Abstract
Synucleinopathies, including Parkinson’s disease (PD), are associated with the misfolding and mistrafficking of alpha-synuclein (α-syn). Here, using an ascorbate peroxidase (APEX)-based labeling method combined with mass spectrometry, we defined a network of proteins in the immediate vicinity of α-syn in living neurons to shed light on α-syn function. This approach identified 225 proteins, including synaptic proteins, proteins involved in endocytic vesicle trafficking, the retromer complex, phosphatases and mRNA binding proteins. Many were in complexes with α-syn, and some were encoded by genes known to be risk factors for PD and other neurodegenerative diseases. Endocytic trafficking and mRNA translation proteins within this spatial α-syn map overlapped with genetic modifiers of α-syn toxicity, developed in an accompanying study (Khurana et al, 2016). Our data suggest that perturbation of these particular pathways is directly related to the spatial localization of α-syn within the cell. These approaches provide new avenues to systematically examine protein function and pathology in living cells.
Introduction
Neurodegenerative diseases are characterized by the loss of distinct neuronal populations. In most of these diseases, a critical event leading to this neuronal loss is the abnormal folding and mis-trafficking of proteins. Different diseases are defined by the particular culprit proteins and the specific cell-types affected. For example, abnormal intraneuronal accumulation of the protein α-synuclein (α-syn) defines the synucleinopathies, a group of diseases that includes Parkinson’s disease (PD) and dementia with Lewy bodies (DLB)(Goedert et al. 2013). In these diseases, midbrain dopaminergic and cortical glutamatergic neurons are among the neuronal populations that prominently degenerate; their degeneration is associated with clinically important phenotypes. α-syn is encoded by the SNCA gene. The identification in PD of mutations and polymorphisms at the SNCA gene locus has causally tied the misfolding of this protein to disease (Shulman et al. 2010).
α-syn is a highly abundant neuronal protein. Early studies localized α-syn predominantly to the pre-synaptic terminal (Maroteaux et al. 1988). Subsequent studies have concentrated on this synaptic localization, and its apposition to synaptic vesicles(Auluck et al. 2010). Abundant evidence now indicates that α-syn is found on the cytosolic face of vesicles and adopts an amphipathic conformation upon binding phospholipid membranes (Auluck et al. 2010; Davidson et al. 1998; Mizuno et al. 2012). Its localization to other cellular compartments, including the nucleus, remains relatively under-explored but may be relevant to disease (Kontopoulos et al. 2006). Beyond neurons, α-syn is also abundant in other cell types, notably red blood cells (Nakai et al. 2007). While its biological function remains far from fully known, findings in cellular models from yeast to neurons have coalesced around important roles for α-syn in vesicle trafficking and synaptic physiology(Greten-Harrison et al. 2010; Chandra et al. 2005; Auluck et al. 2010; Vargas et al. 2014; Scott et al. 2010; Gitler et al. 2008). These studies have suggested that the normal physiological function of the protein is related to its cellular pathology. Thus, knowledge of α-syn localization and native interactions may also shed light on disease mechanisms when the protein misfolds and mis-traffics.
The elucidation of a protein’s physical interactions can provide essential clues to its function. For α-syn, a number of complementary approaches have been used to identify around 300 physical interactions (http://thebiogrid.org/112506/summary/homo-sapiens/snca.html). First, co-immunoprecipitation has been used in conjunction with mass spectrometry to delineate diverse protein-protein associations(McFarland et al. 2008). While this methodology is unbiased and high-throughput, it depends on lysis of cells that disrupts the native cellular context. Moreover, only stable, protein complexes are recovered by standard co-immunoprecipitation techniques. Second, low-throughput candidate-based experiments have validated biochemical interactions between α-syn and numerous other proteins, and demonstrated that specific interactions with α-syn are required for specific physiological functions. These include the identification of interactions between the C-terminus of α-syn and SNARE proteins such as Synaptobrevin-2/VAMP2 and SNAP-25(Sharma et al. 2012). Complemetary α-syn knockout experiments suggest a role for α-syn in SNARE complex assembly during exocytosis (Burré et al. 2014; Burré et al. 2010) and subsequent studies have extended this function to multiple steps in vesicle trafficking, including endocytosis(Vargas et al. 2014). Additional low-throughput interaction studies, including reconstitution in multi-protein complexes, have revealed biochemical interactions between α-syn and other proteins implicated in Parkinson’s disease, including the microtubule-associated protein Tau (encoded by the MAPT gene)(Jensen et al. 1999) and Parkin (Choi et al. 2001). Finally, it is worth noting that conventional yeast-two-hybrid technologies – in which a prey and bait protein must interact in the nucleus of yeast cells for a positive readout of the assay – have been largely unsuccessful in recovering any interactions for α-syn (Engelender et al. 1999), likely because meaningful interactions for a membrane-associated protein like α-syn may critically depend on subcellular context.
In this study, we built on previous work and defined the α–syn’s physiologic compartments within the cell and its protein-protein interaction network within its native environment using a chemical biology approach. In the presence of H2O2, ascorbate peroxidase (APEX) oxidizes numerous phenol derivatives to phenoxyl radicals. Such radicals are short-lived (<1 msec) and can covalently react with electron-rich amino acids such as Tyr within their small labeling radius (<10 nm). This chemistry combined with mass spectrometry was recently exploited to define the proteome of organelles, including the mitochondrial matrix (Rhee et al. 2013) and intermembrane space (Hung et al. 2014). The overarching advantage of this technique is that the labeling is performed when the cell is still alive, with all membranes and protein-protein interactions preserved, including transient interactions.
Rather than using the APEX tag-based methodology to define the proteome of an organelle or cellular compartment, we used it here to define the proteins in the immediate vicinity of α-syn in mammalian cells, including primary neurons. We then used two orthogonal assays (co-immunoprecipitation and membrane-2-hybrid [MYTH] (Stagljar et al. 1998)) to ask whether proteins identified through APEX interacted with α-syn in physical complexes. In order to address the significance of these proteins and their functional compartments to α-syn proteotoxicity, we compared them to a comprehensive genetic network generated through unbiased genome-wide screens against α-syn toxicity (accompanying manuscript Khurana, Chung et al. 2016). In sum, the use of multiple orthogonal approaches in this study defines the intrinsic localization and protein interaction network of α-syn, and their intimate relationship to its proteotoxicity.
Results
APEX defines the in situ proteome of α–syn in neurons
We first tested the APEX-based approach for defining α-syn’s protein neighborood in HEK-293 cells. The data from HEK-293 cells confirmed that APEX could yield useful information on the localization, neighboring proteome and protein-protein interactions of α-syn in living cells (see Fig. S1, Fig. S2 and Table S1 for complete details).
The misfolding of α-syn causes pathology in neurons, including loss of dopaminergic and cortical neurons. We thus optimized the APEX technique in this more disease-relevant cell type. We utilized primary rat cortical neurons that can be cultured in high abundance at relatively high levels of consistency and homogeneity compared to dopaminergic neurons. We used a “second-generation” APEX with improved catalytic efficiency enzyme (APEX2) (Lam et al. 2015) that is optimized for robust labeling in live neurons when expressed under the synapsin promoter. We tagged APEX2 to the C-terminal, cytosol facing end of α–syn, allowing the enzyme to be directed to the native cellular environment of α–syn (Fig. 1A).
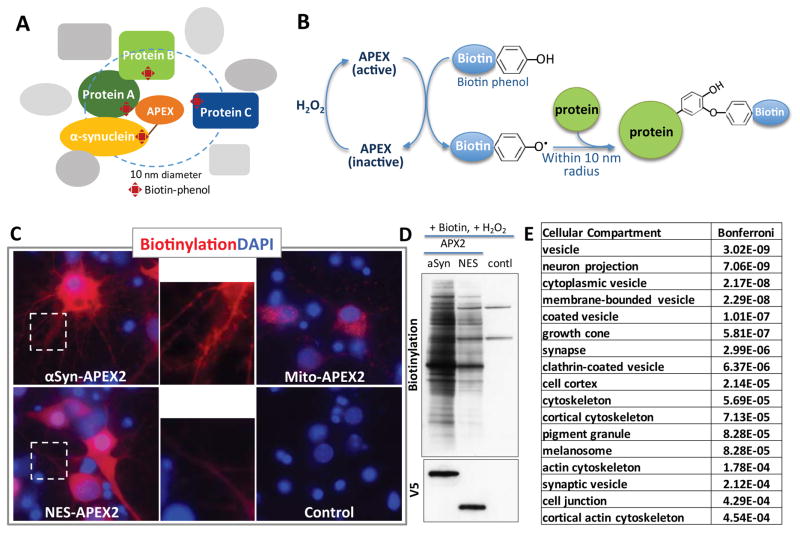
Fig. 1. α syn-APEX2-labeled proteins in primary rat cortical neurons.
(A) Schematic diagram of α–syn-APEX depicting a genetically engineered peroxidase APEX tagged to the C-terminal of wild-type α-syn. Labeled proteins are within an approximately 10nm distance of the tag. Some of these will be in complexes with α-syn. (B) With a short pulse of H2O2, APEX becomes activated and creates a free radical from biotin tyramide, labeling amino acid residues of proteins in the vicinity of APEX C–E) Pattern of APEX2 labeling in primary cortical neurons is distinct for α-syn-APEX2-, Mito-APEX2-, NES-APEX2-infected neurons by imaging (C) and Western blot (D). Inset: distinct labeling patterns highlighted in neuritic processes. Mito: mitochondrial matrix-targeted; NES: Nuclear exclusion sequence. (E) Compartments enriched for proteins labeled in α-syn-APEX2-versus NES-APEX2 conditions (Gene ontology enrichment scores for cellular compartment; DAVID functional annotation clustering ttps://david.ncifcrf.gov)
After 10 days in culture, we transduced α-syn-APEX2 into rat primary cortical neurons using lentivirus. At this timepoint, endogenous α-syn is fully expressed. We have previously shown that at a multiplicity of infection (MOI) of 20, α-syn overexpression from an extremely strong CMV promoter causes overt toxicity only after 2 weeks (Chung et al 2013). To avoid the potentially confounding effects of neurotoxicity on our proteomic analysis, we transduced neurons at an MOI of 5 and expressing α-syn from a synapsin promoter, well below toxic levels. Three days after viral transduction, we activated APEX by a short (1 min) pulse of H2O2 in the presence of biotin-phenol immediately prior to lysing the cells (Fig. 1B).
APEX2 produced robust and specific labeling patterns in neurons. For αSyn-APEX2, this localization was punctate in neurites as expected. In contrast to nucleus-excluded APEX2 (NES-APEX2) there was diffuse cytosolic and neuritic labeling. Mito-APEX2 labeling was consistent with discrete mitochondrial localization (Figures 1C and D). Biotinylated proteins from αSyn-APEX2, NES-APEX2 and APEX2-negative neurons were quantified using iTRAQ mass spectrometry Fig. S3). More than 2000 proteins were identified by MS, and after filtering the dataset for nonspecific cytosolic labeling by comparing enrichment in the αSyn-APEX2 condition versus the NES-APEX2 control (see Supp. Fig. 3 and Methods section for full details) we identified 225 enriched proteins proximal to α-syn in cortical neurons (Table S2).
Of the 225, these were enriched for proteins enriched in subcellular compartments previously identified as relevant to a-syn, including vesicles and the synaptic terminal (Fig. 1E, Fig. S5 and Table S3). Cellular processes such as protein transport and vesicle trafficking (including clathrin-mediated endocytosis and retromer-mediated retrograde trafficking) were among the top pathways enriched (Fig. 1E).
Beyond the expected localization at the synaptic terminal and vesicle compartments, the α-syn-APEX2 analysis also implicated other compartments, pathways and processes including actin and microtubule cytoskeleton and serine/threonine-specific dephosphorylation (Table 1). The cytoskeletal proteins included the microtubule-associated protein tau (MAPT) that has been heavily implicated in distinct forms of parkinsonism. MAPT polymorphisms are independent risk factors for PD(Shulman et al. 2010), and physical interactions between MAPT and α-syn have been well described in the literature(Jensen et al. 1999). Particularly prominent classes of proteins significantly enriched among the α-syn-APEX2-labeled proteins were mRNA binding, processing and translation proteins. These were explored in more detail below.
Table 1. Biological processes enriched for proteins labeled in α-syn-APEX2 versus NES-APEX2.
Protein trafficking, transport and endocytosis were the top category labeled by α-syn-APEX2. In addition, cytoskeleton (actin and microtubule), protein phosphatases and mRNA metabolism (RNA binding and protein synthesis) are the processes that are labled by α-syn-APEX2.
Gene ontology enrichment scores for biological process; DAVID functional annotation clustering ttps://david.ncifcrf.gov.
| Biological Process | No. | Genes | Bonferroni |
|---|---|---|---|
| membrane trafficking | 9 | SYP, RAB3A, SYT1, CPLX2, RAB3B, RAB8A, COPB1, RAB6A, DNM1 | 4.03E-10 |
| protein transport | 27 | VPS29, RAB3A, XPO1, RAB3B, RAB3C, AP1B1, NAPG, AP2B1, STX12, TRIM3, COPB1, VPS35, STAM, RAB6A, TNPO2, SEC24C, RAB8A, SCAMP3, ACTN4, | 2.67E-09 |
| transport | 45 | VPS29, RAB3A, XPO1, CPLX2, RAB3B, OSBP, ATP1B1, CPLX1, RAB3C, NAPG, AP1B1, CACNB1, CACNB3, ATP6V1B2, AP2B1, STX12, TRIM3, COPB1, VPS35, PAFAH1B1, STAM, SV2B, RAB6A, TNPO2, SEC24C, SCAMP3, RAB8A, ACTN4, RAB4B, G3BP2, ATP1A1, RPH3A, HNRNPA1, CADPS, AP2A2, RABEP1, VCP, LASP1, RAB35, TXN, KHSRP, RAB15, ATP6V0A1, OSBPL11, ERC1 | 2.65E-06 |
| Endocytosis | 8 | PACSIN1, AP2A2, RABEP1, AP1B1, SYNJ1, DNM1, SH3GL2, SH3GL1 | 1.76E-02 |
| cytoskeleton | 29 | SHROOM2, TLN1, ACTR3B, WASF1, ABI2, ABI1, ARPC4, CTNNB1, TPM3, CTTN, PFN2, CRMP1, MAPT, PAFAH1B1, ACTN1, DCTN3, DCTN2, PPP1R9B, LASP1, MAP2, TMOD2, SPTBN2, SPTBN1, MAPRE1, ERC2, SEPT6, DNM1, ADD2, SPTAN1 | 4.03E-08 |
| actin-binding | 16 | SHROOM2, ACTN4, ACTR3B, WASF1, ACTN1, ARPC4, TPM3, PPP1R9B, PFN2, SYN1, LASP1, TMOD2, SPTBN2, SPTBN1, ADD2, SPTAN1 | 1.51E-05 |
| microtubule | 12 | SHROOM2, MAP1LC3A, DYNLL2, MAPT, KIF5C, MAP2, PAFAH1B1, ARPC4, MAPRE1, MAPRE3, DNM1, DCTN2 | 8.99E-03 |
| serine/threonine-specific phosphatase | 5 | PPP1CA, PPP3CB, PPP3CA, PPP1CC, PPP1CB | 3.65E-04 |
| RNA-binding | 19 | XPO1, RNMT, SYNJ1, HNRNPA2B1, G3BP2, SYNCRIP, ILF3, CDC5L, HNRNPA1, HNRNPR, DDX17, TIA1, HTATSF1, KHSRP, CIRBP, PABPC1, MARS, HNRNPAB, DDX42 | 4.47E-03 |
| protein biosynthesis | EIF3C, EIF3D, CARS, EEF1B2, DARS, EIF3L, EEF2, RPS10, EEF1D, MARS | 4.37E-02 |
MYTH and genetic interaction analysis confirm a direct effect of α-syn on endocytic and retrograde trafficking pathways
α-syn-APEX2 prominently labeled clathrin-mediated endocytic proteins, as well as two proteins within the retromer complex that traffics proteins from endosomes to late Golgi, VPS29 and VPS35. These are pathways and proteins that have been strongly implicated in human studies of PD. Indeed, the VPS35 gene is also known as the PARK17 locus because mutation of its coding sequences causes Mendelian PD (Zimprich et al. 2011; Hunn et al. 2015).
The APEX2 data alone cannot distinguish whether α-syn was simply located in the endocytic and retrograde trafficking compartments, or if it was bound in macromolecular complexes to these proteins. So, for each protein in the endocytic or retrograde trafficking pathway that emerged in our APEX dataset, we performed a binary α-syn interaction assay with Membrane Yeast Two-Hybrid (MYTH, see STAR methods, Fig S2 and Table S5). We tested all isoforms available in the ORFeome for 19 endocytosis/retromer proteins suggested by APEX. Of these, 10/19 were positive with MYTH. These included Clathrin, Dynamin and VPS29 (Fig. 2A, Table S5). Another retromer-associated protein, SNX1, also formed a complex with α–syn (Fig. 2A) but the closely related retromer proteins, VPS35 and SNX5, did not. Our data suggests that α-syn impacts endocytic and retrograde trafficking through direct physical interactions with key proteins in these pathways.
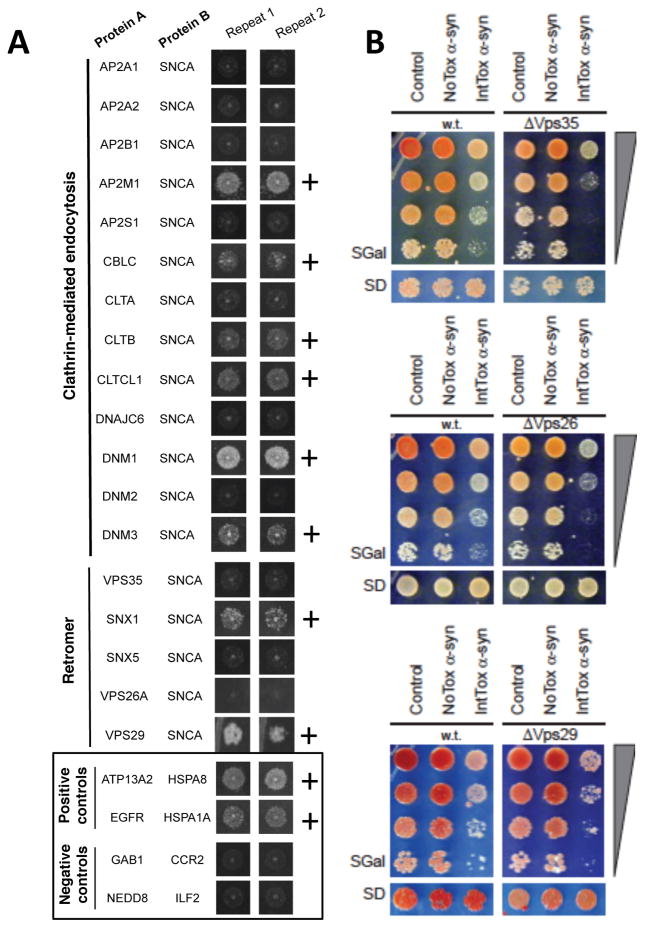
Fig. 2. Endocytic and retromer trafficking proteins interact with α-syn physically and genetically.
(A) Endocytic and retromer trafficking proteins enriched in α-syn-APEX2-labeled neurons are tested for physical interaction with a-syn using the membrane-2-hybrid (MYTH) technique (Fig. S2). Interactions scored as positive are marked “+.” Positive and negative controls are shown in the box. (B) Spotting assays on yeast agar plates indicate that deleting each of the three core retromer components (VPS26, VPS29 and VPS35) enhances toxicity in a strain expressing moderately toxic levels of α-syn (IntTox) but does not enhance toxicity in Control or NoTox α-syn yeast strains.
While α-syn-APEX2 in conjunction with MYTH can uncover physical interactions between proteins, as noted above, complementary genetic approaches are required to reveal the biological significance of these interactions. In an accompanying manuscript (Khurana, Chung et al.), we executed three genome-wide screens in yeast to identify genes that impact α-syn toxicity. Homologs of a number of human PD genes, including VPS35, were recovered in these screens. Loss of VPS35 enhances α-syn toxicity, confirmed here for a strain that expresses moderately toxic levels of α-syn (IntTox) (Fig. 2B). The convergence of both physical and genetic interaction data on the same protein complexes substantiates these direct interactions between α-syn and its neighboring proteins as inciting events in α-syn toxicity.
Our genetic screens did not recover the two other core retromer components, VPS26 or VPS29, as modifiers of α-syn toxicity. However, high-throughput screens are never comprehensive. In this case, the identification of VPS29 in our spatial assays (α-syn-APEX2 and MYTH) led us to test the effects of deleting each of the retromer genes on α-syn toxicity in a candidate-based experiment. In fact, deletion of VPS26 or VPS26 also enhanced α-syn toxicity (Fig. 2B). Thus, these independent genetic and physical interaction analyses were mutually reinforcing and complementary. In this case, the retromer emerges as a multi-protein complex that interacts physically with α-syn and plays a key role in its toxicity. These connections to α-syn and its toxicity provide important biological context for VPS35 (PARK17) as a PD gene. Fig. S6 shows an adaptation of the entire endocytosis pathway from the Kegg database demonstrating the substantial data linking this pathway to α-syn in both our genetic (red star) and spatial (green for APEX2; yellow for MYTH) analyses.
Convergence of genetic and spatial maps on vesicle trafficking and mRNA translation
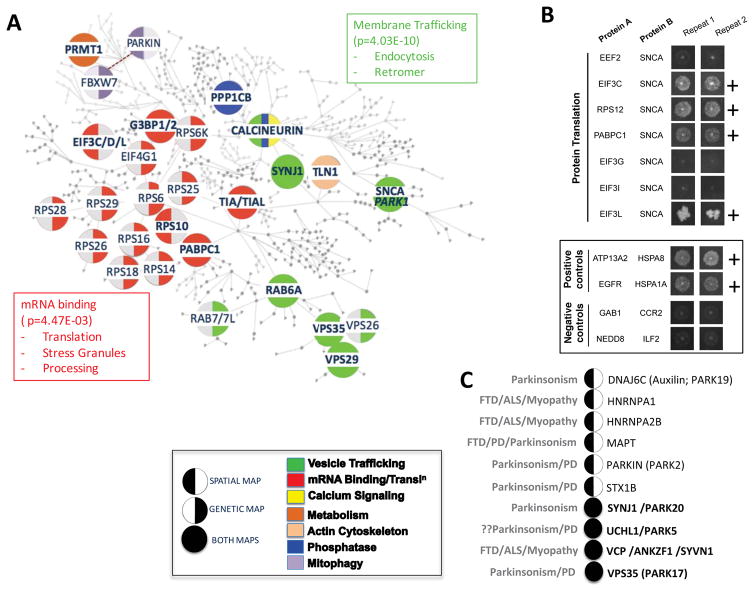
Taking our lead from the retromer-α-syn interaction, we looked more broadly at the relationship of genetic modifiers of α-syn toxicity to the spatial α-syn-APEX2 map. In the accompanying manuscript, we developed computational methods that could assemble genetic modifiers of α-syn toxicity into molecular networks and place these networks squarely in the context of the human proteome. This network is represented in Fig. 3A. Many core components of the genetic network encode proteins that our α-syn-APEX2 and MYTH analysis revealed were in the immediate vicinity of α-syn (Fig. 3A, filled circles). Beyond the retromer complex, these include proteins involved in other trafficking steps (RAB6A, Synaptojanin/SYNJ1), mRNA translation factors and other mRNA binding proteins (PABPC1, G3BP1/2, TIA1/TIAL1), signaling proteins and phosphatases (Calcineurin/PPP3C, PPP1C).
Fig. 3. Convergence of genetic interaction map and spatial (α-syn-APEX2-labeled) map on vesicle trafficking and mRNA metabolism.
A) α-syn-APEX2-labeled proteins are overlaid on to a “humanized” genetic interaction network map. Each circular node of the genetic network depicts a human protein related to a genetic modifier of α-syn toxicity in yeast (full details in accompanying manuscript Khurana et al. 2016), or an α-syn-APEX2-labeled protein that is in a complex with a genetic hit. Enlargement of certain nodes is purely for emphasis and readability. Nodes that belong to both the genetic and α-syn-APEX2 maps are indicated fully shaded. These include G3BP1/2, TIA/TIAL1, PABPC1, VPS29, vPS35, RAB6A, PPP1CB, PPP3C (Calcineurin), TLN1 (Talin 1) and PRMT (Protein Arginine Methyl Transferase). Nodes that were only recovered as genetic modifiers are marked with a left semi-circle. Conversely, nodes that were only recovered in the spatial α-syn-APEX2 map are indicated with a right semi-circle. Pathways/processes that are enriched in both genetic and spatial maps include vesicle trafficking, mRNA metabolism and mRNA binding proteins and phosphatases (Gene ontology enrichment scores for cellular compartment; DAVID functional annotation clustering ttps://david.ncifcrf.gov). B) mRNA translation-related proteins are tested for physical interaction with α-syn using the membrane-2-hybrid (MYTH) technique. Interactions scored as positive are marked “+.” Positive and negative controls are shown in the box. C) Neurodegenerative disease-associated genes emerge among α-syn-APEX2-labeled proteins. Some of these are also recovered in the genetic network of α-syn toxicity modifiers (Khurana, Chung et al., accompanying paper; full circles). Others are not (left semi-circle). VCP was among the α-syn-APEX2-labeled proteins. While VCP was not directly recovered as a genetic modifier of α-syn toxicity, two proteins that work closely with VCP in endoplasmic reticulum- and mitochondria-associated degradation - SYVN1 and ANKZF1 - were recovered in genetic screens. Notably, a gene no longer considered a Parkinson’s disease gene (UCHL1/PARK5) was recovered in both the spatial and genetic α-syn maps.
The overlap between genetic and spatial maps extends beyond these specific proteins. For example, while the spatial map identified α-syn in the immediate vicinity of Rps10, the genetic map identified multiple other small ribosomal protein complex components as genetic modifiers. Likewise, while many EIF3 subunit components emerged from the spatial map, other translation initiation factors (including PABPC1, EIF4G1 and ATXN2) emerged in the genetic map. Indeed, there was a convergence of the genetic and spatial maps at the pathway enrichment level (DAVID functional annotation clustering ttps://david.ncifcrf.gov), particularly for vesicle trafficking (p=4.03E-10; and mRNA translation/binding proteins (p=4.47E-03; Fig. 3A and Table S4). Of the mRNA translation hits in the APEX neuron study, 5 were available for testing by MYTH in the ORFeome library. Of these, 4/5 were confirmed (EIF3C, EIF3L, RPS12 and PABPC1; Fig. 3B; Table S5 and Fig. S7). These data imply that, as for vesicle trafficking proteins, mRNA translation and binding proteins are proximal interactors with α-syn that are integrally involved in its mechanisms of toxicity.
It is worth noting that, while the genetic map of α-syn toxicity was generated in yeast, the spatial α-syn-APEX map was established in mammalian neurons. While the yeast genetic map was “humanized” (that is, placed in the context of the human proteome), a number of important relationships would likely be missed, most likely for proteins that are neuron-specific. This indeed was the case. For example, the spatial α–syn map recovered multiple RAB3 proteins. These are neuron-specific RAB proteins involved in synaptic vesicle exocytosis and do not feature among our genetic screen hits in yeast. However, multiple previous studies, including from our own lab, have established that modulating the levels of these RAB proteins can modify α-syn toxicity in neurons, shown for both RAB3A (Gitler et al. 2008) and RAB3B (Chung et al. 2009).
Finally, another measure of the biological significance of our spatial α-syn map comes from human genetic studies. Our genetic and spatial maps converged on specific human neurodegenerative disease genes (Fig. 3C), including two Mendelian parkinsonism genes, VPS35 (PARK17)(Zimprich et al. 2011) and SYNJ1 (PARK20)(Quadri et al. 2013; Olgiati et al. 2014; Krebs et al. 2013). Also recovered in the spatial map was PARKIN (PARK2). While PARKIN does not have a direct yeast homolog, a related ubiquitin ligase in yeast, Cdc4 (FBXW7)(Ivatt et al. 2014; Ekholm-Reed et al. 2013), was recovered in our genetic screens. A third PARK gene, DNAJC6 (PARK19)(Köroğlu et al. 2013; Olgiati et al. 2015; Edvardson et al. 2012), was recovered in the spatial map. DNAJC6 is also known as an auxilin, and is a chaperone involved in clathrin uncoating. One PARK gene that is no longer considered a bona fide PD gene because of failure to replicate in human genetic studies is UCHL1 (PARK5). It appeared in both our genetic and spatial maps, suggesting that it may be involved in α-syn toxicity. In fact, a number of mechanistic studies have previously suggested such a relationship (Cartier et al. 2012; Liu et al. 2009; Yasuda et al. 2009) and this association may be of relevance in the broader context of synuclein pathology beyond PD. Beyond Mendelian risk factors, our spatial map further recovered two neuronal genes implicated through common variants in PD, MAPT and Syntaxin 1B (STX1B) (Nalls et al. 2014). Finally, like VCP, two other proteins related to multisystem proteinopathies, were recovered in our spatial map, including HNRNPA1 and HNRNPA2B(Müller-Kuller et al. 2015; Taylor 2015; Kim et al. 2013). These findings collectively deepen our understanding of α-syn toxicity, and specifically establish a key relationship between the native cellular context of the α-syn protein in neurons to pathogenic mechanisms of high relevance to human disease.
Discussion
How the intrinsic localization of a misfolding protein relates to its proteotoxicity has been unclear. The major advance of the APEX2 labeling methodology is its ability to capture the localization of a protein and its proteome context within a living cell. It provides a snapshot of proteins, somewhat analogous to immunocytochemistry, except that APEX2 provides information at the proteome scale that is independent of the idiosyncrasies of immunohistochemistry. In short APEX2 captures subcellular complexity and spatial context. This is not captured by techniques that require cell lysis, like co-IP, or with heterologous systems such as MYTH, in which a bait protein is tethered to the ER membrane. However, APEX2 will also label proteins that are in the vicinity of α-syn but not necessarily complexed to it directly. There are other differences between APEX2 labeling and standard protein interaction methodologies. For example, co-IP will only recover stable complexes and the MYTH method requires an interaction to take place at the ER membrane in a yeast cell. In this context, it is important to consider that the APEX2 tag could impede protein-protein interactions or the entry of the tagged protein to specific subcellular compartments. Moreover, proteins without surface-accessible Tyr or other electron-rich residues will not be detected with the APEX technique. These different methodologies are thus complementary. When they are convergent, they are mutually reinforcing. When they are divergent, interactions are worthy of investigation with yet additional methods.
At a high level, this study and the accompanying work (Khurana et al 2016) systematically explore α-syn toxicity through a variety of complementary, independent methodologies: physical and spatial mapping techniques like APEX2 and MYTH, multiple genetic screens, computational tools, physical and spatial mapping techniques and ribosomal footprinting. Proteins that emerge in common between these approaches, proximal as they are to α-syn itself, are likely to reflect direct and inciting events in α-syn cytotoxicity. These include vesicle trafficking proteins (RAB6A, VPS35, SYNJ1), mRNA translation factors (PABPC1) and key signaling molecules (PPP3/Calcineurin). The correlation with entirely independent human genetic studies reinforces the significance of these proteins and pathways to human disease. Most broadly, the building of molecular networks with our various approaches provides a view of how seemingly disparate genes, proteins and pathways interconnect to cause proteotoxicity.
While these methods are powerful, they clearly do not replace deeper, mechanistic studies or studies in more complex models. For example, in the accompanying study the emergence of mRNA translation factors in our spatial and genetic networks allowed us to uncover new pathologic phenotypes in patient-derived neurons. Hence, the main strength of these studies is to identify important directions for future investigations. While more detailed mechanistic work within a neuronal context is required for a more detailed understanding of clinical observations, our studies provide a systems-level view of how intrinsic biology might relate to the pathology of misfolded and mis-trafficked proteins. These approaches can now be applied to other proteinopathies in which a relationship of intrinsic biology to disease pathology may well emerge as a common theme.
Supplementary Material
Acknowledgments
Research was supported by an HHMI Collaborative Innovation Award (V.K., C.Y.C., A.Y.T., and S.L.), the JPB Foundation (V.K., C.Y.C., S.L.), NIH K01AG038546 (C.Y.C.), U01CA184898 and R01GM089903 (E.F.), R01GM081871 (B.B.) and HG001715 (M.V. and D.E.H), an American Brain Foundation and Parkinson’s Disease Foundation Clinician-Scientist Development Award (V.K.), the Harvard Neurodiscovery Center Pilot Project Program (V.K.), the Multiple System Atrophy Coalition (V.K.), the Eleanor Schwartz Charitable Foundation (S.L.), NIH., We thank P. Thiru, B. Yuan and E. Spooner from the Whitehead Institute for expert assistance. We dedicate this manuscript to Dr Susan Lindquist – our incomparable mentor and colleague – who passed away while this manuscript was in its final stages of revision.
Footnotes
Author Contributions
Conceptualization: C.Y.C., V.K. and S.L.; Methodology: C.Y.C., V.K., S.Y., N.S., N.D.U., and K.H.L.; Investigation: C.Y.C., V.K., S.Y., N.S., P.K.A., V.B. and Y.F.; Resources: K.H.L. and A.Y.T.; Writing-Original Draft and review and editing: C.Y.C., V.K. and S.L.; Supervision: C.Y.C, V.K., S.L., A.Y.T., D.E. H., M.V. and S.A.C.
Potential Conflicts of Interest:
V.K., C.Y.C., and S.L. are scientific co-founders of Yumanity Therapeutics, a company focused on developing neurodegenerative disease therapeutics.
References
- Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annual review of cell and developmental biology. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- Burré J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(40):E4274–83. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier AE, et al. Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. P. J. Kahle, ed. PLoS ONE. 2012;7(4):e34713. doi: 10.1371/journal.pone.0034713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, et al. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Choi P, et al. Co-association of parkin and alpha-synuclein. Neuroreport. 2001;12(13):2839–2843. doi: 10.1097/00001756-200109170-00017. [DOI] [PubMed] [Google Scholar]
- Chung CY, et al. Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22474–22479. doi: 10.1073/pnas.0912193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, et al. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273(16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Edvardson S, et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. C. Wider, ed. PLoS ONE. 2012;7(5):e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S, et al. Parkin-dependent degradation of the F-box protein Fbw7β promotes neuronal survival in response to oxidative stress by stabilizing Mcl-1. Mol Cell Biol. 2013;33(18):3627–3643. doi: 10.1128/MCB.00535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S, et al. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nature genetics. 1999;22(1):110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- Gitler AD, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, et al. 100 years of Lewy pathology. Nature reviews. Neurology. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Greten-Harrison B, et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung V, et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Molecular cell. 2014;55(2):332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunn BHM, et al. Impaired intracellular trafficking defines early Parkinson’s disease. Trends in Neurosciences. 2015;38(3):178–188. doi: 10.1016/j.tins.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivatt RM, et al. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(23):8494–8499. doi: 10.1073/pnas.1321207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, et al. alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274(36):25481–25489. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- Kim HJ, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Human molecular genetics. 2006;15(20):3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- Köroğlu Ç, et al. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism & related disorders. 2013;19(3):320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Krebs CE, et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Human mutation. 2013;34(9):1200–1207. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SS, et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nature methods. 2015;12(1):51–54. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(12):4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MA, et al. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Molecular & cellular proteomics: MCP. 2008;7(11):2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, et al. Remodeling of lipid vesicles into cylindrical micelles by α-synuclein in an extended α-helical conformation. J Biol Chem. 2012;287(35):29301–29311. doi: 10.1074/jbc.M112.365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Kuller U, et al. A minimal ubiquitous chromatin opening element (UCOE) effectively prevents silencing of juxtaposed heterologous promoters by epigenetic remodeling in multipotent and pluripotent stem cells. Nucleic Acids Res. 2015;43(3):1577–1592. doi: 10.1093/nar/gkv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, et al. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358(1):104–110. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nature genetics. 2014:1–7. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgiati S, et al. DNAJC6 mutations associated with early-onset Parkinson’s disease. Annals of neurology. 2015 doi: 10.1002/ana.24553. [DOI] [PubMed] [Google Scholar]
- Olgiati S, et al. PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics. 2014;15(3):183–188. doi: 10.1007/s10048-014-0406-0. [DOI] [PubMed] [Google Scholar]
- Quadri M, et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Human mutation. 2013;34(9):1208–1215. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- Rhee HW, et al. Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging. Science. 2013 doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, et al. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(24):8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, et al. CSPα knockout causes neurodegeneration by impairing SNAP-25 function. Embo J. 2012;31(4):829–841. doi: 10.1038/emboj.2011.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. Parkinson’s Disease: Genetics and Pathogenesis. Annual review of pathology. 2010 doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Stagljar I, et al. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci U S A. 1998;95(9):5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP. Multisystem proteinopathy: Intersecting genetics in muscle, bone, and brain degeneration. Neurology. 2015;85(8):658–660. doi: 10.1212/WNL.0000000000001862. [DOI] [PubMed] [Google Scholar]
- Udeshi ND, et al. Large-scale identification of ubiquitination sites by mass spectrometry. Nature Protocols. 2013;8(10):1950–1960. doi: 10.1038/nprot.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas KJ, et al. Synucleins regulate the kinetics of synaptic vesicle endocytosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(28):9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, et al. Effects of UCH-L1 on alpha-synuclein over-expression mouse model of Parkinson’s disease. J Neurochem. 2009;108(4):932–944. doi: 10.1111/j.1471-4159.2008.05827.x. [DOI] [PubMed] [Google Scholar]
- Zimprich A, et al. A Mutation in VPS35, Encoding a Subunit of the Retromer Complex, Causes Late-Onset Parkinson Disease. American journal of human genetics. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.