Abstract
Objectives
The primary objective of this study was to characterize the pharmacokinetics of etonogestrel (ENG) released from a contraceptive implant in Ugandan women living with HIV who were receiving efavirenz (EFV)- or nevirapine (NVP)-based antiretroviral therapy (ART), compared to ART-naïve women over 24 weeks.
Design
Non-randomized, parallel-group study with 3 arms: ART-naïve, NVP-, or EFV-based ART (N=20 per group).
Methods
Sparse pharmacokinetic sampling of ENG, NVP or EFV were performed at screening, entry, and then 1, 4, 12 and 24 weeks post-implant insertion. The primary endpoint was ENG concentrations at week 24, compared between the ART-naïve group and each ART group, using geometric mean ratio (GMR) with 90% confidence intervals.
Results
Sixty participants competed the study and data from 58 participants are included; one participant each was excluded from the NVP group and EFV group due to a sample processing error and ART nonadherence, respectively. At week 24, geometric mean (GM) ENG was 362, 341, and 66 pg/mL in the ART-naïve, NVP, and EFV groups, respectively [GMR: NVP:ART-naïve 0.94 (0.90–1.01); EFV:ART-naïve 0.18 (0.17–0.20)]. NVP and EFV concentrations were lower at week 24 compared to pre-implant [NVP: GM 5.7 versus 6.8 mg/L, respectively, GMR 0.84 (0.83–0.85); EFV: GM 3.6 versus 4.9mg/L, respectively, GMR 0.73 (0.69–0.80)].
Conclusions
After 24 weeks of combined use, ENG exposure was 82% lower in women using EFV-based ART compared to ART-naïve women. In contrast, NVP did not significantly impact ENG exposure. These results raise concerns about reduced effectiveness of implantable contraception for women taking EFV-based ART.
Keywords: contraceptive implant, etonogestrel, efavirenz, nevirapine, family planning, antiretroviral therapy, drug-drug interaction
INTRODUCTION
Family planning services, including hormonal contraception, are essential to improving women’s reproductive health. Each year, effective contraception prevents 188 million unintended pregnancies, thereby averting 1.1 million newborn and 150,000 maternal deaths, as well as 112 million abortions worldwide [1, 2]. Absence of effective contraception carries even greater risk for the over 16 million women living with HIV worldwide, in whom prevention of unintended pregnancy through use of effective contraception is a successful and cost-effective strategy to decrease the rate of perinatal HIV transmission [3, 4]. Progestin-only contraceptive implants are highly effective, with <1% failure rate over 12-months in a pooled survey of 43 countries [5]. Recently, contraceptive implants are increasingly used in sub-Saharan Africa with high rates of acceptability and there were an estimated 21 million implant users in Africa by the end of 2015 [6]. Two forms of contraceptive implants are available, the etonogestrel (ENG) implant, approved for 3-years of use, and the levonorgestrel (LNG) implant, approved for 5-years of use.
Despite clear benefits of hormonal contraception, significant drug-drug interactions with some antiretroviral medications create a critical barrier to successful contraceptive use in women living with HIV. The progestin hormone concentration released from the implant is low relative to other systemic hormonal contraceptive methods, making this method particularly vulnerable to contraceptive failure due to drug-drug interactions. Hormonal contraceptives are primarily metabolized by the cytochrome P450 (CYP) enzyme system, and some non-nucleoside reverse transcriptase inhibitors (NNRTIs), such as efavirenz (EFV) and nevirapine (NVP), are CYP inducers [7, 8]. Nevirapine and EFV are widely prescribed in sub-Saharan Africa, and EFV-based ART is currently the preferred first-line treatment regimen recommended by the World Health Organization (WHO) for adults living with HIV, including women of reproductive age [10]. In addition, international guidelines recommend that all adults living with HIV should be treated with ART regardless of CD4 count, thus approximately 13 million women living with HIV in sub-Saharan Africa are candidates for EFV-based ART [9].
Of concern, several pharmacokinetic studies of hormonal contraceptives have demonstrated a significant decrease in LNG and ENG exposure, after either oral or subdermal administration, when combined with EFV compared to when used alone, including LNG emergency contraception [10], desogestrel combined oral contraceptive pill [11], and ENG and LNG implants [12, 13]. Vieira and colleagues reported when the ENG implant was used with EFV-based ART, the ENG median minimum concentration over 24 weeks was 61.9 pg/mL [12], which is below the reported ovulation suppression threshold for ENG of 90 pg/mL [14]. These pharmacokinetic data are supported by an additional six case reports of ENG implant contraceptive failures with combined EFV use [15–18], as well as three recent clinical studies that reported pregnancy rates of 5.5% to 15% in women using LNG contraceptive implants while taking EFV-based ART [13, 19, 20]. A large retrospective cohort reported a pregnancy rate of 3.0 per 100 woman-years (95% CI 1·4–4·7) among women using EFV-based ART with the ENG implant [19]. These findings are in stark contrast to the <1% pregnancy rate reported in women using the contraceptive implant without a co-administered interacting drug [5, 21].
The primary aim of this study was to estimate the effect of EFV- or NVP-based ART on the pharmacokinetics of ENG released from a subdermal contraceptive implant in women living with HIV over 24 weeks of combined use. Our secondary objective was to characterize the pharmacokinetics of EFV and NVP pre- versus post-insertion of the ENG implant.
METHODS
We conducted a nonrandomized, open-label, parallel group, pharmacokinetic study of Ugandan women living with HIV. All study procedures occurred at the Infectious Disease Institute (IDI) in Kampala, Uganda and were approved by the University of Pittsburgh (PRO14010195), the Joint Clinical Research Centre, and Uganda National Council of Science and Technology (HS 1618). This study followed the Declaration of Helsinki and was registered at clinicaltrials.gov (NCT02082652).
Participants
Family planning services are integrated into HIV care at IDI, thus all women receiving care at IDI receive comprehensive contraceptive counseling. Women at least 18 years of age who desired the contraceptive implant were offered study participation and informed consent was obtained before the initiation of screening procedures. Women were included if they were: 1) not yet taking ART (ART-naïve group), 2) receiving NVP-based ART (NVP group; NVP 200mg twice daily + two nucleoside reverse transcriptase inhibitors (NRTIs)), or 3) receiving EFV-based ART (EFV group; EFV 600mg once daily + two NRTIs). Participants in the ART groups were on stable ART for at least 1 month prior to enrollment. Participants using EFV-based ART were additionally required to use a copper intrauterine device (IUD) to minimize the risk of unintended pregnancy, due to accumulating data of observed pregnancies in women receiving contraceptive implants plus EFV-based ART that was not observed with NVP-based ART [13, 20]. The IUD was placed by a trained member of the study team according to the WHO Medical Eligibility Criteria for Contraceptive Use [22].
We excluded participants from the study if they had plasma HIV RNA greater than 400 copies per milliliter (ART groups only); had laboratory evidence of anemia (hemoglobin less than 9.0 gm/dL), liver disease (serum alanine transaminase above 5 times the upper limit of normal), or renal disease (serum creatinine above 2.5 times the upper limit of normal); or if they were currently pregnant or less than thirty days postpartum. Participants were also excluded if they were taking a medication contraindicated for use with ENG, EFV (EFV group only), or NVP (NVP group only); other medications with CYP-inducing (e.g. rifampin) or -inhibiting (e.g. ritonavir) potential; or other hormonal contraceptive medications [21, 23].
Study Procedures
Within 30 days of screening, participants who met the study eligibility criteria returned for the enrollment visit. After a negative pregnancy test, a trained study team member placed an ENG implant (Implanon® 68 mg) subdermally in the upper inner arm [21]. The most recent CD4 count was abstracted from the participant’s medical record. Study visits occurred 1, 4, 12 and 24 weeks after implant placement. During each visit, staff assessed adverse events, updated a record of concomitant medications, and performed a urine pregnancy test. Participants were asked about implant-associated side effects using a structured questionnaire. Adherence to ART was assessed at each visit by participant report. Condom use was encouraged for all study groups at every visit to provide a second form of non-hormonal contraception and to prevent HIV transmission. At the completion of the study, participants could choose to keep the ENG implant and/or the IUD (as applicable), or have them removed by a study clinician.
Pharmacokinetic Assessment
We collected blood samples to analyze ENG concentration at weeks 1, 4, 12 and 24 after ENG implant placement. For participants on ART, a single, timed blood sample was collected to analyze the NNRTI concentration at the screening and enrollment visits (two samples prior to implant placement were collected in each participant), then at 4, 12, and 24 weeks post-implant insertion. For participants on NVP-based ART, the pharmacokinetic sampling was performed 11–13 hours after taking their last NVP dose. For participants on EFV-based ART, blood sampling was performed 12–14 hours after their last EFV dose. For each sample, whole blood was collected into vacutainers containing ethylenediaminetetraacetic acid anticoagulant. Samples were transported by a dedicated laboratory runner from the Private Ward in Mulago Hospital to the Makerere-University Johns Hopkins University Core Laboratory, where they were centrifuged at 3000 revolutions per minute for 10 minutes to separate plasma within one hour of collection. Plasma aliquots were stored at −70° Celsius until they were analyzed.
Etonogestrel plasma samples were shipped on dry ice to the University of Pittsburgh Small Molecule Biomarker Core Laboratory. ENG levels were quantitated using a modified method of high performance liquid chromatography (HPLC) linked to mass spectrometry (LC-MS/MS) [24]. The assay was validated over the calibration range of 25–1000 pg/mL. The interday relative standard deviations were 12.4% for the 35 pg/ml quality control (QC), 6.5% for the 175 pg/ml QC and 4.2% for the 875 pg/ml QC.
Antiretroviral plasma concentrations were analyzed at the Infectious Diseases Institute Translational Laboratory, Makerere University, using previously described HPLC methods with ultraviolet detection [25, 26]. The EFV assay was validated over a calibration range of 0.2–10 mg/L. The EFV interday precision was between 2.82% and 5.25%; accuracy was between 93.6% and 107%. The NVP assay was validated over a calibration range of 0.05–16.1mg/L. The interday NVP precision was between 1.15% and 4.64%; accuracy was between 94.3% and 108.4%. The laboratory participates in an external quality assurance program for antiretroviral assays: Kwaliteitsbewaking Klinische Geneesmiddelanalyse en Toxicologie, the Netherlands (http://kkgt.nl/). All pharmacokinetic assays were validated in accordance with guidance from the US Food and Drug Administration [27].
Statistical Analysis
A sample size of 20 participants per group provided 80% power to detect a 35% change (one standard deviation) between groups in the ENG concentration at week 24, using an alpha level of 0.05, and allowed for up to 20% participant attrition (leaving at least 16 participants per group). This calculation considers a previously reported ENG mean concentration of 297.5 pg/mL (SD 104.5 pg/mL) with reported interpatient variability of 35%, from 15 women at 6 months post-implant insertion [28].
The ENG geometric mean (GM) concentration with 90% confidence intervals (CI) was summarized by study visit and compared between the ART-naïve group and each ART group as a GM ratio (GMR) with 90% CI. Each participant’s ENG area under the concentration-time curve from weeks 0 to 24 (AUC0–24) was determined using the trapezoidal rule (Phoenix WinNonlin, Certara®). To evaluate the impact of ENG on the NNRTI concentration, the EFV or NVP GM concentration was compared pre- (at screening) to post- (at week 24) implant insertion as a GMR with 90% CI. NNRTI and ENG concentrations were considered bioequivalent if the 90% CI of the GMR fell between 0.8 and 1.25 in accordance to guidance by the Food and Drug Administration [27]. All safety and adverse event data were characterized using the DAIDS severity tables [29], descriptively summarized and compared using the Kruskal–Wallis or Wilcoxon rank sum tests for continuous data, and a χ2 test for discrete data. All statistical analyses were conducted using IBM SPSS Statistics.
RESULTS
Demographic Characteristics
Between September 2014 and July 2015, 64 participants were screened for the study. Four participants were excluded; three participants for detectable HIV RNA (>400 copies/mL) while taking EFV- or NVP-based ART, and one participant for unprotected intercourse within two weeks prior to enrollment. Of the 60 participants enrolled, all completed the primary endpoint at week 24; however, one participant was excluded from the NVP group (missing sample) and one participant was excluded from the EFV group (ART nonadherence), leaving 58 participants eligible for the primary analysis (Figure 1). Overall, the median age of the study population was 29 years (interquartile range [IQR]: 25–34), and 62% of the participants were married with a median parity of 3 (IQR: 2–4). Overall, the median weight and body mass index was 57.8 kg (IQR: 51.3–67.8) and 23.0 kg/m2 (IQR: 20.8–26.7), respectively. The ART-naïve group had a higher median body weight than EFV group (p=0.045). The demographic characteristics of the study population by group are summarized in Table 1.
Figure 1.
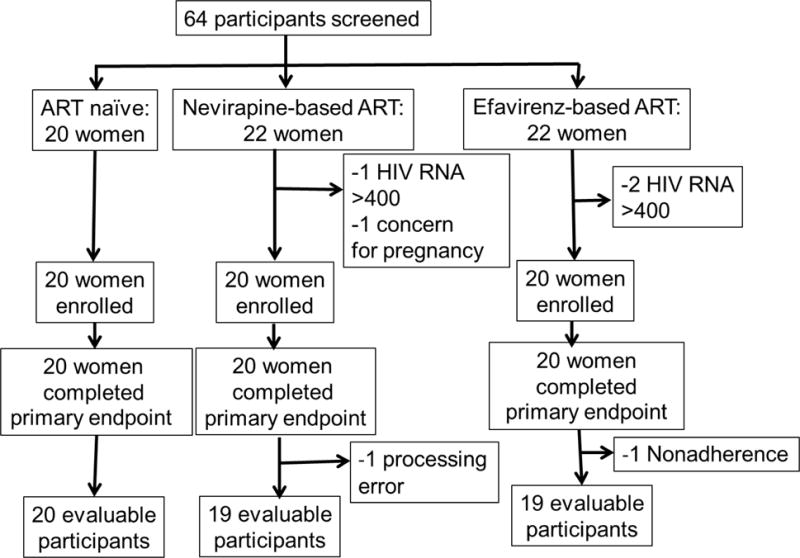
Study Flow Chart
Table 1.
Baseline Characteristics of Participants by Study Group1
| ART-Naïve Group (N=20) |
NVP Group (N=19) |
EFV Group (N=19) |
|
|---|---|---|---|
| Age (year) | 27.5 (25.0–30.0) |
32.0 (28.0–34.5) |
29.0 (25.0–34.0) |
| Weight (kg) | 65.5 (55.3–71.5) |
56.0 (52.5–69.5) |
57.0 (48.5–59.5) |
| BMI (kg/m2) | 25.1 (21.5–28.8) |
22.1 (21.3–26.5) |
22.3 (20.7–23.6) |
| Married, n (%) | 8 (40%) | 14 (74%) | 14 (74%) |
| Prior Live Births | 2.0 (1.8–3.0) |
3.0 (2.0–4.0) |
3.0 (2.0–4.0) |
| CD4 Count (cells/mL) | 884 (690–1125) |
552 (439–719) |
549 (378–990) |
| Duration on current ART regimen (Months) | ----- | 32.1 (27.0–53.6) |
23.3 (17.8–25.5) |
Data are represented either as n (%) or median (interquartile range), as appropriate
ART= Antiretroviral Therapy, NVP=Nevirapine, EFV=efavirenz, BMI = body mass index
Etonogestrel Pharmacokinetics
ENG pharmacokinetic data are summarized in Table 2 and Figure 2. In the EFV group, ENG concentrations were significantly lower at each visit compared to the ART-naïve group [Week 24 GMR: 0.18 (0.17–0.20)]. In contrast, there were no significant differences in the ENG concentrations in the NVP group compared to the ART-naïve group at weeks 12 and 24 after implant placement [Week 24 GMR: 0.94 (0.90–1.01)]. The ENG GM AUC0–24 was 11.12, 10.47, and 1.80 ng*wk/mL in the ART-naïve, NVP, and EFV groups, respectively [AUC0–24 GMR: NVP:ART-naïve 0.94 (0.94–0.94); EFV:ART-naïve 0.16 (0.16–0.16)]. The number of participants in the EFV group with ENG concentrations below 90 pg/mL (reported threshold for ovulation suppression [14]) was 9 (47%), 16 (84%), and 18 (95%) at weeks 4, 12 and 24, respectively. In contrast, all participants in the ART-naïve and NVP groups had ENG concentrations above 90 pg/mL throughout the 6-month study duration.
Table 2.
Etonogestrel Plasma Concentrations over 24 weeks (pg/mL)
| Weeks from Implant Insertion | ART-Naïve Group (N=20)1 |
NVP Group (N=19)1 |
EFV Group (N=19)1 |
GMR NVP:ART-Naive2 | GMR EFV:ART-Naive2 |
|---|---|---|---|---|---|
| 1 | 831 (738, 924) |
647 (548, 746) |
137 (118, 156) |
0.78 (0.74–0.81) |
0.16 (0.16–0.17) |
| 4 | 507 (445, 568) |
457 (394, 521) |
87 (74, 99) |
0.90 (0.88–0.91) |
0.17 (0.17–0.17) |
| 12 | 441 (382,500) | 453 (398, 509) |
63 (55, 71) |
1.03 (1.02–1.04) |
0.14 (0.14–0.14) |
| 24 | 362 (308, 415) |
341 (310, 373) |
66 (60, 72) |
0.94 (0.90–1.01) |
0.18 (0.17–0.20) |
Geometric mean with 90% confidence intervals
Geometric mean ratio (GMR) with 90% confidence intervals
ART= Antiretroviral Therapy, NVP=Nevirapine, EFV=efavirenz
Figure 2.
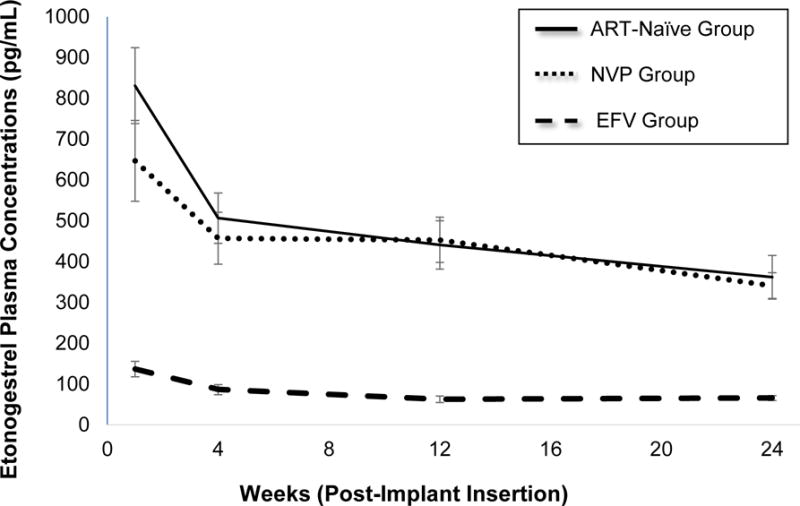
Etonogestrel (ENG) geometric mean concentrations (pg/mL) with 90% confidence intervals over 24 weeks
Antiretroviral Pharmacokinetics
NVP and EFV geometric mean concentrations measured throughout the study are summarized in Table 3. NVP concentrations remained bioequivalent from baseline (screening) to week 24 [GMR 0.84 (0.83–0.85)]. However, EFV concentrations were not bioequivalent from baseline to week 24 [GMR 0.73 (0.69–0.80)]. Despite this decrease in EFV concentrations, there were no participants in the EFV group with plasma EFV concentrations less than 1 mg/L, the proposed minimum threshold for mid-dosing interval EFV concentrations [30].
Table 3.
Geometric mean nevirapine (NVP) and efavirenz (EFV) plasma concentrations (mg/L) before and after etonogestrel implant insertion
| Time from implant insertion | NVP (90%CI) | EFV (90%CI) |
|---|---|---|
| Pre-insertion1 | 6.8 (5.5–8.0) | 4.9 (3.2–6.5) |
| Week 4 | 6.7 (5.6–7.8) | 4.0 (2.6–5.5) |
| Week 12 | 6.1 (4.9–7.3) | 3.7 (2.8–4.7) |
| Week 24 | 5.7 (4.7–6.8) | 3.6 (2.6–4.5) |
| GMR2 | 0.84 (0.83–0.85) | 0.73 (0.69–0.80) |
Geometric mean of antiretroviral concentration at screening
Geometric mean ratio (GMR) of week 24:pre-insertion.
Adverse Events
There were 298 reported adverse events, 297 were mild intensity (Grade 1) and one of moderate intensity (Grade 2). The one adverse event of moderate intensity was due to menorrhagia in the NVP group. The cumulative adverse events that were possibly related to the ENG implant are summarized in the supplemental table. None of the participants discontinued study participation or implant use due to an adverse event.
DISCUSSION
In this study, we found that women living with HIV who were using the ENG contraceptive implant in combination with EFV-based ART had 82% lower ENG plasma concentrations at week 24 compared to ART-naïve women. Importantly, the reported ENG concentration threshold for ovulation suppression is 90 pg/mL [14], and we observed GM ENG concentrations below this threshold by week 4. These results suggest that the combined use of EFV-based ART and the ENG implant could decrease contraceptive efficacy of the implant as early as one month after implant placement in women taking concomitant EFV-based ART. Our findings are similar to a Brazilian study by Vieira et al. who noted 63.4% lower serum ENG AUC over 24 weeks for women using the ENG implant with EFV compared to those not on ART [12]. These findings are also similar to the Ugandan study of the LNG implant, which found 47% lower LNG concentrations for women on EFV-based ART when compared to those that were ART-naïve [13].
In contrast, NVP-based ART did not affect the ENG exposure from the implant at weeks 12 and 24 after implant placement. We noted a decrease in the ENG concentrations at 1 and 4 weeks after implant placement (21% and 10%, respectively) when comparing the ART:naïve group to the NVP group; however, this small magnitude of change, so early in the product life when concentrations are highest, is not likely to have a clinically meaningful effect on the contraceptive efficacy. This study provides reassurance that the ENG implant may be safely combined with NVP-based ART regimens. These findings are also similar to those reported with the LNG implant in Ugandan women; LNG plasma concentrations at week 24 were similar for women on NVP-based ART compared to ART-naïve women [13]. Despite this encouraging finding, NVP-based ART is no longer a preferred first-line ART strategy due to an increased risk of serious and potentially fatal hypersensitivity reactions and decreased efficacy when compared to EFV [31]. Unfortunately, many women living with HIV must choose between a more effective and better tolerated EFV-based ART regimen and an effective and highly desired implantable contraceptive method.
Etonogestrel is primarily metabolized by the CYP3A4 enzyme [32], and both EFV and NVP are CYP3A4 inducers [8, 32]. Therefore, the decreased ENG exposure observed with concomitant EFV-based ART use is likely due to CYP3A4 enzyme induction and thus increased metabolism of ENG. Interestingly, NVP is also a CYP3A4 inducer, and it remains unclear why a similar effect is not observed with concomitant NVP-based ART and either ENG- or LNG-based contraceptive implants [32]. It is possible that EFV is a more potent CYP3A4 inducer than NVP. Alternatively, other complex drug metabolic or drug transport pathways for ENG and LNG could be responsible for the interaction observed with EFV, yet not with NVP. Our study is limited by not evaluating ENG metabolites that perhaps could clarify the mechanism for this interaction, such as any additional role of glucuronidation. These mechanisms have yet to be fully elucidated, and indicate a knowledge gap in steroid contraceptive metabolism.
We observed a greater reduction in ENG in our Ugandan population as compared to a Brazilian population [12] (84 vs 63% reduction in AUC over 24 weeks, respectively). This result may be due to inter-individual variability among two small pharmacokinetic studies (n=20 and 15 per group, respectively), analytic differences between detecting ENG in the plasma versus serum, variation in drug disposition between study populations, or body weight differences. Higher body weight has been associated with decreased ENG exposure [33], and the ART-naïve group had a higher median body weight than the EFV group in our study. However, the lower body weight noted in the EFV group relative to the ART-naïve group would imply that our estimates for impact of EFV on ENG exposure are conservative. With regard to genetic variations between study populations, Neary et al. recently identified that Ugandan women expressing pharmacogenetic variations in CYP2B6 resulting in slow metabolism of EFV, and therefore higher overall EFV exposure, had lower LNG exposure when LNG-subdermal implant use was combined with EFV-based ART compared to Ugandan women without pharmacogenetic CYP2B6 variations [34]. Given that polymorphisms influencing CYP2B6 are more common in some African populations, these women may be at greater risk of drug-drug interactions that jeopardize the contraceptive effectiveness of LNG and ENG implants with concomitant EFV use. Future evaluations are needed to confirm the influence of pharmacogenetic diversity on both LNG and ENG disposition.
Our study was a pharmacokinetic evaluation and did not include pharmacodynamic biomarkers of contraceptive efficacy such as inhibition of ovulation or cervical mucus thickening; thus, the clinical correlation between contraceptive efficacy and these pharmacokinetic observations cannot be confirmed. The participants in the EFV group had a copper IUD in place during the study to avoid unintended pregnancies. However, blood progestin concentrations have commonly been used as a marker for contraceptive efficacy for implantable and other hormonal contraceptives. For example, three pregnancies were reported in a total of twenty study participants on EFV-based ART while using the LNG implant and these pregnancies were associated with low plasma LNG concentrations [13]. In addition, retrospective analyses confirm that contraceptive failures are more frequent in women receiving EFV-based ART in combination with contraceptive implants [19, 20, 35]. Thus, decreased ENG exposure in the setting of concomitant EFV-based ART reported here is concerning for increased risk of contraceptive failures.
We also found a decrease in EFV exposure when comparing EFV concentrations measured prior to implant insertion to those at 24 weeks after ENG implant insertion. Notably, three participants had significant decreases in EFV concentrations over the study duration, and six others had modest decreases in EFV concentrations of at least 1 mg/L from screening to week 24 post-implant placement. None of the participants in the EFV group ever had EFV concentrations less than 1 mg/L, which is the proposed mid-dosing interval concentration related to antiretroviral effectiveness [30]. This indicates that the decrease in EFV concentration is likely not clinically significant. The reason for this finding is unclear. Lower EFV concentrations at 24 weeks post-implant insertion could be due to non-adherence, incorrect timing of drug doses, or a drug-drug interaction. One other study found statistically lower EFV concentrations when given with an oral contraceptive pill containing ethinyl estradiol/desogestrel (EFV concentrations: 3.3 vs. 2.7 mg/L; p=0.03) [36]. However, Cohn et al. showed no change in EFV exposure before depot medroxyprogesterone acetate injection compared to 4 or 12 weeks after injection [37]. Scarsi et al. reported that there was no change in either EFV or NVP concentrations before and after LNG implant insertion [13]. Unfortunately, we do not have HIV viral load data at week 24 after implant insertion to more fully evaluate the clinical significance of this decline in EFV concentration following insertion of the ENG implant.
Though our study relied on self-reported adherence to ART, the measured EFV and NVP concentrations from participants reflect recent adherence to these antiretrovirals, although we cannot confirm perfect ART adherence over the entire study duration. Notably, ART non-adherence may result in underestimation of the effect of EFV or NVP on ENG.
In summary, we present a pharmacokinetic evaluation of the subdermal ENG implant used concomitantly with two first-line ART regimens commonly used in sub-Saharan Africa. Our study of Ugandan women is of critical public health importance to women in sub-Saharan Africa who are at greatest risk for living with HIV, as well as for pregnancy-related mortality or complications. Contraceptive implants represent a highly desirable method of contraception due to their high efficacy and tolerability; thus, their combined use with EFV-based ART is expected to increase in sub-Saharan Africa. Of grave concern is the consistency of these and other similar published pharmacokinetic results, combined with the published clinical findings of increased contraceptive failures in women using EFV-based ART and contraceptive implants. Future studies are urgently needed and should explore alternate strategies for provision of safe, effective implantable contraception for women on EFV-based ART.
Supplementary Material
Acknowledgments
FUNDING
This study was supported by the Society of Family Planning Research Fund grant #SFPRF14-12 and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health [5R21HD074462 and 1R01HD085887 (Scarsi) and by NIH/ORWH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) NIH K12HD043441 to Chappell. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors gratefully acknowledge the contributions of the women who participated in this study and express sincere appreciation to the members of the study team including Isabella Kyohairwe, Frank Mubiru and Henry Onen.
CAC is receiving research funding from Gilead Sciences through Magee-Womens Research Institute (MWRI) and has served as a consultant for Gilead Sciences. ML has received research funding from Janssen Pharmaceutica and ViiV. BAC is receiving research funding from Merck, Bayer and Medicines360 through MWRI. SAR is receiving research funding from Gilead Sciences and GlaxoSmithKline managed through the University of Pittsburgh. SLA has been a consultant for Merck and is receiving research funding from PRA Health Sciences through MWRI.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
The other authors have no conflicts of interest to disclose.
These data were presented at HIV Drug Therapy Glasgow on October 26, 2016 in Glasgow, Scotland and at the North American Forum on Family Planning on November 5, 2016 in Denver, CO.
Authors’ contributions: CAC, KKS, ML, SN, SLA, BAC, SEC, SAR and KMD were involved with conception and design. SN, ML, CAC, SLA, BAC and HM were involved with performance of the study. CAC performed the descriptive statistics and KKS preformed the pharmacokinetic analysis. CAC drafted the manuscript. KKS, ML, SLA, BAC, SEC, KMD and SAR edited the text. All authors have read the manuscript and approved the text.
References
- 1.Darroch JE. Trends in contraceptive use. Contraception. 2013;87:259–263. doi: 10.1016/j.contraception.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Darroch JE, Singh S. Trends in contraceptive need and use in developing countries in 2003, 2008, and 2012: an analysis of national surveys. Lancet. 2013;381:1756–1762. doi: 10.1016/S0140-6736(13)60597-8. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds HW, Janowitz B, Homan R, Johnson L. The value of contraception to prevent perinatal HIV transmission. Sexually transmitted diseases. 2006;33:350–356. doi: 10.1097/01.olq.0000194602.01058.e1. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds HW, Janowitz B, Wilcher R, Cates W. Contraception to prevent HIV-positive births: current contribution and potential cost savings in PEPFAR countries. Sexually transmitted infections. 2008;84(Suppl 2):ii49–53. doi: 10.1136/sti.2008.030049. [DOI] [PubMed] [Google Scholar]
- 5.Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception. 2016;94:11–17. doi: 10.1016/j.contraception.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvall S, Thurston S, Weinberger M, Nuccio O, Fuchs-Montgomery N. Scaling up delivery of contraceptive implants in sub-Saharan Africa: operational experiences of Marie Stopes International. Global health, science and practice. 2014;2:72–92. doi: 10.9745/GHSP-D-13-00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert opinion on drug metabolism & toxicology. 2013;9:559–572. doi: 10.1517/17425255.2013.772579. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JA, Jamshidi R, Burke AE. Contraception for the HIV-positive woman: a review of interactions between hormonal contraception and antiretroviral therapy. Infectious diseases in obstetrics and gynecology. 2012;2012:890160. doi: 10.1155/2012/890160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretroviral therapy for treating and preventing HIV infection: Recommendations for a public health approach. second. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 10.Carten ML, Kiser JJ, Kwara A, Mawhinney S, Cu-Uvin S. Pharmacokinetic interactions between the hormonal emergency contraception, levonorgestrel (Plan B), and Efavirenz. Infectious diseases in obstetrics and gynecology. 2012;2012:137192. doi: 10.1155/2012/137192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landolt NK, Phanuphak N, Ubolyam S, Pinyakorn S, Kerr S, Ahluwalia J, et al. Significant decrease of ethinylestradiol with nevirapine, and of etonogestrel with efavirenz in HIV-positive women. Journal of acquired immune deficiency syndromes. 2014;66:e50–52. doi: 10.1097/QAI.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 12.Vieira CS, Bahamondes MV, de Souza RM, Brito MB, Rocha Prandini TR, Amaral E, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. Journal of acquired immune deficiency syndromes. 2014;66:378–385. doi: 10.1097/QAI.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 13.Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika-Kibwika P, Else LJ, et al. Unintended Pregnancies Observed With Combined Use of the Levonorgestrel Contraceptive Implant and Efavirenz-based Antiretroviral Therapy: A Three-Arm Pharmacokinetic Evaluation Over 48 Weeks. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62:675–682. doi: 10.1093/cid/civ1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz S, Pavez M, Moo-Young AJ, Bardin CW, Croxatto HB. Clinical trial with 3-keto-desogestrel subdermal implants. Contraception. 1991;44:393–408. doi: 10.1016/0010-7824(91)90030-j. [DOI] [PubMed] [Google Scholar]
- 15.Lakhi N, Govind A. Implanon failure in patients on antiretroviral medication: the importance of disclosure. The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2010;36:181–182. doi: 10.1783/147118910791749164. [DOI] [PubMed] [Google Scholar]
- 16.Matiluko AA, Soundararjan L, Hogston P. Early contraceptive failure of Implanon in an HIV-seropositive patient on triple antiretroviral therapy with zidovudine, lamivudine and efavirenz. The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2007;33:277–278. doi: 10.1783/147118907782101724. [DOI] [PubMed] [Google Scholar]
- 17.McCarty EJ, Keane H, Quinn K, Quah S. Implanon(R) failure in an HIV-positive woman on antiretroviral therapy resulting in two ectopic pregnancies. International journal of STD & AIDS. 2011;22:413–414. doi: 10.1258/ijsa.2009.009469. [DOI] [PubMed] [Google Scholar]
- 18.Leticee N, Viard JP, Yamgnane A, Karmochkine M, Benachi A. Contraceptive failure of etonogestrel implant in patients treated with antiretrovirals including efavirenz. Contraception. 2012;85:425–427. doi: 10.1016/j.contraception.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Patel RC, Onono M, Gandhi M, Blat C, Hagey J, Shade SB, et al. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: a retrospective cohort study. The lancet HIV. 2015;2:e474–482. doi: 10.1016/S2352-3018(15)00184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry SH, Swamy P, Preidis GA, Mwanyumba A, Motsa N, Sarero HN. Implementing the Jadelle implant for women living with HIV in a resource-limited setting: concerns for drug interactions leading to unintended pregnancies. AIDS. 2014;28:791–793. doi: 10.1097/QAD.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 21.Implanon® [package insert] Roseland, NJ: Organon USA Inc.; Nov, 2008. [Google Scholar]
- 22.World Health Organization. Medical eligibility criteria for contraceptive use. Fourth. Geneva: World Health Organization; 2009. [Google Scholar]
- 23.HIV Drug Interactions. University of Liverpool; http://hiv-druginteractions.org. Accessed February 3, 2014. [Google Scholar]
- 24.Moser C, Zoderer D, Luef G, Rauchenzauner M, Wildt L, Griesmacher A, et al. Simultaneous online SPE-LC-MS/MS quantification of six widely used synthetic progestins in human plasma. Analytical and bioanalytical chemistry. 2012;403:961–972. doi: 10.1007/s00216-011-5612-0. [DOI] [PubMed] [Google Scholar]
- 25.Almond LM, Edirisinghe D, Dalton M, Bonington A, Back DJ, Khoo SH. Intracellular and plasma pharmacokinetics of nevirapine in human immunodeficiency virus-infected individuals. Clinical pharmacology and therapeutics. 2005;78:132–142. doi: 10.1016/j.clpt.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Almond LM, Hoggard PG, Edirisinghe D, Khoo SH, Back DJ. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. The Journal of antimicrobial chemotherapy. 2005;56:738–744. doi: 10.1093/jac/dki308. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Bioanalytical Method Validation. 2013 Sep; Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107. Accessed January 21, 2017.
- 28.Korver T, et al. Merck Internal Document. 1996 Sep; [Google Scholar]
- 29.US Department of Heath and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. 2014 Version 2.0. Available at: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed 21 February 2017.
- 30.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 31.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed: 9 Feb 2016. [Google Scholar]
- 32.Korhonen T, Tolonen A, Uusitalo J, Lundgren S, Jalonen J, Laine K. The role of CYP2C and CYP3A in the disposition of 3-keto-desogestrel after administration of desogestrel. British journal of clinical pharmacology. 2005;60:69–75. doi: 10.1111/j.1365-2125.2005.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mornar S, Chan LN, Mistretta S, Neustadt A, Martins S, Gilliam M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. American journal of obstetrics and gynecology. 2012;207:110e111–116. doi: 10.1016/j.ajog.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Neary M, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, et al. The Effect of Gene Variants on Levonorgestrel Pharmacokinetics when Combined with Antiretroviral Therapy containing Efavirenz or Nevirapine. Clinical pharmacology and therapeutics. 2017 doi: 10.1002/cpt.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug-Drug Interactions, Effectiveness, and Safety of Hormonal Contraceptives in Women Living with HIV. Drug safety. 2016;39:1053–1072. doi: 10.1007/s40264-016-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landolt NK, Phanuphak N, Ubolyam S, Pinyakorn S, Kriengsinyot R, Ahluwalia J, et al. Efavirenz, in contrast to nevirapine, is associated with unfavorable progesterone and antiretroviral levels when coadministered with combined oral contraceptives. J Acquir Immune Defic Syndr. 2013;62:534–539. doi: 10.1097/QAI.0b013e31827e8f98. [DOI] [PubMed] [Google Scholar]
- 37.Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clinical pharmacology and therapeutics. 2007;81:222–227. doi: 10.1038/sj.clpt.6100040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


