Abstract
Hypertensive adults demonstrate atypical increases in blood pressure (BP) and muscle sympathetic nerve activity (MSNA) at the immediate onset of static muscle contraction. However, it is unknown whether these abnormal responses occur in young, otherwise healthy adults at risk for developing future disease, such as those with a family history of hypertension (+FH). We tested the hypothesis that +FH young women have exaggerated increases in BP and MSNA at the onset of static muscle contraction compared to those without a family history of hypertension (-FH). We retrospectively examined beat-by-beat BP and MSNA during the initial 30 seconds of isometric handgrip exercise (30% of maximum voluntary contraction) in 16 +FH (22±2yrs, 22±3kg•m-2) and 16 -FH (22±3yrs, 22±3kg•m-2) women. Resting mean arterial pressure (MAP; +FH 80±11 vs. -FH 84±13mmHg), MSNA burst frequency (+FH 7±3 vs. -FH 9±5bursts•min-1), and burst incidence (+FH 12±4 vs. -FH 12±8bursts•100 heartbeats -1) were similar between groups (all P>0.05). Within the first 10 seconds of exercise, changes in MAP (+FH Δ8±6 vs. -FH Δ3±2 mmHg, P<0.05) and HR (+FH Δ8±5 vs. -FH Δ4±4 mmHg, P<0.05) were greater in +FH women. Absolute MSNA burst frequency during the first 30 seconds of exercise was not different between groups (-FH: 7±5 vs. +FH: 9±3 bursts/min). Cardiovascular and sympathetic responses during cold pressor test were not different between groups. These data demonstrate that young women at risk for developing cardiovascular disease exhibit greater changes in BP at the onset of static muscle contraction.
Keywords: Handgrip Exercise, Blood Pressure, Muscle Sympathetic Nerve Activity
Introduction
Excessive increases in blood pressure (BP) during exercise, even in otherwise healthy adults, predicts future cardiovascular disease (Berger et al., 2015). There are emerging data indicating aberrant neural-cardiovascular control in young adults with a family history of hypertension (+FH) (Greaney et al., 2015b; Fonkoue et al., 2016). We previously reported exaggerated pressor and sympathetic responses during the last minute of submaximal static handgrip in young women +FH (Greaney et al., 2015b), a finding consistent with results in older adults with essential hypertension (Delaney et al., 2010). However, recent data demonstrate augmented cardiovascular responses within the first 30 sec of isometric exercise in adults with hypertension (Greaney et al., 2015a), peripheral arterial disease (Muller et al., 2012) and heart failure (Middlekauff et al., 2004), suggesting that impairments in BP regulation at the onset of exercise may be characteristic of multiple cardiovascular pathologies.
Sustained submaximal isometric exercise elicits robust increases in BP (Mark et al., 1985), which are noted within the first 60 seconds. However, in healthy young adults, BP does not typically increase at the immediate onset (i.e., within the first 20s) of moderate-intensity isometric handgrip because muscle sympathetic nerve activity (MSNA) is not yet activated and systemic vascular resistance is reduced (Lind et al., 1964; Lalande et al., 2014). Importantly, most activities of daily living do not require sustained intense muscular work, but are instead short in duration and submaximal in intensity. Thus, the BP response to the immediate onset of exercise (i.e., within the first 20s of submaximal isometric exercise) may be especially critical, and may be additionally informative for predicting future cardiovascular risk (Greaney & Farquhar, 2012). Such immediate pressor responses to daily activities may result in serial episodic surges in BP (increased BP variability) and result in greater cardiac and vascular damage (Parati et al., 1987; Mancia et al., 2001) and increased incidence of cardiovascular events (Mancia et al., 2007).
Young adults with a family history of hypertension are at an increased risk for developing future hypertension, and are therefore at risk for future cardiovascular disease (Hunt et al., 1986). Although prior studies have documented abnormal increases in BP at the onset of exercise in patients with established cardiovascular disease, to our knowledge, no studies have examined potential alterations in the timecourse of cardiovascular responses to isometric exercise in young, otherwise healthy, women with increased cardiovascular risk. With this background in mind, it is reasonable to postulate that young healthy normotensive women +FH may also exhibit altered cardiovascular responses at the onset of exercise. Therefore, the purpose of this study was to retrospectively examine cardiovascular responses at the immediate onset of submaximal isometric exercise (within the first 10s) in young women with and without a family history of hypertension. We hypothesized that +FH young women would exhibit greater increases in BP and MSNA at exercise onset compared to young women without a family history of hypertension (-FH).
Methods
Ethical Approval
The protocols utilized in this study conform to the Declaration of Helsinki (except for registration in database) and were approved by the University of Delaware Institutional Review Board (approvoal #224730-16). All women signed written informed consents prior to participation. To test our novel hypothesis regarding the immediate onset of exercise, data were analyzed from 32 women that have participated in both previous (Greaney et al., 2015b) and ongoing studies in our laboratory. All women completed a screening visit and experimental visit. Resting BP and a 12-lead resting ECG were measured during the screening visit. Women were classified as +FH through self-report by identifying if either their mother and/or father have high blood pressure; this was done using a standard clinical medical history form provided by the University of Delaware Nurse Managed Health Center. Recent studies classifying participants using self-report have demonstrated clear, robust differences in a variety of sympathetic stimuli (Greaney et al., 2015b; Fonkoue et al., 2016). All women were normotensive and free of co-morbidities (cardiovascular disease, diabetes, kidney disease, pulmonary disease, etc.). Women were excluded if they were obese (body mass index > 30 kg/m2), used nicotine containing products, or medications with cardiovascular effects. Twelve +FH and 10 -FH women reported engaging in aerobic exercise at least 3 days per week. Women were tested during the early follicular phase of the menstrual cycle, or during placebo days of hormonal contraception (+FH n=8, -FH n=10). Women were familiarized with the experimental protocol during the screening visit.
Experimental Measurements
Heart rate was monitored via electrocardiogram (ECG; Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI, USA). Beat-by-beat BP was measured using a servo-controlled finger photoplethysmograph (Finometer; Finapres Medical Systems, Amsterdam, Netherlands) on the non-dominant hand. Cardiac output and total peripheral resistance were derived using Modelflow (Finometer). Brachial BP (Dinamap Dash 2000; GE Medical Systems) was used to verify absolute Finometer-derived arterial BP. Respiration was monitored using a strain-gauge pneumograph (Pneumotrace; UFI, Morro Bay, CA, USA).
MSNA was recorded from the peroneal nerve using standard microneurographic methods, as previously described (Vallbo et al., 1979; Greaney et al., 2015b). Briefly, a unipolar tungsten microelectrode was inserted through the skin and positioned into the peroneal nerve. A reference microelectrode was inserted approximately 3 cm away. Signals were amplified (70,000-fold), bandpass filtered (700-2,000 Hz), rectified, and integrated (0.1 s time constant) to obtain a mean voltage neurogram (Nerve Traffic Analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA, USA). The signal was confirmed as MSNA by the presence of spontaneously occurring bursts with pulse synchronicity, responsiveness to an end-expiratory breath hold or Valsalva maneuver, and lack of responsiveness to arousal stimuli or skin stroking. Heart rate, BP, and MSNA were continuously recorded throughout the experimental.
Experimental Protocol
All women refrained from food intake for at least 4 hours, and alcohol, caffeine, and strenuous physical activity for at least 24 hours prior to the experimental visit. Women were tested in the supine position in a temperature controlled laboratory (20-22°C). The maximal voluntary contraction (MVC) of the dominant hand was determined by having participants squeeze a commercially-available handgrip device (ADInstruments, Bella Vista, NSW, Australia) at maximal effort; 3-5 trials were performed, and the highest value was utilized as their maximum. Women rested quietly for 10 minutes after instrumentation and obtaining MSNA recordings. Following this overall baseline, an additional 5-minute baseline was measured prior to the isometric exercise trial. Women were instructed to squeeze the handgrip device at 30% MVC for 2 minutes; visual feedback was provided. After completion of the isometric exercise trial, a minimum of 10 minutes of rest was given to ensure cardiovascular variables returned to resting levels. A second baseline period was recorded, and then the women placed their dominant hand in ice water for 2 minutes (cold pressor test, CPT). The CPT was used as a non-exercise, sympatho-excitatory stimulus (Victor et al., 1987) to determine if rapid cardiovascular responses were exercise specific.
Data Analysis
Data were recorded at 1,000 Hz (Powerlab and Chart; ADInstruments, Bella Vista, NSW, Australia). We analyzed cardiovascular and sympathetic responses during the first 30s of exercise onset. In anesthetized cats, electrically-evoked muscle contraction resulted in an increase in BP within 6.4s (range: 2-16s) and an increase in HR within 8.3s after ventral root stimulation (Kaufman et al., 1983). Given these findings, and based upon recent studies in humans (Middlekauff et al., 2004; Muller et al., 2012; Greaney et al., 2015a), we elected to further analyze our hemodynamic data in 10s increments at the beginning of isometric handgrip (HG) and the CPT. However, because the reliability and validity of MSNA analysis is reduced with time intervals <30s (Notay et al., 2016), we did not analyze MSNA in shorter (10s) time segments. A custom LabVIEW program with set criteria (Fairfax et al., 2013) was used to detect MSNA bursts from the mean voltage neurogram. In brief, the custom program generated synchronized beat-by-beat data for BP and MSNA accounting for the latency from the R-wave of the ECG. Analyzable recordings with no discernable shift in electrode placement were obtained in 8 +FH and 10 -FH women during handgrip exercise, and 7 +FH and 8 –FH during the CPT. MSNA was quantified as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total activity (AU/min). Both burst frequency and burst incidence are reproducible within a participant over time (Kienbaum et al., 2001).
Statistical Analysis
Participant characteristics were compared using unpaired t-tests. Cardiovascular responses (i.e. change from the preceding baseline) for BP and HR were initially assessed with unpaired t-tests between +FH and -FH women for the average of the first 30s of both isometric exercise and CPT. In order to determine the time-course of these responses, a two-way mixed-model ANOVA with repeated measures (SPSS v24) was used to evaluate hemodynamic data in 10s intervals (for exercise and CPT), and Fisher's LSD post-hoc tests were used when appropriate. Absolute MSNA in the first 30s of exercise and CPT were compared using unpaired t-tests. Results are reported as means ± SD, and the alpha level was set at P<0.05.
Results
Women were well matched for age (-FH: 22±3 vs. +FH: 22±2yrs), BMI (-FH: 22±3 vs. +FH: 22±3kg•m-2), resting mean arterial BP (MAP; -FH: 84±13 vs. +FH: 80±11 mmHg), and heart rate (-FH: 66±6 vs. +FH: 61±9 bpm) (all P>0.05). Resting MSNA burst frequency (-FH: 9±5 vs. +FH: 7±3 bursts/min), burst incidence (-FH: 12±8 vs. +FH: 12±4 bursts/100 heartbeats), and total MSNA (-FH: 506±267 vs. +FH: 398±139 AU/min) were not different between groups (all P>0.05).
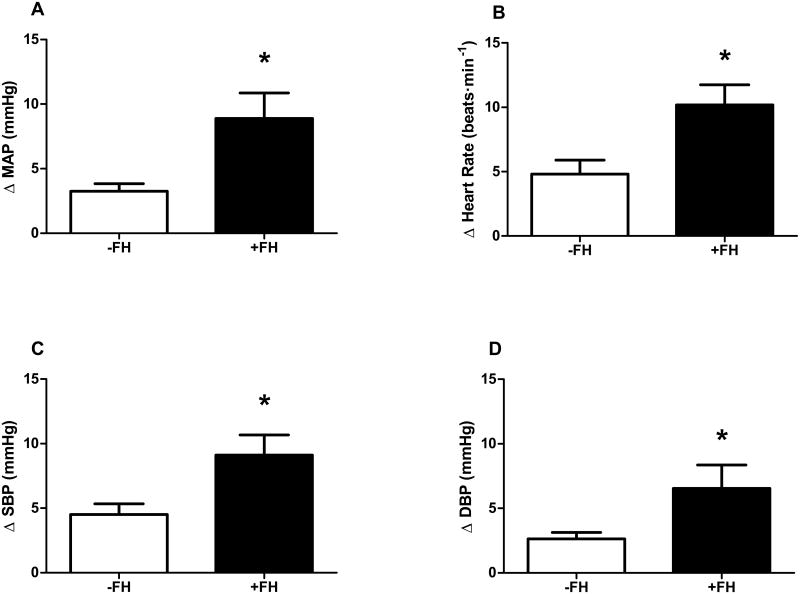
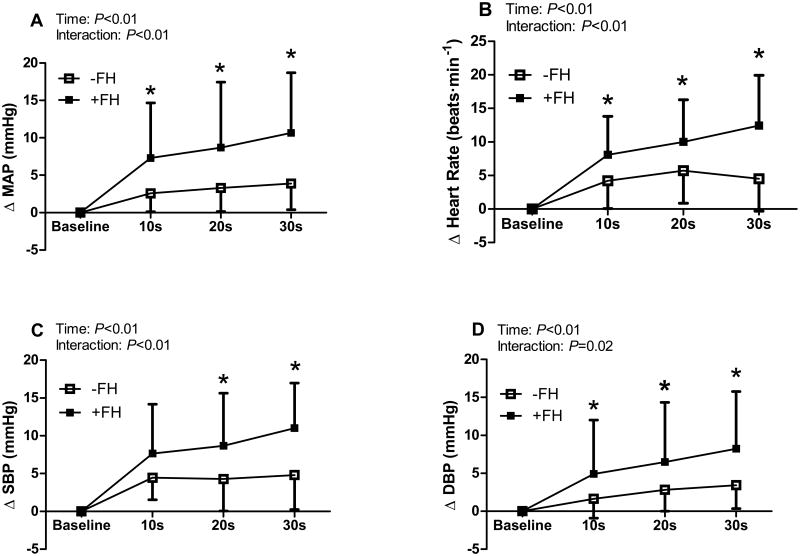
Hemodynamic responses to exercise are presented in Table 1. BP and HR increased during handgrip in both groups (Table 1); however, +FH women exhibited a greater pressor and HR response to the initial 30s of isometric exercise (Fig. 1). When further examining the timecourse of responsiveness, greater increases in MAP and DBP were observed within the first 10s of handgrip in women with a +FH (Fig. 2). Women with a +FH also demonstrated a greater increase in HR at the immediate onset of exercise (Fig. 2B). There were no group differences in the increase in cardiac output or the decrease in TPR during the first 30s of exercise (Table 1).
Table 1. Hemodynamic Responses at Exercise Onset.
| Baseline | 10s | 20s | 30s | ANOVA | |
|---|---|---|---|---|---|
| MAP (mmHg) | Time P<0.01 | ||||
| -FH | 84 ± 13 | 87 ± 13* | 87 ± 13* | 88 ± 14* | Interaction P<0.01 |
| +FH | 80 ± 11 | 87 ± 11* | 89 ± 12* | 91 ± 12* | |
|
| |||||
| SBP (mmHg) | Time P<0.01 | ||||
| -FH | 127 ± 19 | 130 ± 19* | 130 ± 19* | 130 ± 20* | Interaction P<0.01 |
| +FH | 122 ± 17 | 129 ± 16* | 130 ± 18* | 133 ± 17* | |
|
| |||||
| DBP (mmHg) | Time P<0.01 | ||||
| -FH | 64 ± 11 | 65 ± 11 | 66 ± 12 | 67 ± 12* | Interaction P<0.01 |
| +FH | 61 ± 13 | 66 ± 11* | 68 ± 12* | 70 ± 13* | |
|
| |||||
| HR (bpm) | Time P<0.01 | ||||
| -FH | 66 ± 6 | 70 ± 7* | 72 ± 7* | 70 ± 8* | Interaction P<0.01 |
| +FH | 61 ± 9 | 69 ± 10* | 71 ± 10* | 74 ± 11* | |
|
| |||||
| CO (L/min) | |||||
| -FH | 4.8 ± 0.9 | 5.2 ± 1.0 | 5.2 ± 1.0 | 5.2 ± 1.0 | Time P<0.01 |
| +FH | 5.0 ± 1.3 | 5.5 ± 1.7 | 5.5 ± 1.7 | 5.5 ± 1.7 | Interaction P=0.90 |
|
| |||||
| TPR (cgs) | |||||
| -FH | 1671 ± 351 | 1603 ± 311 | 1623 ± 311 | 1642 ± 304 | Time P=0.04 |
| +FH | 1751 ± 606 | 1651 ± 522 | 1654 ± 494 | 1682 ± 501 | Interaction P=0.86 |
MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CO, cardiac output; TPR, total peripheral resistance; -FH, without family history of hypertension; +FH, positive family history of hypertension.
P<0.05 vs. baseline. Data presented as mean±SD.
Figure 1.
Blood pressure and heart rate responses during the initial 30-seconds of isometric handgrip exercise in young women with (+FH) and without (-FH) a family history of hypertension. Changes in mean arterial pressure (MAP, panel A), heart rate (panel B), systolic blood pressure (SBP, panel C), and diastolic blood pressure (DBP, panel D) were all greater in +FH women. * P<0.05 between groups. Data expressed as mean±SD.
Figure 2.
Changes in blood pressure and heart rate in 10-second intervals at exercise onset in young women with (+FH) and without (-FH) a family history of hypertension. In +FH women exaggerated increases in mean arterial pressure (MAP, panel A), heart rate (panel B), and diastolic blood pressure (DBP, panel D) were observed as early as 10 seconds, whereas differences in systolic blood pressure (SBP, panel C) were observed as early as 20 seconds into the exercise bout. * P<0.05 between groups. Data expressed as mean±SD.
Absolute MSNA burst frequency (-FH: 7±5 vs. +FH: 9±3 bursts/min), burst incidence (-FH: 8±6 vs. +FH: 13±4 bursts/100 heartbeats) and total activity (-FH: 500±510 vs. +FH: 474±256 AU/min) were not different between groups during the first 30s of handgrip exercise (all P>0.05). Maximal voluntary contraction (-FH: 229±98 vs. +FH: 252±71N, p=0.46), along with absolute and relative force production during handgrip exercise, were not different between groups (ANOVA P>0.40 for all).
There were no group differences in resting hemodynamics prior to the CPT (Table 2). Moreover, the hemodynamic responses to the first 30s of the CPT were not different in women with a +FH (Table 2). Absolute MSNA burst frequency (-FH: 16±10 vs. +FH: 14±4 bursts/min), burst incidence (-FH: 20±14 vs. +FH: 20±9 bursts/100 heartbeats), and total activity (-FH: 494±361 vs. +FH: 477±204 AU/min) during the CPT were not different between groups.
Table 2. Hemodynamic Responses to Cold Pressor Test.
| Baseline | 10s | 20s | 30s | ANOVA | |
|---|---|---|---|---|---|
| Δ MAP (mmHg) | Time P<0.01 | ||||
| -FH | 86 ± 11 | 87 ± 11 | 86 ± 11 | 90 ± 13 | Interaction P=0.46 |
| +FH | 87 ± 14 | 91 ± 13 | 90 ± 15 | 92 ± 14 | |
|
| |||||
| Δ SBP (mmHg) | Time P=0.01 | ||||
| -FH | 127 ± 14 | 129 ± 17 | 128 ± 19 | 131 ± 22 | Interaction P=0.49 |
| +FH | 132 ± 19 | 138 ± 18 | 136 ± 19 | 138 ± 17 | |
|
| |||||
| Δ DBP (mmHg) | Time P<0.01 | ||||
| -FH | 67 ± 10 | 67 ± 9 | 67 ± 9 | 70 ± 10 | Interaction P=0.69 |
| +FH | 66 ± 13 | 68 ± 14 | 67 ± 15 | 70 ± 15 | |
|
| |||||
| Δ HR (bpm) | Time P<0.01 | ||||
| -FH | 65 ± 6 | 69 ± 5 | 69 ± 5 | 69 ± 3 | Interaction P=0.04 |
| +FH | 64 ± 12 | 68 ± 13 | 71 ± 13 | 73 ± 14 | |
|
| |||||
| CO (L/min) | |||||
| -FH | 4.5 ± 0.9 | 4.9 ± 1.0 | 4.9 ± 1.1 | 4.9 ± 1.1 | Time P<0.01 |
| +FH | 5.1 ± 1.4 | 5.5 ± 1.5 | 5.6 ± 1.6 | 5.7 ± 1.8 | Interaction P=0.10 |
|
| |||||
| TPR (cgs) | |||||
| -FH | 1770 ± 290 | 1677 ± 266 | 1659 ± 261 | 1740 ± 253 | Time P=0.10 |
| +FH | 1733 ± 605 | 1700 ± 578 | 1613 ± 528 | 1641 ± 532 | Interaction P=0.08 |
MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CO, Cardiac Output; TPR, total peripheral resistance; -FH, without family history of hypertension; +FH, positive family history of hypertension. Data presented as mean±SD.
Discussion
The main novel finding of the current study is that the BP responses to isometric handgrip are significantly different in young healthy normotensive women with a +FH compared to those with a -FH, and these differences are evident within the first 10s of exercise. These findings are important because BP does not typically increase at the onset (i.e., within the first 20-30s) of submaximal isometric exercise (Lalande et al., 2014). This rapid and robust BP response to the onset of exercise onset is consistent with findings in older hypertensive adults (Greaney et al., 2015a) and patients with established cardiovascular disease (Middlekauff et al., 2004; Muller et al., 2012). Importantly, we are the first to extend these findings of altered cardiovascular control at exercise onset to young, otherwise healthy, adults at risk for future disease due to familial predisposition. Taken together with previous studies (Middlekauff et al., 2004; Muller et al., 2012; Greaney et al., 2015a), this rapid onset pressor response to isometric exercise may be an early clinical characteristic of future hypertension and cardiovascular disease (Greaney & Farquhar, 2012).
Cardiovascular responses to exercise are regulated through a precise interplay between feed forward (central command) and feedback (exercise pressor reflex; baroreflex) neural mechanisms. The exercise pressor reflex consists of both mechanoreceptors (largely comprised of group III afferent fibers) and metaboreceptors (primarily group IV afferent fibers) and contributes to the increases in BP during exercise via activation of the sympathetic nervous system (Coote et al., 1971; Goodwin et al., 1972; Kaufman et al., 1983). However, increases in MSNA do not typically occur in healthy humans during submaximal static exercise until ∼1 min, and can largely be attributed to stimulation of the metaboreflex (Mark et al., 1985). Given the unique analytical approach used in the current study (i.e., within 10 seconds of exercise onset), and due to the time-course of the responses we observed, it is unlikely that the metaboreflex is the primary mechanism contributing to the altered BP response at exercise onset in +FH women.
Mechanoreceptors are activated by contracting skeletal muscle at the onset of exercise, as previously demonstrated by Kaufman et al. (Kaufman et al., 1983) in anesthetized cats. In response to static muscle contraction evoked by electrical stimulation, the authors reported a ‘sudden explosive burst of impulses’ from group III fibers (Kaufman et al., 1983). This activity occurred in less than 1 second, and had returned to baseline values within 20 seconds of the contraction (Kaufman et al., 1983). Notably, there was a corresponding increase in BP that occurred between 2-16 seconds after contraction (Kaufman et al., 1983). We observed a similar BP pattern at the onset of handgrip exercise in +FH women. This temporal pattern of responsiveness suggests potential alterations in mechanoreflex function in +FH women. Although muscle mechanoreflex dysfunction has been noted in cardiovascular diseases such as hypertension and heart failure (Middlekauff et al., 2004; Greaney & Farquhar, 2012; Greaney et al., 2015a), the degree to which it is involved in adults at risk for developing cardiovascular disease remains unclear.
In addition to the augmented BP response at exercise onset, we also observed greater changes in HR within the first 10 seconds of muscle contraction in +FH women. Because increases in HR at the onset of exercise occur, at least in part, due to activation of central command (Mark et al., 1985), it is possible that +FH women have a greater central drive. Recent data demonstrates that central command plays a crucial role in the altered cardiovascular control during exercise in hypertensive animal models (Liang et al., 2016). However, in experimental models using either passive muscle stretch or electrically-induced muscle contraction to eliminate central command, an increase in HR still occurs (Gladwell & Coote, 2002), suggesting that other mechanisms (most notably the muscle mechanoreflex) may be involved in the cardioaccelerator response to exercise. Thus, it is plausible that alterations in mechanoreflex function may explain the abnormal temporal pattern of both BP and HR responsiveness to the immediate onset of isometric exercise in +FH women; this warrants further investigation.
It is plausible that the greater cardio-acceleration that occurred at the beginning of exercise contributed to the greater change in BP in +FH women. In this manner, a larger change in BP may primarily be related to increases in cardiac output. In young adults, increases in HR and cardiac output – as opposed to total peripheral resistance – are primary contributing factors mediating BP changes at the beginning of exercise (Lalande et al., 2014). However, this effect was reported at higher (≥40% MVC) exercise intensities (Lalande et al., 2014). In our data, we report greater changes in HR and BP in +FH women at submaximal intensities within 10s of isometric exercise, but not changes in cardiac output. It is unclear if this may be linked to the variability associated with Modelflow-derived cardiac output or due to the lower intensity used in the current study as changes in cardiac output appear to be intensity dependent (Lalande et al., 2014).
Limitations
Because the baroreflex remains active during exercise to further modulate BP (Gallagher et al., 2006; Michelini et al., 2015), it is possible that alterations in baroreflex function also contributes to the abnormal cardiovascular responses at exercise onset in +FH women. The limited studies examining baroreflex function in adults with a family history of hypertension are equivocal, with reports of both reduced (Parmer et al., 1992) and preserved (Boutcher et al., 2009) baroreflex sensitivity at rest in men with a family history of hypertension, and to our knowledge, no studies have examined baroreflex function during exercise in those with a +FH. Although the current study was not designed to discern the precise mechanisms mediating altered cardiovascular control during the early temporal phase of exercise in +FH women, these data provide an important foundation for future studies in this clinical population. Second, we recognize that self-reporting parental hypertensive history is a limitation. Although there is a possibility that some women may have misidentified their family history, we observe robust differences in BP at the onset of exercise between groups. Furthermore, recent studies classifying participants using self-report have demonstrated clear, robust differences in a variety of sympathetic stimuli (Greaney et al., 2015b; Fonkoue et al., 2016). Finally, we obtained analyzable nerve recordings in ∼50% of the participants; it is possible that this low yield coupled with analyzing MSNA in a short time window introduced error. Short sampling durations (15 and 30 seconds) may be less reliable with small sample sizes, and can either underestimate or overestimate MSNA (Notay et al., 2016).
In conclusion, we show that +FH women display rapid increases in BP at the onset of isometric exercise. Our data are consistent with findings in older hypertensive adults, and extend those findings in that we observed similar pressor responses in young normotensive women at risk for future cardiovascular disease. Many activities of daily living require short duration submaximal isometric muscle contractions, such as opening a jar or carrying groceries (Greaney & Farquhar, 2012). Such muscular activities can cause exaggerated blood pressure responses in adults with cardiovascular disease (Greaney & Farquhar, 2012). The long-term clinical implications of these regularly occurring episodic surges in blood pressure are not completely known. Because cardiovascular disease is the leading cause of death in women, and the prevalence of hypertension is greater in women compared to men later in life (Lima et al., 2012), it is important to understand factors that may increase the risk for, or predict, future hypertension and cardiovascular disease in women.
New Findings.
What is the central question of this study?
Alterations in blood pressure control at exercise onset are apparent in older adults with established cardiovascular disease. It is currently not known if these alterations are evident in young adults with a family history of hypertension.
What is the main finding and its importance?
We demonstrate that young women with a family history of hypertension display a larger change in blood pressure within the first 10 seconds of isometric exercise. These data suggest altered BP control in young women with a family history of hypertension.
Acknowledgments
The authors wish to thank Andrew Kuczmarski and Kelly Sebzda for assistance with data analysis, Ryan Pohlig, PhD, for statistical support, the University of Delaware Nurse Managed Health Center, and the participants for their time.
Grants: This work was supported in part by NIH Grant U54-GM104941 (PI: Binder-Macleod)
Footnotes
Competing Interests: None
Author Contributions: E.L.M., J.L.G., and M.M.W. all participated in the conception/design of this study, data collection/analysis/interpretation, and the writing the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship; all those who qualify for authorship are listed.
References
- Berger A, Grossman E, Katz M, Kivity S, Klempfner R, Segev S, Goldenberg I, Sidi Y, Maor E. Exercise blood pressure and the risk for future hypertension among normotensive middle-aged adults. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutcher YN, Park YJ, Boutcher SH. Vascular and baroreceptor abnormalities in young males with a family history of hypertension. Eur J Appl Physiol. 2009;107:653–658. doi: 10.1007/s00421-009-1170-y. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol. 2013;304:H759–766. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkoue IT, Wang M, Carter JR. Sympathetic neural reactivity to mental stress in offspring of hypertensive parents: 20 years revisited. Am J Physiol Heart Circ Physiol. 2016 doi: 10.1152/ajpheart.00378.2016. ajpheart 00378 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Stromstad M, Ide K, Secher NH, Raven PB. The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol. 2006;91:79–87. doi: 10.1113/expphysiol.2005.032110. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens. 2015a;29:402–408. doi: 10.1038/jhh.2014.106. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Farquhar WB. Skeletal muscle contraction triggers rapid onset pressor responses in cardiovascular disease. J Physiol. 2012;590:5933–5934. doi: 10.1113/jphysiol.2012.247155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Matthews EL, Wenner MM. Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol. 2015b;308:H816–822. doi: 10.1152/ajpheart.00867.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalande S, Sawicki CP, Baker JR, Shoemaker JK. Effect of age on the hemodynamic and sympathetic responses at the onset of isometric handgrip exercise. J Appl Physiol (1985) 2014;116:222–227. doi: 10.1152/japplphysiol.01022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Mitchell JH, Smith SA, Mizuno M. Exaggerated sympathetic and cardiovascular responses to stimulation of the mesencephalic locomotor region in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2016;310:H123–131. doi: 10.1152/ajpheart.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14:254–260. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AR, Taylor SH, Humphreys PW, Kennelly BM, Donald KW. The Circulatiory Effects Of Sustained Voluntary Muscle Contraction. Clin Sci. 1964;27:229–244. [PubMed] [Google Scholar]
- Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2001;19:1981–1989. doi: 10.1097/00004872-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Michelini LC, O'Leary DS, Raven PB, Nobrega AC. Neural control of circulation and exercise: a translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2015;309:H381–392. doi: 10.1152/ajpheart.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol. 2012;590:6237–6246. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notay K, Seed JD, Incognito AV, Doherty CJ, Nardone M, Burns MJ, Millar PJ. Validity and reliability of measuring resting muscle sympathetic nerve activity using short sampling durations in healthy humans. J Appl Physiol (1985) 2016;121:1065–1073. doi: 10.1152/japplphysiol.00736.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]