Abstract
Objective
To investigate proteins associated with neuronal damage in plasma neuron-derived exosomes (NDE) of HIV-infected subjects as a liquid biomarker for cognitive impairment.
Methods
Plasma NDE were isolated using precipitation and immunoadsorption with antibody to a cell surface specific neuronal marker. Total exosomes and NDE were enumerated, characterized and proteins extracted and targets quantified by ELISA.
Results
Plasma NDE from 23 HIV seropositive individuals of which 11 had mild cognitive impairment, and 12 HIV seronegative controls of which 3 had cognitive impairment were isolated. NDE were enriched for the neuronal markers neurofilament-light (NF-L) and synaptophysin (SYP). Neuropsychologically impaired (NPI) individuals had fewer NDE compared to neuropsychological normal subjects (NPN). NDE from NPI subjects had significantly higher levels of high mobility group box 1 (HMGB1), NF-L and amyloid beta (Aβ) proteins compared to NPN individuals. NDE HMGB1 protein significantly decreased with age in HIV-infected individuals.
Conclusion
Plasma NDE were altered in several ways in HIV infection. Elevated HMGB1, NF-L and Aβ proteins could distinguish cognitive impairment. NDE contents reflect neuronal health in “real-time” and may be useful for following cognitive impairment and response to therapy in HIV infection.
Keywords: HIV, HAND, biomarker, exosomes, neurological impairment, aging
Introduction
In the setting of antiretroviral therapy (ART), viral loads for most HIV-infected individuals remain undetectable; however, for some individuals on therapy, spikes in viral load in the periphery and chronic inflammation remain as well as a spectrum of neurological deficits termed HIV-associated neurocognitive disorder (HAND). HAND develops in a portion of patients ranging from 20–69% and for the most part, is more frequent in subjects who are not virally suppressed [1]. Biomarkers to assess neurological involvement should be objective, inexpensive, easily accessible, noninvasive tools to monitor the course of infection and identify those at risk for neurological damage. While cerebrospinal fluid (CSF) is thought to be closest to the neuropathology, it is an invasive procedure and like plasma will have a complex protein profile comprised of different cell types. With increased aging of HIV-infected subjects, biomarkers also need to separate normal cognitive changes due to age from that associated with HIV infection. Exosomes are 30–150 nm microvesicles formed in late endosomes and collected as multivesicular bodies prior to fusion with the plasma membrane [2–4]. They are shed from various cells under normal as well as pathological conditions into the surrounding milieu including plasma, urine, saliva and inflammatory tissues. The cellular cargo packaged into exosomes can be significantly altered depending on the physiological state of the parent cell including immune activation [4]. Most cells in the (CNS) nervous system including neurons, astrocytes, oligodendrocytes and microglia shed exosomes (Review [5]). These extracellular vesicles are secreted by neural cells under normal and pathological conditions and have been isolated from the CSF [6], adult human brain [7] and recently plasma [8–11]. Exosomes can reflect the host cell proteins and nucleic acids at the time of secretion and can be taken up by recipient cells thereby altering their function and setting off a cascade of events that alter homeostasis. Exosomes can diffuse across the blood brain barrier (BBB) into the periphery and be captured by antibodies directed against the cell surface proteins embedded in the vesicle membrane. This strategy has been used to isolate neuron-derived exosomes (NDE) [10].
In this pilot study, our goal was to begin to identify a plasma biomarker profile to determine the functional state of neurons in HIV infection. We report that NDE from cognitively impaired subjects, regardless of HIV infection, have an increase in proteins associated with neuronal damage. We also show that there are decreases in these NDE proteins with age in HIV-infected subjects compared to normal controls.
Materials and Methods
Subject characteristics and plasma collection
Individuals were recruited from the San Francisco Veterans Affairs Medical Center (SFVAMC). All participants provided written consent approved by the University of California, San Francisco Committee on Human Research. HIV-infected subjects were on stable ART while controls were healthy individuals receiving care at the SFVAMC. Exclusion criteria included psychoactive drug use, alcoholism, clinical depression or psychiatric disease, seizure disorder or history of head injury. Whole blood was collected using BD Vacutainer Heparin tubes (BD, Franklin Lakes, New Jersey) and centrifuged at 300g for 10min. Plasma was collected, aliquoted and frozen at −80°C.
Neuropsychological evaluation
All subjects were tested for neuropsychological performance within 2–3 days of the blood draw. Neuropsychological testing was performed by the same trained psychologist (L.A.). All subjects underwent 7 domains of neuropsychological testing as previously described [12]. The 7 domains tested included: attention working memory, information processing speed, executive function, fine motor skills, verbal fluency and verbal learning and memory. Neuropsychological evaluation results were demographically corrected per educational level, gender and age. Raw individual test scores were converted to standard T scores. Two or more domains below 1.5 standard deviation from the norm was determined as NPI, otherwise, NPN.
Isolation of exosomes
One milliliter frozen plasma was thawed, centrifuged at 1000g for 15 min at 4°C. Exosome isolation followed Goetzl et. al. protocol [13]. Briefly, 0.75 mL plasma was incubated with 0.3 mL thromboplastin-D (Thermo-Fisher Scientific, Inc., Waltham, Maine, USA) for 1 h at room temperature (RT). After addition of 0.45 mL PBS with 3X protease inhibitors (Sigma-Aldrich Corp. St. Louis, Missouri, USA) and phosphatase inhibitors (Thermo), samples were mixed and centrifuged at 3000g for 20 min. The supernatants were mixed with 378 μL of ExoQuick (System Biosciences, Inc., Palo Alto, California, USA) and incubated for 1 h at 4°C. Exosomes were collected and resuspended in water with protease and phosphatase inhibitors. These were total exosomes. The exosome suspension (700 μL) was incubated with 4 μg mouse anti-human L1CAM-biotinylated antibody (CD171, clone 5G3, eBiosciences, San Diego, California, USA) and bound to 20 μL Streptavidin-plus ultra-link resin (Thermo) for 1 h at 4°C. Following centrifugation at 400g for 10 min at 4°C, each pellet was resuspended with 200 μL 50 mM glycine-HCl (pH 3.0). Supernatants were neutralized with 20 μL of 1 M Tris-HCl (pH 8.0). These were neuron-derived exosomes. Each 100 μL NDE were lysed with 365 μL M-PER (Thermo) with 25 μL of 3% BSA and protease and phosphatase inhibitors. The samples were mixed and subjected to 2 cycles of free-thaw and stored at −20°C.
NanoSight analysis
Exosome size and count were determined by a nanoparticle characterization system, NanoSight LM10 instrument (Malvern Instruments, Malvern, United Kingdom) with a 405nm laser-equipped sample chamber, as described previously [14]. The samples were diluted in PBS to 108–109 particles/mL. Camera shutter, gain and software detection threshold were manually adjusted for optimal detection. Each sample was analyzed three times. Each analysis consisted of three recordings of 40 seconds each. Since the distributions of the sizes were highly skewed, the modes were reported for particle size. The mean of the triplicate recordings was reported for size and concentration.
ELISA
The samples were thawed, vortexed and centrifuged before ELISA analysis. Neuronal markers were quantified using ELISA kits for human neurofilament protein L (NF-L, MyBioSource, San Diego, California, USA) and synaptophysin (SYP, American Research Products-Cusabio, Waltham, Maine, USA). Tetraspanin exosome markers were tested using human CD81 (American Research Products-Cusabio) and CD63 (MyBioSource). Mediators of neuronal dysfunction included high mobility group protein B1 (HMGB1, LifeSpanBioSciences, Inc., Seattle, Washington, USA) and human amyloid beta 42 (Aβ) Ultrasensitive ELISA Kit (Thermo).
Statistical analysis
The differences of the means between groups were tested using 2-tail unpaired Student’s t test assuming unequal variances. Log10 transformation was performed before tests to obtain Gaussian distribution. When concentrations of ELISA data contained zero values, zeros were replaced with half of the otherwise minimum data point to perform log transformation. Contingency tables were analyzed with Chi-squared test or Fisher’s exact test where appropriate. When comparing multiple groups, ANOVA was used. Significance was determined by P < 0.05. P values for multiple tests were corrected using Benjamini and Hochberg’s adjustment [15]. All statistical analyses were performed in R statistical software version 3.3.2.
Results
Study population
The characteristics and clinical and laboratory data for the participants are summarized in Table 1. Subjects were all male except for 1 female, representing the demography of patients cared for at the SFVAMC. The ages ranged from 35 to 67 years (IQR 50.5–60). There was no difference in age among the groups. There were 12 HIV negative controls including 9 NPN and 3 impaired. All controls were recruited without prior knowledge of their neuropsychological status. Three controls, after testing and in spite of exclusion criteria, were found to have non-HIV related mild impairment. The 23 HIV-infected subjects included 11 undetectable HIV viral load (UD, <40 copies/mL) and 12 with plasma HIV viral load at the time of blood draw. The number of subjects in each group was not significantly different. There was no difference in viral load between the NPI and NPN HIV-infected groups. The subjects were primarily Caucasian in all groups. Plasma CD4 cell counts were lower in the HIV NPI group compared to controls; however, there was no significant difference in CD4 counts between NPN and NPI HIV-infected individuals.
Table 1.
Patient characteristics
| Control | HIV | P value | |||
|---|---|---|---|---|---|
|
|
|
||||
| NPN* | NPI | NPN | NPI | ||
| log10 viral load 1 | NA | NA | 3.2 (1.7) | 3.3 (1.7) | 0.903 |
| N 2 | 9 | 3 | 12 | 11 | 0.282 |
| Ethnicity 3 | 0.599 | ||||
| African-American | 3 | 1 | 1 | 4 | |
| Asian | 1 | 0 | 0 | 1 | |
| Caucasian | 4 | 1 | 10 | 5 | |
| Hispanic | 1 | 1 | 1 | 1 | |
| Age 4 | 57.3 (4.5) | 58.0 (5.6) | 54.4 (9.2) | 52.1 (9.4) | 0.467 |
| CD4 count 5 | NA | 995.5 (350.0) | 533.1 (442.1) | 306.3 (236.9)# | 0.049 |
NPN, neuropsychologically normal; NPI, neuropsychologically impaired, defined as 2 or more domains of the neuropsychological tests were below the norm. NA, not applicable or not available. Data displayed as mean (sd).
Log10 transformed HIV viral load (copies/mL), two-tailed Student’s t test was used.
No difference in number of patients (N) for each group (Fisher’s exact test).
No difference in ethnicity for each group (χ2 test).
No difference in age (ANOVA test).
CD4 lower than Control NPI (ANOVA test, # P<0.05). No difference between HIV NPN and HIV NPI (Student’s t test P=0.14).
Exosomes are decreased in HIV-infected and cognitively impaired subjects
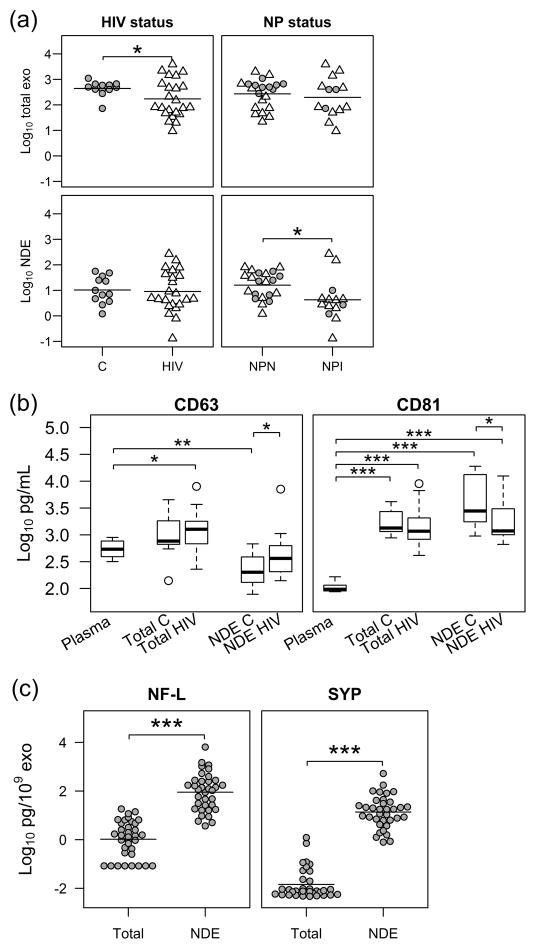
Exosomes were counted using the NanoSight system as previously described [14]. The size of total exosomes (108±30 nm) and NDE (106±28 nm) were similar (P=0.73). The size of NDE were similar between impaired (103±29 nm) and non-impaired (108±28 nm, P=0.65) subjects. The number of total exosomes was fewer in HIV-infected subjects compared to controls (Fig. 1a top-left panel, 2.2±0.7 vs. 2.6±0.3, log10 transformed, x109 particles/mL, P=0.027). The number of NDE was fewer in neurocognitively impaired subjects compared to non-impaired (Fig. 1a bottom-right panel, 0.6±0.8 vs. 1.2±0.5, log10 transformed, x109 particles/mL, P=0.034) whereas the number of total exosomes was similar between NPN and NPI subjects (P=0.57).
Fig. 1. Exosome characterization and normalization.
(a) Exosomes were counted with a nanoparticle tracking system NanoSight LM10. The range of total exosome counts was 9.4 – 3900 x109/mL. The range of neuron-derived exosome (NDE) counts was 0.13 – 270 x109/mL, about 5% of the total exosomes. Individuals with HIV (N=23) had fewer total exosomes than controls (C, N=12); neuropsychologically impaired (NPI) individuals (N=14) had fewer NDE than neuropsychologically normal individuals (NPN, N=21). The units are log10 transformed exosome counts x109/mL plasma. HIV (△); Controls (
 ). Horizontal bars indicate group means. (b) Box-whisker plots show tetraspanins CD63 and CD81 concentrations measured by ELISA. Plasma contained high levels of CD63 (ranged 317–896 pg/mL, N=5). Total exosomes were enriched with CD63. Although NDE showed low levels of CD63, the number of NDE was only 5.2% of total exosomes. CD63 was also enriched in NDE but different in HIV compared to C. Total exosomes and NDE from all subjects (N=35) were enriched with CD81. NDE from HIV infection showed higher levels of CD63 and lower levels of CD81. Therefore, exosome counts were used for normalization. Plasma data included 2 controls and 3 HIV-infected subjects for CD63 and 2 controls, 5 HIV-infected subjects for CD81 analyses. Because no difference was found between plasma control and HIV levels for either CD63 or CD81, the two groups were combined. Concentrations of CD63 and CD81 were log10 transformed. The box-whisker plot indicates the 25th, 50th and 75th percentile within the box, whiskers extending from the boxes indicate extreme values within 1.5 inter-quartile range from the 25th and 75th percentile respectively and the circles indicate outliers. (c) The source of NDE was further confirmed with neuronal markers. Neurofilament-light (NF-L) and synaptophysin (SYP) were both enriched in NDE compared to total exosomes. Concentrations of NF-L and SYP were normalized to exosome counts and log10 transformed. Horizontal bars indicate group means. Two-tailed Student’s t test with Benjamini and Hochberg multiple test correction was used. *P<0.05, **P <0.01, ***P< 0.001.
). Horizontal bars indicate group means. (b) Box-whisker plots show tetraspanins CD63 and CD81 concentrations measured by ELISA. Plasma contained high levels of CD63 (ranged 317–896 pg/mL, N=5). Total exosomes were enriched with CD63. Although NDE showed low levels of CD63, the number of NDE was only 5.2% of total exosomes. CD63 was also enriched in NDE but different in HIV compared to C. Total exosomes and NDE from all subjects (N=35) were enriched with CD81. NDE from HIV infection showed higher levels of CD63 and lower levels of CD81. Therefore, exosome counts were used for normalization. Plasma data included 2 controls and 3 HIV-infected subjects for CD63 and 2 controls, 5 HIV-infected subjects for CD81 analyses. Because no difference was found between plasma control and HIV levels for either CD63 or CD81, the two groups were combined. Concentrations of CD63 and CD81 were log10 transformed. The box-whisker plot indicates the 25th, 50th and 75th percentile within the box, whiskers extending from the boxes indicate extreme values within 1.5 inter-quartile range from the 25th and 75th percentile respectively and the circles indicate outliers. (c) The source of NDE was further confirmed with neuronal markers. Neurofilament-light (NF-L) and synaptophysin (SYP) were both enriched in NDE compared to total exosomes. Concentrations of NF-L and SYP were normalized to exosome counts and log10 transformed. Horizontal bars indicate group means. Two-tailed Student’s t test with Benjamini and Hochberg multiple test correction was used. *P<0.05, **P <0.01, ***P< 0.001.
Exosomal proteins are differentially expressed in HIV infection
To further characterize exosomes, tetraspanins CD63 and CD81 were evaluated using ELISA. The levels of CD63 and CD81 were enriched in both total exosomes and NDE (Fig. 1b). Plasma contained measurable levels of CD63 (range 317–896 pg/mL) and CD81 (range 87–165 pg/mL). Total exosomes isolated from plasma were enriched for CD63 and CD81. Although the total amount of CD63 was lower in the NDE, the number of NDE was only 5.2% of total exosomes, this indicates CD63 was actually highly enriched in NDE. CD81 was also highly enriched in NDE. We found that the level of CD63 from NDE was increased in HIV individuals (P=0.045) while CD81 was decreased (P=0.048) (Fig. 1b). Since exosome counts were not changed between controls and HIV subjects (Fig. 1a, bottom-left), we chose exosome counts as the normalization tool. To confirm the exosomes were derived from neurons, we evaluated neuronal markers using ELISA. The levels of the neuronal markers, NF-L and SYP were enriched significantly in NDE by 86-fold and 951-fold, respectively, compared to total exosomes (Fig. 1c).
Markers of neuronal damage increased in NDE from impaired subjects
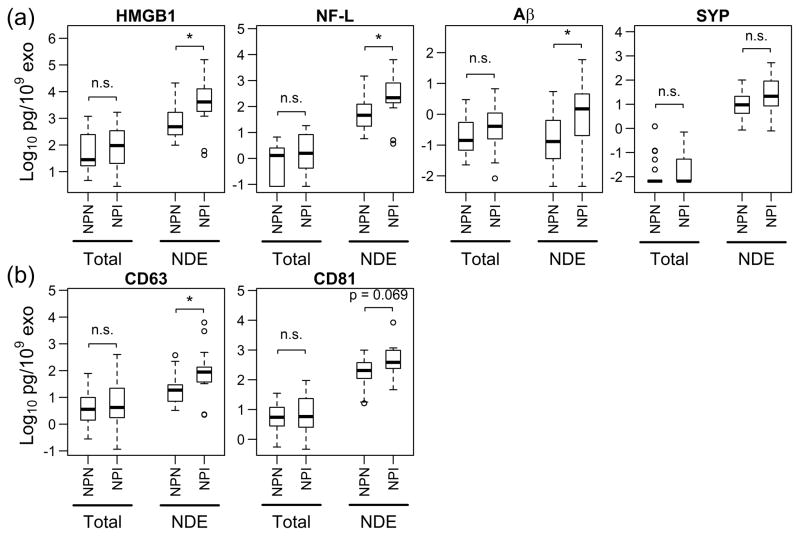
To investigate the effects of neurocognitive impairment, we chose 4 protein targets to assess neuronal damage, HMGB1, NF-L, Aβ and SYP. There were no significant differences in protein levels of NDE between HIV-infected and uninfected subjects (data not shown). However, NDE from NPI subjects showed significant increases in HMGB1 (5.1-fold change, P=0.023), NF-L (3.8-fold change, P=0.041) and Aβ (6.2-fold change, P=0.048) compared to NPN subjects (Fig. 2a). SYP showed no difference (P=0.11) between these 2 groups. Interestingly, tetraspanin CD63 increased 5-fold (P=0.023) and CD81 had a trending increase of 2.3-fold (P=0.069). The levels of all these analytes showed no difference between NPI and NPN in total exosomes. This strongly suggests that plasma total exosomes may not adequately reflect neuronal damage. In addition, the NPI group was a mixture of HIV-infection status and included not only HIV controlled subjects (N=5), but also subjects with a viral load (N=6) and HIV negative subjects (N=3).
Fig. 2. NDE markers for neuropsychological impairment (NPI).
Levels of markers in exosome lysates were quantified with ELISA. (a) NPI individuals (N=14) had significantly elevated levels of high mobility group box 1 (HMGB1), neurofilament-light (NF-L) and amyloid beta (Aβ) compared with neuropsychologically normal individuals (NPN, N=21). Synaptophysin (SYP) was not significant. (b) CD63 was increased in NPI and CD81 was trending elevated in NPI subjects. Total exosomes showed similar levels from NPN and NPI subjects in all analytes tested. n.s., not significant; Total, total exosomes; NDE, neuron-derived exosomes. Data are shown as box-whisker plots as explained in Fig. 1b. Concentrations of analytes were normalized to exosome counts of each sample. Two-tailed Student’s t test was used, *P< 0.05.
Aging changes exosomal proteins in HIV-infected subjects
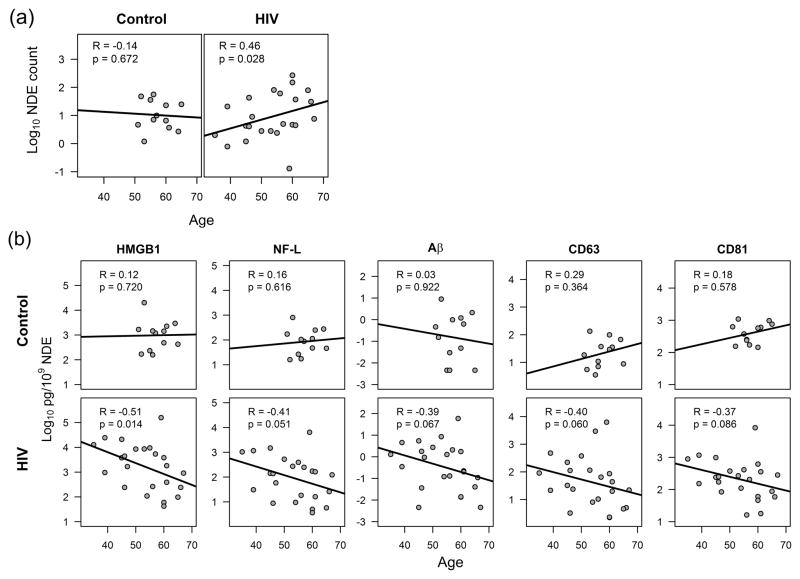
The age range of our subjects was 35 to 67 years and would not be considered in the older age range associated with Alzheimer’s disease. HIV-infected subjects showed a positive correlation between age and NDE counts (R=0.46, P=0.028) while control subjects showed no correlation (Fig. 3a). The controls in this study were older although not significantly (57.5 vs. 53.3 years in HIV, P=0.079) and had a relative narrower range (51–65 years, IQR: 54–60.5) compared to the HIV-infected subjects (35–67 years, IQR:46–60). To investigate the effects of age on each of the markers, we performed linear regression analysis. There were no correlations with the target proteins in NDE and age in the controls although the limited age distribution may affect this conclusion. HMGB1 levels in NDE showed a negative correlation with age in HIV-infected patients (R=−0.51, P=0.014) (Fig. 3b). A trending negative correlation was observed between age and NF-L (R=−0.41, P=0.051) and Aβ (R=−0.39, P=0.067) in NDE. Furthermore, the tetraspanins CD63 and CD81 also showed a trending decrease with age.
Figure 3. Aging and neuron-derived exosome (NDE) proteins.
(a) Aging correlated with higher exosome counts in NDE from individuals with HIV. The units were log10 transformed exosome counts x109 per milliliter plasma. (b) High mobility group box 1 (HMGB1) decreased significantly with age in NDE from HIV infection; neurofilament-light (NF-L), amyloid beta (Aβ) and tetraspanins CD63 and CD81 were trending decrease with age in HIV infection. No effect was seen in NDE isolated from controls. The range of ages in controls (51–65 years) was narrower than HIV (35–67 years). Exosome protein levels were log10 transformed. Non-parametric Spearman correlation was used.
Discussion
This small pilot study showed that neuron-derived exosomes are influenced by both HIV infection and neurocognitive impairment. The study was initiated with the expectation that HIV infection would alter neuronal function and be different from HIV negative controls. What was surprising was that the impaired subjects tended to cluster together and HIV infection appeared to influence the numbers of total and neuron-derived exosomes. Our findings showed that total exosomes isolated from plasma did not show differences in several distress proteins between HIV-infected and control subjects (data not shown). When we grouped the subjects into cognitive impairment versus normal cognition, there still was no difference in the levels of targeted proteins in total exosomes. However, we began to see these elevated stress proteins in isolated NDE from impaired subjects compared to normal individuals. This implies that NDE may be a better reflection of cognitive impairment than plasma exosomes and more accurate biomarker of neuronal damage.
We utilized a two-step purification technique to isolate exosomes secreted from neurons. After total exosome isolation from plasma, neuron-derived exosomes were captured using the neuron specific L1CAM monoclonal antibody. This procedure has been successfully used to predict Alzheimer’s disease (AD) [10, 16, 17]. We found that total exosome counts were lower in HIV infection but the same in NDE counts. Total plasma exosomes can originate from all blood cell types [18] but tend to be enriched from platelets, dendritic cells and monocytes [19]. Fewer total exosomes may reflect a loss in one of these cells types. On the other hand, NDE from neuropsychologically impaired subjects were significantly fewer (52%) than unimpaired subjects. We speculate that neurons in neurocognitively impaired individuals may secrete less exosomes because they are stressed or it may be due to loss of neurons.
To further characterize the NDE, we quantified several tetraspanins. The role of tetraspanins has evolved from markers of transmembrane domains used to identify exosomes to functional regulators of migration and signaling (Review [20]). We showed that NDE from HIV-infected individuals were increased in CD63 and decreased in CD81; however, both tetraspanins were increased in cognitively impaired subjects compared to cognitively normal subjects. While there is scant data as to the role of these tetraspanins in HIV infection, reports show that HIV acquires CD63 upon budding from cells [21]. Likewise, HIV can be internalized in astrocyte CD81 vesicles that can trans-infect other cells without de novo virus production [22]. Although the functions of these tetraspanins in neurons are unclear, these studies suggest that CD63 and CD81 may participate in HIV infection.
We chose several target proteins associated with HIV and stress to analyze neuronal injury. HMGB1 is a danger-associated molecular patterns (DAMP) molecule and an alarmin, which is released from necrotic cells and serves as a “danger signal” to the immune system. It is a nuclear DNA binding protein expressed in all cell types including neurons and glial cells [23]. Passively released HMGB1 by necrotic or injured cells results in inflammation initiation while actively secreted HMGB1 by immune cells, once released into extracellular space, can promote neuroinflammation [23–25]. HMGB1 is a pro-inflammatory mediator and directly involved in HIV replication and reactivation [26]. HMGB1 binds to receptors TLR-2, TLR-4 and RAGE and propagates inflammation in a positive feedback manner in traumatic brain injury [27–29]. Also, HMGB1 was elevated in CSF after traumatic brain injury (TBI) [29]. Investigations of acute death from TBI found that HMGB1 was partly lost in neurons and increased in the cytoplasm of infiltrating phagocytic microglia [27]. HMGB1 was recently reported to be elevated in the CSF from HIV-infected subjects on stable ART, strongly suggesting persistent inflammation in the CNS and potentially a biomarker for early cognitive impairment [30]. HMGB1 was increased in the brains of subjects with Alzheimer’s disease (AD), colocalized with Aβ in senile plaques and thought to promote neuronal death and synaptic malfunction [31]. Collectively, HMGB1 is associated with neuronal damage and neurocognitive impairment. It is consistent with our findings that HMGB1 increased in neuron-derived exosomes of NPI subjects. We posit that chronically injured neurons release exosomes containing higher levels of HMGB1. Neurons may utilize this route to remove cytotoxic HMGB1 from the cytoplasm or passively signal neuronal cell death. The NDE containing HMGB1 may also behave as a vehicle disseminating HMGB1 in the brain and activating astrocytes and microglia with further damage to neurons.
NF-L is an axonal degeneration marker. CSF NF-L was the most sensitive neuronal marker from several targets tested including total and phosphorylated tau, soluble amyloid precursor proteins (APP) and Aβ fragments in a study evaluating 8 different degrees of HIV infection [32]. They also reported CSF NF-L was over 10-fold higher in only HIV-associated dementia (HAD) [32]. In another study, CSF NF-L levels were recognized as a sensitive neuronal injury marker in milder neurocognitive impairment in HIV infection [33–35]. CSF NF-L increased in HIV and correlated with MRS markers of neuronal injury [33]. Our results showed that NDE from impaired subjects had increased NF-L, which indicates neuronal damage.
Aβ has been associated with Alzheimer disease since its characterization. Aβ can trigger neuroinflammation, oxidative stress and cell signaling (Review [36]). We along with others have reported that Aβ is increased in the brains of HIV-infected subjects [37–39] and might contribute to an Alzheimer-like pathology or the development of HAND. However, to our knowledge there are no reports of an increase in AD among HIV-infected subjects. Therefore, we suggest that neuronal accumulation of Aβ may impair memory by direct toxicity or disruption in synaptic plasticity or the dissemination of Aβ in NDE. In HIV-related neurodegeneration, the role of Aβ42 in CSF is not clear and the reports are less consistent [40–42]. Aβ42 increased in CSF from HIV individuals with BBB disruption [43]. A recent report showed increased exosome secretion and increased exosomal Aβ cargo in HIV-infected brain endothelial cells [44]. Neuron-derived exosomes were initially studied to identify AD-related proteins Aβ42 and forms of tau that distinguished cognitively normal controls from patients with frontal temporal dementia or AD [8]. In a recent study, NDE showed increased Aβ in mild cognitive impairment and pre-AD patients [8]; mild cognitive impairment converting to AD had the highest Aβ level that decreased when a diagnosis of AD was made [45]. In the present study, we showed that Aβ increased in NDE from NPI subjects and this difference was not associated with HIV infection but rather with neurocognitive impairment.
Tetraspanins CD63 and CD81 are involved with γ-secretase activity and neurocognitive dysfunction [46]. Knockout of CD81 or use of CD81-deficient mice strongly decreased the activity of γ-secretase and subsequently decreased Aβ production [46]. CD63 was also found associated with the γ-secretase complex and presenilin interacting proteins [47, 48]. Our increased levels of CD63 and CD81 in NDE from NPI subjects were concomitant with increased Aβ levels.
When looking at exosomes as biomarkers of disease, one must consider normal aging and how this will affect the composition of exosomes. It will be important to investigate concentration and content changes in exosomes in age-related pathologies. A recent report showed that the concentration of extracellular vesicles (EV) from normal subjects decreased with aging [49]. Our data from controls showed no correlation; however, this discrepancy may due to the age of our controls, which spanned a relatively narrow range of middle age. In addition, the source of the exosomes would greatly influence concentration and content. With HIV infection, although the number of subjects observed was small, there appeared to be an increase in the concentration of NDE with age that may be specific to HIV infection. An increase in neuronal exosome secretion in vitro was reported to follow extensive synaptic activation [50]. Another report showed that premature cellular senescence caused an increase in secretion of exosomes [51]. Despite the increased concentration, we observed a significant decrease in HMGB1 protein and trending decrease in NF-L and Aβ with age. A recent report on aging found that EV content might change with normal aging [49]. While the function of HMGB1 in brain is little understood, it appears that it can regulate DNA repair mechanisms. In mouse brain, HMGB1 gradually decreased in neurons during aging, which was reported to be from an accumulation of DNA double-strand breaks [52]. In contrast, HMGB1 was increased in astrocytes with aging, with no demonstrable accumulation of DNA double-strand breaks. It’s interesting to speculate that a decrease in neuronal HMGB1 with aging may be associated with the accumulation of DNA damage guiding neuronal dysfunction.
The goal of this study was to begin to characterize blood neuron-derived exosomes that might identify neurocognitive changes associated with HIV infection. While the subject numbers are small, we are beginning to see some changes both in HIV-infected and normal subjects and more importantly, between cognitively impaired and unimpaired subjects. Our results did not show a direct correlation between HIV status and impairment nor an increase in stress markers in NDE only from HIV-infected subjects. This is encouraging since cognitive impairment occurs in only some individuals; not all individuals with HIV infection are impaired. Interestingly, in this study, even a few subjects with breakthrough viral load were not impaired showing that viral load did not always correlate with cognitive impairment. This study involves a small number of subjects and target proteins and needs further investigation. Factors that drive HIV-associated neurological involvement are probably not specific to HIV infection alone but rather a general neurodegenerative process. This study demonstrated the utility of isolating and characterizing NDE in HIV infection as a reflection of neuronal function in living subjects. By studying NDE, new neuronal stress proteins or profile can be identified to give an emerging liquid biopsy that can be followed throughout infection and with new therapies.
Acknowledgments
The authors thank all the blood donors for their participation. The authors also thank Edward J. Goetzl (UCSF) for providing NDE isolation protocols and helpful discussions and Archana Gupta (SBI) for exosome technical support. This study was supported by the National Institute of Health awards R01MH085538 and R21MH112483 (LP).
L.P. and B.S. designed the study. B.S. performed the study and analyzed the data. N.T. setup NanoSight parameters and P.D. performed analysis. L.A. performed neuropsychological tests. B.S. and N.T. refined exosome isolation procedure. L.P. and B.S. wrote the manuscript and all authors revised.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res. 2017;6:312. doi: 10.12688/f1000research.10651.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 4.Pulliam L, Gupta A. Modulation of cellular function through immune-activated exosomes. DNA and cell biology. 2015;34(7):459–463. doi: 10.1089/dna.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. Journal of neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. Journal of translational medicine. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J, et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PloS one. 2013;8(1):e48814. doi: 10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2015;11(6):600–607. e601. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(2):589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85(1):40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(12):4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. Journal of acquired immune deficiency syndromes. 2013;62(2):190–196. doi: 10.1097/QAI.0b013e31827b61f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Annals of clinical and translational neurology. 2015;2(7):769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(9):3097–3106. doi: 10.1096/fj.201600368RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 16.Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum Brain Mapp. 2017;38(4):1933–1940. doi: 10.1002/hbm.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A, et al. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13(5):541–549. doi: 10.1016/j.jalz.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 19.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PloS one. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seipold L, Saftig P. Corrigendum: The Emerging Role of Tetraspanins in the Proteolytic Processing of the Amyloid Precursor Protein. Front Mol Neurosci. 2017;10:37. doi: 10.3389/fnmol.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thali M. Tetraspanin functions during HIV-1 and influenza virus replication. Biochem Soc Trans. 2011;39(2):529–531. doi: 10.1042/BST0390529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray LR, Turville SG, Hitchen TL, Cheng WJ, Ellett AM, Salimi H, et al. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PloS one. 2014;9(2):e90620. doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Molecular neurobiology. 2012;45(3):499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 24.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature reviews Immunology. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 25.Venegas C, Heneka MT. Danger-associated molecular patterns in Alzheimer’s disease. J Leukoc Biol. 2017;101(1):87–98. doi: 10.1189/jlb.3MR0416-204R. [DOI] [PubMed] [Google Scholar]
- 26.Thierry S, Gozlan J, Jaulmes A, Boniface R, Nasreddine N, Strauss F, et al. High-mobility group box 1 protein induces HIV-1 expression from persistently infected cells. AIDS. 2007;21(3):283–292. doi: 10.1097/QAD.0b013e3280115b50. [DOI] [PubMed] [Google Scholar]
- 27.Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, et al. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J Trauma Acute Care Surg. 2012;72(3):643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Wang H, Zhao J, Pan H, Mao L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-kappaB pathway after traumatic brain injury in the rat. Mediators of inflammation. 2011;2011:807142. doi: 10.1155/2011/807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62(1):26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gougeon ML, Poirier-Beaudouin B, Durant J, Lebrun-Frenay C, Saidi H, Seffer V, et al. HMGB1/anti-HMGB1 antibodies define a molecular signature of early stages of HIV-Associated Neurocognitive Isorders (HAND) Heliyon. 2017;3(2):e00245. doi: 10.1016/j.heliyon.2017.e00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takata K, Kitamura Y, Kakimura J, Shibagaki K, Tsuchiya D, Taniguchi T, et al. Role of high mobility group protein-1 (HMG1) in amyloid-beta homeostasis. Biochem Biophys Res Commun. 2003;301(3):699–703. doi: 10.1016/s0006-291x(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 32.Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PloS one. 2014;9(12):e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. The Journal of infectious diseases. 2013;207(11):1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, et al. Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire JL, Gill AJ, Douglas SD, Kolson DL group CHA-RTER. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. Journal of neurovirology. 2015;21(4):439–448. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 37.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E Neurobehavioral Research C. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4(2):190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4(2):163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 39.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19(2):127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 40.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65(9):1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 43.Calcagno A, Atzori C, Romito A, Vai D, Audagnotto S, Stella ML, et al. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. Journal of neurovirology. 2016;22(1):88–92. doi: 10.1007/s13365-015-0371-x. [DOI] [PubMed] [Google Scholar]
- 44.Andras IE, Leda A, Contreras MG, Bertrand L, Park M, Skowronska M, et al. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol Cell Neurosci. 2017;79:12–22. doi: 10.1016/j.mcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakabayashi T, Craessaerts K, Bammens L, Bentahir M, Borgions F, Herdewijn P, et al. Analysis of the gamma-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol. 2009;11(11):1340–1346. doi: 10.1038/ncb1978. [DOI] [PubMed] [Google Scholar]
- 47.Seipold L, Damme M, Prox J, Rabe B, Kasparek P, Sedlacek R, et al. Tetraspanin 3: A central endocytic membrane component regulating the expression of ADAM10, presenilin and the amyloid precursor protein. Biochim Biophys Acta. 2017;1864(1):217–230. doi: 10.1016/j.bbamcr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Jeon AH, Bohm C, Chen F, Huo H, Ruan X, Ren CH, et al. Interactome analyses of mature gamma-secretase complexes reveal distinct molecular environments of presenilin (PS) paralogs and preferential binding of signal peptide peptidase to PS2. The Journal of biological chemistry. 2013;288(21):15352–15366. doi: 10.1074/jbc.M112.441840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eitan E, Green J, Bodogai M, Mode NA, Baek R, Jorgensen MM, et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Scientific reports. 2017;7(1):1342. doi: 10.1038/s41598-017-01386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376(1):128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]