Abstract
Background
Antipsychotic combinations (CA) are prescribed in schizophrenia despite limited evidence of efficacy. To explore the effect of switching from CA to monotherapy, we performed an exploratory analysis of the PROACTIVE study data, in which 305 patients with schizophrenia and schizoaffective disorder were followed for 30-months after randomization to long-acting-injectable (LAI) risperidone or oral 2nd-generation antipsychotic (OA).
Methods
Patients who entered the PROACTIVE study on CA (n=50), LAI (n=20) or OA (n=206), were compared in terms of time to relapse and clinical measures.
Findings
The OA group had significantly fewer hospitalizations than the CA group (p=0.009) at baseline. In the CA group, 68% patients relapsed vs. 53% in the LAI, and 52% in the OA groups. While there was no significant difference in the relapse rate among groups on chi-square test (χ2 = 3.85, d.f. = 2, p = 0.146), the log-rank test showed a significant difference among the groups in time to first relapse (χ2 = 6.81, d.f. = 2, p = 0.033), with significantly longer time to relapse in the OA group (mean 562.8 days) than in the CA group (mean 409.5, p = 0.011). The LAI group’s mean time to first relapse (594 days) was not significantly different from the other groups. However, after adjusting for number of hospitalizations, group was no longer significant (hazard ratio = 1.541, p = 0.052).
Implications
Based on our exploratory analysis, taking antipsychotic combinations predicts earlier relapse and calls for additional treatment guidance in schizophrenia.
Keywords: schizophrenia, antipsychotic, combinations, prognosis, relapse
Introduction
Combination antipsychotics (CA) are prescribed in 10–30% cases of schizophrenia notwithstanding the risks and limited evidence of efficacy1, 2, 3. The American Psychiatric Association Schizophrenia Patient Outcomes Research Team and the UK National Institute for Health and Clinical Excellence treatment guidelines for schizophrenia do not offer guidance beyond clozapine augmentation4, 5, 6, 7. The Texas Medication Antipsychotic Algorithm mentions that two antipsychotics with different receptor profiles can be used after failure of clozapine combination with either a 1st or 2nd generation antipsychotic8. Royal Australian and New Zealand College of Psychiatrists guidelines for schizophrenia management9 cautiously recommend CA for patients who fail clozapine and advise careful monitoring due to high risk for side effects, increased hospitalizations and increased mortality. The rate of CA prescribing in schizophrenia varied from 15% –55% in the US in 20021, to 17–31% in a sample from the Danish health registry10, 30.7% in Norway11 and 19.6% in a review of studies published worldwide between 1970 and 200912. CA use was associated with higher number of patients per psychiatrist, younger age, living alone, treatment with clozapine, long acting injectable antipsychotics, antidepressants and anticholinergic medications, more hospital admissions, and higher Positive and Negative Syndrome Scale and lower Global Assessment of Function scores10, 11. Antipsychotic combinations are associated with high side effect burden (Parkinsonian side effects, hyperprolactinemia, sexual dysfunction, hyper-salivation, sedation, cognitive impairment and diabetes), but are often prescribed because of possible pharmacodynamic rationale, including optimizing dopamine D2 receptor occupancy, achieving a broad receptor coverage and minimizing side effects from high dose of a single drug1, 13. Beyond consistent association with inpatient treatment10, 11, 12, longitudinal progression of antipsychotic treatment leading to initiation of CA (rather than switching over to another antipsychotic or clozapine) is poorly understood. A chart-review of 100 antipsychotic-naïve outpatients with schizophrenia in Japan showed that, over two years, 17.8% of patients were placed on CA after a median of 84 days following the initiation of monotherapy and, in many cases (47.4%), before a presumably full effective dose was achieved14. There is limited information about the outcome of switching from CA to monotherapy. In a case study of 25 patients on CA who were switched to monotherapy, Suzuki et al.15 reported significant reduction in antipsychotic dose, lower number of total psychotropic medications and progress towards discharge. Essock et al.16 studied the effectiveness of switching from CA to antipsychotic monotherapy in people with schizophrenia over 6 months and found that patients randomized to monotherapy discontinued treatment (i.e., switched to another monotherapy agent or returned to antipsychotic polypharmacy) significantly sooner than those who remained on CA. However, the 69% of study participants who switched and remained on monotherapy lost weight (0.5 BMI units on average) and their symptoms and number of hospitalizations were not significantly different from the CA group. In a meta-analysis of 1216 patients on CA performed by Correll et al.17, antipsychotic polypharmacy was more effective than monotherapy and more patients dropped out in the monotherapy groups than in the polypharmacy groups. This was particularly the case when treatment lasted longer than 10 weeks, when one of the antipsychotics was clozapine, and when treatment was initiated simultaneously with both antipsychotics in the combination, rather than a 2nd antipsychotic being added due to lack of response. In summary, although there is evidence of increased risk of side effects and consistent association with inpatient admission, the practice of combining antipsychotics is broadly utilized and appears to be effective in a group of patients who are sicker, but not clearly recognized as having “treatment refractory” schizophrenia.
To elucidate the long-term outcome of switching from CA to monotherapy, we performed an exploratory analysis of the NIH dataset from the Preventing Relapse in Schizophrenia: Oral Antipsychotics Compared with Injectables: Evaluating Efficacy (PROACTIVE) study18, which followed 305 patients with schizophrenia and schizoaffective disorder for up to 30 months after randomization to long acting injectable (LAI) risperidone or any oral 2nd generation antipsychotic chosen by their treating physician to assess relapse prevention and course of clinical outcome. The primary outcome of the study was time to first relapse, defined as substantial clinical deterioration, increase in level of care or psychiatric hospitalization. PROACTIVE’s pragmatic elements, i.e., acceptance of patients on LAI, oral or combination antipsychotics at baseline, flexibility in allowing clinician’s expertise in implementing the intervention (type and dose of oral antipsychotic, dose of LAI risperidone), inclusion of new antipsychotics as they entered the market, inclusion of all patients in the analysis through intent to treat and survival analysis, as well as the long duration of the study (up to 30 months) allowed for an analysis of the patients who entered the study on CA18. The goal of our exploratory analysis was to examine the effects of medication status (CA, OA and LAI) at study entry on outcomes and to evaluate whether that status moderated the effect of being randomized to oral 2nd generation antipsychotic or LAI risperidone microspheres monotherapy. This secondary analysis study is hypothesis-generating and has limitations since the patient assignment to these groups was not randomized or blinded. Based on clinical experience (AF) we expected that patients receiving CA would be the most impaired clinically and those receiving LAI would be the least impaired. Further, we expected that when treated with LAI risperidone microspheres monotherapy, patients on CA would do better than those who entered the study on antipsychotic monotherapy, since non-adherence might have contributed to their clinical presentation at study entry, while LAI patients switched to oral monotherapy would do worse.
Materials and methods
This secondary analysis of the limited access dataset distributed from the NIH-supported PROACTIVE study was approved by the Florida International University IRB as exempt. Details about the inclusion and exclusion criteria and the methodology used to establish relapse in the PROACTIVE study were described elsewhere18. Based on the antipsychotic medication prescribed at study entry, the 305 patients with schizophrenia and schizoaffective disorder in the PROACTIVE study were divided into the following groups: (1) LAI: first or 2nd generation long acting injectable (n = 20), (2) OA: any single oral antipsychotic (n = 206), and (3) CA: combination of two or more antipsychotics (n = 50). Consistent with the PROACTIVE study medication manual, which allowed quetiapine up to 200 mg daily to be added to the randomization antipsychotic to facilitate sleep, patients who were taking quetiapine up to 200 mg daily in addition to another antipsychotic at study entry were included in the OA or LAI, rather than the CA group. Data were analyzed separately for each of the following clinical measures: BPRS19 anxiety depression, BPRS activation excitement, BPRS psychosis cluster, BPRS anergia - negative symptoms, BPRS total score, and Scale of Functioning20 overall level of function. Comparisons were made in terms of these outcomes among the three groups. Because of the extreme skewness in the clinical measures data, nonparametric methods based on ranks were used for analysis. For each outcome, the three groups were compared at baseline and at study endpoint using the Kruskal-Wallis test, followed by nonparametric pairwise comparisons comparing LAI vs. OA, LAI vs. CA, and OA vs. CA. In addition, in order to account for any baseline differences among groups, "change scores" were calculated for each outcome for each subject. These were defined in terms of improvement in the clinical measure between baseline and study endpoint, so that a positive value indicated that the patient improved in terms of that clinical measure, whereas a negative value indicated that the patient worsened. The Kruskal-Wallis test, together with nonparametric pairwise comparisons, was then used to compare the three groups in terms of improvement in the clinical measures over the course of the study. The chi-square test was used to compare the three groups in terms of the percentage in each group who experienced a relapse. The Kaplan-Meier method was used to construct survival curves for time until first relapse in the three groups and the log-rank test was used to perform a comparison of the groups in terms of overall "survival." The log-rank test with Bonferroni adjustment was used to perform pairwise comparisons among the three groups in terms of time to relapse. The Kruskal-Wallis test was used to compare the three groups in terms of baseline patient characteristics measured as continuous or ordinal variables (e.g., age, education), and the chi-square test was used to compare the three groups in terms of patient characteristics measured as nominal variables (e.g., race). Any characteristics that differed significantly between groups at baseline were considered for possible inclusion as confounders in the group comparisons of the clinical measures and time until first relapse. Unless otherwise specified, two-tailed tests with a significance level of 0.05 were used. Summary statistics for continuous and ordinal variables are given as mean ± S.D., and as percentages for nominal variables. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, 2012) and IBM SPSS Statistics, Version 21 (IBM Corp, 2012). A power calculation indicated that the observed sample sizes (LAI, n = 20; OA, n = 206; CA, n = 50) would yield 80% power for detecting an effect size of 0.19 when comparing the three groups in terms of a continuous outcome (e.g., any of the BPRS subscales) using the Kruskal-Wallis test, or when comparing the groups in terms of a nominal outcome (e.g., relapse) using the chi-square test, with a significance level of 0.05. An effect size of 0.19 is classified as "small to medium" according to the Cohen criteria21.
Results
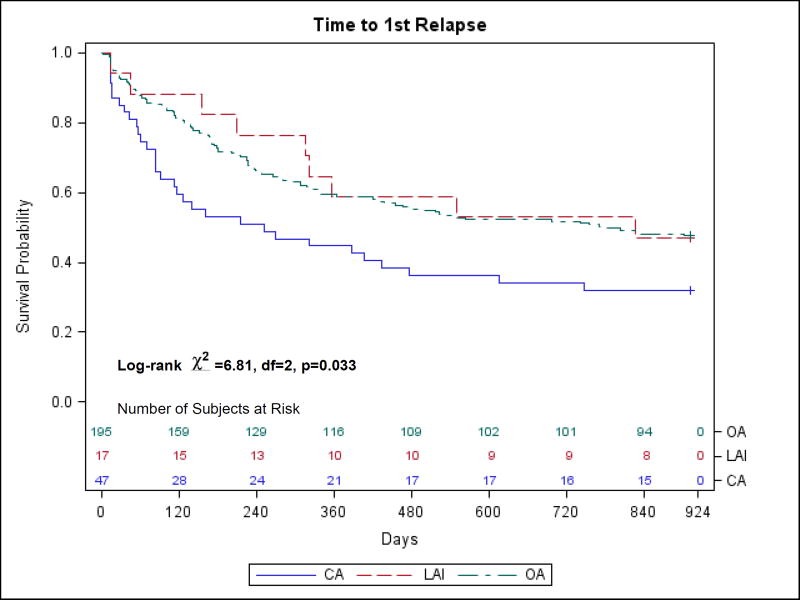
As shown in Table 1, 50 study participants (18%) were prescribed CA, 206 were prescribed OA and 20 were prescribed LAI, for a total of 276 included in the present analysis. Patients who were not taking an antipsychotic at study entry (13) or those with missing medication data (16) were not included in the analysis. Risperidone was the most frequently prescribed drug in all three groups (CA, OA and LAI) at study entry. The most frequent antipsychotic combinations were risperidone and quetiapine (10) and risperidone and olanzapine (7). Four patients were taking three antipsychotics at study entry (all antipsychotic combinations are shown in Supplemental Data Content Table 1). The patient characteristics at baseline are summarized in Supplemental Data Content Table 2. The only significant differences when comparing the three groups of patients were for age at study entry (p = 0.039) and number of hospitalizations prior to baseline (p = 0.011). There were no significant pairwise comparisons among the age groups; however, the OA group had significantly fewer hospitalizations than the CA group (p = 0.009). Slightly more than 50% of both the LAI and CA groups were randomized to LAI-R (Supplementary Data Content Table 2) but the three groups did not differ significantly in percent randomized. In regards to clinical measures data at baseline and endpoint (see Supplementary Data Content Table 3), the only significant difference at baseline was for BPRS anxiety/depression (p = 0.009). Both the OA group (p = 0.018) and the CA group (p = 0.012) had significantly more anxiety/depression than the LAI group, but the OA and CA groups were not significantly different. At endpoint, BPRS psychosis cluster (p = 0.049), BPRS anergia - negative symptoms (p = 0.044) and BPRS total score (p = 0.002) differed significantly among the groups. For BPRS total score, the CA group was significantly worse than the OA group (p = 0.003). For BPRS psychosis cluster and BPRS anergia - negative symptoms, there were no significant pairwise differences at the 0.05 level. With regard to improvement in clinical outcome (defined in terms of change scores), the only significant difference when comparing the three groups in terms of improvement was for BPRS anxiety/depression (p = 0.009). There was significantly less improvement in BPRS anxiety/depression in the LAI group than in the OA group (p = 0.009) (see Table 2). Of the 17 patients in the LAI group for whom relapse data were available, 9 (53%) suffered a relapse during the study. In the OA and CA groups, the percentages were 52% (102/195) and 68% (32/47), respectively. The chi-square test indicated no significant difference among the groups in terms of relapse rate (χ2 = 3.85, d.f. = 2, p = 0.146). However, the log-rank test did indicate a significant difference among the three groups in terms of overall time to first relapse (mean time to first relapse was 594 days in LAI group, 562 in OA group and 409 days in CA group; log-rank χ2 = 6.81, d.f. = 2, p = 0.033). Bonferroni pairwise comparisons indicated that the overall time to relapse was significantly longer in the OA group than in the CA group (p = 0.011). The survival curves for time until first relapse for the three groups are shown in Figure 1. Cox regression was used to model the time until relapse as a function of group (OA vs. CA only) and the only potential confounding variable that differed significantly between the OA and CA groups, number of hospitalizations prior to baseline (see Supplemental Data Content Table 4). Risk of relapse was significantly associated with group (hazard ratio = 1.669, p = 0.012) and number of hospitalizations (hazard ratio = 1.013, p = 0.012). However, after adjusting for number of hospitalizations, group was no longer significant (hazard ratio = 1.541, p = 0.052), just barely missing the usual 0.05 cutoff for statistical significance. The results for number of hospitalizations were not materially affected by adjusting for group. We performed further analysis to incorporate the treatment group as randomized (oral antipsychotic vs. risperidone microspheres) into the comparison of the CA and OA groups; the Kaplan-Meier plots did not change materially when we stratified by treatment group.
Table 1.
Distribution of antipsychotics taken by PROACTIVE participants at study entry
| Antipsychotic drug at study entry (N) | ||
|---|---|---|
| Long acting injectable (LAI) | First generation antipsychotic: haloperidol decanoate, fluphenazine decanoate | 7 |
| Risperidone microspheres | 13 | |
| Oral antipsychotic (OA) | Risperidone | 91 |
| Olanzapine | 37 | |
| Aripiprazole | 24 | |
| Quetiapine | 21 | |
| Ziprasidone | 16 | |
| Paliperidone | 5 | |
| First generation antipsychotic: haloperidol, fluphenazine, loxapine, chlorpromazine | 12 | |
| Combination antipsychotics (CA) | 50 | |
| No antipsychotic | 13 | |
| Missing data | 16 | |
| Total | 305 | |
PROACTIVE: Preventing Relapse in Schizophrenia: Oral Antipsychotics Compared with Injectables: Evaluating Efficacy
Table 2.
Improvement in Clinical Measures from Baseline to Endpoint as Measured by Change Scores
| Clinical Measure | LAI (n = 20) |
OA (n = 206) |
CA (n = 50) |
p-value |
|---|---|---|---|---|
| BPRS§ anxiety depression | ||||
| Improvement, mean ± SD | −0.1 ± 0.5 (n = 17) | 0.5 ± 0.9 (n = 183) | 0.4 ± 0.9 (n = 45) | 0.009 |
| BPRS activation excitement | ||||
| Improvement, mean ± SD | 0.2 ± 0.7 (n = 15) | 0.1 ± 0.5 (n = 181) | 0.1 ± 0.4 (n = 40) | 0.371 |
| BPRS psychosis cluster | ||||
| Improvement, mean ± SD | 0.5 ± 0.8 (n = 17) | 0.6 ± 1.1 (n = 184) | 0.4 ± 1.1 (n = 45) | 0.449 |
| BPRS anergia - negative symptoms | ||||
| Improvement, mean ± SD | 0.1 ± 0.8 (n = 15) | −0.1 ± 0.7 (n = 180) | −0.1 ± 0.9 (n = 41) | 0.587 |
| BPRS total score | ||||
| Improvement, mean ± SD | 4.4 ± 6.7 (n = 15) | 5.3 ± 8.7 (n = 180) | 3.1 ± 8.3 (n = 41) | 0.249 |
| SOF overall level of function | ||||
| Improvement, mean ± SD | 0.0 ± 0.6 (n = 18) | −0.2 ± 0.7 (n = 187) | −0.3 ± 0.9 (n = 45) | 0.418 |
LAI=long acting injectable, OA=oral antipsychotic; CA=combination antipsychotics
The 18-item Brief Psychiatric Rating Scale (BPRS) was used; items were measured from 1–7, with 1=not present and 7=extremely severe.
Fig. 1.
A comparison of time to first relapse between patients on oral antipsychotic (OA), patients on long acting injectable antipsychotic (LAI), and patients on two or more antipsychotics (CA).
Discussion
As predicted, there was a significant difference in patients’ number of hospitalizations at baseline as a function of antipsychotic medication status; patients on oral monotherapy had significantly fewer hospitalizations than those on CA at study entry. Once patients entered the study, there was a difference in time to relapse among the groups (mean time to 1st-relapse for people on CA at baseline was 409.5 days vs. those on OA, 562.8 days, and those on LAI, 594 days). However, after adjusting for the number of prior hospitalizations the difference between the OA and the CA groups in terms of time to 1st-relapse was no longer significant. The pragmatic nature of PROACTIVE, which included patients on monotherapy, those on CA and patients on no antipsychotics at study baseline, allowed us to perform a secondary analysis in order to create a “switch trial” of antipsychotic combinations to monotherapy. Among CA patients, who entered the 30-month PROACTIVE study with a history of significantly more hospitalizations, randomization to oral or long acting injectable monotherapy did not have a significant effect. Based on our exploratory analysis, caution must be taken when switching from antipsychotic polypharmacy to monotherapy. This study confirms the findings of Essock16 in a sample of patients followed for up to 30 months, and it appears to validate the clinical decision-making of the treating physicians, although our symptom rating scales did not reflect greater severity of illness in the patients on CA. Beyond the general acceptance of the fact that patients who are treated with antipsychotic combinations represent a vulnerable group, clinical guidance for treating this group of patients is lacking. Studies are warranted to explore whether psychosocial intervention to help patients recognize relapse, or early introduction of clozapine, could be effective in breaking the cycle of relapse in this patient group. In addition, systematic investigation of combinations of antipsychotics carefully selected to augment each other’s pharmacodynamic profile and avoid exacerbating adverse effects in patients who do not respond to one antipsychotic, appears necessary in order to further validate and guide clinical practice.
Our study had substantial limitations, as expected in any secondary data analysis. The groups that we compared were defined by medication status at PROACTIVE study entry, rather than by randomization, and the CA and LAI groups were relatively small. Further, once patients entered the study, clinicians treating those randomized to oral 2nd generation antipsychotics were allowed to make medication changes as dictated by the patient’s clinical status, whereas the patients in the LAI group could only have dose adjustments or leave treatment if LAI risperidone needed to be discontinued. Therefore, the differences between groups could have been confounded by these factors.
In conclusion, there was a significant difference in subjects’ number of prior hospitalizations at baseline, with the highest number in the group taking combination antipsychotics. People on combination antipsychotics at baseline relapsed considerably sooner than those on oral and those on long acting injectable antipsychotics when switched to antipsychotic monotherapy, although this difference was not statistically significant when we adjusted for the number of hospitalizations at baseline. Within the limitations described, our analysis confirms the clinical experience that being on two or more antipsychotics indicates greater risk of relapse and predicts earlier relapse in schizophrenia. Treatment guidance for this group of patients still needs to be established.
Supplementary Material
Acknowledgments
Peter Buckley has received grant and research support from Ameritox, Auspex Pharmaceuticals, Inc., Alkermes, Inc., Avanir Pharmaceuticals, Inc., Otsuka Pharmaceuticals, NIMH and is a consultant for NIMH.
John Lauriello participated in clinical research as a site on a study headed and paid by Columbia University—sponsored by Sunovion, as a clinical research site for a study headed and paid by Florida Atlantic University—sponsored by Otsuka, is a member of the Event Monitoring Board for Janssen-(Services funded through a contract with the University of Missouri), member of the Event Monitoring Board for Alkermes–(Services funded through a contract with the University of Missouri) and a member of the Advisory Panel for Sunovion, Janssen, Otsuka, Reckitt Benckiser, Teva.
Nina Schooler has received grant support from Genentech and Otsuka; she has served on advisory boards or provided consultation to Alkermes, Allergan, Forum, Roche and Sunovion.
Data used in the preparation of this article were obtained from the limited access datasets distributed from the NIH-supported “Preventing Relapse in Schizophrenia: Oral Antipsychotics Compared with Injectables: Evaluating Efficacy” (PROACTIVE). This is a multi-site, clinical trial of persons with Schizophrenia comparing effectiveness of randomly assigned medication treatment. The study was supported by NIMH grant #s: U01 MH070007-01, U01 MH070023, U01 MH070011, U01 MH070009, U01 MH070008, U01 MH070017, U01 MH070010, U01 MH070016, U01 MH070012. The ClinicalTrials.gov identifier is NCT00330863. This manuscript reflects the views of the authors and may not reflect the opinions or views of the PROACTIVE Study Investigators or the NIH.
Footnotes
Disclosures:
Adriana Foster has nothing to disclose.
Stephen Looney has nothing to disclose.
Contributor Information
Adriana Foster, Department of Psychiatry and Behavioral Health, Herbert Wertheim College of Medicine, Florida International University, 11200 SW 8th Street, AHC1 343, Miami, FL 33199, adfoster@fiu.edu, 305.348.8463|Fax 305.348.4331.
Peter Buckley, Virginia Commonwealth University School of Medicine.
John Lauriello, Department of Psychiatry, University of Missouri, Medical Director of the Missouri Psychiatric Center LaurielloJ@health.missouri.edu, 573.882.8913.
Stephen Looney, Dept. of Biostatistics and Epidemiology, Dept. of Oral Health and Diagnostic Sciences, Augusta University, slooney@augusta.edu, 706.721.4846.
Nina Schooler, Schizophrenia Research Program, Department of Psychiatry, Suny Downstate Medical Center, nina.schooler@gmail.com, 718.270.4483.
References
- 1.Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand. 2002;106:323–330. doi: 10.1034/j.1600-0447.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher JE, Makela EH, Griffin HR. Multiple antipsychotic medication prescribing patterns. Ann Pharmacother. 2003;37:951–955. doi: 10.1345/aph.1C420. [DOI] [PubMed] [Google Scholar]
- 3.Kreyenbuhl JA, Valenstein M, McCarthy JF, et al. Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatr Serv. 2007;58:489–95. doi: 10.1176/appi.ps.58.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2004;161(2 SUPPL):40. [PubMed] [Google Scholar]
- 5.Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations. Schizophr Bull. 2009;36:94–103. doi: 10.1093/schbul/sbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psychosis and schizophrenia in adults: prevention and management. [Accessed November 26, 2016];2014 Feb; https://www.nice.org.uk/guidance/cg178.
- 7.Moore T. Schizophrenia Treatment Guidelines in the United States. Clinical Schizophrenia & Related Psychoses. 2011;5:40–49. doi: 10.3371/CSRP.5.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751–62. doi: 10.4088/jcp.v68n1115. [DOI] [PubMed] [Google Scholar]
- 9.Galletly C, Castle D, Dark F, et al. The Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for the Management of Schizophrenia. Aust N Z J Psychiatry. 2016;50:410–472. doi: 10.1177/0004867416641195. [DOI] [PubMed] [Google Scholar]
- 10.Sneider B, Pristed SG, Correll C, et al. Frequency and correlates of antipsychotic polypharmacy among patients with schizophrenia in Denmark: A nation-wide pharmacoepidemiological study. Eur Neuropsychopharmacol. 2015;25:1669–1676. doi: 10.1016/j.euroneuro.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Bolstad A, Andreassen OA, Rossberg JI, et al. Previous hospital admissions and disease severity predict the use of antipsychotic combination treatment in patients with schizophrenia. BMC Psychiatry. 2011;11:126. doi: 10.1186/1471-244X-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallego JA, Bonetti J, Zhang J, et al. Prevalence and Correlates of Antipsychotic Polypharmacy: A Systematic Review and Meta-regression of Global and Regional Trends from the 1970s to 2009. Schizophr Res. 2012;138:18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallego JA, Nielsen J, De Hert M, et al. Safety and Tolerability of Antipsychotic Polypharmacy. Expert Opin Drug Saf. 2012;11:527–542. doi: 10.1517/14740338.2012.683523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsumi C, Uchida H, Suzuki T, et al. The evolution of antipsychotic switch and polypharmacy in natural practice-a longitudinal perspective. Schizophr Res. 2011;130:40–46. doi: 10.1016/j.schres.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Uchida H, Watanabe K, et al. A clinical case series of switching from antipsychotic polypharmacy to monotherapy with a second-generation agent on patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:361–369. doi: 10.1016/j.pnpbp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Essock SM, Schooler NR, Stroup S, et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168:702–708. doi: 10.1176/appi.ajp.2011.10060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr bull. 2009;35:443–57. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley PF, Schooler NR, Goff DC, et al. Comparison of SGA Oral Medications and a Long-Acting Injectable SGA: The PROACTIVE Study. Schizophr Bull. 2015;41:449–59. doi: 10.1093/schbul/sbu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 20.Rapaport MH, Bazzetta J, McAdams LA, et al. Validation of the scale of functioning in older outpatients with schizophrenia. Am J Geriatr Psychiatry. 1996;4:218–228. doi: 10.1097/00019442-199622430-00005. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.) Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.