Abstract
Background
Microneedle patches provide an alternative to conventional needle-and-syringe immunization, and potentially offer improved immunogenicity, simplicity, cost-effectiveness, acceptability and safety. We describe safety, immunogenicity and acceptability of the first-in-human study on single, dissolvable microneedle patch vaccination against influenza.
Methods
The TIV-MNP 2015 study was a phase 1, partially blinded, placebo-controlled, randomized clinical trial conducted at Emory University that enrolled non-pregnant, immunocompetent adults (age 18–49 years) from Atlanta (USA) and naïve to 2014–2015 influenza vaccine. Participants were equally randomized among four groups and received a single dose of inactivated influenza vaccine 1) by microneedle patch or 2) by intramuscular injection, or received 3) placebo by microneedle patch, all administered by an unblinded healthcare worker; or received 4) inactivated influenza vaccine by microneedle patch self-administered by study participants. Primary safety outcomes were reactogenicity, grade 3 adverse events and serious adverse events within 8, 28 and 180 days and secondary safety outcomes were new-onset chronic illnesses within 180 days and unsolicited adverse events within 28 days all analyzed by intention to treat. Secondary immunogenicity outcomes were antibody titers at day 28 as well as seroconversion and seroprotection rates all determined by hemagglutination inhibition antibody. The trial is completed and registered with ClinicalTrials.gov, NCT02438423.
Findings
Twenty-five participants per group were enrolled between June 23 and September 25, 2015. There were no related serious adverse events, no related grade 3 or higher adverse events and no new-onset chronic illnesses. Overall incidence of solicited and unsolicited events was similar among vaccinated groups. Reactogenicity was mild, transient and most commonly reported as tenderness at 60% (95% CI, 39– 79%) and pain at 44% (95% CI, 24–65%) after intramuscular injection and tenderness at 66% (95% CI, 51–79%), erythema at 40% (95% CI, 26–55%) and pruritus at 82% (95% CI, 69–91%) after vaccination by microneedle patch application The geometric mean titers were comparable at day 28, between the microneedle patch administered by healthcare worker and the intramuscular route with values of 1197 (95% CI, 855– 1675) and 997 (95% CI, 703–1415) (p=0.5), respectively, for the H1N1 strain; 287 (95% CI, 192–430) and 223 (95% CI, 160–312) (p=0.4), respectively, for the H3N2 strain and 126 (95% CI, 86–184) and 94 (95% CI, 73–122) (p=0.06), respectively, for the B strain. Similar GMT titers were observed in participants who self-administered the microneedle patch. The seroconversion rates were significantly higher at day 28 after microneedle patch vaccination compared to placebo and were comparable to intramuscular injection.
Interpretation
Use of dissolvable microneedle patches for influenza vaccination was well-tolerated and generated robust antibody responses.
Funding
National Institutes of Health.
Keywords: dissolvable polymer microneedle patch, safety, immunogenicity, acceptability, skin reactogenicity, influenza vaccine, intradermal vaccination
Introduction
Even with the recommendation for universal vaccination,1 influenza illness continues to be a major cause of morbidity and mortality resulting in up to 48,000 deaths annually in the United States.2 Influenza prevention through immunization in adults is hindered by low vaccination coverage,3 high immunization costs4 and suboptimal vaccine effectiveness.5,6 While many types of influenza vaccines are currently licensed, improved delivery methods are needed to address these limitations.
In this study, we examine influenza vaccination using microneedle patches (MNPs), which are micron-scale solid conical structures made of dissolvable excipients on a patch backing that deliver vaccine antigens across the stratum corneum barrier into the viable epidermis and dermis of the skin. The intradermal route for immunization offers several immunologic advantages due to the presence of large numbers of antigen-presenting cells (e.g., Langerhans cells and other dendritic cells) in the skin.7,8 In mice, influenza antigens delivered by MNP resulted in a more robust immune response with greater longevity, increased breadth of immunity and potential for dose sparing when compared to the intramuscular (IM) route.9,10
MNP immunization also has the potential to overcome many factors affecting influenza vaccine uptake in adults such as needle phobia,11 lack of time, cost and vaccine access.12,13 MNPs for vaccine delivery are economically advantageous with an expected low manufacturing cost; elimination of sharps waste; reduction or elimination of cold chain requirements through increased thermostability; decreased storage, transport and disposal costs through smaller packaging volume; and lower healthcare-associated administration costs through self-administration by patients.14 MNPs for vaccine administration have also been shown to have greater acceptability when compared to traditional IM hypodermic injection.15
Dissolvable MNPs are used in a number of cosmetic products16 and other MNPs have been in human trials, most notably for administration of parathyroid hormone drugs.17–19 However, vaccination using MNPs has been studied mostly in animals for delivery, for example, of polio, measles, and human papilloma virus as well as influenza antigens. Here, we conducted a first-in-human, partially blinded, placebo-controlled, randomized phase 1 clinical trial comparing the safety, reactogenicity, immunogenicity, and acceptability of inactivated influenza vaccine (IIV) delivered using a dissolvable MNP applied by a healthcare worker (HCW) or through self-administration, to traditional IM delivery by hypodermic needle.
Methods
Study design and participants
At Emory University, a partially blinded, randomized, placebo-controlled study was conducted in which non-pregnant, immunocompetent adults aged 18–49 years were recruited from the community in Atlanta, GA (USA). Healthy participants who previously had not received the influenza vaccine during the 2014–2015 influenza season and without any significant dermatologic conditions were enrolled in the study. Additional inclusion and exclusion criteria are detailed on ClinicalTrials.gov (NCT02438423). All participants provided written consent for study participation prior to enrollment. The study was approved by Emory University and Georgia Institute of Technology institutional review boards and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Randomization and masking
Participants were equally randomized to one of four groups receiving: IIV by MNP (MNPIIV-HCW), IIV by IM injection (IMIIV), or placebo by MNP (MNPplacebo), all applied by an unblinded healthcare worker (HCW); or IIV by MNP self-administered by study participants (MNPIIV-self).
The randomization code was prepared by a research pharmacist using a computer-generated randomization schedule (Research Randomizer Form V4.0) with a block size of 4, and provided to an unblinded HCW. Once the study products were administered, the unblinded HCW and the research pharmacists were not involved in subsequent study procedures. Participants were unaware if the MNP applied by the unblinded HCW contained IIV or placebo, and investigators were unaware if MNPs were applied by unblinded HCW or by participants. Because of the nature of the study, patients were not masked to the type of vaccination method (i.e., MNP vs. IM injection). Laboratory staff conducting the haemagglutination inhibition antibody assays was masked to the group assignment.
Procedures
The licensed 2014–2015 seasonal trivalent influenza vaccine (Fluvirin®) was provided by Seqirus Inc. (formerly NVS Influenza Vaccines, Cambridge, MA) in single-dose, pre-filled syringes for IM injection reported in the product insert to contain 15 μg of each the following three influenza vaccine strains: A/Christchurch/16/2010, NIB-74 (H1N1), A/Texas/50/2012, NYMC X-223 (H3N2), B/Massachusetts/2/2012, NYMC BX-51(B).
The MNPs were designed at the Georgia Institute of Technology and manufactured by the Global Center for Medical Innovation (Atlanta, GA) using Phase 1 Good Manufacturing Practice (Figure 1). The formulation and fabrication methods have been previously described.22 Seqirus also provided concentrated monobulks of each antigen, which were formulated into microneedle patches (MNPIIV) to contain an equivalent dose of each of the three influenza vaccine strains in the microneedles as found in the IM injection product, Vaccine potency was determined by single radial immunodiffusion (SRID) assay.21 Placebo patches contained the same formulation excipients, but without addition of the vaccine monobulks (MNPplacebo). MNPIIV stability was assessed for 12 months at 5°C, 25°C and 40°C by SRID. Residual vaccine content in MNPIIV was also measured by SRID in used patches to determine the actual dose delivered.
Figure 1.
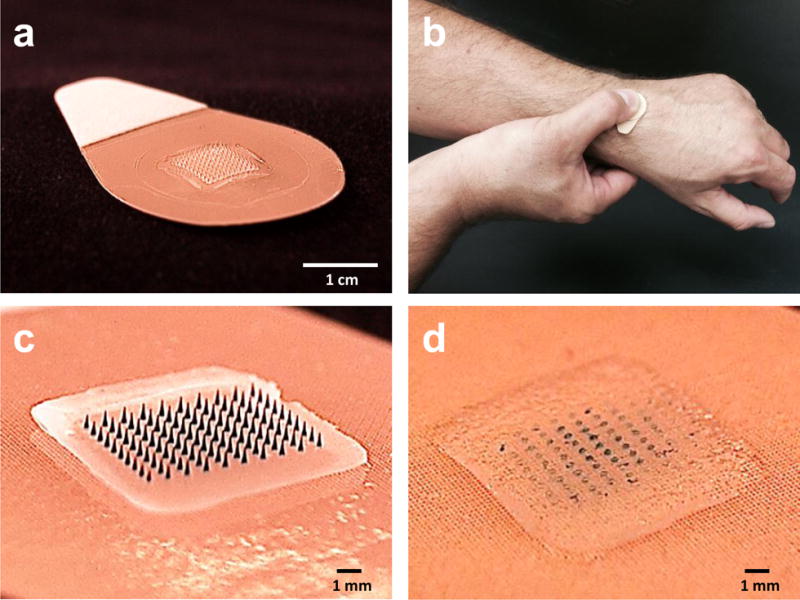
Microneedle patch (MNP) for influenza vaccination. (a) The MNP contains an array of 100 microneedles measuring 650 μm tall that is mounted on an adhesive backing. (b) The MNP is manually administered to the wrist, enabling self-administration by study subjects. (c) Microneedles encapsulate influenza vaccine (represented here by blue dye) within a water-soluble matrix. (d) After application to the skin, the microneedles dissolve, thereby depositing vaccine in the skin and leaving behind a patch backing that can be discarded as non-sharps waste.
IMIIV was administered by hypodermic needle in the deltoid muscle of the arm preferred by the participant and the MNPs were applied for 20 minutes to the dorsal aspect of the wrist of the non-dominant arm.
For the MNPIIV-self group, instructions were provided using a brief audiovisual presentation, and participants applied the patch under the unblinded HCW’s supervision, but without physical intervention. Snap components were incorporated into the back of MNPs to guide MNP application by providing audible and tactile feedback to the user when sufficient force was applied.
After study product administration on day 0, participants were assessed on days 2, 8, 28, 56, and 180. Solicited injection-site and systemic reactogenicity events were collected for 7 days after study product administration using a subject’s diary and by subject interview and examination by study staff. Unsolicited adverse events were collected for 28 days. Serious adverse events, new onset of chronic medical conditions and concomitant medication use were collected for the duration of the study. Grading of adverse events was based on Food and Drug Administration toxicity grading.20 Blood samples were obtained at all 6 clinic visits for safety and/or immunogenicity testing. Hemagglutination inhibition (HAI) assays were performed by blinded Hope Clinic Laboratory staff on samples from day 0, 28, and 180 for all three influenza strains. For these assays, the H1N1 virus reference strain was obtained from the National Institute for Biological Standards and Control (Potters Bar, Hertfordshire, United Kingdom). The H3N2 and B virus reference strains were obtained from the Influenza Reagent Resource of the Centers for Disease Control and Prevention (Atlanta, GA). These reference strains are the ones contained in the 2014–2015 trivalent influenza vaccine. Influenza viruses were propagated in MDCK.2 cells and MDCK.2 SIAT1 cells in the presence of TPCK trypsin (Sigma Aldrich, Saint Louis, MO). HAI assays were performed and described in the WHO Influenza Surveillance Network laboratory manual.23 Sera were treated overnight with receptor destroying enzyme (RDE) (Sigma Aldrich) at 37°C, inactivated at 56° C for 30 minutes, and diluted in PBS for an initial dilution of 1:10. A 0.5% solution of Turkey red blood cells (RBCs) (Fisher, Hampton, NH) was prepared in HA buffer (Becton Dickinson, Franklin Lakes, NJ). RDE and diluted sera were tested for non-specific agglutination, and if activity was detected, they were pre-absorbed with RBCs. HA titers of H1, H3, and B viruses were determined with turkey RBCs, diluted to 8 HA units per 50 μl, and back titrated to confirm the dilution titer. Treated and diluted sera were serially diluted 2-fold in HA buffer, mixed with 4 HA units of virus, and incubated at room temperature for 30 minutes. After incubation, turkey RBCs were added, mixed, and incubated at room temperature for 30 minutes. Hemagglutination or inhibition was then recorded for each serum dilution and virus mixture. HAI titers were summarized as geometric mean titers (GMT), seroprotection and seroconversion rates.
Participants completed questionnaires at 0, 8, and 28 days after enrollment to assess vaccination acceptability outcomes via continuous scales (0–10 likelihood). We also measured vaccination knowledge, attitudes, perceptions, and beliefs using Likert-type scales (1–5 agreement levels).
Outcomes
Primary safety outcome measures are the incidence of study product-related serious adverse events within 180 days, grade 3 solicited or unsolicited adverse events within 28 days and solicited injection site and systemic reactogenicity on the day of study product administration through 7 days after administration.
Secondary safety outcome measures are the incidence of new-onset chronic illnesses within 180 days and unsolicited adverse events within 28 days from enrollment. Secondary immunogenicity outcome measures are the geometric mean titer (GMT) of HAI antibody, proportion of subjects achieving seroprotection (defined as a HAI antibody titer of 1:40 or greater) and percentage of subjects achieving seroconversion (defined as either a pre-vaccination HAI titer <1:10 and a post-vaccination HAI titer ≥1:40, or a pre-vaccination HAI titer ≥1:10 and a minimum four-fold rise in post-vaccination HAI antibody titer) approximately 28 days following receipt of study products in the following groups MNPIIV-HCW and IMIIV.
Exploratory immunogenicity outcome measures are the GMT, seroprotection and seroconversion within 28 days and 180 days between each of the MNPIIV groups and between the MNPIIV groups and the IMIIV group.
Another exploratory outcome measure is the preference for administration method of future influenza vaccination as determined by written survey by study participants on days 0, 8 and 28.
Statistical analysis
For the primary safety endpoint, with 25 subjects per group, if the true adverse event rate was 5% (10%), we would have 34% (38%) chance to observe one AE and 12% (34%) chance to observe more. For secondary immunogenicity endpoints, the sample size of 25 per group allowed 80% power to detect a difference of 1.2 (Cohen’s d) in HAI GMT between groups at the alpha level of 0.05 using a two-sided t-test. The sample size confers 80% power to detect difference in seroconversion rate between a vaccine group and the placebo group when the difference in proportions is 0.42 or higher. These effect sizes are powered for the comparison between a vaccinated group and the placebo group, but not for the comparison between the vaccinated groups (e.g., non-inferiority test between MNPIIV and IMIIV), which are not the primary aims of this study. Descriptive data are presented for reactogenicity, safety, acceptability and immunogenicity. The reactogenicity, safety and acceptability populations included all participants who received a study product. The immunogenicity population included all participants who provided serum samples at baseline and at least 28 or 180 days after study product administration. The 95% confidence interval of GMT was calculated based on normal distribution of log-transformed data, and the Clopper-Pearson exact confidence interval was calculated for seroprotection, seroconversion, adverse events (AEs) and acceptability rates in each group. The Wilcoxon test was used to compare GMT of each vaccinated group with the placebo group, and Fisher’s exact test was used to compare the frequencies of seroprotection, seroconversion and AEs rate between each vaccinated group and the placebo group. The frequencies of AEs were compared between the four groups using Fisher’s exact test. Analyses were performed using the R statistical software version 3.2.2.24 ANOVA and correlational analyses were used for the acceptability assessments (SAS 9.2, Cary, NC). An independent safety monitor oversaw the safety of the study. The trial was registered with ClinicalTrials.gov (NCT02438423).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The MNP developer provided MNP patches and was involved in discussions of study design; in study monitoring; and training unblinded clinic staff on MNP application. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for journal publication.
Results
Between June 23 and September 25, 2015, 100 participants were enrolled, underwent randomization, and received study product administration (Table 1). The demographics of the four groups were similar. Five participants (with 3 in the placebo group) missed either the day 28 or the day 180 visit (Figure 2).
Table 1.
Demographic Characteristics of participants*
| Characteristics | IMIIV | MNPIIV-HCW | MNPIIV-self | MNPplacebo | All |
|---|---|---|---|---|---|
|
| |||||
| Age, year | |||||
| Mean | 29.6+/− 6.9 | 27.4+/−5.9 | 31.4+/−8.4 | 29.3+/−8.4 | 29.4+/−7.5 |
| Median | 29 | 26 | 26 | 26 | 26 |
| Range | 21–49 | 18–43 | 22–47 | 21–49 | 18–49 |
|
| |||||
| Sex, no. (%) | |||||
| Male | 14 (56%) | 13 (52%) | 13 (52%) | 13 (52%) | 53 (53%) |
| Female | 11 (44%) | 12 (48%) | 12 (48%) | 12 (48%) | 47 (47%) |
|
| |||||
| Ethnic Origin**, no. (%) | |||||
| White | 12 (48%) | 11 (44%) | 14 (56%) | 12 (48%) | 49 (49%) |
| Black | 8 (32%) | 8 (32%) | 8 (32%) | 7 (28%) | 31 (31%) |
| Other | 5 (20%) | 6 (24%) | 3 (12%) | 6 (24%) | 20 (20%) |
|
| |||||
| BMI, kg/m2 | |||||
| Mean | 24.9 +/− 4.3 | 25.0+/−4.5 | 25.7+/−4.0 | 24.6+/−4.6 | 25.0+/−4.3 |
| Median | 24.8 | 24.5 | 25.7 | 23.8 | 24.6 |
| Range | 17.4–34.2 | 19.2–35.0 | 18.7–34.2 | 18.4–34.1 | 17.1–35.0 |
|
| |||||
| Prior IIV | |||||
| 2013–2014 season, no. (%) | 4 (16%) | 6 (24%) | 7 (28%) | 6 (24%) | 23 (23%) |
| 2012–2013 season, no. (%) | 6 (24%) | 3 (12%) | 6 (24%) | 4 (16%) | 19 (19%) |
| Any of these 2 seasons, no. (%) | 8 (32%) | 7 (28%) | 9 (36%) | 9 (36%) | 33 (33%) |
BMI: body mass index
IIV: inactivated influenza vaccine
Plus–minus values are means+/− SD.
All participants were vaccinated between June 23 and September 25, 2015
Ethnic origin was self-reported.
Figure 2.
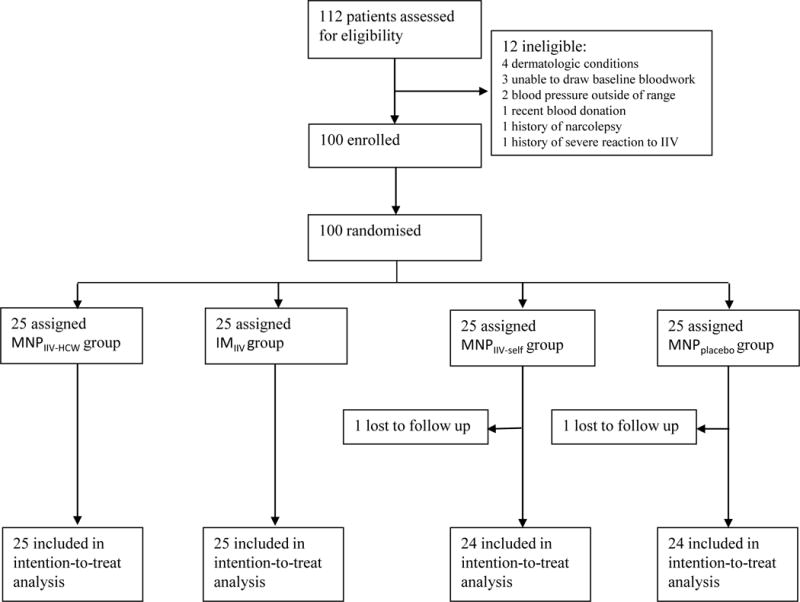
Trial profile.
No serious adverse events related to the study products were reported during the study. Stopping rules were not triggered, and there were no withdrawals because of adverse events.
Reactogenicity events observed in the MNPIIV-HCW and the MNPIIV-self groups were similar and mostly mild (p=0.2). Compared to either of the MNPIIV groups, the IMIIV group had two-fold higher incidence of grade 2 and 3 reactogenicity events (2% (95%CI, 0–11%) [1/50] vs 12% (95%CI, 26–31%) [3/25] respectively (p=0.02). Significantly more local reactogenicity events (Figure 3a) were observed in the MNPIIV groups than the IMIIV group, for pruritus (82% (95%CI, 69–91%) [41/50] vs 16% (95%CI, 5–36%) [4/25], p<0.0001) and erythema (40% (95%CI, 26–55%) [20/50] vs 0% (95%CI, 0–14%) [0/25], p=0.0002) (Figure 4). The most common vaccination site reaction for the two MNPIIV groups was pruritus; 87% [36/41] of these reactions were mild and self-limited, lasting 2–3 days on average. In the IMIIV group, injection site pain reported over the days following vaccination was twice as frequent (44% (95%CI, 24–65%) [11/25] vs 20% (95%CI, 10–34%) [10/50], p=005) and more severe (≥ grade 2) (12% (95%CI, 3– 31%) [3/25] vs 2% (95%CI, 0–10%) [1/50], p=0.1) compared to the MNPIIV groups combined. The rate and severity of systemic reactogenicity events (Figure 3b) did not differ among the groups receiving IIV.
Figure 3.
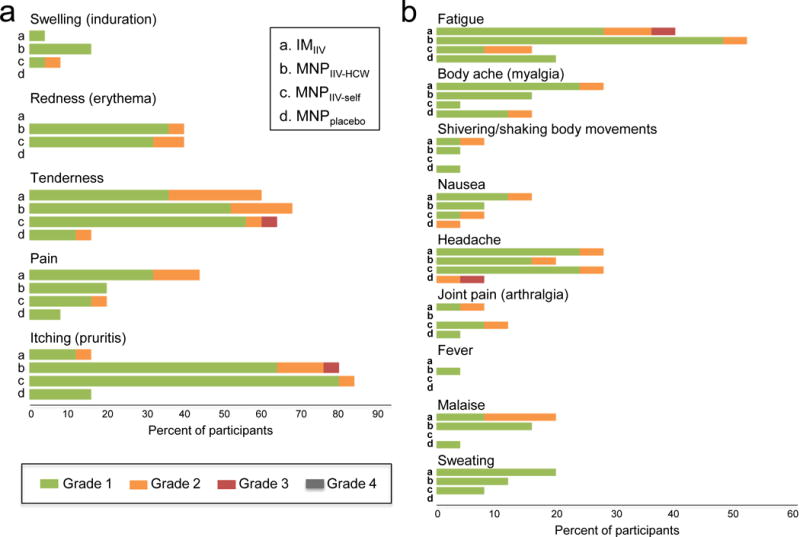
Solicited reports of adverse events 7 days after vaccination. Local (a) and systemic (b) adverse events associated with vaccination are shown in different groups.
Figure 4.
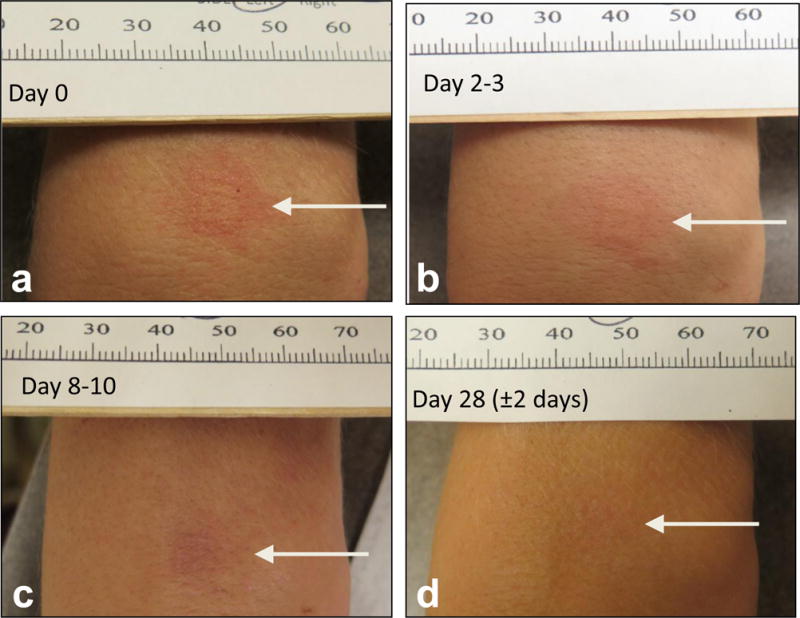
Typical local reaction seen with MNPIIV application after patch removal at day 0 (a), at day 2–3 (b), day 8–10 (c), day 28 after vaccination (d).
No new chronic medical illnesses or influenza-like illnesses were reported. Sixty-one unsolicited adverse events after study product administration were reported by 41% [41/100] of participants. Few unrelated grade 3 events were observed. One participant in the MNPIIV-self group developed acute enteritis requiring hospitalization, and another participant in the MNPplacebo group developed grade 3 hypertension while off her hypertensive medications. One participant in the MNPIIV-self group had rhabdomyolysis due to strenuous exercise at baseline prior to receipt of study product and another participant in the IMIIV group had a grade 3 elevation in liver function test due to exercise and excessive alcohol and acetaminophen consumption 30 days after vaccination. These laboratory abnormalities resolved spontaneously. There were 13 related AEs (7 in MNPplacebo group, 3 in IMIIV group and 3 in MNPIIV-HCW group) observed in 8 participants. These AEs were mostly grade 1 laboratory events (thrombocytopenia, leukopenia, and neutropenia), all of which resolved during study follow up.
The geometric mean titers determined by HAI antibody assay were comparable at day 28 between the MNPIIV-HCW and the IMIIV groups with values of 1197 (95% CI, 855– 1675) and 997 (95% CI, 703–1415) (p=0.5), respectively, for the H1N1 strain; 287 (95% CI, 192–430) and 223 (95% CI, 160–312) (p=0.4), respectively, for the H3N2 strain and 126 (95% CI, 86–184) and 94 (95% CI, 73–122) (p=006), respectively, for the B strain. GMT titers similar to these were observed in the MNPIIV-self group (see Supporting Information Table S1).
When comparing immune response in the MNPIIV-HCW and the IMIIV groups, seroprotection and seroconversion rates at day 28 were comparable and significantly higher for all three strains contained in the influenza vaccine groups compared to placebo (p<0.01) ((see Supporting Information Table S1 and Figure 5) (with the exception of a similar day 28 seroprotection rate against H3N2 influenza strain between the 3 groups). There was a higher seroconversion rate against the B strain for the MNPIIV-HCW and MNPIIV-self groups combined (65% (95%CI, 60–78%) [31/48]) compared to the IMIIV group (32% (95%CI, 15–54%) [8/25]) (p=0.01). Seroprotection against the three influenza strains 6 months after vaccination was seen in 83–100% [20 to 24/24] of participants in the MNPIIV-HCW group as well as 80–100% [20 to 25/25] in the IMIIV group. The MNPIIV-self group had similar seroprotection with 75–100% [18 to 24/24] of participants having a HAI titer of 1:40 or above at 180 days later (see Supporting Information Table S1).
Figure 5.
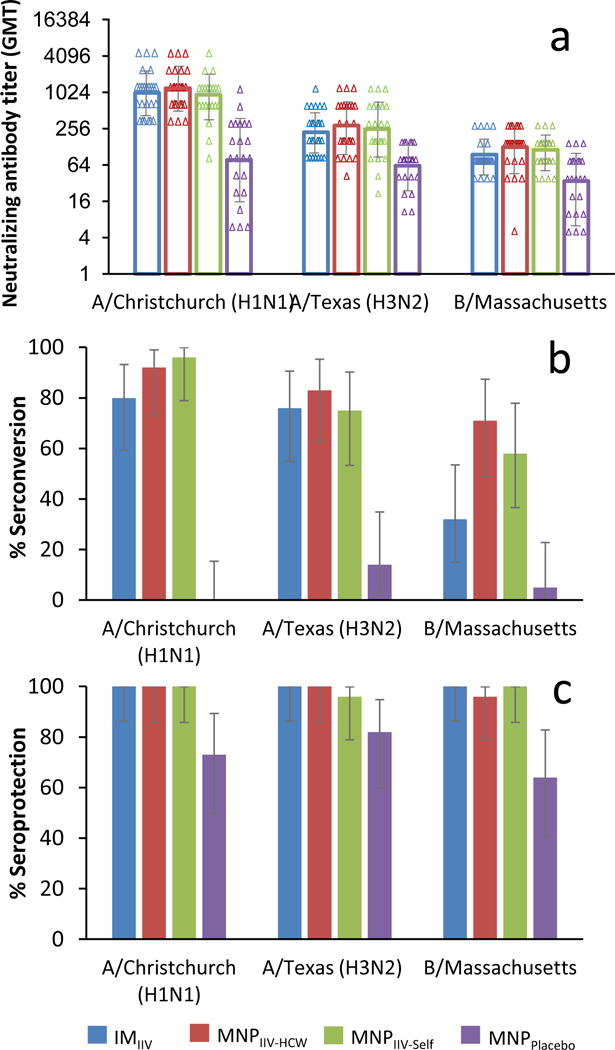
Hemagglutination inhibition (HAI) geometric mean titers (GMT) (log 2) (a), seroconversion rate (b) and seroprotection rate (c) against A/Christchurch/16/2010, NIB-74 (H1N1), A/Texas/50/2012, NYMC X-223 (H3N2), B/Massachusetts/2/2012, NYMC BX-51(B) strains for MNPIIV-HCW, MNPIIV-self, MNPplacebo, IMIIV 28 days after vaccination with 95% confidence interval (CI; vertical bars).
IM vaccination delivered at least 15 μg of each influenza antigen. Measurement of residual antigens in the 50 MNPIIV patches used in the study showed that the average dose (± standard error) delivered by MNPIIV was 11.3 ± 0.5 μg, 14.4 ± 0.5 μg, and 13.1 ± 0.4 μg for H1N1, H3N2, and B strains, respectively. No significant difference was observed between the dose of each strain delivered by the MNPIIV-HCW and MNPIIV-self groups (p>0.60), indicating that the participants were able to correctly self-administer MNPs. After vaccination, imaging of used MNPs showed that the microneedles had dissolved in the skin (Figure 1d), suggesting that the used patches could be discarded as non-sharps waste. After storage in desiccated packaging at 5, 25, and 40°C for 12 months, IIV potency for all three strains in the MNPIIV remained within product specifications in the IND (see Supporting Information Figure S1), which supports the storage of patches without refrigeration.
Right after vaccination, 96% (95% CI, 86–100%) [48/50] of participants who received MNPIIV reported no pain during MNP application, but only 82% (95% CI, 60–95%) [18/22] of participants reported that IM injection was painless (p=0.04). On a scale of 1 (negative experience) to 5 (positive experience), participants in the MNP groups reported high acceptability for MNP vaccination, with mean scores between 4.5 and 4.8 across the three MNP groups. Those receiving IMIIV reported a mean score 4.4, which was not significantly different between the IM and MNP groups (p=0.07; see Supporting Information Table S2). When asked on Day 28 (i.e., thereby assessing the complete vaccination and post-vaccination experience), ≥70% (95% CI, 55–83%) [33/47] of MNPIIV recipients preferred MNP vaccination over IM or intranasal vaccination (19% (95% CI, 9–33%) [9/47] as a delivery method for future influenza vaccination (p<0.0001) (see Supporting Information Table S3), indicating a positive experience with MNP vaccination.
Discussion
This study shows for the first time in a human clinical trial that influenza vaccination using a MNP was well-tolerated, immunogenic, and preferred after a single-dose vaccination administered by HCW or by the participants themselves.
MNPs were well tolerated without any safety issues detected in this phase 1 study, although certain local, mild, self-limited reactions were more commonly observed with MNPIIV compared to IMIIV. The higher rates of these local events are consistent with prior experience with intradermal influenza vaccination.25,26 Observed reactions in skin could reflect a local immune response that is visible on the skin surface. Pain was more commonly reported in muscle after IMIIV vaccination at rates consistent with prior experience.27
Both MNPIIV groups met all FDA immunogenicity criteria for licensure28 for all strains, except the B strain lower bound confidence interval (CI) criterion for seroconversion for the MNPIIV-self group ((see Supporting Information). This weaker response to the B strain was also observed in the IMIIV group (which failed to meet lower bound confidence interval criteria for seroconversion and seroprotection), similar to prior studies of influenza vaccination.25 These findings are consistent with prior animal studies showing strong immune responses to skin vaccination using MNPs.9,10 While the pre-clinical studies in naïve animals have in some cases shown superior immunogenicity and efficacy after MNP vaccination (e.g., due to targeting of antigen-presenting cells in the skin), this human trial was not powered to show such differences.
In our population, MNPs were well accepted and strongly preferred over traditional IM injection for influenza vaccination, consistent with previous results.15 This finding may be significant, because increased acceptability could enable increased rates of influenza vaccination, which are currently less than 50% among adult populations.3 Moreover, because participants were able to self-vaccinate and preferred it in ≥70% of participants, there could be significant cost savings enabled by MNPs, due to reduction in HCW time devoted to vaccination.
There is limited prior experience with MNPs in human participants in the published literature. Parathyroid hormone has been administered in clinical trials using non-dissolvable, metal MNPs, and has shown good safety and efficacy.17–19 A prior study examined influenza vaccination using a dissolving MNP, but did not include self-administration or a negative control group.28 That study also differed from the present study in that MNPs were worn for 6 h, microneedles were difficult to insert into the skin such that only 37% of MNPs delivered at least half of the vaccine on the first patch application, local skin reactions were more pronounced (e.g., purpura, pigmentation, and longer-lasting erythema), and MNPs were not stable during extended storage at elevated temperature.
Our study showed a number of advantages compared to other studies on self-administration of influenza vaccine by intradermal injection or nasal spray (currently not recommended by the Advisory Committee on Immunization Practices for the 2016–2017 influenza season in the USA).29,30 In our study, self-immunization by MNP was achieved in all participants with only brief training using audiovisual materials and without HCW intervention. The participants also had no specific medical background and therefore reflected the general adult population.
Self-administration using MNPs may be further facilitated by the strong patient acceptance and preference of MNPs as well as the lack of sharps waste; lack of refrigerated storage; generally painless vaccination and only infrequent, minor pain afterwards; small package size; and expected cost-savings. In the future, self-vaccination using MNPs could occur in a clinical or workplace vaccination setting with HCW supervision, at home after purchasing at the pharmacy or after distribution by mail in a pandemic scenario.
There are several limitations of the study. The subjects enrolled were probably less inclined to receive influenza vaccination by hypodermic injection since only those who did not receive the 2014–2015 influenza vaccine were included in the study. Other comparative arms were considered for the study (such as intranasal and intradermal injection), however we elected, for logistical reasons, to focus on comparing delivery by MNP to the most widely approved administration method (i.e., IM injection). The study population had very high titers at baseline prior to vaccination, which makes differentiating the effects of the different routes of administration difficult (e.g., only the B strain showed a significant difference between IMIIV and MNPIIV immune response, possibly because it had the lowest pre-vaccination titers). A detailed analysis of the immunologic mechanisms of MNPIIV is needed. In our study, additional blood samples were collected in a subset of participants for exploratory outcome measures of immune response; their analysis will be the subject of a future publication. Additional studies testing the acceptability and reliability of MNP self-application in larger populations are also warranted. The next generation of MNP formulation may be optimized to further reduce local reactogenicity and increase delivery efficiency. Larger human trials are needed to confirm the findings of this study with greater power.
We conclude that influenza vaccination using MNPs is well tolerated, well accepted, and results in robust immunologic responses, whether administered by HCW or by the participants themselves. These results provide evidence that MNP vaccination is an innovative new approach with the potential to improve current vaccination coverage and reduce immunization costs.
Panel: research in context
Evidence before this study
There have been a number of studies on intradermal influenza vaccination using a hollow microneedle injection, and there is an approved product using this approach (e.g., Fluzone Intradermal Quadrivalent Influenza Vaccine, Sanofi Pasteur). Although safe and effective, this microneedle device is not dissolvable (i.e., does not eliminate sharps waste), is not thermostable outside the cold chain and requires administration by trained healthcare providers. A dissolvable microneedle patch for influenza vaccination was recently assessed in a small clinical study. This study did not include self-administration or a negative control group, it required multiple vaccinations and data from 63% of study participants were discarded due to product failure. No additional human studies were found after searching PubMed for reports published with the terms “influenza vaccine” and “microneedle”.
Added value of this study
This first-in-humans study shows that the use of a single dissolvable microneedle patch for influenza vaccination was well-tolerated, resulted in robust antibody responses and was preferred over conventional influenza vaccination using needles and syringes. It also shows that the microneedle patches were reliably self-administered by study participants, were stable for at least one year at 40°C and generated no sharps waste.
Implications of all the available evidence
This study suggests that dissolvable microneedle patches could simplify delivery of influenza vaccines, thereby enabling distribution and storage outside the cold chain (e.g., on the pharmacy shelf), disposal as non-sharps waste and possible self-administration under medical supervision or possibly at home. These advances could reduce cost of influenza vaccination, increase patient access to influenza vaccine and thereby increase vaccination coverage and protection from influenza morbidity and mortality. Animal studies have shown improved immunogenicity of influenza vaccination by microneedle patch compared to intramuscular injection; although the present study was not powered or designed to show it, future clinical studies may similarly show that influenza vaccination by microneedle patch also enhances immune response. Once confirmed by larger trials, the use of microneedle patches for influenza vaccination can have major public health implications on vaccination coverage and protection from disease.
Supplementary Material
Acknowledgments
Olivia Antao, Jaya Arya, Kristy Bachman, Dawn Battle, Mary Bower, Elaine Brock, Yunmi Chung, Diane Choi, Laura Clegg, Justin Colwell, Lyngail Cooper, Ellen DeStefano, Briyana Domjahn, Francine Dyer, Vickie Grimes, Xin Dong Guo, Mari Hart, Melanie Hartsough, Dennis Hannigan, Sarah Lapp, Jamie MacKelfresh, Amy Morris, Melinda Ogilvie, Eileen Osinski, Peter Patriarca, Emily Presmanes, Courtney Purcell, Madison Raese, Bruce Ribner, Marly Richter-Roche, Nayoka Rimann, David Rimland, Jo Ann Sadowski, Lynn Schaich, Jay Schamel, Mark Schwade, Vidisha Singh, Talib Sirajud-Deen, Pamela Turner, Andrea Trementozzi, Tiffany Wilson, Jianguo Xu, Wells Yang.
Funding: This study was funded by a grant from the National Institute of Biomedical Imaging and Bioengineering (U01 EB012495); SK received funding from a training grant from the National Institute of Allergy and Infectious Diseases (T32 AI074492); and instrumentation support was provided to MJM by the Georgia Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registry number NCT02438423
Conflict of Interest:
SH, DVM and MRP are inventors on licensed patents and have ownership interest in companies developing microneedle products (Micron Biomedical). SH, DVM and WP are currently employed by Micron Biomedical. These potential conflicts of interest have been disclosed and are overseen by Georgia Institute of Technology and Emory University.
All other authors declare no conflict.
Contributors
All authors contributed to the study concept and design, and approved the final version of the report. NGR wrote the manuscript with significant contributions from MRP and MJM. NGR, MP, PMF and TW analyzed and interpreted data. NGR, MP, RM, SK, MJM and TIV-MNP 2015 Study Group collected clinical data. DVM, HK and WP prepared and analyzed microneedle patches. NJT, LL and EVV optimized and performed immunologic assays. NGR and MJM supervised the study. MP and TY provided statistical analyses. NGR and MRP did the literature search.
Contributor Information
Nadine G Rouphael, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Michele Paine, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Regina Mosley, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Sebastien Henry, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States of America.
Devin V McAllister, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States of America.
Haripriya Kalluri, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States of America.
Winston Pewin, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States of America.
Paula M Frew, Division of Infectious Diseases, Emory University, Atlanta, Georgia, United States of AmericaRollins School of Public Health, Emory University, Atlanta, Georgia, United States of America.
Tianwei Yu, Rollins School of Public Health, Emory University, Atlanta, Georgia, United States of America.
Natalie J. Thornburg, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Sarah Kabbani, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Lilin Lai, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Elena V Vassilieva, Department of Microbiology and Immunology and Emory Vaccine Center, Emory University, Atlanta, Georgia, United States of America.
Ioanna Skountzou, Department of Microbiology and Immunology and Emory Vaccine Center, Emory University, Atlanta, Georgia, United States of America.
Richard W Compans, Department of Microbiology and Immunology and Emory Vaccine Center, Emory University, Atlanta, Georgia, United States of America.
Mark J Mulligan, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States of America.
Mark R Prausnitz, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States of America.
References
- 1.http://www.cdc.gov/mmwr/preview/mmwrhtml/rr59e0729a1.htm
- 2.Estimates of deaths associated with seasonal influenza — United States 1976–2007. MMWR Morbidity and mortality weekly report. 2010;59:1057–62. [PubMed] [Google Scholar]
- 3.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of Vaccination Coverage Among Adult Populations - United States, 2014. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 2016;65:1–36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 4.Lee BY, Bartsch SM, Mvundura M, et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine. 2015;33:4727–36. doi: 10.1016/j.vaccine.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. The Lancet Infectious diseases. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 6.http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
- 7.Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Hung IF, Levin Y, To KK, et al. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine. 2012;30:6427–35. doi: 10.1016/j.vaccine.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Skountzou I, Compans RW. Skin immunization with influenza vaccines. Current topics in microbiology and immunology. 2015;386:343–69. doi: 10.1007/82_2014_407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Human vaccines & immunotherapeutics. 2016:0. doi: 10.1080/21645515.2016.1171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Bednarczyk RA, Chu SL, Sickler H, Shaw J, Nadeau JA, McNutt LA. Low uptake of influenza vaccine among university students: evaluating predictors beyond cost and safety concerns. Vaccine. 2015;33:1659–63. doi: 10.1016/j.vaccine.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Prematunge C, Corace K, McCarthy A, Nair RC, Pugsley R, Garber G. Factors influencing pandemic influenza vaccination of healthcare workers–a systematic review. Vaccine. 2012;30:4733–43. doi: 10.1016/j.vaccine.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. Journal of controlled release : official journal of the Controlled Release Society. 2015 doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrudden MT, McAlister E, Courtenay AJ, Gonzalez-Vazquez P, Singh TR, Donnelly RF. Microneedle applications in improving skin appearance. Experimental dermatology. 2015;24:561–6. doi: 10.1111/exd.12723. [DOI] [PubMed] [Google Scholar]
- 17.Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1–34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharmaceutical research. 2011;28:159–65. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 18.Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Therapeutic delivery. 2012;3:357–71. doi: 10.4155/tde.12.13. [DOI] [PubMed] [Google Scholar]
- 19.Hirobe S, Azukizawa H, Hanafusa T, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–8. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm
- 21.Williams MS. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Vet Microbiology. 1993;37:253–62. doi: 10.1016/0378-1135(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 22.Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP, Pulit-Penaloza JA, Prausnitz MR, Compans RW, Skountzou I. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature, Drug Delivery and Translational Research. 2015;5(4):360–71. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. 2011:1–139. [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. URL https://www.R-project.org/ [Google Scholar]
- 25.Kieninger D, Sheldon E, Lin WY, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged >/=18 years. BMC infectious diseases. 2013;13:343. doi: 10.1186/1471-2334-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. The New England journal of medicine. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 27.http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM123694.pdf
- 28.Hirobe S, Azukizawa H, Matsuo K, et al. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharmaceutical research. 2013;30:2664–74. doi: 10.1007/s11095-013-1092-6. [DOI] [PubMed] [Google Scholar]
- 29.Coleman BL, et al. A randomized control trial comparing immunogenicity, safety, and preference for self-versus nurse-administered intradermal influenza vaccine. Vaccine. 2012;30:6287–93. doi: 10.1016/j.vaccine.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Burgess TH, et al. Self-administration of intranasal influenza vaccine: Immunogenicity and volunteer acceptance. Vaccine. 2015;33:3894–9. doi: 10.1016/j.vaccine.2015.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


