psychiatry Res 136:211–221. [DOI] [PubMed] [Google Scholar]