Abstract
To examine the role of transient receptor potential vanilloid type 1 (TRPV1) ion channel in the vascular function of human skeletal muscle feed arteries (SMFAs) and if activation of this heat-sensitive receptor could be involved in modulating vascular function, SMFAs from 16 humans (63±5, 41–89 yrs) were studied using wire myography with capsaicin (TRPV1 agonist) and without (control). Specifically, phenylephrine (PE, α1-adrenergic receptor agonist), dexemedetomidine (DEX, α2-adrenergic receptor agonist), acetylcholine (ACh), and sodium nitroprusside (SNP) concentration response curves (CRCs) were performed to assess the role of TRPV1 channels in α-receptor-mediated vasocontraction as well as endothelium-dependent and independent vasorelaxation, respectively. Compared to control, capsaicin significantly attenuated maximum vasocontraction in response to PE (control: 52±8; capsaicin: 21±5 %length-tensionmax (LTmax)) and DEX (control: 29±12; capsaicin: 2±3 %LTmax), while robustly enhancing maximum vasorelaxation with ACh (control: 78±8; capsaicin: 108±13 %vasorelaxation), and less clearly enhancing the SNP response. Denudation of the endothelium greatly attenuated the maximum ACh-induced vasorelaxation equally in the control and capsaicin conditions (~17 %vasorelaxation), and abolished the attenuating effect of capsaicin on the maximal PE response (denuded+capsaicin: 61±20 %LTmax). Immunohistochemistry identified a relatively high density of TRPV1 channels in the endothelium compared to the smooth muscle of the SMFAs, but, due to the far greater volume of smooth muscle, total TRPV1 protein content was not significantly attenuated by denudation. Thus, SMFAs ubiquitously express functional TRPV1 channels, which alter vascular function, in terms of α1-receptors, in a predominantly endothelium dependent manner, conceivably contributing to the functional sympatholysis and unveiling a therapeutic target.
Keywords: heat, blood flow, sympatholysis
INTRODUCTION
The skeletal muscle feed artery (SMFA) has been recognized as a key site of skeletal muscle blood flow regulation in both animals (Segal & Duling, 1986; Williams & Segal, 1993; Lash, 1994; Segal, 2000; VanTeeffelen & Segal, 2003, 2006), and humans (Ives et al., 2012b). Thus, understanding the factors contributing to the basic function of the feed artery is important in terms of understanding the mechanisms that regulate muscle blood flow. However, currently there is a paucity of studies that address the basic physiology of human SMFAs. Recent work from our group has provided evidence of a regulatory role for human SMFAs (Ives et al., 2012b) and that these vessels may be susceptible to “metabolic inhibition” of sympathetically-mediated vasocontraction via metabolic by-products such as heat (Ives et al., 2011a; Ives et al., 2012a) and acidosis (Ives et al., 2013). Furthermore, we (Ives et al., 2012a) and others (Harris et al., 2003), have found that the endothelium and the endothelial nitric oxide synthase (eNOS) pathway appear to be integral components of this heat-induced sympatholysis, however, the mechanisms and receptors contributing to NOS activation in human SMFAs are still not well understood.
There is a growing interest in the transient receptor potential (TRP) family of ion channels and their role in the vasculature (Ramsey et al., 2006; Venkatachalam & Montell, 2007; Baylie & Brayden, 2011). Specifically, although the vanilloid type TRP channels (TRPV), have classically been suggested to play a role in the afferent neuronal sensation of temperature (Wang et al., 2006b; Light et al., 2008; Yang et al., 2010b) or noxious stimuli (Ramsey et al., 2006; Baylie & Brayden, 2011), recent work by our group (Gifford et al., 2014), investigated the role of TRPV channels, particularly the TRPV4 channel, in the heat induced sympatholysis of SMFAs. Whether using non-specific TRPV inhibition (Ruthenium Red, RR) or TRPV4 specific inhibition (RN-1734), the heat-induced suppression of the vasocontractile response to PE was abolished, suggesting TRPV channels, particularly the TRPV4 channels, are activated by heat and antagonize adrenergic-mediated vascular responses. Interestingly, TRPV inhibition also blunted the ACh response, indicative of an endothelial-dependent TRPV-mediated response, which was confirmed by denuding the endothelium. While it appears likely that the TRPV4 channel accounts for the majority of the functional sympatholytic effect of heat from muscle contraction, it is important to recognize that several factors associated with exercise (e.g. elevated reactive oxygen species (ROS), temperature, levels of anandamide, and decreased pH), probably acting synergistically (Ho et al., 2008; Roy et al., 2012), would also activate the vascular TRPV1 channels (Chuang & Lin, 2009; Mergler et al., 2010). Therefore, given the effect that non-specific TRPV inhibition, which includes TRPV1 inhibition, had on vascular function (Gifford et al., 2014) and the established relationship between the TRPV1 channels and endothelial function in mice (Yang et al., 2010a), it is conceivable that the TRPV1 channels play a similar role to the TRPV4 channel in modulating vascular function in the SMFAs of humans. Indeed, coupled with epidemiological data that suggests regular ingestion of capsaicin-rich foods (e.g. peppers from capsicum frutescent plants) reduces cardiovascular-related mortality (Lv et al., 2015), there is the implication that TRPV1 channels may play a significant role in vascular function. However, there is actually very little known about the potential role of the TRPV1 channels in modulating endothelial or vascular smooth muscle function in humans.
Consequently, the purpose of this study was to determine if human SMFAs express functional TRPV1 channels, what, if any, potential role they may play in vascular function, and, using an agonist approach, look for evidence that activation of TRPV1 channels could, conceivably, play a role in the functional sympatholysis associated with exercise. Additionally, by denuding the endothelium, we sought to determine if the endothelium plays a role in mediating any of the observed TRPV1 responses. We hypothesized that human SMFAs will express TRPV1 channels, which, upon activation, will significantly alter vascular function, specifically, reducing adrenergic mediated vasocontraction in a similar fashion to heat-induced sympatholysis, and this phenomenon would be predominantly endothelium-dependent.
METHODS
Subjects and General Procedures
A heterogeneous group of subjects agreed to have their vessels harvested during melanoma-related surgeries and used in this study (Table 1). Although medical conditions and medications were noted, by means of medical records, there were no exclusions based on this information. All subjects included in this study had not received chemotherapy, as this was a contraindication for surgery. All protocols were approved by the Institutional Review Boards of the University of Utah (IRB#32786) and the Salt Lake City VA Medical Center, and written informed consent was obtained from all subjects. This study was performed in accordance with the latest revision of the Declaration of Helsinki, except for registration in a database.
Table 1.
Subject Characteristics.
| Mean ± SE | Normal Range | |
|---|---|---|
| Age (yr) | 63 ± 5 (41–89) | -- |
| Males/Females (n) | 9/7 | |
| Height (cm) | 169 ± 9 | -- |
| Body Mass (kg) | 93 ± 10 | -- |
| BMI (kg/m2) | 33 ± 3 | < 30 |
| Systolic Blood Pressure (mmHg) | 133 ± 5 † | ≤ 120 |
| Diastolic Blood Pressure (mmHg) | 82 ± 3 † | ≤ 80 |
| MAP (mmHg) | 99 ± 4 | -- |
| Glucose (mg/dl) | 100 ± 8 | 65 – 110 |
| Blood Urea Nitrogen (mg/dl) | 16 ± 1 | 6 – 21 |
| Creatinine (mg/dl) | 0.8 ± 0.1 | 0.52 – 0.99 |
| Albumin (g/dl) | 3.9 ± 0.1 | 3.3 – 4.8 |
| Bilirubin (mg/dl) | 0.4 ± 0.1 | 0.2 – 1.3 |
| Lactate Dehydrogenase (U/L) | 458 ± 39 | 300 – 600 |
| WBC (K/uL) | 6.3 ± 1.1 | 3.2 – 10.6 |
| Platelets (K/uL) | 230 ± 18 | 150 – 400 |
| RBC (M/uL) | 4.9 ± 0.1 | 4 – 5.2 |
| Hemoglobin (g/dl) | 14 ± 0.4 | 12 – 16 |
| Hematocrit (%) | 42 ± 0.8 | 36 – 46 |
| Medications (users/n) | ||
| Cardiovascular | ||
| Statin | 6/16 | |
| Ca++ Channel Blocker | 1/16 | |
| Beta Blocker | 1/16 | |
| ACE inhibitor | 2/16 | |
| Diuretic | 2/16 | |
| Other | ||
| Hypothyroid | 2/16 | |
Data obtained during pre-operative examination, (n = 16).
Vessel Harvest
Human SMFAs from the axillary and inguinal regions were obtained during melanoma-related node dissection surgeries at the Huntsman Cancer Hospital, University of Utah. Patients were anaesthetized using a standard protocol including: propofol, fentanyl, benzodiazepines, and succinylcholine. After removal of sentinel lymph nodes or lymph node dissection, SMFAs in the axillary (e.g. serratus anterior, or latissimus dorsi) or inguinal (e.g. hip adductors, or quadriceps femoris) regions were identified and classified as SMFAs based on entry into a muscle bed, structure, coloration, and pulsatile bleed pattern. The vessels were ligated, excised, and immediately placed in iced normal physiological saline solution (PSS) and brought to the laboratory within 15 minutes of harvesting.
Wire Myography
Vessels were dissected under a stereo microscope in cold (~4°C) normal physiological saline solution (NPSS) (125 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 18 NaHCO3, 0.026 Na2EDTA, and 11.2 Glucose mM). All NPSS solutions and drugs were prepared fresh daily. Vessel internal diameter was measured using a calibrated micrometer eyepiece and reported in micrometers (μm). Perivascular adipose tissue was dissected from the SMFAs. The NPSS was continuously aerated with carbogen gas (95% oxygen, 5% carbon dioxide), and pH was monitored at regular intervals and maintained at pH 7.35 – 7.45 by altering the amount of aeration (Orion 3 Star, Thermo Scientific, Waltham MA).
Vessels were cut into four rings measuring approximately 2 mm in length, and mounted in wire myography baths (700 MO, DMT Systems, Aarhus, DK) to be studied using the isometric tension technique, as previously utilized by our group (Ives et al., 2011b). Once the vessels were mounted, the vessel baths were also aerated with the same carbogen gas mixture, and the media in the bath was exchanged at 10 minute intervals, except during the assessment of cumulative drug dose responses. Vessel baths were warmed to 37°C over a 30 minute equilibration period prior to the start of a protocol.
All vessel segments underwent length tension procedures at 37°C under control conditions to determine the length at which the vessels produced the greatest tension in response to a single dose of 100mM KCl (LTmax) (Symons et al., 2002). LTmax was operationally defined as less than a 10% improvement in developed tension in response to 100mM KCl.
Capsaicin and Vascular Reactivity
An overview of the experimental procedures is presented in Figure 1. To activate TRPV1 channels, capsaicin (1 μM) was added to two of the four baths 15 minutes prior to the start of the concentration response curves (CRCs), and was maintained in the designated baths throughout the experiment. This concentration of capsaicin was chosen as it is a commonly used dose sufficient to elicit significant TRPV1 channel activation in the vasculature (Poblete et al., 2005; Czikora et al., 2012). The capsaicin was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1mM, from this concentrated stock, 8 uL was added to the 8mL bath, resulting in a low concentration (0.1%/vol.) of DMSO, a concentration that has previously been determined to have no dilatory effect on isolated arteries (Pitts et al., 1986). In a balanced order, the following CRCs were performed: PE (10−9 – 10−3 log M), DEX (10−10 – 10−3 log M), ACh (10−7 – 10−3 log M), and SNP (10−9 – 10−4 log M) to determine α-receptor-mediated vasocontraction as well as endothelium-dependent and independent vasorelaxation. The CRCs were performed in a balanced manner, minimizing an order effect, and overall reducing the impact of neuronal TRPV1 receptors, due to repeated exposure of capsaicin and subsequent depletion of sensory neurotransmitters such as calcitonin gene related peptide (Zygmunt et al., 1999). It also should be noted that each bath contained, originally contiguous, vessel rings which were simultaneously exposed to the capsaicin or control conditions for each CRC. This approach was adopted to minimize the effect of time on a given CRC. To normalize vasocontraction data to the individual maximal response, as described elsewhere (Jarajapu et al., 2001; Wareing et al., 2002; Kluess et al., 2005; Ives et al., 2013), all vasocontractile responses are expressed as a percent of the individual maximal response to 100mM KCl during the length tension procedure (%LTmax) obtained during the length tension protocol, which typically yields the greatest tension development (unpublished observations). All vasorelaxation responses are expressed as percent relaxation (%) from approximately 60–70% PE pre-contraction (Ives et al., 2011a). All chemicals were obtained from Sigma Aldrich (St Louis, MO). All data were acquired at 4Hz using an analog to digital data acquisition system (Biopac Systems, Goleta, CA) to monitor vessel tension and allow later offline analyses.
Figure 1. Overview of the Experimental Approaches.
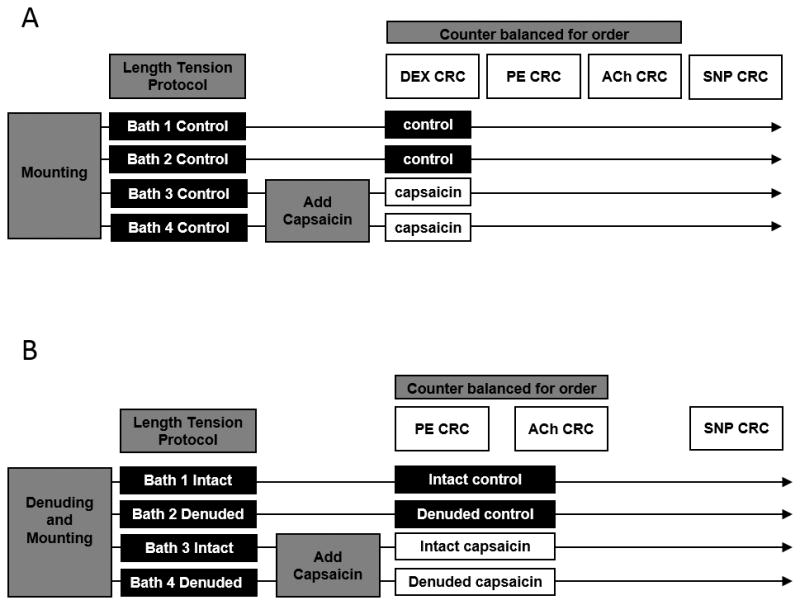
PE; Phenylephrine, DEX; Dexemedetomidine, ACh; Acetylcholine, SNP; Sodium Nitroprusside, CRC; Concentration Response Curve.
The Role of the Endothelium in Capsaicin-Mediated Responses
To determine the potential role of the endothelial TRPV1 channels, additional control and capsaicin experiments were performed in vessel rings, from the same subject, that either had an intact endothelium or had been denuded of endothelial cells. Denudation was achieved by passing 2 ml of air through the lumen of the artery before it was dissected and mounted onto the myograph chambers, as described previously (Gifford et al., 2014). Specifically, CRCs for PE, ACh, and SNP were performed on these intact and denuded vessels with and without capsaicin.
TRPV1 mRNA and Protein Expression
Using quantitative PCR (q-PCR) analysis, samples of the human SMFAs were probed for the gene expression of TRPV1 channels, α1A-, and α2B-adrenergic receptors (Rudner et al., 1999; Kable et al., 2000) using GAPDH as the normalizing gene. After homogenization in ice cold buffer containing RNAse inhibitors, RNA was extracted using RNAeasy mini kits (Qiagen, Valencia, CA) and was immediately converted to a cDNA library and stored at -20°C until further analysis. After which, cDNA libraries were analyzed using the ABI quantitative real time PCR system on the ABI Sequence detection system (SDS) platform (v 2.4.1, ABI, Foster City, CA). Using ABI Taqman master mix and Taqman gene expression assay primer probes for TRPV1, α1A-, α2B-adrenergic receptors, and GAPDH for a control gene, as well as no template controls, q-PCR was performed. Gene expression was quantified using the comparative Ct (2−Δct) method.
Frozen endothelium intact and endothelium denuded vessels were thawed on ice and then homogenized in 200 μL of ice-cold homogenization buffer containing a protease and phosphatase inhibitor cocktail (Sigma, St. Louis, MO)] and centrifuged for 15 min at 13,800 g at 4°C. The supernatant was then collected and total protein concentrations determined using the bicinchoninic acid (BCA) method with bovine serum albumin (BSA) as a standard (Pierce Chemical Company, Rockford, IL). Supernatants were stored at -80°C. Using standard western blotting methods membranes were probed with primary antibodies for TRPV1 receptor (sc12498, Santa Cruz, San Juan Ranch, CA), and GAPDH (ab9485, Abcam, Cambridge, MA) and visualized with enhanced chemiluminescence (ECL) (Pierce detection kit, Rockford, IL) using a digital imaging system (BioRad Chemidoc XRS Imager, Bio-Rad Laboratories, Hercules, CA). Membranes were stained with Coomassie blue (BioRad Laboratories, Hercules, CA) for a loading/transfer control. The TRPV1 signal was then normalized to the loading control signal (GAPDH).
To determine the locus of the TRPV1 channels, immunohistochemistry of the vessel sections was performed. Again, the primary antibodies for TRPV1 receptor (ab3487, Abcam, Cambridge, MA) were used to visualize the distribution of TRPV1 channels.
Statistical Analyses
Two way repeated measures ANOVA were used to determine significant responses for each CRC, between conditions (control vs. capsaicin) (SPSS v.16, Chicago, IL). Where significant main effects were identified, Tukey’s HSD post hoc tests were utilized (α = 0.05). Vasocontraction was calculated as follows: vasocontraction (%LTmax) = (Dose induced change in tension (change from pre-drug baseline)/maximal change in tension observed during LT)*100. Vasorelaxation (%) was calculated as follows: vasorelaxation (%) = (Dose induced change in tension (change from pre-contraction)/pre-contraction induced change in tension (pre-contraction − baseline)) *100. For the CRCs, to account for potential differences in the concentration which elicited the greatest response, maximal responses were determined on an individual basis and compared between conditions. The logEC50 was individually calculated using a sigmoidal parameter to estimate vascular sensitivity to each agonist (biodatafit v.1.02, Castro Valley, CA) and was compared between conditions using t-tests. To compare the effects of TRPV1 activation on adrenergic receptor subtype, percent sympatholysis was calculated (%Sympatholysis = (control response − capsaicin response)/control response*100) and compared using t-tests. All data are expressed as mean ± SEM1 for better visual clarity in the figures, and thus, for consistency, throughout the paper.
RESULTS
Subject Characteristics
SMFAs were successfully harvested from 16 volunteers (63 ± 5 yrs, 9 male, 7 female) (Table 1). None of these subjects had overt coronary artery, peripheral vascular, or cerebrovascular disease, or a history of MI/stenting/angioplasty. Due to the heterogeneity of the subjects and the origin of the vessels, this study was inadequately powered to detect differences in vascular function between sex, age, vessel location, medication use, complete blood count, or blood chemistry in any of the outcome variables. The average basal internal diameter for these SMFAs was 652 ± 96 μm. Twelve of the sixteen vessels obtained were harvested from the inguinal region, and 4 were obtained from the axillary region.
TRPV1 Activation and Adrenergic Mediated Vasocontraction
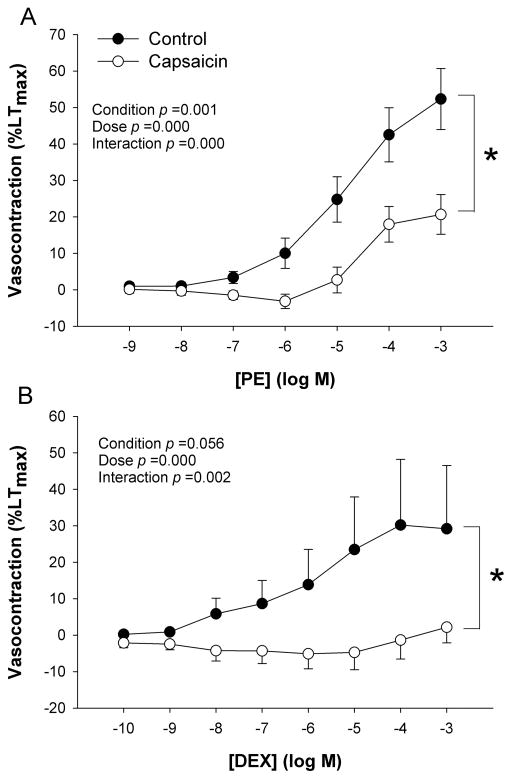
Baseline vascular tension was modestly, and transiently, increased by capsaicin, after which capsaicin tended to reduce baseline tension (588 ± 211 vs. 334 ± 117 mg, control vs. capsaicin, p > 0.05). Vessel function protocols revealed a significant main effect of concentration on the contraction response to the α1-receptor agonist PE and the α2-receptor agonist DEX (Figure 2A and B). For the PE CRC, there was a significant condition (control vs. capsaicin) by concentration interaction effect, indicating a reduced responsiveness with increasing concentrations of PE in the presence of capsaicin (Figure 2A). Here, there was also a significant main effect for condition (control vs. capsaicin, p < 0.05), indicating an overall blunted response to PE in the presence of capsaicin such that the individual maximal response to PE was attenuated (52 ± 8; 21 ± 5 %LTmax, control vs. capsaicin, respectively). The α2-receptor CRC assessments also displayed a significant condition by concentration interaction, revealing a blunted vascular response to increasing concentrations of DEX in the presence of capsaicin (Figure 2B). The main effect for condition, indicating an overall reduction in the vascular response to DEX, tended to be attenuated, but did not reach statistical significance (p = 0.06). The individual maximal response to α2-receptor stimulation was significantly blunted in the capsaicin condition (30 ± 13 vs. 4 ± 2 %LTmax, control vs. capsaicin, p < 0.05). The logEC50 was unaffected by capsaicin in response to either PE (-4.7 ± 0.3 vs. -4.7 ± 0.2 log M) or DEX (-5.8 ± 0.3 vs. -5.9 ± 0.7 log M) (control vs. capsaicin, respectively). Calculation of percent sympatholysis, revealed a significantly greater attenuating effect of TRPV1 activation on α2-receptor versus α1-receptor-mediated vasocontraction (PE 50 ± 10 vs. DEX 79 ± 16 % Sympatholysis).
Figure 2. The effect of TRPV1 activation on vasocontraction in human skeletal muscle feed arteries.
A) Phenylephrine (PE) concentration response curve (CRC) for α1-mediated vasocontraction, B) Dexemedetomidine (DEX) CRC for α2-mediated vasocontraction (n=16). * p < 0.05 control vs. capsaicin for maximal responses. Data are presented as mean ± SE.
TRPV1 Activation and Endothelium-Dependent and -Independent Vasorelaxation
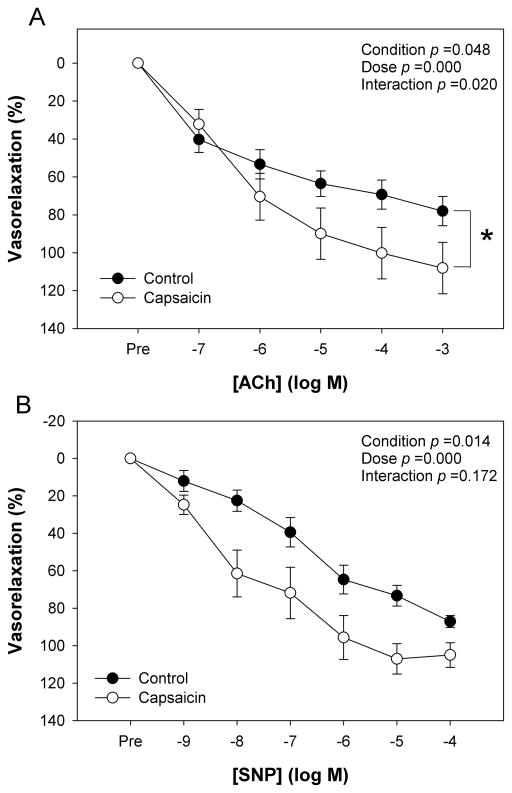
The SMFAs exhibited significant vasodilation (p < 0.05) in response to both the endothelium dependent agonist ACh and the endothelium-independent agonist SNP (Figure 3A and B). For the ACh CRC, there was a significant concentration by condition interaction effect, indicating an enhanced endothelial responsiveness with increasing concentrations of ACh in the presence of capsaicin. Here, there was also a significant main effect for condition (control vs. capsaicin, p < 0.05), indicating an overall greater endothelial response to ACh in the capsaicin condition such that the maximal response to ACh was increased (78 ± 8 vs. 108 ± 13 % vasorelaxation, capsaicin vs. control, respectively, Figure 3A). The logEC50 for ACh was not significantly altered with capsaicin. On a post hoc basis, as capsaicin was determined to alter PE-induced vasocontraction, the level of pre-contraction and maximal ACh-induced relaxation were entered into a simple linear regression, and there was no evidence of a relationship (r2 = 0.07, p > 0.05), suggesting that the level of pre-contraction was not influential in determining the relaxation response.
Figure 3. The effect of TRPV1 activation on vasorelaxation in human skeletal muscle feed arteries.
A) Acetylcholine (ACh) concentration response curve (CRC) for endothelium dependent vasodilation, B) Sodium Nitroprusside (SNP) CRC for endothelium independent vasodilation, (n=16). * p < 0.05 control vs. capsaicin for maximal responses. Data are presented as mean ± SE.
For the SNP CRC, there was not a significant interaction effect between condition and concentration (p = 0.2) (Figure 3B). However, there was a significant main effect for condition (control vs. capsaicin, p < 0.05), indicating an overall greater vasorelaxation response to SNP in the capsaicin condition, but the difference in the maximal response between conditions did not achieve statistical significance (87 ± 3 vs. 105 ± 6 %vasorelaxation, control vs. capsaicin, respectively, Figure 3B, p = 0.07). The logEC50 for SNP was significantly increased in the presence of capsaicin (-6.9 ± 0.4 vs. -7.6 ± 0.3 log M, control vs. capsaicin, respectively), indicating an enhanced vascular sensitivity to exogenous NO.
The Role of the Endothelium in the Capsaicin-Mediated Responses
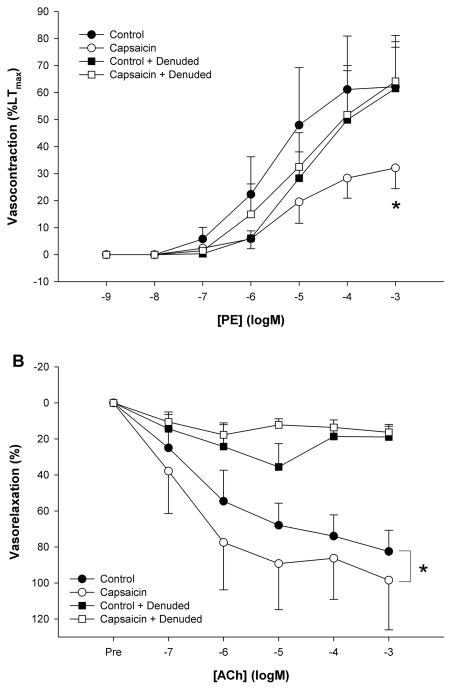
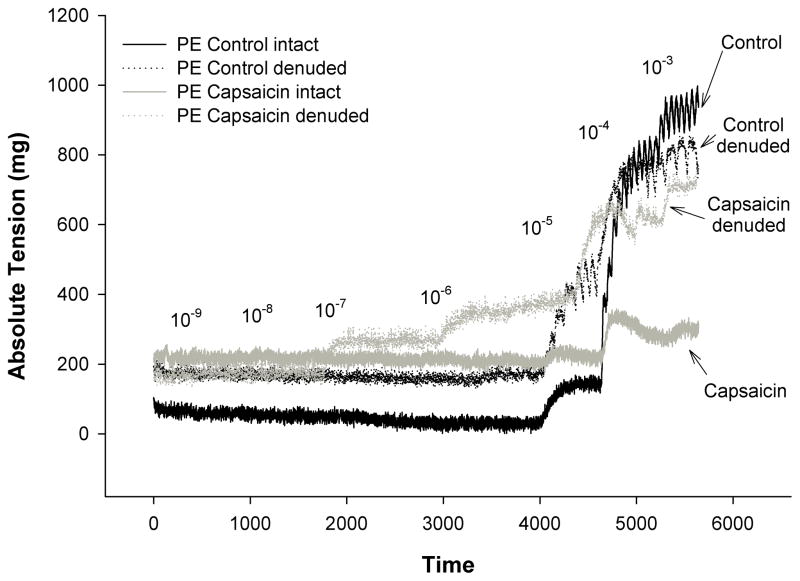
Removal of the endothelium abolished the attenuating effect of capsaicin on PE (capsaicin: 32 ± 8 vs. denuded+capsaicin: 61 ± 20 %LTmax) and restored vasocontraction in the control condition (62 ± 16%LTmax) (Figure 4A). Denudation of the endothelium significantly reduced the maximal vasorelaxation response to ACh in both the control (control: 82 ± 12 vs. control+denuded: 19 ± 6 % vasorelaxation) and capsaicin (capsaicin: 98 ± 27 vs. capsaicin+denuded: 16 ± 4 % vasorelaxation) conditions (Figure 4B). An original tracing of the impact of Capsaicin and/or endothelial denuding on the vasocontraction response to Phenylephrine in human skeletal muscle feed arteries is presented in Figure 5.
Figure 4. Endothelial contribution to the effect of TRPV1 channel activation on α1-receptor mediated vasocontraction and vasorelaxation in human skeletal muscle feed arteries.
A) Phenylephrine (PE) concentration response curve (CRC) for α1-receptor mediated vasocontraction. * p < 0.05 control vs. capsaicin. B) Acetylcholine (ACh) concentration response curve (CRC) for endothelium dependent vasodilation. * p < 0.05 endothelium intact vs. endothelium denuded (n=6). Data are presented as mean ± SE.
Figure 5.
Original tracings of the impact of Capsaicin and/or endothelial denuding on the vasocontraction response to Phenylephrine in human skeletal muscle feed arteries.
TRPV1 mRNA, Protein Expression, and Immunohistochemistry
Using quantitative PCR analysis, the SMFAs were determined to express significant levels of TRPV1 mRNA (0.05 ± 0.01 mRNA relative to GAPDH), which was on par with the level of adrenergic receptor mRNA (α1A 0.03 ± 0.00 and α2B 0.02 ± 0.01 mRNA relative to GAPDH). Immunohistochemistry of the SMFAs revealed ubiquitous expression of TRPV1 in both the endothelial and smooth muscle layers, however, notably, there appeared to be a relatively high TRPV1 prevalence in the relatively small volume of the endothelium (Fig 6A and B). Correspondingly, Western blots indicated expression of TRPV1 channels which was not greatly reduced following denudation (Figure 6).
Figure 6. Immunohistochemistry and protein expression of TRPV1 receptors in human skeletal muscle feed arteries (SMFA).
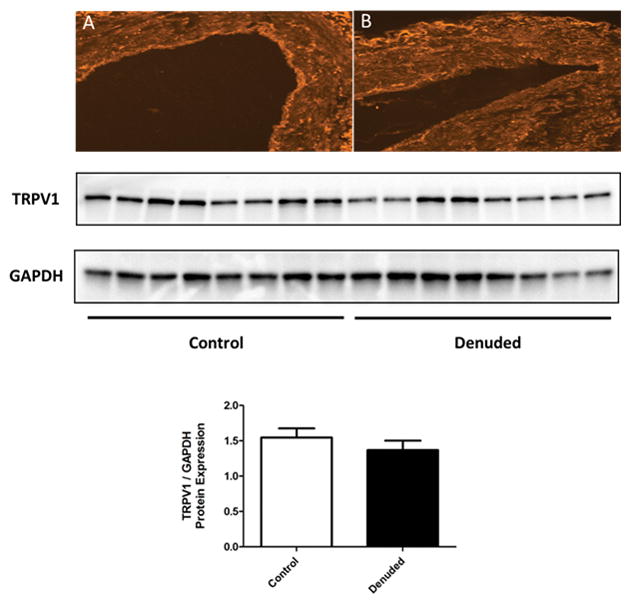
A) TRPV1 receptors (bright signal) evident both in the endothelium and smooth muscle in a control (endothelium intact) SMFA. B) TRPV1 receptors (bright signal) evident only in the smooth muscle in a denuded SMFA. Western blot analysis for TRPV1 protein content in control and denuded SMFAs with GAPDH as a loading control (n=8). Mean ± SE.
DISCUSSION
The main finding of this study is that human skeletal muscle feed arteries express functional TRPV1 channels, in both the endothelial and smooth muscle layers, which are capable of significantly altering vascular function. Specifically, pharmacological activation of vascular TRPV1 channels with capsaicin, blunted sympathetic vasocontraction mediated through either the α1- or α2-receptors. There was apparent receptor specificity, such that the α2-receptors were far more suppressed by TRPV1 activation than the α1-adrenergic receptors. The mechanism responsible for these findings is, at least in part, mediated by the endothelium, as denudation of this layer abolished the effects of capsaicin on α1-receptor mediated vasocontraction. In support of this, capsaicin enhanced endothelium-dependent vasorelaxation, as measured by ACh-induced vasorelaxation, which was also ablated by denudation of the endothelium. These findings suggest an important role of vascular endothelial TRPV1 channels in modulating vascular function, likely through an endothelium dependent increase in NO bioavailability, which can oppose α1-receptor mediated vasocontraction. Given the well documented sensitivity of TRPV1 channels to heat and other physiological activators such as those encountered during exercise (e.g. elevated reactive oxygen species, anandamide, and decreased pH), and the likely synergy of these stimuli, the results of this study suggest that these ion channels have the potential to contribute to sympatholysis during exercise. Furthermore, pharmacological activation of these channels via capsaicin might also be of therapeutic value.
The role of TRPV1 channels on vasocontraction
Previous investigations have revealed that TRPV1 activation can significantly alter myogenic tone (Scotland et al., 2004), induce vasodilation (Poblete et al., 2005; Wang et al., 2006a; Hoi et al., 2007; Bratz et al., 2008) or vasoconstriction (Lizanecz et al., 2006; Cavanaugh et al., 2011), depending upon capsaicin dose (10−8 vs. 10−6) (Kark et al., 2008), vessel location, and location of TRPV1 channels (neural compared to vascular) (Cavanaugh et al., 2011; Czikora et al., 2012). Using a dose of capsaicin (1 μM) that has been previously reported to induce either vasoconstriction (Kark et al., 2008; Czikora et al., 2012) or vascular NO release (Poblete et al., 2005), this study revealed no sustained effect of capsaicin alone on baseline tension and a blunted vasocontractile response to both α1- and α2-adrenergic receptors. Finally, removal of the endothelium prevented the capsaicin-induced reduction in α1-receptor mediated vasocontraction, suggesting that the TRPV1 activity is likely endothelium-dependent.
Interestingly, much of the work investigating the effects of TRPV1 activation on vascular responses, utilizing capsaicin, implies a neural mechanism, specifically though perivascular sensory neuronal release of vasoactive substances (Wang et al., 2006a; Kark et al., 2008). Thus, the possibility of neuronal TRPV1 channel involvement, and subsequent capsaicin-induced release of substance P or calcitonin gene related peptide (Kark et al., 2008) cannot be definitively excluded. However, the response, at least for α1-receptors, appears to be endothelium dependent (Figure 4A), which strongly implicates the vascular, not neuronal, TRPV1 receptors in modulating vascular function.
The current study revealed a significant effect of activating vascular TRPV1 channels, via capsaicin, on vasocontractile function in human feed arteries in the form of blunting adrenergic receptor mediated vasocontraction. These results suggest antagonism of G-protein coupled receptor function and subsequent signaling which is likely the result of antagonistic vasorelaxation initiated by the endothelium, as denuding restored PE-induced vasocontraction, and the endothelium specific agonist ACh, was enhanced in the presence of capsaicin. This observation is in agreement with the findings of others suggesting that the activation of TRPV1 channels in vascular endothelial cells activates the eNOS pathway, through both the activation (Yang et al., 2010a) and the removal of inhibition (Ching et al., 2013) of eNOS. Therefore, this study suggests that TRPV1 channels reduce adrenergic mediated vasocontraction in human feed arteries, in which multiple TRPV1 channel stimuli (i.e. heat, ROS, anandamide, and reductions in pH) may contribute to the functional sympatholysis associated with exercise, but this warrants further investigation.
The role of TRPV1 channels in vasorelaxation
Previous investigations focusing on the role of TRPV1 channels on vasorelaxation and the endothelium, provide convincing evidence of a role for endothelial TRPV1 channels in producing NO. Specifically, TRPV1 channels are Ca++ conducting channels, allowing Ca++ influx, and potentially activating Ca++ sensitive pathways. Given the well described role for Ca++ in activating eNOS (Dimmeler et al., 1999), it stands to reason that TRPV1 activation, either through exogenous capsaicin or endogenously through anandamide, heat, or both, would result in endothelial NO production (Poblete et al., 2005). In agreement with this concept, this study revealed that capsaicin enhanced the vasorelaxation response to ACh, suggestive of a synergistic effect of the endothelial receptor-mediated second messenger and TRPV1 channel-induced calcium signaling pathways, leading to greater endothelial NOS activity and NO bioavailability. In addition to documenting functional responses, this study followed up with gene and protein expression, and immunohistochemistry assays. Immunohistochemistry identified a relatively high density of TRPV1 channels in the endothelium compared to the smooth muscle of the SMFAs, but, due to the far greater volume of smooth muscle, total TRPV1 protein content was not significantly attenuated by denudation (Figure 6). Interestingly, SNP-induced vasorelaxation tended to also be augmented with capsaicin (Figure 3B), implying that the vascular response to exogenous NO had been sensitized, likely due to mechanisms aside from eNOS derived NO. One such mechanism could be the TRPV1-mediated release of endothelial derived hyperpolarizing factors (Baylie & Brayden, 2011), or direct hyperpolarization of the vascular smooth muscle (Bratz et al., 2008) through TRPV1 channels in the smooth muscle. Clinically, such knowledge may be of use in terms of targeting TRPV1 to improve endothelial function, which might help to explain the recently documented link between ingesting capsaicin-rich foods and reduced cardiovascular mortality (Lv et al., 2015), and to that end capsaicin has been used to prevent hypertension in mice (Yang et al., 2010a), though this remains to be explored in humans.
TRPV1 versus TRPV4 Ion channels in SMFAs
In a previous study we examined the role of the TRPV4 ion channels in modulating vascular function in human feed arteries (Gifford et al., 2014). Although the methods by which the ion channels were activated differed in these two studies, the current study utilizing a ligand and the other using heat, the results still offer an opportunity to compare the roles of these two ion channels in feed arteries. First, it should be noted that the activation of either TRPV1 or TRPV4 ion channels resulted in attenuated α1 adrenergic vasocontraction, but despite a tendency for decreased α2-induced contraction under TRPV4 activated conditions, only TRPV1 channel activation with capsaicin significantly inhibited both α1 and α2-receptor mediated vasocontraction. Second, it should be noted that while TRPV4 channel activation, with heat, inhibited adrenergic contraction in an endothelium-dependent manner, there was only a weak tendency to potentiate ACh-induced vasorelaxation while TRPV1 activation with capsaicin significantly augmented ACh-induced vasorelaxation. Thus, while activation of both types of ion channels results in a similar sympatholytic response, TRPV1 activation may present a more potent stimulus. This, teleologically, makes sense because temperatures known to activate TRPV1 channels (40+°C) are much higher and more noxious than those that activate the TRPV4 channels (25–39°C) (Baylie & Brayden, 2011), perhaps demanding a greater response. Alternatively, unlike TRPV4 channels, TRPV1 are known to be sensitized by other metabolic factors aside from temperature, such as reductions in pH (Faisy et al., 2007; Gao et al., 2007), ROS, as well as the endogenous ligand anandamide (Poblete et al., 2005; Chuang & Lin, 2009; Mergler et al., 2010). During exercise pH can be reduced and the circulating levels of ROS (Bailey et al., 2003) and anandamide increased (Heyman et al., 2012), both of which likely lower the activation threshold of TRPV1 (Ho et al., 2008; Roy et al., 2012), and/or increase TRPV1 activity under more physiological conditions than previously thought. Therefore, the activation of TRPV1 ion channels with heat, ROS, acidosis, and/or anandamide may, in combination with the TRPV4 channels (Gifford et al., 2014), also contribute to functional sympatholysis, but this speculation awaits further investigation.
Experimental Considerations
The subjects who took part in this study were certainly heterogeneous in terms of age, gender, and health, but, although exhibiting a tendency to be overweight and some evidence of systolic hypertension (although it should be noted that these measurements were obtained during pre-operative examination), they were taking minimal medications and had normal blood chemistry and complete blood count data (Table 1). Thus, interpretation of the current data must be taken in this context and may not apply to young healthy humans. Additionally, given the disparate vessel harvest location it is possible that differences in muscle fiber type might complicate the current results. However, unlike murine models, where muscle fiber type is far more dichotomous, human muscle is quite mosaic, likely minimizing this as a confounding issue. Thus, despite a group of heterogeneous subjects, varied vessel harvest location (i.e. axillary and inguinal), and potential pathology, the notion that TRPV1 activation elicited profound effects on vascular function speaks to the robust nature of these findings as they relate to vascular function and blood flow regulation in humans. However, at present, the potential role of age in these findings cannot be ruled out, as previously, albeit in subjects a decade older than the current average subject age, our group has documented age-related differences in vascular function using pressure myography (Park et al., 2016). Therefore, it would be useful for future studies to explore TRPV1 involvement in vascular function across the lifespan. Additionally, due to the relative scarcity of human SMFAs for research and the apparent predominant role of the α1-adrenergic receptors at this point in the vascular tree (Figure 2), it should be noted that the role of the α2-adrenergic receptors was not assessed with and without an intact endothelium. Thus, although we expect a similar important role for the endothelium in the TRPV1 modulation of α2-adrenergic receptor vasoconstriction, this remains to be confirmed.
Conclusion
Utilizing an isolated in vitro approach to study human SMFA function, this study reveals that these arteries express functional vascular TRPV1 channels in both the endothelial and smooth muscle layers. Activation of these channels significantly increased endothelium-dependent vasorelaxation and opposed α-adrenergic-mediated vasocontraction, effects that could be reversed with denudation of the endothelium, as assessed by α1-receptor mediated responses. Thus, skeletal muscle feed arteries ubiquitously express functional TRPV1 channels, which alter vascular function in a predominantly endothelium-dependent manner, perhaps presenting as a novel therapeutic target, and could, conceivably, contribute to the functional sympatholysis associated with exercise.
NEW FINDINGS.
This study sought to determine if human skeletal muscle feed arteries (SFMAs) express TRPV1 channels and what role do they play in modulating vascular function. Human SMFAs do, in fact, express functional TRPV1 channels that modulate vascular function, specifically opposing α-adrenergic receptor-mediated vasocontraction and potentiating vasorelaxation, in an endothelium-dependent manner, as evidenced α1-receptor mediated responses. Thus, the vasodilatory role of TRPV1 channels, and their ligand, capsaicin, could be a potential therapeutic target for improving vascular function. Additionally, given the “sympatholytic” effect of TRPV1 activation and known endogenous activators (anandamide, ROS, H+, etc.) TRPV1 channels may contribute to functional sympatholysis during exercise.
Acknowledgments
The authors would like to thank the volunteers for their participation, the surgical staff at the Huntsman, and financial support by NIH PO1 HL-091830 (R.S.R), VA Merit Grants E6910R (R.S.R) and E1697-R (R.S.R), VA SPiRe Grant E1433-P, and a VA Advanced Fellowship in Geriatrics (S.J.I). We would also like to thank Alan Light, Ron Hughen, and Tim VanHaitsma for performing the q-PCR analyses.
Footnotes
All data are expressed as mean ± SEM for better visual clarity in the figures
CONFLICT OF INTEREST: The authors have no disclosures or conflicts of interest to report.
AUTHOR CONTRIBUTIONS: The data were collected in the Utah Vascular Research Laboratory in the Salt Lake City VA Medical Center. S.I., R.R., contributed to the conception or design of the work, S.I., S.P., O.K., J.R., R.A., J.H. and R.R. were involved in acquisition, analysis, or interpretation of data for the work, and S.I., S.P., O.K., J.R., R.A., J.H. and R.R. drafted the work or revised it critically for important intellectual content.
References
- Bailey D, Davies B, Young I, Jackson M, Davison G, Isaacson R, Richardson R. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. Journal of Applied Physiology. 2003;94:1714. doi: 10.1152/japplphysiol.01024.2002. [DOI] [PubMed] [Google Scholar]
- Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2011;203:99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. AJP: Heart and Circulatory Physiology. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. Journal of Neuroscience. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching LC, Zhao JF, Su KH, Shyue SK, Hsu CP, Lu TM, Lin SJ, Lee TS. Activation of transient receptor potential vanilloid 1 decreases endothelial nitric oxide synthase phosphorylation at Thr497 by protein phosphatase 2B-dependent dephosphorylation of protein kinase C. Acta Physiol (Oxf) 2013 doi: 10.1111/apha.12157. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Chuang H-h, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proceedings of the National Academy of Sciences. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czikora Á, Lizanecz E, Bakó P, Rutkai I, Ruzsnavszky F, Magyar J, Pórszász R, Kark T, Facskó A, Papp Z, Édes I, Tóth A. Structure-activity relationships of vanilloid receptor agonists for arteriolar TRPV1. British Journal of Pharmacology. 2012;165:1801–1812. doi: 10.1111/j.1476-5381.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P. Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor. Life Sciences. 2007;81:1094–1102. doi: 10.1016/j.lfs.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. Journal of Applied Physiology. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- Gifford JR, Ives SJ, Park SY, Andtbacka RH, Hyngstrom JR, Mueller MT, Treiman GS, Ward C, Trinity JD, Richardson RS. alpha1- and alpha2-adrenergic responsiveness in human skeletal muscle feed arteries: the role of TRPV ion channels in heat-induced sympatholysis. Am J Physiol Heart Circ Physiol. 2014;307:H1288–1297. doi: 10.1152/ajpheart.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285:H333–340. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. British Journal of Pharmacology. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM, Visintin C, Okuyama M, Gardiner SM, Kaup SS, Bennett T, Baker D, Selwood DL, Hiley CR. Vascular pharmacology of a novel cannabinoid-like compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16) in the rat. British Journal of Pharmacology. 2007;152:751–764. doi: 10.1038/sj.bjp.0707470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Kwon SH, Shiu YT, Ruan T, Noyes RD, Zhang QJ, Symons JD, Richardson RS. Heat and alpha1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. Journal of Applied Physiology. 2012a;113:1690–1698. doi: 10.1152/japplphysiol.00955.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Noyes RD, McDaniel J, Amann M, Witman MA, Symons JD, Wray DW, Richardson RS. Human skeletal muscle feed arteries studied in vitro: the effect of temperature on alpha(1)-adrenergic responsiveness. Experimental Physiology. 2011a;96:907–918. doi: 10.1113/expphysiol.2011.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA, Richardson RS. Human skeletal muscle feed arteries: evidence of regulatory potential. Acta Physiol (Oxf) 2012b;206:135–141. doi: 10.1111/j.1748-1716.2012.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RHI, Noyes RD, McDaniel J, Amann M, Witman MAH, Symons JD, Wray DW, Richardson RS. Human skeletal muscle feed arteries studied in vitro: the effect of temperature on α1-adrenergic responsiveness. Experimental Physiology. 2011b;96:907–918. doi: 10.1113/expphysiol.2011.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RHI, Noyes RD, Morgan RG, Gifford JR, Park S-Y, Symons JD, Richardson RS. α1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Experimental Physiology. 2013;98:256–267. doi: 10.1113/expphysiol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarajapu YP, Coats P, McGrath JC, Hillier C, MacDonald A. Functional characterization of alpha(1)-adrenoceptor subtypes in human skeletal muscle resistance arteries. British Journal of Pharmacology. 2001;133:679–686. doi: 10.1038/sj.bjp.0704130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. In Vivo Gene Modification Elucidates Subtype-Specific Functions of α2-Adrenergic Receptors. Journal of Pharmacology and Experimental Therapeutics. 2000;293:1–7. [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Molecular Pharmacology. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Acidosis attenuates P2X purinergic vasoconstriction in skeletal muscle arteries. Am J Physiol Heart Circ Physiol. 2005;288:H129–132. doi: 10.1152/ajpheart.00574.2004. [DOI] [PubMed] [Google Scholar]
- Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. Journal of Applied Physiology. 1994;76:1512–1519. doi: 10.1152/jappl.1994.76.4.1512. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. Journal of Neurophysiology. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizanecz E, Bagi Z, Pasztor ET, Papp Z, Edes I, Kedei N, Blumberg PM, Toth A. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Molecular Pharmacology. 2006;69:1015–1023. doi: 10.1124/mol.105.015644. [DOI] [PubMed] [Google Scholar]
- Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, Bian Z, Sun D, Du J, Ge P, Tang Z, Hou W, Li Y, Chen J, Chen Z, Li L China Kadoorie Biobank Collaborative G. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942. doi: 10.1136/bmj.h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, Pleyer U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Experimental Eye Research. 2010;90:758–770. doi: 10.1016/j.exer.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD, Richardson RS. Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol. 2016;310:H217–225. doi: 10.1152/ajpheart.00716.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts LH, Young AR, McCulloch J, MacKenzie E. Vasomotor effects of dimethyl sulfoxide on cat cerebral arteries in vitro and in vivo. Stroke. 1986;17:483–487. doi: 10.1161/01.str.17.3.483. [DOI] [PubMed] [Google Scholar]
- Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. The Journal of Physiology. 2005;568:539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. British Journal of Pharmacology. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. AN INTRODUCTION TO TRP CHANNELS. Annual Review of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Roy A, Mandadi S, Fiamma M-N, Rodikova E, Ferguson EV, Whelan PJ, Wilson RJA. Anandamide modulates carotid sinus nerve afferent activity via TRPV1 receptors increasing responses to heat. 2012;112 doi: 10.1152/japplphysiol.01303.2010. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype Specific Regulation of Human Vascular {alpha}1-Adrenergic Receptors by Vessel Bed and Age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circulation Research. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiologica Scandinavica. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circulation Research. 1986;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- Symons JD, Mullick AE, Ensunsa JL, Ma AA, Rutledge JC. Hyperhomocysteinemia evoked by folate depletion: effects on coronary and carotid arterial function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:772–780. doi: 10.1161/01.atv.0000014588.71807.0a. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annual Review of Biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. Journal of Hypertension. 2006a;24:2399–2408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiology of Aging. 2006b;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing M, Crocker IP, Warren AY, Taggart MJ, Baker PN. Characterization of small arteries isolated from the human placental chorionic plate. Placenta. 2002;23:400–409. doi: 10.1053/plac.2002.0825. [DOI] [PubMed] [Google Scholar]
- Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010a;12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proceedings of the National Academy of Sciences. 2010b;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H-h, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]