Abstract
The risk of several cancers, including colorectal cancer, is increased in patients with obesity and type 2 diabetes, conditions characterized by hyperinsulinemia and insulin resistance. Because hyperinsulinemia itself is an independent risk factor for cancer development, we examined tissue-specific insulin action in intestinal tumor formation. In vitro, insulin increased proliferation of primary cultures of intestinal tumor epithelial cells from ApcMin/+ mice by over 2-fold. Surprisingly, targeted deletion of insulin receptors in intestinal epithelial cells in ApcMin/+ mice did not change intestinal tumor number or size distribution on either a low or high-fat diet. We therefore asked whether cells in the tumor stroma might explain the association between tumor formation and insulin resistance. To this end, we generated ApcMin/+ mice with loss of insulin receptors in vascular endothelial cells. Strikingly, these mice had 42% more intestinal tumors than controls, no change in tumor angiogenesis, but increased expression of vascular cell adhesion molecule-1 (VCAM-1) in primary culture of tumor endothelial cells. Insulin decreased VCAM-1 expression and leukocyte adhesion in quiescent tumor endothelial cells with intact insulin receptors and partly prevented increases in VCAM-1 and leukocyte adhesion after treatment with tumor necrosis factor-α. Knockout of insulin receptors in endothelial cells also increased leukocyte adhesion in mesenteric venules and increased the frequency of neutrophils in tumors. We conclude that although insulin is mitogenic for intestinal tumor cells in vitro, its action on tumor cells in vivo is via signals from the tumor microenvironment. Insulin resistance in tumor endothelial cells produces an activated, proinflammatory state that promotes tumorigenesis. Improvement of endothelial dysfunction may reduce colorectal cancer risk in patients with obesity and type 2 diabetes.
Keywords: hyperinsulinemia, colorectal cancer, adhesion molecules, endothelial cell function
Introduction
Obesity and type 2 diabetes are independent risk factors for the development of colorectal cancer and other malignancies [1–3]. Hyperinsulinemia is a central feature of these metabolic diseases and is itself an independent risk factor for tumor formation [4], including colorectal cancer [5] and colorectal adenomas [6]. Since a mitogenic action of insulin is well-described [4, 7–9] it has been proposed that the link between obesity and cancer can be explained by a growth-promoting effect of increased insulin action directly on tumor cells [4, 10, 11]. However, this hypothesis has not been directly tested in the context of intestinal tumor formation.
Chronic inflammation has often been proposed as another possible contributor to the relationship between obesity and cancer [12]. Diet-induced obesity changes the immune cells resident in the intestine [13] and myeloid cells and T cells can promote tumor growth by releasing cytokines like tumor necrosis factor-α (TNF-α) or interleukin-6 or by production of reactive oxygen or nitrogen species [14]. We previously found that suppression of the proinflammatory factor prostaglandin E2 suppressed the stromal inflammatory response and reduced intestinal tumor formation [15]. Anti-inflammatory treatment has been effective in clinical translation of such findings as shown by prevention of colorectal adenomas with the cyclooxygenase-2 inhibitor celecoxib [16] and reduction of colorectal cancer risk with long-term aspirin treatment [17].
Intriguingly, impaired insulin signaling may contribute to the chronic inflammation observed in obesity. We previously demonstrated that endothelial-specific deletion of the insulin receptor results in a marked increase in the burden of atherosclerosis in the Apoe null mouse [18]. Loss of vascular endothelial cell insulin signaling also resulted in a pronounced increase in leukocyte rolling and adhesion in the intestinal microcirculation observed during in vivo observation of mesenteric venules [18]. This supports a pro-inflammatory effect of endothelial cell insulin resistance in the intestine akin to that observed in atherosclerotic plaques. Importantly, endothelial cell insulin resistance occurs early in the development of diet-induced obesity in animal models [19, 20] and is present in humans with obesity or type 2 diabetes [21–24]. Therefore, impaired insulin signaling in endothelial cells could contribute to the increased risk of colon cancer in obesity by promoting chronic inflammation.
In this study, we examined the contribution of epithelial and endothelial insulin signaling to the development of endogenous intestinal tumor formation. Tumor-prone ApcMin/+ mice were modified by tissue-specific knockout of the insulin receptor in intestinal epithelium or in vascular endothelial cells. Remarkably, tumor burden was not affected by loss of epithelial cell insulin signaling in lean animals or in the context of hyperinsulinemia induced by high-fat diet feeding. In contrast, loss of the endothelial insulin receptor enhanced intestinal tumor formation. Moreover, vascular cell adhesion molecule-1 (VCAM-1), a key mediator of vascular inflammation and immune cell recruitment, was upregulated by loss of the insulin receptor in primary tumor endothelial cells. We conclude that insulin resistance in vascular endothelial cells promotes vascular inflammation and intestinal tumorigenesis.
Results
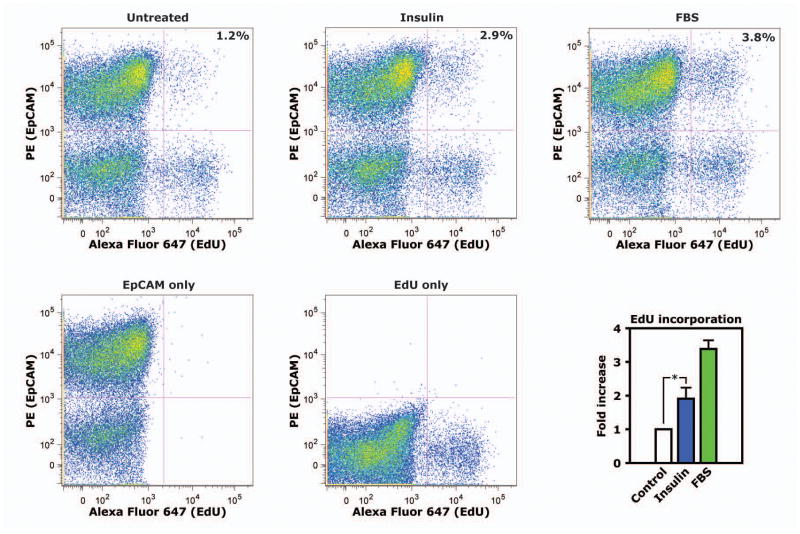
Insulin has been shown to promote proliferation in a range of cancer cell lines [4, 7–9]. To determine whether insulin has this effect in primary tumor cells from mice with the multiple intestinal neoplasia (Min) mutation (ApcMin/+ mice), we enzymatically dissociated polyps from the small intestine of ApcMin/+ mice and maintained mixed tumor cells in short-term culture. Tumor cells were serum-starved and treated with 10 nM insulin for 16 hours, then labeled with 5-ethynyl-2′-deoxyuridine (EdU) and analyzed by flow cytometry. An antibody against EpCAM, a marker of epithelial cells, stained 70.1± 7.8% of the cell population cultured from polyps (Fig. 1). In EpCAM+ tumor epithelial cells, insulin treatment increased EdU incorporation by 1.9±0.3 fold, a considerable increase given that treatment with FBS increased EdU incorporation by 3.4±0.3 fold (Fig. 1). Therefore, insulin clearly increased DNA synthesis in transformed epithelial cells from ApcMin/+ mice during serum-starved conditions in culture.
Figure 1. Insulin increases proliferation of serum-starved primary polyp epithelial cells in culture.
Polyps were isolated from ApcMin/+ mice and tumor cells dissociated by enzymatic digestion were grown in culture. Cells were serum-starved overnight, then stimulated with insulin for 16 hours. In the final 4 hours of this period, cultures were labeled with EdU. The proportion of cells double-positive for EpCAM, an epithelial cell marker, and EdU were analyzed by flow cytometry. Scatter plots show representative data from flow cytometry. The graph shows mean values from independent experiments using primary culture from 4 different animals. *, p<0.05.
Whole-body glucose tolerance is not altered by Insr knockout in intestinal epithelial cells
Despite the well-known mitogenic effect of insulin on tumor cells it has not been directly assessed whether insulin action on normal or transformed epithelial cells contributes to intestinal tumor formation in vivo. We therefore generated ApcMin/+ mice with or without knockout of the insulin receptor gene (Insr) specifically in intestinal epithelial cells. In these Vil1-cre Insrlox/lox ApcMin/+ (VILIRKO-Min) mice, insulin receptor mRNA was reduced by 97% and 93% in lysate of normal epithelium and intestinal tumors, respectively, compared to Insrlox/lox ApcMin/+ controls (Fig. 2A).
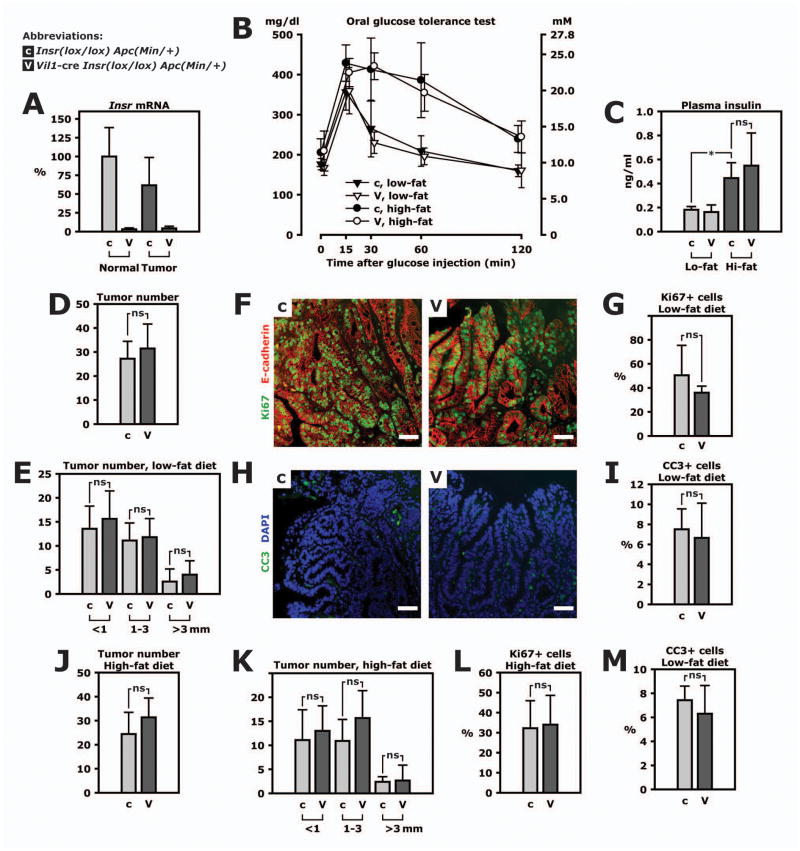
Figure 2. Loss of the insulin receptor in intestinal epithelial cells does not change tumorigenesis.
ApcMin/+ mice with insulin receptor knockout targeted to intestinal epithelial cells were studied (genotype Vil1-cre Insrlox/lox ApcMin/+, abbreviated VILIRKO-Min and labeled “V” in this figure). They were compared to littermate controls (genotype Insrlox/lox ApcMin/+ and labeled “c” in this figure). A. Insr mRNA was measured by real-time PCR in lysate of tumors or normal jejunum. B. Mice were fed a low-fat or high-fat diet for 8 weeks and subjected to an oral glucose tolerance test at 12 weeks of age. For the test, mice fasted overnight and were given 2 mg glucose per g body weight by gavage. Glucose concentration was measured in plasma from tail blood. C. Insulin was measured in plasma in fasted animals. D. Tumor number was counted in the entire small intestine of 9 control mice and 11 VILIRKO-Min mice fed a low-fat diet. E. Tumor number in 3 categories of tumor size. F. Representative microphotos of Ki67 (green) and E-cadherin (red) immunohistofluorescence performed on sections of paraffin-embedded tissue. Scale bars represent 50 μm. G. Frequency of Ki67+ cells in tumor tissue. H. Representative microphotos of cleaved caspase-3 (CC3) immunohistofluorescence (green) and DAPI (blue). Scale bars represent 50 μm. I. Frequency of CC3+ cells in tumor tissue. J. Tumor number in the entire small intestine of 9 control mice and 11 VILIRKO-Min mice fed a high-fat diet. K. Size distribution of small intestinal tumors in mice fed a high-fat diet. L. Frequency of Ki67+ cells in tumor tissue mice fed a high-fat diet. M. Frequency of CC3+ cells in tumor tissue in mice mice fed a high-fat diet. All panels: *, p<0.05.
VILIRKO-Min and control mice were fed a high-fat or control diet containing 60% or 22% of calories provided by fat, respectively. An oral glucose load administered by gavage showed that animals with diet-induced obesity had glucose intolerance compared to lean animals (Fig. 2B). However, glucose tolerance was not different between VILIRKO-Min and control mice within the same diet group (Fig. 2B). Similarly, plasma insulin was 2.4-fold higher in obese versus lean control mice but not different between the genotypes (Fig. 2C).
Loss of insulin signaling in intestinal epithelial cells does not change tumor formation
Surprisingly, loss of insulin signaling in intestinal epithelial cells did not change tumor formation in lean animals (Fig. 2D) and tumor size distribution was unchanged (Fig. 2E). Consistent with this result, proliferation was similar in tumors from mice of either genotype with similar values for the relative frequency tumor cells positive for Ki67 (Fig. 2F and G). In addition, the relative frequency of cells positive for cleaved caspase 3, a measure of apoptosis, was similar in the two groups (Fig. 2H and I).
Diet-induced obesity and hyperinsulinemia failed to reveal any difference in tumor number and size distribution between VILIRKO-Min and control mice (Fig. 2J and K). Again, frequency of cells positive for Ki67 or cleaved caspase 3 in tissue sections of tumors was similar in VILIRKO-Min and control mice when both groups were fed a high-fat diet (Fig. 2L and M). Thus, insulin does not affect tumor cell proliferation or apoptosis in these intestinal tumors. We therefore concluded that even though insulin increases proliferation of adenoma epithelial cells during serum-starved conditions in vitro, insulin action on tumor epithelium plays a minor role in vivo where it is only one of many growth factors.
Loss of insulin signaling in vascular endothelial cells does not change systemic glucose tolerance
We wondered whether insulin resistance might be more closely related to tumorigenesis than hyperinsulinemia and whether tumor stroma could mediate an effect of abnormal insulin signaling. We have previously demonstrated that insulin resistance in vascular endothelial cells produces an activated endothelium with increased leukocyte adhesion and accelerated recruitment of leukocytes to atherosclerotic plaques [18]. We therefore hypothesized that endothelial cell insulin resistance can promote tumor progression by facilitating chronic inflammation.
We generated ApcMin/+ mice with insulin resistance in vascular endothelial cells by use of mice with a cre recombinase transgene under control of the VE-cadherin (Cdh5) promoter [25]. To characterize the specificity of cre-mediated recombination we bred mice carrying the Cdh5-cre transgene with ApcMin/+ mice and double-fluorescent (mT/mG ) cre reporter mice [26]. These reporter mice express a red fluorescent protein (membrane-targeted tandem dTomato) in most cells. In cells expressing cre, however, the transgene is recombined and expression of dTomato is replaced by a green fluorescent protein (membrane-targeted enhanced GFP) [26]. In normal intestine (fig. 3A) and in polyps (fig. 3B), GFP fluorescence was clearly confined to vascular structures whereas epithelial cells in villi and polyps had uniform red fluorescence (fig. 3A and B). As Cdh5-cre transgene activity was specific for our tissues of interest, we then bred Cdh5-cre mice with Insrlox/lox and ApcMin/+ mice to generate Cdh5-cre Insrlox/lox mice (EndoIRKO) and their Insrlox/lox controls as well as Cdh5-cre Insrlox/lox ApcMin/+ (EndoIRKO-Min) mice and their Insrlox/lox ApcMin/+ controls.
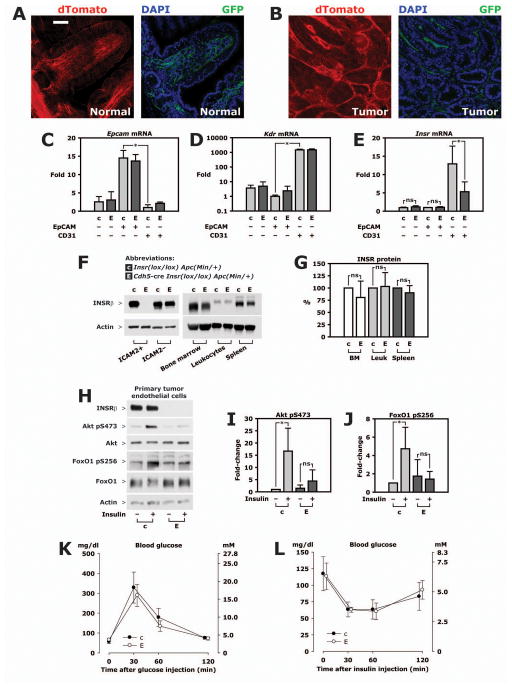
Figure 3. Characterization of EndoIRKO-Min mice.
A–B. Cdh5-cre ApcMin/+ mice were crossed with mT/mG mice, a double-fluorescent reporter of cre recombinase activity. Offspring have ubiquitous expression of a red fluorescent protein, i. e. membrane-targeted tandem dTomato (dTomato), except in cells with cre-mediated recombination of the mT/mG transgene where dTomato expression is replaced with expression of membrane-targeted enhanced green fluorescent protein (GFP). 80 μm cryosections of formalin-fixed tissue was stained with DAPI only and imaged with confocal microscopy. Scale bar, 50 μm. A. Normal intestine. B. Polyp. C–E. Tumors from 3 EndoIRKO-Min mice and 3 controls were enzymatically digested and cells sorted by FACS using antibodies against EpCAM or CD31; status as double-negative or single-positive for this antigens is indicated below graphs. mRNA expression was measured by real-time PCR and normalized to expression of Rplp0, a ribosomal RNA. C. Enrichment of epithelial cells in EpCAM+ CD31− fraction shown by expression of Epcam mRNA. D. Enrichment of endothelial cells EpCAM− CD31+ fraction shown by expression of Kdr mRNA. Please note log scale. E. Expression of Insr mRNA. F. Tumor cells from Cdh5-cre Insrlox/lox ApcMin/+ mice (EndoIRKO-Min, “E”) or their littermate Insrlox/lox ApcMin/+ controls (“c”) were grown in a mixed culture and endothelial cells isolated by magnetic selection using ICAM-2 conjugated Dynabeads at the first and second passage. ICAM-2-negative cells were collected at the first of these immuno-magnetic sortings while tumor endothelial cells were used after passage 3. Bone marrow, peripheral blood leukocytes and spleen were isolated and lysed. Representative Western blots of lysate is shown. G. Quantitation based on densitometry of Western blots using material from 3 pairs of animals. H. Isolated tumor endothelial cells were serum-starved overnight, then treated with 10 nM insulin for 5 minutes. Representative Western blots are shown. I–J. Quantitation based on lysate from 4 independent experiments. K. glucose tolerance test. Plasma glucose concentrations were measured after intraperitoneal injection of 2 mg/g glucose in 5 EndoIRKO-Min mice and 5 control animals. L. Insulin tolerance test. Plasma glucose concentrations after intraperitoneal injection of 0.75 mU/g Humulin R in 5 EndoIRKO-Min mice and 5 control animals. All panels: *, p<0.05.
To validate that insulin receptor knockout was restricted to endothelial cells in polyps, we used flow-activated cell sorting (FACS) to separate endothelial cells and epithelial cells from enzymatically dissociated tumors and measured insulin receptor expression in the sorted cells. Sorting was efficient. Thus, in control animals, mRNA expression of the epithelial cell marker Epcam was 14.5±2.1 fold higher in EpCAM+ cells than CD31+ cells (fig. 3C). In contrast, Kdr mRNA, another marker for endothelial cells, was 1465±206 fold higher in CD31+ cells than EpCAM+ cells (fig. 3D). Epcam and Kdr mRNA was not different between EndoIRKO-Min mice and controls in the same category of cell marker (fig. 3C–E). Although not a substitute for quantitating number of insulin receptors per cell it is interesting that Insr mRNA, normalized to Rplp0 which encodes a ribosomal RNA, was 12.5 fold higher in CD31+ (endothelial) cells than EpCAM+ (epithelial) cells (fig. 3E). Insr mRNA was reduced by 57% in CD31+ cells in EndoIRKO mice but not different in EpCAM+ cells or double-negative cells (fig. 3E), demonstrating that Cdh5-cre mediated recombination had no effect on tumor epithelial cells or, as a group, other tumor cell types.
We then evaluated the efficiency of the targeted gene deletion at the protein level by culturing the mixed tumor cell population and isolating primary endothelial cells by immunomagnetic selection. We have previously shown that this method yields cultures with a very high purity of endothelial cells [18]. In primary tumor endothelial cells cultured from control animals with intact Insr, insulin receptor protein was clearly present whereas no protein band was detected in cultures from EndoIRKO-Min mice (Fig. 3F). In contrast, insulin receptor expression was similar in tumor cells negatively selected by Dynabeads conjugated with ICAM-2 antibody (Fig. 3F). Insulin receptor expression was also unchanged in bone marrow, peripheral blood leukocytes and spleen from EndoIRKO-Min and control mice (Fig. 3F and G). These data show that the insulin receptor was efficiently deleted in vascular endothelial cells without signs of reduction in hematopoietic lineages where endothelial-specific cre promoters can have off-target activity [18]. In cultured primary tumor endothelial cells from control mice with intact insulin receptors, treatment with 10 nM insulin increased Akt phosphorylation by 17-fold (fig. 3H and I) and FoxO1 phosphorylation by 4.7-fold (fig. 3H and J). By contrast, insulin had no significant effect in primary tumor endothelial cells from EndoIRKO-Min mice (Fig. 3H–J).
Endothelial cell Insr knockout did not change measures of systemic insulin regulation of glucose metabolism. Plasma insulin concentrations were not different in EndoIRKO-Min and control mice, 0.60±0.20 and 0.78±0.36 ng/ml, respectively. There was no difference in whole-body glucose tolerance (Fig. 3K) or insulin tolerance (Fig. 3L).
Endothelial cell Insr knockout causes increased intestinal tumor formation
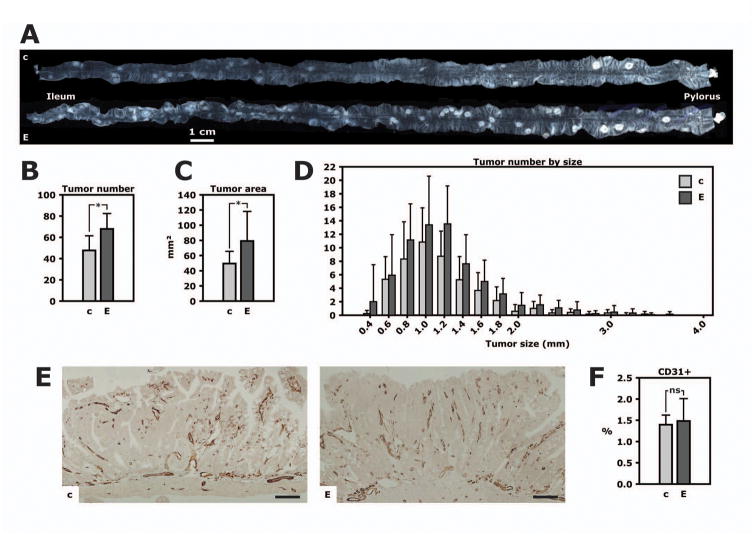
At 16 weeks of age, the total tumor number in the small intestine was 42% higher and the combined tumor area 60% higher in EndoIRKO-Min than control mice (Fig. 4A–C). There were 67.8±14.6 and 47.7±13.8 tumors in EndoIRKO-Min and control mice, respectively (Fig. 4B, p=0.002). This difference was manifest without changes in tumor size distribution (Fig. 4D), indicating an effect on tumor initiation or early tumor progression. Specifically, 52±21 and 47±17% of tumors were >1.0 mm in diameter in EndoIRKO-Min and control mice, respectively; 8±6 and 7±5% were ≥2.0 mm (both p>0.4).
Figure 4. Loss of the vascular endothelial cell insulin receptor enhances tumor formation.
EndoIRKO-Min mice (“E”) and their littermate controls (“c”) were fed a regular chow diet and sacrificed at 16 weeks of age. A. The entire small intestine from two littermates. Adenomas are clearly visible as pale, circular lesions. B. Total number of tumors in the small intestine from EndoIRKO-Min mice (n=12) and controls (n=13). *, p=0.002. C. Total tumor area. *, p=0.02. D. Distribution of tumor number by diameter. E. Representative microphotos of CD31 immunohistochemistry in small intestinal tumors. Scale bar, 100 μm. F. Quantitation of tumor vascular density based on CD31 immunohistochemistry.
Loss of insulin signaling in vascular endothelial cells can impair angiogenesis [27]. Therefore, endothelial cell Insr knockout, as in EndoIRKO-Min mice, would be expected to decrease tumor angiogenesis and impair tumor growth whereas we in fact found increased tumor formation in EndoIRKO-Min mice. Vascular density in tumors measured by CD31 immunohistochemistry revealed no changes between EndoIRKO-Min and control mice (Fig. 4E and F). Therefore, although tumor angiogenesis could be limited by endothelial cell Insr knockout, such a mechanism is unlikely to affect the phenotype of EndoIRKO-Min mice.
Loss of endothelial cell insulin signaling increases VCAM-1 expression in tumor endothelial cells and increases neutrophil recruitment to tumors
Using in vivo microscopy of postcapillary venules in the intestinal mesentery, we have previously shown an increase in leukocyte adhesion to endothelial cells in mice with knockout of the Insr gene in endothelial cells [18]. The increase in leukocyte adhesion to endothelial cells in vivo was completely reversed by a VCAM-1 blocking antibody. We therefore hypothesized that a similar mechanism could increase VCAM-1 expression and promote chronic inflammation and tumor formation in EndoIRKO-Min mice. In primary tumor endothelial cells from EndoIRKO-Min mice, VCAM-1 protein expression was 56% higher than in cultures from control mice (Fig. 5A and B). Conversely, in quiescent cultures from control mice insulin treatment decreased VCAM-1 expression by 18% (Fig. 5C and D). Treatment with TNF-α, on the other hand, induced VCAM-1 expression by 3.8-fold and 10 nM insulin prevented 56% of this induction (Fig. 5C and D). Therefore, insulin decreases VCAM-1 expression in quiescent primary tumor endothelial cells and partly prevents VCAM-1 induction when these cells are activated.
Figure 5. VCAM-1 expression and leukocyte recruitment associated with insulin resistant tumor endothelium.
Primary tumor endothelial cells were isolated by immuno-magnetic selection from a mixed culture of tumor cells. Cultures were used at passage 3 or 4. After overnight serum starvation, cells were treated with 10 nM insulin for 24 hours and in some experiments with or without 1 ng/ml TNF-α. A. Representative Western blot from tumor endothelial cells cultured from EndoIRKO-Min or control mice. B. Quantitative analysis based on densitometry from 3 independent experiments. C. Representative Western blot from tumor endothelial cells cultured from control animals with intact insulin receptors. D. Quantitative analysis based on densitometry from 3 independent experiments. E. Representative image frames from video of intravital microscopy of mesenteric venules. Leukocytes were labeled by intravenous injection of rhodamine 6G. Arrows point to rolling leukocytes. Scalebar, 100 μm. F. Quantitation of leukocyte rolling in 3 EndoIRKO mice and 4 controls. G. Results from a representative experiment using flow cytometry of enzymatically dissociated tumor cells. H. Quantitation of the frequency of neutrophils (Ly-6G+ cells) in 6 tumors per genotype, analyzed by flow cytometry. I. Quantitation of the frequency of macrophages (F4/80+ cells) in 6 tumors per genotype. All panels: *, p<0.05.
In our previous study, Insr knockout in endothelial cells was achieved using a Tie2-cre transgene and was complicated by reduction of insulin receptor expression in hematopoietic cells [18]. Insr knockout using a Cdh5-cre transgene in the current study is more specific in this regard (fig. 3F–G). We therefore characterized leukocyte adhesion in EndoIRKO mice. During intravital microscopy, rolling of leukocytes on the endothelium of mesenteric venules in EndoIRKO mice was increased by 2.2-fold compared with their controls (fig. 5E–F). This result extends the finding of a proinflammatory activation of insulin resistant endothelium to the mice generated for the current study.
We then measured the frequency of tumor-associated neutrophils and macrophages. In tumors from EndoIRKO-Min mice the frequency of Ly-6G+ cells was 5.6±1.1% compared to 3.3±1.6% in control mice (fig. 5G and H, p=0.02). The frequency of F4/80+ cells, 4.3±1.3 and 3.7±1.3%, respectively, was not different (fig. 5G and I, p>0.4). These data suggest that insulin resistant endothelium promotes recruitment of neutrophils to tumors where they may promote tumorigenesis.
Discussion
Insulin resistance and ensuing compensatory hyperinsulinemia are central features of obesity and type 2 diabetes. Hyperinsulinemia has been implicated in the pathogenesis of a range of cancers which are more common in these metabolic diseases [4]. However, in the present study we demonstrate that tumor progression is unaffected by hyperinsulinemia via insulin action on intestinal epithelial cells. Our data further show that insulin resistance in vascular endothelial cells can promote tumor formation, possibly through mechanisms involving chronic inflammation.
In 1924, three years after the discovery of insulin, George Gey published that this hormone promotes proliferation of cells in culture [28]. Since then, his suggestion that the observed effect could have relevance for malignant growth has been supported by numerous publications reporting that insulin increase proliferation of cancer cells in vitro [4, 7, 9, 29–31]. This has led to the widely held belief that hyperinsulinemia in obesity or type 2 diabetes promotes tumor progression by acting directly on tumor cells. We extend these observations by showing that insulin increases proliferation of primary adenoma cells in culture. However, we find no evidence that the mitogenic action of insulin is relevant to intestinal tumor formation in vivo. Indeed, deletion of the insulin receptor in normal intestinal epithelium and in tumor cells did not limit tumor formation, proliferation or apoptosis on a regular chow diet or in the presence of hyperinsulinemia caused by high-fat feeding. Thus, we expect that the role of insulin is minor in the context of a growth factor replete tumor microenvironment. An alternative explanation is that hyperinsulinemia may not actually result in enhanced insulin signaling in normal or transformed epithelial cells because of insulin resistance associated with a high-fat diet. Indeed, impaired insulin signaling has been demonstrated in enterocytes from obese individuals [32, 33].
How our finding relates to other cancers associated with obesity or hyperinsulinemia remains to be determined. DeRoith and colleagues have implicated insulin in the progression of breast cancer using mice expressing a dominant-negative insulin-like growth factor-1 (IGF-1) receptor in skeletal muscle (MKR mice). These animals have marked hyperinsulinemia in the absence of obesity. In mice with breast tumors induced by transgenic expression of the polyomavirus middle T oncogene, the MKR mutation causes accelerated tumor growth [34]. In addition, enhanced growth of tumors implanted in MKR mice was inhibited by insulin receptor knockdown in tumor cells [35]. Therefore, the role of insulin action on tumor cells may be different in tumors from different tissues and at different stages of malignant transformation.
It is recognized that neutrophils participate in tumor initiation and progression in many cancers [36, 37]. In ApcMin/+ mice, depletion of neutrophils by anti- Ly-6G antibody reduced tumor formation [38]. It is possible that upregulation of VCAM-1 in insulin resistant tumor endothelium changes homing of other immune cells to the tumor microenvironment and future studies should further characterize how endothelial cells activated by insulin resistance affects interaction with different leukocyte subpopulations. It will also be important to provide evidence for causality in this relationship by showing that recruitment of neutrophils or other immune cells is responsible for accelerated tumor progression in EndoIRKO-Min mice or models of obesity or type 2 diabetes, for example by showing that treatment with anti-VCAM-1 antibody inhibits excess tumorigenesis.
To date, vascular biology has made a profound contribution to our understanding of cancer. Tumor angiogenesis is now understood as a key driver of tumor progression and vasculostatic agents have been employed as successful clinical therapies [39]. In contrast, relatively little is known about how endothelial cell recruitment of immune cells contributes to tumorigenesis [40, 41]. Vascular inflammation is present in a number of chronic inflammatory diseases [42] and inhibiting immune cell adhesion to the endothelium has shown promise as a treatment for inflammatory bowel disease, suggesting that vascular inflammation is a primary event in intestinal inflammation [43, 44]. Blocking α4 integrin, the VCAM-1 ligand on leukocytes, is efficacious in the treatment of inflammatory bowel disease [45] consistent with the notion that endothelial VCAM-1 upregulation due to endothelial cell insulin resistance promotes intestinal inflammation. To further understand this mechanism it will be important to evaluate whether insulin resistant endothelial cells selectively recruit certain immune cell populations and characterize how such recruitment is regulated by molecules expressed on or secreted from endothelial cells.
Loss-of-function mutations in the adenomatous polyposis colon (APC) gene cause familial adenomatous polyposis. Similar mutations are present in >80% of sporadic colorectal cancer and APC mutations are a very frequent if not mandatory event in intestinal tumor formation in humans. Loss of Apc in intestinal stem cells is sufficient to initiate tumor formation in mice [46] and restoration of Apc leads to sustained regression of intestinal tumors caused by loss of Apc [47]. Mice with the multiple intestinal neoplasia (Min) mutation (ApcMin/+ mice) therefore are a widely used model of intestinal tumor formation. Removal of adenomas can reduce risk of colorectal cancer [48], supporting the notion that in most patients, colorectal carcinoma develops from adenoma. The increased tumor formation in EndoIRKO mice has relevance for patients with obesity, as this patient group has increased for colorectal adenoma [49–51]. However, to test whether endothelial cell insulin resistance also affects malignant disease, experiments similar to the ones presented in the current manuscript should be performed in animal models of colon adenocarcinoma. Future studies should also characterize insulin sensitivity in different types of tumor cells in metabolic disease. Impaired insulin signaling is present in intestinal tumors in mice with diet-induced obesity, including in ApcMin/+ mice, when measured in tumor lysate [52]. Insulin signaling is impaired in large [53] and small [54] vessels in animal models of obesity and in both arteriolar [23] and venous endothelial cells [24] in insulin resistant humans. Regardless, it will be important to directly demonstrate insulin resistance in intestinal or tumor-associated endothelial cells in diet-induced obesity or other models of insulin resistance.
Our findings demonstrate that insulin resistance in endothelial cells, a characteristic of endothelial dysfunction in obesity and type 2 diabetes [21–24], can promote tumor development. In addition, we find little effect of intestinal epithelial cell insulin signaling on intestinal tumorigenesis. These findings provide an impetus to re-examine the dogma of insulin as a tumor promoter in obesity and type 2 diabetes and draw attention to the fact that important regulatory actions of insulin may be lost in obesity-induced insulin resistance [55]. We propose that insulin resistance, not increased insulin stimulation, may drive tumor development in certain tissues in these metabolic diseases. Improving endothelial function could decrease cancer risk in obesity.
Materials and Methods
Animals
LIRKO mice [56] were backcrossed >10 times to the C57BL/6 background strain. Insrlox/lox mice negative for cre from this colony were cross-bred with ApcMin/+ mice [57] (Jackson Labs stock number 002020) and with mice harboring either a cre transgene under control of the villin-1 (Vil1) promoter [58] (Jackson Labs stock number 004586) or the VE-cadherin (Cdh5) promoter [25] (Jackson Labs stock number 006137). ApcMin/+ mice had been backcrossed 95 generations to C57BL/6, Vil1-cre mice at least 6 generations and Cdh5-cre at least 13 generations (although a single nucleotide polymorphism panel analysis of Cdh5-cre at Jackson Labs indicates less extensive backcrossing, see www.jax.org). Cdh5-cre and ApcMin/+ mice were also cross-bred with mT/mG mice [26] (Jackson Labs stock number 007676), backcrossed to the C57BL/6 strain for at least 5 generations.
Mice were fed either a control chow diet with 9.0% fat by weight (22% of calories provided by fat, Mouse Diet 9F, LabDiet) or a high-fat rodent diet containing 34.9% fat by weight (60% of calories provided by fat, Research Diets cat. no. D12492). Insr knockout animals used for experiments were caged with littermate controls from birth to sacrifice. All protocols for animal use and euthanasia were reviewed and approved by the Animal Care Committee of the Joslin Diabetes Center and were in accordance with NIH guidelines.
Tumor number and size
Animals were sacrificed by CO2 inhalation and perfused with 4% paraformaldehyde in PBS (formalin) through the left ventricle. The intestine was flushed with formalin and mesenteric fat removed by dissection. The intestine was opened longitudinally and flatmounted in formalin under a strip of transparency copy film (Staples) for 30 minutes. The tissue was subsequently fixed in formalin for 24 hours at room temperature, then stored at 4 °C in PBS. The small intestine was photographed through a stereomicroscope producing overlapping images, 15–20 per intestine, which were stitched together (Adobe Photoshop version CS4). Circles were drawn to fit tumor outline and the number and size circles were quantified automatically (ImageJ version 1.43u). In the images shown in Figure 4, for presentation purposes only, the image area representing the intestine was selected in PhotoShop and placed on a uniformly black background.
Cell Culture
Primary tumor cells were isolated and cultured using methods described previously [18]. Briefly, polyps were removed from a single animal by microdissection, then incubated for up to one hour at 37 °C in DMEM containing 500 U/ml collagenase type 1 (Worthington Biochemical Corporation), dispase 3 mg/ml Dispase (Roche) and 10% FBS. The tissue digest was passed through a 70 μm strainer and centrifuged at 200 × g. Pelleted cells were cultured on collagen-coated tissue culture dishes in DMEM with 10% (v/v) FBS. If the culture was used for EdU incorporation, the medium was supplemented with 50 ng/ml mouse epidermal growth factor (Gold Biotechnology) and used without subculture. If the culture was used for isolation of tumor endothelial cells, the medium was instead supplemented with 50 μg/ml endothelial cell growth substance (Alfa Aesar) and 100 μg/ml heparin. After Trypsin treatment in the first and second passages cultures were sorted with Dynabeads conjugated with sheep anti-rat IgG (Thermo Fisher Scientific) and complexed to ICAM-2 rat anti-mouse monoclonal antibody (BD Pharmingen) in DMEM containing 0.1% bovine serum albumin (BSA) for 30 minutes at 4 °C. These primary endothelial cells were used at passage 2–4. Lung endothelial cells were isolated similarly to tumor endothelial cells as described previously [18].
EdU incorporation
Tumor cells were grown to subconfluence, typically for 4 days, then starved in DMEM containing 0.1% BSA overnight before treatment with 10 nM insulin or 10% FBS for 16 hours. Cultures were labeled with 10 μM of 5-ethynyl-2-deoxyuridine (EdU) (Life Technologies) for 4 hours. A single-cell suspension was made by trypsin treatment and stained by Click-iT EdU Alexa Fluor 647 Kit (Life Technologies) and with an epithelial cell adhesion molecule (EpCAM) antibody conjugated to PE (Biolegend). Stained cells were analyzed by flow cytometry using an LSRII instrument (BD Biosciences).
Intravital microscopy of leukocyte-endothelial cell interaction
Animals were anaesthetized by intraperitoneal injection of avertin 480 mg/kg, supplemented as necessary. Circulating leukocytes were fluorescently labeled by intravenous injection of 0.3 mg/kg rhodamine 6G (Sigma-Aldrich). The abdomen was opened in the midline, a loop of ileum exteriorized and the mouse placed on a heated stage (Harvard Apparatus WP-10). Second- or third-order mesenteric venules were observed by fluorescence microscopy using an Axio Observer D1 microscope with inverted configuration and a 20× objective, resulting in a final magnification of 200×. Images were acquired at 60 frames per second at high resolution by a digital video camera (Hamamatsu C11440-22CU CMOS Camera) and Zeiss software (ZEN Pro 2012). Rolling leukocytes was measured as the total number of leukocytes crossing a 100 μm venular segment in 60 seconds at a velocity significantly lower than the centerline velocity.
Flow cytometry and fluorescence-activated cell sorting (FACS) of dissociated tumor cells
Intestinal tumors were isolated by dissection under a stereomicroscope, minced by scalpels and digested for one hour in DMEM containing 2 mg/ml (560 U/ml) collagenase I (Worthington) and 3 mg/ml Dispase (Roche). Cell suspensions were washed and passed through a 40 μm filter and Fc receptors were blocked using TruStain fcX (anti-mouse CD16/32) antibody (Biolegend cat. no. 101320), then stained with EpCAM antibody conjugated to Brilliant Violet 421 (BioLegend cat. no. 118225), F4/80 antibody conjugated to Alexa Fluor 488 (BioLegend cat. no. 123119), and Ly-6G antibody conjugated to APC-Cy7 (BioLegend cat. no. 127623). Dead cells were stained with propidium iodide (PI) immediately before analysis. Filtered cells were stained with antibodies and sorted on a Moflo Legacy (Cytomation, currently Beckman Coulter) or analyzed on an LSRII instrument (BD Biosciences). For FACS, 100 000–300 000 cells were sorted directly into TRI Reagent (Molecular Research Center). For all analysis, populations were first gated for cells (as opposed to debris) in a FSC-A vs. SSC-A graph, for single cells (as opposed to doublets/aggregates) in a FSC-A vs. FSC-W graph and for live (PI-negative) cells. Compensation was performed using single-stained cells and gating was aided by fluorescence minus one (FMO) controls.
Other analyses
Real-time PCR, Western blotting, glucose and insulin tolerance tests, insulin ELISA and immunostaining were performed as described previously [18].
Statistical analysis
Comparisons were made using paired t test for the cell culture studies and unpaired t test when comparing variables in mice with p < 0.05 considered statistically significant. In text and graphs, data are presented as the mean ± standard deviation.
Acknowledgments
This work was supported by NIH grant R21 CA185196 (to CRM), the National Natural Science Foundation of China grant 81370936 (XW), the Diabetes Research and Wellness Foundation (SML), the Danish Diabetes Academy which receives funding from the Novo Nordisk Foundation (DS), as well as NIH grants DK031036, DK033201 and the Mary K. Iacocca Professorship (CRK). TR was an employee of Novo Nordisk and was supported by the STAR Programme. Measurement of plasma insulin and flow cytometry was done using cores in the Diabetes Research Center, supported by NIH grant 5P30DK036836, at Joslin Diabetes Center. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest
Thomas Rathjen is an employee of Novo Nordisk A/S as a participant in the company’s “STAR Programme” for postdoc training. Christian Rask-Madsen receives research support from Novo Nordisk as part of this program. The company had no role in the design of this study, collection and analysis of data or decision to publish. The remaining authors disclose no financial, professional, or personal conflict of interest.
References
- 1.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? The American journal of gastroenterology. 2011;106:1911–21. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727–48. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. Journal of the National Cancer Institute. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 6.Yoon YS, Keum N, Zhang X, Cho E, Giovannucci EL. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism. 2015;64:1324–33. doi: 10.1016/j.metabol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci U S A. 1976;73:4536–40. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ish-Shalom D, Christoffersen CT, Vorwerk P, Sacerdoti-Sierra N, Shymko RM, Naor D, et al. Mitogenic properties of insulin and insulin analogues mediated by the insulin receptor. Diabetologia. 1997;40(Suppl 2):S25–31. doi: 10.1007/s001250051393. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E. Insulin and colon cancer. Cancer causes & control : CCC. 1995;6:164–79. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 11.Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr Cancer. 2000;37:19–26. doi: 10.1207/S15327914NC3701_2. [DOI] [PubMed] [Google Scholar]
- 12.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–95. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 13.Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carothers AM, Moran AE, Cho NL, Redston M, Bertagnolli MM. Changes in antitumor response in C57BL/6J-Min/+ mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res. 2006;66:6432–8. doi: 10.1158/0008-5472.CAN-06-0992. [DOI] [PubMed] [Google Scholar]
- 16.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 17.Chan AT, Arber N, Burn J, Chia WK, Elwood P, Hull MA, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5:164–78. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–89. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–8. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, et al. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond) 2015;129:1025–36. doi: 10.1042/CS20150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–83. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rask-Madsen C, Ihlemann N, Krarup T, Christiansen E, Kober L, Nervil Kistorp C, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611–8. doi: 10.2337/diabetes.50.11.2611. [DOI] [PubMed] [Google Scholar]
- 24.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, et al. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–67. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 27.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–80. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gey GTW. Observations on The Effects of Insulin Introduced Into The Medium of Tissue Cultures. JAMA. 1924;82(20):1609. [Google Scholar]
- 29.Temin HM. Carcinogenesis by avian sarcoma viruses. X. The decreased requirement for insulin-replaceable activity in serum for cell multiplication. International journal of cancer Journal international du cancer. 1968;3:771–87. doi: 10.1002/ijc.2910030611. [DOI] [PubMed] [Google Scholar]
- 30.Raizada MK, Perdue JF. Mitogen receptors in chick embryo fibroblasts. Kinetics, specificity, unmasking, and synthesis of 125I-insulin binding sites. J Biol Chem. 1976;251:6445–55. [PubMed] [Google Scholar]
- 31.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80:51–8. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makinen J, Hannukainen JC, Karmi A, Immonen HM, Soinio M, Nelimarkka L, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia. 2015;58:1055–62. doi: 10.1007/s00125-015-3501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro-Sepulveda M, Touch S, Mendes-Sa C, Andre S, Poitou C, Allatif O, et al. Jejunal T Cell Inflammation in Human Obesity Correlates with Decreased Enterocyte Insulin Signaling. Cell Metab. 2015;22:113–24. doi: 10.1016/j.cmet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–51. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostoker R, Abelson S, Bitton-Worms K, Genkin I, Ben-Shmuel S, Dakwar M, et al. Highly specific role of the insulin receptor in breast cancer progression. Endocr Relat Cancer. 2015;22:145–57. doi: 10.1530/ERC-14-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312–28. doi: 10.1111/imr.12444. [DOI] [PubMed] [Google Scholar]
- 37.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–44. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 40.Fukumura D, Salehi HA, Witwer B, Tuma RF, Melder RJ, Jain RK. Tumor necrosis factor alpha-induced leukocyte adhesion in normal and tumor vessels: effect of tumor type, transplantation site, and host strain. Cancer Res. 1995;55:4824–9. [PubMed] [Google Scholar]
- 41.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2:992–7. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- 42.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–49. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, MacDonald JK, Khanna R, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;8:CD007571. doi: 10.1002/14651858.CD007571.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer A, Zundler S, Atreya R, Rath T, Voskens C, Hirschmann S, et al. Differential effects of alpha4beta7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut. 2015 doi: 10.1136/gutjnl-2015-310022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–83. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 46.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 47.Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, et al. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell. 2015;161:1539–52. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okabayashi K, Ashrafian H, Hasegawa H, Yoo JH, Patel VM, Harling L, et al. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. The American journal of gastroenterology. 2012;107:1175–85. doi: 10.1038/ajg.2012.180. quiz 86. [DOI] [PubMed] [Google Scholar]
- 50.Davenport JR, Su T, Zhao Z, Coleman HG, Smalley WE, Ness RM, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. 2016 doi: 10.1136/gutjnl-2016-312893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JC, Weiss JE, Robinson CM, Butterly LF. Adenoma Detection Rates for Screening Colonoscopies in Smokers and Obese Adults: Data From the New Hampshire Colonoscopy Registry. J Clin Gastroenterol. 2017 doi: 10.1097/MCG.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores MB, Rocha GZ, Damas-Souza DM, Osorio-Costa F, Dias MM, Ropelle ER, et al. Obesity-induced increase in tumor necrosis factor-alpha leads to development of colon cancer in mice. Gastroenterology. 2012;143:741–53. e1–4. doi: 10.1053/j.gastro.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 53.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–8. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 54.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–57. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052–9. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 57.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 58.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]