Abstract
Purpose
To characterize intra- and inter-day variation in central corneal thickness (CCT) in healthy and glaucomatous subjects.
Methods
In this prospective cohort study, 40 healthy subjects and 42 subjects with primary open-angle glaucoma underwent CCT measurements by ultrasonic pachymetry on five days over one year: first at baseline, then at one week and one, six and twelve months after baseline. On one visit, CCT was measured every two hours from 0800 to 2000. Intra-day CCT variance was compared with inter-day CCT variance.
Results
Mean CCT at all visits and time points ranged from 561-574 microns in healthy eyes and from 548-563 microns in glaucomatous eyes. The mean intra-day CCT range (highest minus lowest readings) was 21 ± 10 microns in healthy participants and 21 ± 10 microns in glaucoma patients. The mean inter-day CCT range was 27 ± 13 microns in healthy participants and 24 ± 14 microns in glaucoma patients. Within-subject variance was significantly larger among the inter-day CCT measurements than the intra-day CCT measurements in both healthy subjects and glaucoma patients (P<0.0001 for both) demonstrating that measurements taken on separate days are more different than measurements taken on the same day. Inter-day CCT differences of 30 microns or greater were seen in 27.7% of normal subjects and 30% of glaucoma patients.
Conclusions
CCT measurements exhibit intra-day and inter-day variation, with the latter being significantly greater than the former. A single CCT assessment inadequately characterizes CCT and may impact risk assessment in patients with suspect and/or diagnosed glaucoma.
Introduction
It has been well established that central corneal thickness (CCT) is an independent risk factor for the progression from ocular hypertension to open-angle glaucoma1, 2. Further studies have indicated that CCT is linked to glaucoma risk and glaucoma progression3-8. Because CCT is utilized in global risk assessment of both suspected and established glaucoma, accurate assessment of CCT values is fundamentally important in the management of patients with ocular hypertension as well as glaucoma.
The consistency of CCT measurements over time has not been established, and some data suggest that significant inter-visit variation in CCT measurement exists.9,10 The purpose of this study was to characterize the intra-day and inter-day variability of CCT in eyes with and without open-angle glaucoma.
Methods
This was a post hoc analysis of data collected during the Diurnal Intraocular Pressure (IOP) Variability Assessment study. This study was approved by the institutional review board at West Virginia University, and all subjects provided written informed consent to participate. Participating subjects were either healthy subjects or had primary open-angle glaucoma (POAG). For the purposes of enrollment, patients with POAG had glaucomatous optic discs and repeatable visual field loss. A glaucomatous optic disc was defined as having evidence of excavation, diffuse or focal thinning or notching of the neuroretinal rim, visible nerve fiber layer defects, or asymmetry of the vertical cup-disc ratio of > 0.2 between eyes. An abnormal visual field was defined as having a pattern standard deviation (PSD) outside the 95% normal limits for Swedish Interactive Threshold Algorithm fields, or a corrected PSD outside the 95% normal limits for Humphrey Full Threshold algorithm tests, or an abnormal glaucoma hemifield test using either test strategy. Visual field loss was reproducible over at least two consecutive visual field tests. Subjects with POAG were eligible if they were using identical therapy in both eyes (topical eye drop medications and/or bilateral laser trabeculoplasty); POAG subjects on topical therapy remained on unchanged therapy during their participation in this study. Healthy subjects had IOP of 21mmHg or less (and no history of IOP above 21 mmHg), open angles, normal-appearing optic discs, normal visual fields, and no family history of glaucoma. Healthy subjects were excluded if they had another ocular condition that might affect IOP or corneal pathology that might limit the value of tonometry or pachymetry readings.
All subjects attended five visits at baseline and one week, one month, six months and one year after baseline. At each visit, IOP was measured using Goldmann tonometry (Haag Streit, Mason, Ohio) at two-hour intervals from 0800 to 2000. CCT was assessed immediately following the 0800 IOP measurement on each of the five days (inter-day measurements), and immediately following IOP measurement at each diurnal time point on one of the five days (intra-day measurements). CCT was measured in the seated position using the DGH Pachette 2 ultrasound pachymeter (DGH Technology, Exton, Pennsylvania). Right eyes were measured before left eyes, and each measurement represented the average of five values taken from different regions of the central cornea overlying the pupil. All subjects underwent assessment by the same examiner using the same pachymeter at all visits and time points.
The primary objective of this study was to characterize the magnitude of intra-day and inter-day CCT variation and to compare the two. Descriptive statistics were generated to characterize mean CCT at each visit and time point by group. Mean age and gender/race distribution were examined and compared between healthy and POAG subjects via a t-test and chi-square tests, respectively. Linear mixed models were utilized to estimate between-subject (σb2) and within-subject variances (σw2) of both intra-day and inter-day CCT measurements, calculated separately for healthy subjects and glaucoma patients. Various correlation models were initially examined and the resulting Akaike Information Criteria (AIC) values compared to verify that the assumption of a constant correlation across time points was appropriate. Intraclass correlation coefficients (ICCs; ICC = σb2 / (σb2 + σw2)) were calculated for both inter-day and intra-day measurements, separately for healthy and POAG patients. Testing the hypothesis that mean intra-day CCT variation and mean inter-day CCT variation were comparable was accomplished using a likelihood-ratio test of equality of within-subject variance between intra-day and inter-day CCT measurements. The ICC is a type of correlation that can be used to describe the degree of relatedness between measurements; the closer the ICC is to 1, the less within-subject variability relative to the between-subject variability. ICC(3,1) was utilized to describe a random set of subjects and a fixed set of time points. 1000 bootstrap samples were generated to establish 95% confidence intervals for the ICC's. Only data from right eyes were included in this analysis.
Results
Participants were 40 healthy people and 42 people with primary open-angle glaucoma. Demographic information is given in Table 1. Overall, the mean age of participants was 66.3 ± 11.9 yr, 95% were Caucasian and 66% were women. Healthy participants and glaucoma patients were demographically similar (Table 1). Glaucoma patients used an average of 1.4 topical medications for IOP control.
Table 1.
Subject demographic data by group.
| All Subjects (n=82) | Healthy Subjects (n=40) | POAG Subjects (n=42) | P-value | |
|---|---|---|---|---|
| Age, yr (mean ± SD) | 66.3 ± 11.9 | 68.3 ± 12.9 | 64.5 ± 10.8 | 0.146 |
| Race (% Caucasian) | 95.1 | 100 | 90.5 | 0.116 |
| Gender (% female) | 65.9 | 67.5 | 64.3 | 0.759 |
| Glaucoma treatment | ||||
| Number of topical medications | ||||
| 0 | -- | -- | 5 (11.9) | -- |
| 1 | -- | -- | 23 (54.8%) | -- |
| 2 | -- | -- | 9 (21.4%) | -- |
| 3 | -- | -- | 5 (11.9%) | -- |
| Drug classes | ||||
| Prostaglandin analogues | -- | -- | 26 (61.9%) | -- |
| Beta-blockers | -- | -- | 16 (38.1%) | -- |
| Carbonic anhydrase inhibitors | -- | -- | 13 (31.0%) | -- |
| Adrenergic agonists | -- | -- | 4 (9.5%) | -- |
| Trabeculoplasty | -- | -- | 5 (11.9%) | -- |
POAG = primary open-angle glaucoma
Mean CCT values at each of the five visits and at each of the seven diurnal time points are given in Table 2. CCT values in healthy subjects were consistently numerically higher than for subjects with glaucoma. Only CCT at 1000 and at the Month 6 visit were statistically significantly different between healthy and glaucomatous participants at the 0.05 level, but the significance of these findings was marginal (p=0.036 and p=0.027, respectively).
Table 2.
Descriptive statistics for mean central corneal thickness at each visit and time point by group (microns).
| All (n=82) | Healthy (n=40) | Glaucoma (n=42) | ||||
|---|---|---|---|---|---|---|
| Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | |
| Intra-visit | ||||||
| 0800 | 563 (33) | 565 (30) | 570 (27) | 570 (26) | 556 (38) | 560 (33) |
| 1000 | 564 (33) | 565 (30) | 572 (30) | 570 (28) | 556 (35) | 560 (31) |
| 1200 | 564 (36) | 564 (30) | 571 (30) | 569 (26) | 557 (40) | 559 (33) |
| 1400 | 561 (34) | 561 (30) | 567 (27) | 565 (28) | 556 (38) | 557 (31) |
| 1600 | 561 (33) | 564 (30) | 566 (27) | 567 (26) | 556 38) | 560 (33) |
| 1800 | 563 (32) | 562 (31) | 568 (28) | 567 (28) | 558 (36) | 558 (33) |
| 2000 | 562 (32) | 565 (30) | 568 (28) | 567 (28) | 556 (35) | 563 (32) |
| Inter-visit | ||||||
| Day 0 | 556 (32) | 557 (29) | 564 (30) | 563 (28) | 551 (35) | 554 (31) |
| Week 1 | 555 (34) | 556 (29) | 562 (29) | 561 (30) | 548 (35) | 551 (30) |
| Month 1 | 558 (37) | 558 (32) | 562 (33) | 563 (27) | 553 (41) | 553 (34) |
| Month 6 | 562 (35) | 564 (30) | 567 (30) | 564 (29) | 554 (39) | 558 (33) |
| Month 12 | 565 (32) | 567 (30) | 570 (29) | 574 (29) | 560 (36) | 562 (32) |
Mean CCT did not vary significantly throughout the 12-hour diurnal period in either healthy subjects (p=0.059) nor glaucoma patients (p=0.696). Inter-day mean CCT did vary significantly in both healthy subjects (p=0.0001) and glaucoma patients (p=0.005), with CCT generally increasing over time.
Within subjects, there was substantial intra-day and inter-day variation of CCT measurements. The mean range of intra-day CCT measurements (highest minus lowest of 7 intra-day measurements) was 21 ± 10 microns in healthy subjects and 21 ± 10 microns in glaucoma patients. These intra-day CCT differences were statistically significantly different from zero (p<0.0001) in both groups. The distribution of intra-day CCT differences of both healthy subjects and glaucoma patients is given in Figure 1. Among healthy participants, 12.5% of eyes manifested an intra-day CCT difference of 30 microns or more; among glaucomatous participants, 11.9% of eyes did so.
Figure 1.
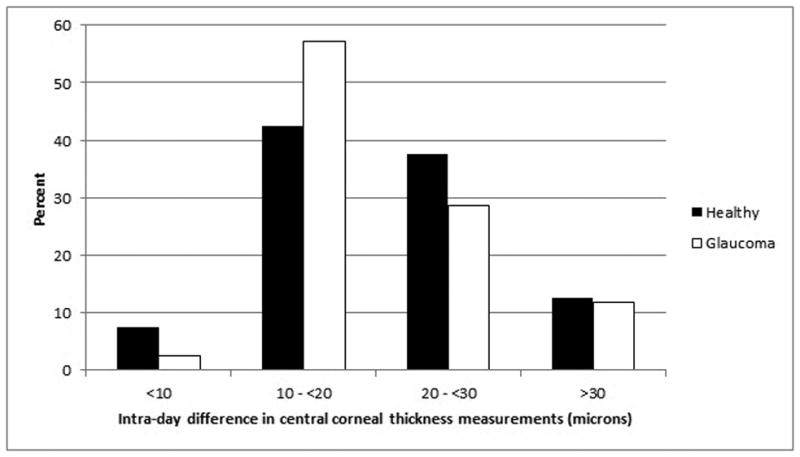
Distribution of variation in intra-day central corneal thickness in healthy subjects and glaucoma patients.
Likewise, there was substantial within-subject inter-day and inter-day variation of CCT measurements. The mean range of inter-day CCT measurements (highest minus lowest of 5 inter-day measurements) was 27 ± 13 microns in healthy subjects and 26 ± 18 microns in glaucoma patients. These inter-day CCT differences were statistically significantly different from zero (p<0.0001) in both groups and their distributions in both healthy subjects and glaucoma patients is given in Figure 2. Among healthy participants, 27.5% of eyes manifested an inter-day CCT difference of 30 microns or more; among glaucomatous participants, 30.9% of eyes did so.
Figure 2.
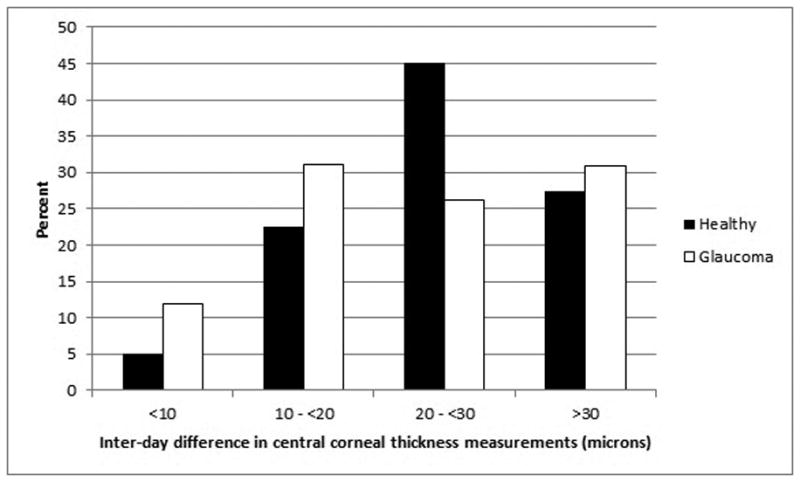
Distribution of variation in inter-day central corneal thickness in healthy subjects and glaucoma patients.
Variance component estimates and comparisons are given in Table 3. The ICCs revealed excellent agreement for both intra-day and inter-day CCT measurements in both healthy subjects and glaucoma patients, although inter-day ICC values were numerically lower than intra-day ICC values for both groups. Comparison of within-subject intra-day versus inter-day variance demonstrated that CCT measurements taken on different days were significantly more variable than CCT measurements taken on the same day in both healthy subjects (p<0.0001) and glaucoma patients (p<0.0001).
Table 3.
Variance of central corneal thickness measurements within and between days.
| Inter-Day Model | Intra-Day Model | |||
|---|---|---|---|---|
| Estimate | Normal Patients | Glaucoma Patients | Normal Patients | Glaucoma Patients |
| Between-Subject Variance (σb2) |
734.8 (487.0, 1235.6) |
1241.6 (833.8, 2044.4) |
720.3 (480.9, 1197.0) |
1315.0 (887.4, 2148.7) |
| Within-Subject Variance (σw2) |
126.7 (102.5, 160.7) |
153.3 (124.7, 192.8) |
70.0 (58.9, 84.6) |
65.1 (54.9, 78.5) |
| Intraclass Correlation Coefficient (95% confidence limits) ICC = σb2 / (σb2 + σw2) |
0.85 (0.79, 0.91) |
0.89 (0.82, 0.94) |
0.91 (0.72, 1.00) |
0.95 (0.77, 1.00) |
Discussion
Accurate and reproducible intra- and inter-day assessment of CCT is necessary to perform global risk assessment of patients with suspected or established glaucoma. The current study demonstrates that CCT measurements vary substantially throughout the day and even more so from day to day. As a result, our data suggest that a single CCT measurement at one moment in time may inadequately characterize CCT. Approximately 30% of both healthy subjects and glaucoma patients exhibited differences in CCT measurements of 30 microns or more on different days.
A change in CCT of this magnitude has significant clinical impact on risk assessment in patients with suspected or known glaucomatous optic neuropathy. In the risk model based on the combined data from the Ocular Hypertension Treatment Study and European Glaucoma Treatment Study, central corneal thickness is the strongest predictor for converting from ocular hypertension to primary open-angle glaucoma.11 Using the online risk calculator based upon this model (http://ohts.wustl.edu/risk/calculator.html) and a typical ocular hypertensive patient profile (age 60 years, untreated IOP 26 mmHg, vertical cup-disc ratio (VCDR) 0.4, Humphrey visual field pattern standard deviation (VFPSD) 0.5dB), a 30-micron change in CCT from 575 to 545 (centered on the average CCT observed in this study of 560 microns) changes the 5-year risk of conversion to POAG from 4.5% to 7.6%, a 67% relative increase in risk. This has the consequence of altering the recommended management from “observe without treatment” to “consider treatment.”12 Song et al demonstrated that longitudinal variability in CCT as well as IOP, VCDR and VFPSD can alter POAG conversion risk estimates by nearly 10-fold (1000%) in ocular hypertensive individuals over time.13 In established POAG, a validated risk model for progression to worse glaucoma has also been described and includes CCT as a risk factor.14 Using the online risk calculator based on this model (http://glaucoma.net/calculator/) and a typical early POAG patient profile (age 60 years, mean untreated IOP 26 mmHg, peak treated IOP 22 mmHg, Humphrey visual field mean deviation -4.5 dB, and no disc hemorrhage, peripapillary atrophy, pseudoexfoliation or prior glaucoma surgery), a similar 30-micron change in CCT from 575 to 545 microns increases the 5-year risk of visual field progression from 9.5% to 12.2%, a 28% relative increase in risk.
The results of the current study indicate that inter-day CCT variation is greater than intra-day variation. Previous studies have characterized intra-day diurnal (day-time hours) and circadian (24-hour) CCT variation (measured by ultrasonic pachymetry) in healthy subjects15-20as well as in subjects with suspected21 or established glaucoma.20 In all of these studies, mean CCT values of the respective samples were compared across time points, precluding evaluation of CCT variation within individuals as reported in the current study. Studies evaluating diurnal CCT variation have reported no significant changes throughout the day in eyes of healthy subjects18-20 or subjects with suspected glaucoma,21 but one study revealed a small but significant 12 micron increase in CCT between 0900 and 1700 hours in glaucomatous eyes.20 In contrast, our data demonstrated that both healthy and glaucomatous eyes manifested an average CCT variation of 21 microns over a 12-hour period, with approximately 12% of eyes manifesting a 12-hour CCT variation in excess of 30 microns.
There are fewer data characterizing inter-day CCT variation measured by ultrasonic pachymetry. An evaluation of CCT in 20 healthy subjects on 2 days separated by a median of 12 days revealed a mean inter-day CCT variation of +1.2 ± 11.6 microns.9 A study of 51 glaucoma patients or suspects reported mean CCT variation between two days separated by a mean of 84 days of 9.6 ± 26.9 microns in right eyes and 19.0 ± 29.2 microns in left eyes.10 The Ocular Hypertension Treatment Study (OHTS) group reported data on longitudinal CCT assessment; among both treated and untreated ocular hypertensive individuals, the mean change in CCT over a 3.8-year period was -2.7 ± 13.4 μ.22 Each of these three reports defined CCT variation as the difference between the two visits (visit 2 minus visit 1 as opposed to higher minus lower) so that values for CCT variation could be positive or negative. This explains why the mean is close to zero in two of the studies,9,22 but does not explain the bias toward significantly higher CCT values at the follow-up visit in the remaining study.10 Interestingly, our data also revealed a trend toward higher CCT at later follow-up visits compared to baseline (table 2 and figure 1). The basis for this trend is unclear and is unlikely to represent a visit-dependent methodologic bias; subjects in the current study attended the five visits of this study in a series of ten groups over a two-year time period, and subjects on a given day often were attending different visits (eg, some were starting the study on the same day others were finishing).
This study is strengthened by several features of its design. All subjects were evaluated by the same study personnel using the same device throughout the study, minimizing errors attributable to inter-operator or inter-device variation. Subjects were evaluated on five days over the course of a year, more often than any previously published report of which we are aware. All subjects were untreated or on stable medical regimens and none underwent any surgical procedures during the study period, thus reducing exogenous sources of CCT variation. However, the analysis was conducted post hoc on an existing data set. While they were not collected specifically to investigate CCT variability over time, these data are suitable for such inquiry. Measurement of CCT after Goldmann tonometry may have affected CCT measurements. However, we followed the methodology of the Ocular Hypertension Treatment Study (in which ultrasound pachymetry was performed after Goldmann tonometry), and we prioritized Goldmann tonometry over CCT in our original data set because the primary outcome of interest was related to IOP. The lack of 24-hour data is also a limitation which precludes fuller characterization of intra-day circadian CCT variation. One potential source of bias is our use of the maximum and minimum CCT values to determine CCT variability; this approach is susceptible to overestimation of CCT variation in the presence of outlying CCT values (which may have been selected as the maximum or minimum CCT value for calculating variability). CCT values at each time point were the mean of five separate readings. For a single outlying measurement to affect a CCT value (mean of five measurements) would require that it differ substantially from the other four measurements. Outliers such as these are typically recognized at the time of CCT measurement. While there were no formal rules for identifying and dealing with outliers among these five measurements, it was our practice to discard such outliers that differ from the remaining four and collect an additional measurement to replace it. Another type of outlier can arise if all five measurements at a given time point are generally close to one another but differ substantially from values at other time points. It is unlikely that all five measurements at a given time point would be similarly erroneous; thus, these values were accepted as true representations of the CCT at that time point. Our handling of these outliers represents real-world handling of such data: if one of five measurements is suspect then replace it, but if all five are consistent with one another, then accept them. Finally, we cannot infer whether the intra-day and inter-day CCT variation observed in our study arises due to true changes in CCT over time, limitations of reproducibility of the pachymetry device, variations in operator technique, or a combination of all three. Because this study was not originally designed to evaluate CCT variability or factors associated with CCT variability, we have not conducted analyses to explore factors associated with CCT variability. Future studies should be specifically designed and adequately powered to test hypotheses related to factors that might be associated with CCT variability.
In summary, CCT measurement by ultrasonic pachymetry exhibited significant intra-day and inter-day variation, with the latter being significantly greater in magnitude than the former. A single CCT assessment in time inadequately characterizes CCT. Inter-day CCT variation is great enough to significantly alter risk assessment of subjects with suspected or established glaucoma. We recommend a series of measurements on different days which provides better characterization of CCT and therefore enables the provider to improve the accuracy of risk assessment in patients with known or suspected glaucoma.
Acknowledgments
This study was supported by National Eye Institute grants EY015682 (TR) and EY018859 (TR), by the National Institute Of General Medical Sciences grant U54GM104942, and by an unrestricted grant from Research to Prevent Blindness, New York, NY (WVU and UCSD).
Footnotes
Previously reported in part at the 2009 annual meeting of the Association for Research in Vision and Ophthalmology, Ft. Lauderdale, FL.
The authors declare no conflicts of interest or relevant financial disclosures pertinent to this study.
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 2.Miglior S, Pfeiffer N, Torri V, et al. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 3.Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004 Jan;122(1):17–21. doi: 10.1001/archopht.122.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Bahamonde JL, Roman-Rodriguez C, Fernandez-Ruiz MC. Central corneal thickness as a predictor of visual field loss in primary opne angle glaucoma for a Hispanic Population. Semin Ophthalmol. 2011 Jan;26(1):28–32. doi: 10.3109/08820538.2010.541317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Congdon NG, Broman AT, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006 May;141(5):868–75. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Kim CY, Seong GJ, Hong YJ. Central corneal thickness and visual field progression in patients with chronic primary angle-closure glaucoma with low intraocular pressure. Am J Ophthalmol. 2007;143:362–363. doi: 10.1016/j.ajo.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Rogers DL, Cantro RN, Catoira Y, Cantor LB, Dunn DW. Central corneal thickness and visual field loss in fellow eyes of patients with open-angle glaucoma. Am J Ophthalmol. 2007;143:159–161. doi: 10.1016/j.ajo.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Marsich MW, Bullimore MA. The repeatability of corneal thickness measures. Cornea. 2000;19:792–5. doi: 10.1097/00003226-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wickham L, Edmunds B, Murdoch IE. Central corneal thickness: will one measurement suffice? Ophthalmology. 2005;112:225–8. doi: 10.1016/j.ophtha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Gordon MO, Torri V, Miglior S, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–67. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Song C, De Moraes CG, Forchheimer I, Prata TS, Ritch R, Liebmann JM. Risk Calculation Variability Over Time in Ocular Hypertensive Subjects. Journal of glaucoma. 2012 doi: 10.1097/IJG.0b013e31825af795. [DOI] [PubMed] [Google Scholar]
- 14.De Moraes CG, Sehi M, Greenfield DS, Chung YS, Ritch R, Liebmann JM. A validated risk calculator to assess risk and rate of visual field progression in treated glaucoma patients. Investigative ophthalmology & visual science. 2012;53:2702–7. doi: 10.1167/iovs.11-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kida T, Liu JH, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2006;47:4422–6. doi: 10.1167/iovs.06-0507. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton KE, Pye DC, Aggarwala S, et al. Diurnal variation of central corneal thickness and Goldmann applanation tonometry estimates of intraocular pressure. J Glaucoma. 2007;16:29–35. doi: 10.1097/IJG.0b013e31802b350f. [DOI] [PubMed] [Google Scholar]
- 17.Harper CL, Boulton ME, Bennett D, et al. Diurnal variations in human corneal thickness. Br J Ophthalmol. 1996;80:1068–72. doi: 10.1136/bjo.80.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotecha A, Crabb DP, Spratt A, Garway-Heath DF. The relationship between diurnal variations in intraocular pressure measurements and central corneal thickness and corneal hysteresis. Invest Ophthalmol Vis Sci. 2009;50:4229–36. doi: 10.1167/iovs.08-2955. [DOI] [PubMed] [Google Scholar]
- 19.Oncel B, Dinc UA, Gorgun E, Yalvac BI. Diurnal variation of corneal biomechanics and intraocular pressure in normal subjects. Eur J Ophthalmol. 2009;19:798–803. doi: 10.1177/112067210901900518. [DOI] [PubMed] [Google Scholar]
- 20.Villas-Boas FS, Doi LM, Sousa AK, Melo LA., Jr Correlation between diurnal variation of intraocular pressure, ocular pulse amplitude and corneal structural properties. Arq Bras Oftalmol. 2009;72:296–301. doi: 10.1590/s0004-27492009000300004. [DOI] [PubMed] [Google Scholar]
- 21.Shah S, Spedding C, Bhojwani R, et al. Assessment of the diurnal variation in central corneal thickness and intraocular pressure for patients with suspected glaucoma. Ophthalmology. 2000;107:1191–3. doi: 10.1016/s0161-6420(00)00090-7. [DOI] [PubMed] [Google Scholar]
- 22.Brandt JD, Gordon MO, Beiser JA, et al. Changes in central corneal thickness over time: the ocular hypertension treatment study. Ophthalmology. 2008;115:1550–6. doi: 10.1016/j.ophtha.2008.02.001. [DOI] [PubMed] [Google Scholar]


