Abstract
Objective
To examine the timing and microbiology of neonatal sepsis in a population-based surveillance in the Indian community setting.
Study Design
All live born infants in 223 villages of Odisha state were followed at home for 60 days. Suspect sepsis cases were referred to study hospitals for further evaluation including blood culture.
Results
Of 12,622 births, 842 were admitted with suspected sepsis of whom 95% were 4–60 days old. Culture confirmed incidence of sepsis was 6.7/1000 births with 51% Gram negatives (Klebsiella predominating) and 26% Gram positives (mostly Staphylococcus aureus). A very high level of resistance to penicillin and ampicillin, moderate resistance to cephalosporins, and extremely low resistance to Gentamicin and Amikacin was observed.
Conclusion
The bacterial burden of sepsis in the Indian community is not high. Judicious choice of empiric antibiotics, antibiotic stewardship, and alternate modalities should be considered for the management or prevention of neonatal sepsis in India.
INTRODUCTION
Neonatal sepsis is a major cause of morbidity worldwide and one of the three primary causes of 2.7 million deaths every year.1 Over 600,000 of these deaths are attributed to infections alone (United nations) and 99% of these deaths take place in developing country settings.2 Of the 6.9 million neonatal sepsis burden, South Asia accounts for 3.5 million cases per year.3 India, with its 1.2 billion population, claims a large proportion of this disease burden. Although no population-based figures are available, the bulk of these sepsis-related neonatal deaths are considered to be taking place in rural India where more than 60% of the Indian population lives. Very little information exists on these infections and deaths due to sub-optimal public health surveillance systems and lack of transportation to appropriate health facilities where culture, other diagnostic tools, and antimicrobial susceptibility testing may be available. In the context of the worldwide threat of antimicrobial resistance (AMR), India’s condition is considered more stark than any other place,4 and neonatal infections form an important piece in the equation. Although there is no consensus on the exact modalities, various home-based regimens of empiric injectable and oral antibiotics are promoted in many developing country settings, including India.5
While these measures are undertaken by national and international bodies, there is widespread acknowledgement of a serious lack of information,6 which, in turn, limits our ability to design and implement appropriate evidence-based interventions. At present, no data are available on timing of neonatal infections, types of infections (bacterial, viral, other), AMR, and precise time of deaths of Indian neonates in the community setting. Due to the overlapping nature of presentation during the first days of life, multiple conditions including prematurity, birth asphyxia, transient tachypnea, hypoglycemia, and other physiologic disturbances may be lumped into the “infection” category and treated with antibiotics as bacteremia. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Global Network for Women’s and Children’s Health Research supported the first population-based, village level surveillance of infections conducted in the first two vulnerable months of life in Indian infants. Here, we report the results of that study where comprehensive clinical assessments, microbiology and antimicrobial susceptibility testing were done in infants in 223 villages of Odisha, an eastern state in India that records one of the highest neonatal mortalities in the country.
METHODS
Preparation of Community Study Sites and Study design
The Integrated Child Development Services scheme is an ongoing Government of India program that has been in place since 1975, and now operates in every state. The services are delivered through a network of Anganwadis (court yards) established for 700–1000 individuals and implemented by a woman from the same village known as an Anganwadi Worker (AWW). The package of services available through an AWW includes pre-school education for children (3 to 6 years), provision of mid-day meals, assessment of nutritional status, minor health checkups, and referral to medical facilities when needed.
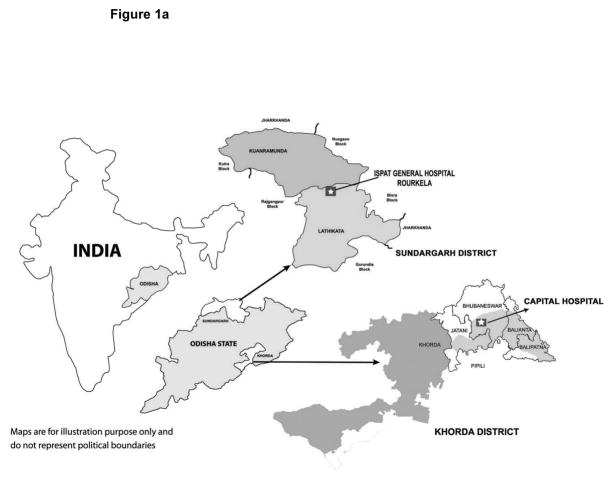
In preparation for the current study, an infrastructure utilizing AWWs to collect data and provide contact with enrolled infants was established. This population-based study was set in the rural area of Odisha, a state with one of the highest infant mortality rates and neonatal mortality rates in India (Figure 1a). The study area included 96 villages in the Khorda district and 127 villages in the Sundargarh district. The population in Khorda district is relatively well-to-do, with irrigated farming as the major source of income. Sundargarh is a less developed hilly district in western Odisha, with a lower population density (one third that of Khorda), and a population that is predominantly tribal, and of lower socio-economic status. Capital Hospital in Bhubaneswar served as the referral hospital in the Khorda district and Ispat General Hospital in Rourkela as the referral hospital in the Sundargarh district.
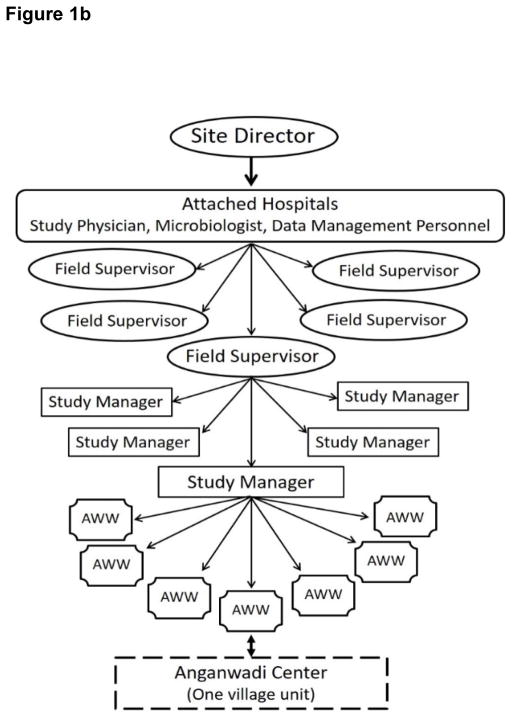
Figure 1.
Figure 1a. Map showing study location in Odisha state in India.
Figure 1b. Study operations and management structure.
For conducting the home-based surveillance, AWWs were trained using a specially designed module on newborns with an emphasis on recognition of signs and symptoms of neonatal sepsis. This included didactic as well as case demonstration in the associated study hospitals. Pre-, immediate, and one-year post-training evaluations were done to assure confidence of AWWs in conducting the triage at the field level.
Each pregnant woman in any given Anganwadi center was registered by the AWW during the seventh month of pregnancy. Every birth was recorded and the infant followed for 60 days by the AWW through daily visits to the child’s home. The AWWs were taught how to use a specially designed card with 12 signs/symptoms of sepsis adapted from the Integrated Management of Childhood Illness guidelines.7 One manager was in-charge of about 8–10 AWWS, and 5–7 managers reported to one supervisor in a three-tier structure of field operations and management (Figure 1b). AWWs visited infants daily for the first 60 days of life and were instructed to refer the infants to associated hospitals in Bhubaneswar or Rourkela via managers for treatment whenever sepsis was suspected; no other intervention was introduced. Transport, treatment, and food cost for parents while at hospital were covered from grant funds.
Local institutional review boards approved the study and a NICHD-convened Data Safety and Monitoring Board examined the data every year during the study to monitor progress.
Patient evaluation
The study pediatrician evaluated referred infants at the hospital and provided in-patient care after admission.
The clinical evaluation included specific questions and physical examination on the 12 signs of sepsis.7 Other signs noted were recorded in a text field. This text field was reviewed and the most frequent signs were separated out, including cough, pustules, abscess, cellulitis, umbilical discharge/infection, vomiting, dehydration, and oral thrush.
Bacterial culture and identification
All centers followed standardized protocols for biospecimen collection and processing. One milliliter of blood was inoculated aseptically into Bactec-Peds Plus bottles (Becton Dickinson, India) and transported immediately to the laboratory. Blood cultures were obtained for each infant immediately after study enrollment and prior to treatment. Blood samples were inoculated in Bactec peds plus/F culture media and processed in a Bactec 9050 detection system (Becton Dickinson). Culture bottles were inspected visually for turbidity and Gram stain- and plated (subcultured) on Blood-agar, MacConkey agar and Chocolate-agar with 1% IsoVitaleX. MacConkey agar plates were incubated in an air incubator at 35–37°C for 24 hours. Blood agar and chocolate agar plates were placed in a candle jar before incubation. Bactec bottles giving positive signals or turning positive on Gram stain, but not producing growth on aerobic culture, were subcultured on BHI-agar and incubated anaerobically in an Anaerobic Jar with a Gas Pak Plus gas generating envelope and an anaerobic indicator strip at 35–37°C for 48 hours. All bacterial isolates appearing after incubation were Gram-stained and identified by use of API tests (aerobes) or API 20A test strips (BioMerieux Inc.). Bactec bottles with no signal within 7 days were considered negative.
Cerebral spinal fluid (CSF) was collected for biochemical and culture analysis when clinically indicated. A Lumbar puncture under aseptic conditions was done to draw about 1 milliliter of CSF which was immediately transported to the microbiology lab. Part of the specimen was used for cell count and biochemical analysis. A two-fold dilution (1:2) of well-mixed CSF with toluidine blue (0.1% in physiological saline) was made and a Neubauer chamber used to determine the white blood cell (WBC) total and differential count. In blood-tinged CSF, both WBC and red blood cell (RBC) counts were performed and reported. If RBCs were noted, the CSF was diluted with 5% acetic acid (1:1) to lyse the RBCs in order to simplify the differentiation of polymorphs and lymphocytes.
For culture, CSF specimens were centrifuged (20min, 3000rpm) and 1–2 drops of sediment was used to prepare a Gram stain and 1–2 drops to streak the primary culture media (chocolate blood agar plate supplemented with 1% IsoVitaleX, blood agar plate, and MacConkey agar). The blood agar plates were incubated in a 5% carbon dioxide incubator or candle-jar. As a back-up culture, 0.5 ml of BHI broth was added to the tube containing the remaining pellet. If there was no growth from the direct culture after 48 hours, the back-up broth was subcultured and served as the primary culture of CSF.
Early onset culture proven sepsis (EOS) was defined by a positive blood culture taken during days 0–3 of life and late onset culture proven sepsis (LOS) by a positive blood culture taken during days 4–60 of life. Meningitis was defined by a positive CSF culture. Coagulase negative Staphylococcus, Corynebacterium, Propionibacterium, Diptheroids, Micrococcus, and Bacillus grown on blood or CSF culture were considered contaminants and the culture was not counted as positive. Clinical sepsis included negative blood/CSF cultures or respiratory symptoms (pneumonia), when the infant was treated with antibiotics for 5 or more days or died within 5 days of enrollment. Pneumonia was defined by site reports of positive chest x-ray findings.
Antimicrobial Susceptibility Test (AST)
ASTs were performed for all isolates recovered from infant cultures following National Committee for Clinical Laboratory Standards 2001 guidelines,8 (now known as the Clinical Laboratory Standards Institute). Organisms were group-tested against panels of antibiotics defined under categories: cephalosporins, first generation (Cef 1: cephalexin, cephalothin, cefazolin); cephalosporins, second generation (Cef 2: cefamandole, cefmetazole, cefonicid, cefaclor, cefotetan, cefoxitin, cefuroxime); cephalosporins, third generation (Cef 3: cefixime, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, cefoperazone); and oxacillin (Ox: oxacillin, cloxacillin). If an isolate showed resistance to more than one antibiotic in a group, it was considered resistant to that group. Similarly, if the organism was intermediate to one or more in a group and sensitive to all others tested it was considered intermediate to that group. AST results from all other antibiotics were analyzed individually.
Microbiology quality control
An overall system of quality control (QC) documentation and certification was maintained at each site, with central oversight to ensure that the microbiology results met the highest standards, including reproducibility and uniformity across the two sites. The QC plan included review of personnel training, equipment function, and media performance. All blood and CSF isolates considered clinically positive were sent to the All India Institute of Medical Sciences reference laboratory where specimens were re-tested for identification of pathogens and antibiotic resistance patterns.
Data management
Study data were recorded on paper forms and then entered into computers at participating hospitals using a Data Management System developed by the study data coordinating center at RTI International. Data entered at each participating hospital was electronically transferred twice weekly to Kalinga Hospital in Bhubaneswar. To maintain confidentiality, all enrolled infants were assigned unique study identifying numbers. A separate form was used to record personal identifiers, separated from all other study forms, and kept locked at the study site by site PIs.
Statistical Analysis
Statistical significance for comparisons was determined by chi square or Fisher’s exact tests for categorical variables or Wilcoxon test for continuous variables. For the purpose of looking at death rates by culture status/final diagnosis, infants with a second admission were classified based on findings at the second episode.
RESULTS
Study population
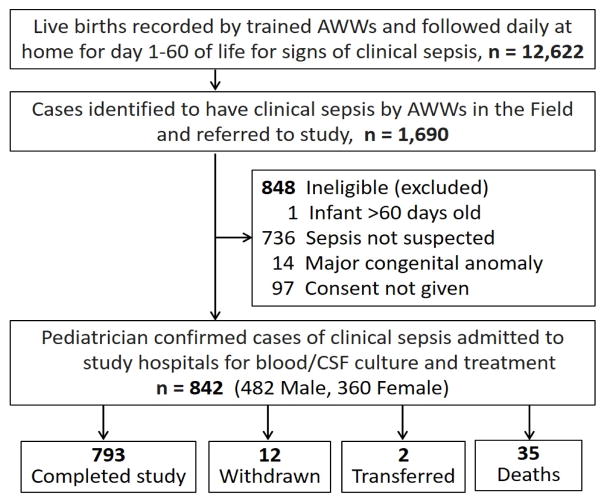
Between April 1, 2002 and March 31, 2005 12,622 live births were recorded in the community surveillance areas of whom 1,690 infants were identified with symptoms suggestive of sepsis by the AWWs and referred to the study hospitals in Bhubaneswar (BBS) and Rourkela (RKL) for further evaluation (Figure 2). Infants were screened up to three times, with most (89%) screened once. Of those screened, 842 (50%) identified by the study pediatrician to have clinical sepsis were enrolled [BBS: 384/903 (43%); RKL: 458/787 (58%)]. Sepsis was not suspected by the screening physician for a majority of the babies brought to the hospital for evaluation who were not enrolled.
Figure 2.
Screening and enrollment of infants in the community sepsis surveillance study. Of the 848 ineligible infants excluded, 75 were screened more than once. Reasons for exclusions are based on the first screening.
Most mothers of enrolled infants lived in villages or tribal villages, a majority had no formal schooling or had completed less than high school (81%), and most reported their occupation as housewife or “none” (94%) (Table 1). A majority of fathers reported their occupation as manual labor (37%) or farm labor (30%). The percent of mothers with no formal schooling was higher in RKL than in BBS (58% vs. 29%, p<0.001), as was the percent of fathers (35% vs. 17%, p<0.001). While the majority of fathers in both sites reported their occupations as manual or farm labor, the percent was higher in RKL (83%) than in BBS (48%). A greater percentage of fathers in BBS worked in business, 16% vs. 1%; did vocational work, 19% vs. 8%; or did technical work, 9% vs. 4%.
Table 1.
Maternal and neonatal characteristics among enrolled infants1/
| Characteristic2/ | Bhubaneswar (N=384) | Rourkela (N=458) | Overall (N=842) |
|---|---|---|---|
|
| |||
| Place of residence** | |||
| Village | 334 (87%) | 92 (20%) | 426 (51%) |
| Tribal village | 0 | 366 (80%) | 366 (43%) |
| Slum | 50 (13%) | 0 | 50 (6%) |
|
| |||
| Age (years) | |||
| Median (p25–p75) | 25 (22–27) | 25 (22–28) | 25 (22–28) |
|
| |||
| Education—mother** | |||
| No formal schooling | 107/374 (29%) | 238/413 (58%) | 345/787 (44%) |
| Less than high school | 174/374 (47%) | 121/413 (29%) | 295/787 (37%) |
| High school completed3/ | 56/374 (15%) | 45/413 (11%) | 101/787 (13%) |
| Beyond high school | 37/374 (10%) | 9/413 (2%) | 46/787 (6%) |
|
| |||
| Education—father** | |||
| No formal schooling | 61/367 (17%) | 146/418 (35%) | 207/785 (26%) |
| Less than high school | 165/367 (45%) | 171/418 (41%) | 336/785 (43%) |
| High school completed3/ | 80/367 (22%) | 81/418 (19%) | 161/785 (21%) |
| Beyond high school | 61/367 (17%) | 20/418 (5%) | 81/785 (10%) |
|
| |||
| Occupation—mother4/ ** | |||
| None/housewife | 350 (91%) | 441/456 (97%) | 791/840 (94%) |
| Professional/executive | 2 (<1%) | 7/456 (2%) | 9/840 (1%) |
| Business | 1 (<1%) | 0 | 1/840 (<1%) |
| Retail trader | 0 | 0 | 0 |
| Vocational work | 7 (2%) | 0 | 7/840 (1%) |
| Technical work | 0 | 0 | 0 |
| Clerk/vendor | 1 (<1%) | 1/456 (<1%) | 2/840 (<1%) |
| Peon | 1 (<1%) | 1/456 (<1%) | 2/840 (<1%) |
| Manual labor | 15 (4%) | 5/456 (1%) | 20/840 (2%) |
| Farm labor | 7 (2%) | 1/456 (<1%) | 8/840 (1%) |
|
| |||
| Occupation—father4/ ** | |||
| None | 1/383 (<1%) | 3/453 (1%) | 4/836 (<1%) |
| Professional/executive | 11/383 (3%) | 7/453 (2%) | 18/836 (2%) |
| Business | 61/383 (16%) | 5/453 (1%) | 66/836 (8%) |
| Retail trader | 5/383 (1%) | 4/453 (1%) | 9/836 (1%) |
| Vocational work | 73/383 (19%) | 34/453 (8%) | 107/836 (13%) |
| Technical work | 35/383 (9%) | 19/453 (4%) | 54/836 (6%) |
| Clerk/vendor | 11/383 (3%) | 3/453 (1%) | 14/836 (2%) |
| Peon | 2/383 (<1%) | 0 | 2/836 (<1%) |
| Manual labor | 117/383 (31%) | 192/453 (42%) | 309/836 (37%) |
| Farm labor | 67/383 (17%) | 186/453 (41%) | 253/835 (30%) |
|
| |||
| Place of delivery** | |||
| Home | 167 (43%) | 379 (83%) | 546 (65%) |
| Study hospital | 95 (25%) | 8 (2%) | 103 (12%) |
| Non-study hospital | 122 (32%) | 71 (15%) | 193 (23%) |
|
| |||
| Interval between rupture of membranes and delivery** | |||
| <12 hrs | 268/372 (72%) | 405/443 (91%) | 673/815 (83%) |
| 12–24 hrs | 77/372 (21%) | 25/443 (6%) | 102/815 (12%) |
| >24 hrs | 27/372 (7%) | 13/443 (3%) | 40/815 (5%) |
|
| |||
| C-section delivery† | 17 (4%) | 9 (2%) | 26 (3%) |
|
| |||
| Preterm | 51/383 (13%) | 82 (18%) | 133/841 (16%) |
|
| |||
| Male† | 238 (62%) | 244 (53%) | 482 (57%) |
|
| |||
| Breastfeeding initiated* | |||
| 0–24 hrs | 277/382 (73%) | 313/387 (81%) | 590/769 (77%) |
| >24 hrs | 95/382 (25%) | 73/387 (19%) | 168/769 (22%) |
| Never | 10/382 (3%) | 1/387 (<1%) | 11/769 (1%) |
|
| |||
| Infant age at enrollment (days) | |||
| 0–3 | 14 (4%) | 24 (5%) | 38 (5%) |
| 4–7 | 41 (11%) | 62 (14%) | 103 (12%) |
| 8–28 | 170 (44%) | 206 (45%) | 376 (45%) |
| 29–60 | 159 (41%) | 166 (36%) | 325 (39%) |
|
| |||
| Admission weight (grams) | |||
| Median (p25–p75) | 2797 (2250–3495) | 2930 (2400–3440) | 2890 (2340–3455) |
| By category* | |||
| < 1250 | 14 (4%) | 10 (2%) | 24 (3%) |
| 1250–1499 | 19 (5%) | 5 (1%) | 24 (3%) |
| 1500–1999 | 35 (9%) | 25 (5%) | 60 (7%) |
| 2000–2499 | 73 (19%) | 91 (20%) | 164 (19%) |
| ≥ 2500 | 243 (63%) | 327 (71%) | 570 (68%) |
n (% of column N) shown unless otherwise noted. Denominator Ns are shown when they differ from total Ns.
Information is missing for mother’s age: 2, mother’s education: 55, father’s education: 57, mother’s occupation: 2, father’s occupation: 6, timing of rupture of membranes: 27, preterm: 1, time breastfeeding initiated: 73.
Schooling completed through 10 years.
Occupation codes were derived from databases in use in some Indian hospitals with definitions as follows: professional—lawyer, physician, nurse, teacher, etc.; executive—businessperson in a leadership role; business—businessperson in a non-leadership role; retail trader—shopkeeper businessman; vocational work—security guard, hatcheck personnel, telephone operator, artisan, assembly line personnel, etc.; technical work—computer-related jobs, electrician, plumber, etc.; clerk—secretary, data entry personnel, etc.; vendor—sales representative, grocery personnel, etc.; peon—individuals working at very low-level repetitive tasks such as messengers, sweepers, houseboys, etc.; manual labor—hard labor such as roadwork, lifting, construction, etc.; farm labor—includes all agricultural workers.
p ≤ 0.001,
p ≤ 0.01,
p ≤ 0.05 for a difference in the proportions by site, by chi-square, Fisher’s exact, or Wilcoxon test.
No mothers received antibiotics in the last month of pregnancy. More infants enrolled at RKL were born at home compared to infants enrolled at BBS (83% vs. 43%, p<0.001). Rupture of membranes occurred 24 hours or less before delivery for most infants (95%). The proportion of cesarean deliveries was low at both sites, but slightly higher at BBS (4% vs. 2%, p=0.05). Overall, 16% of enrolled infants were preterm and 57% were male. Breastfeeding was initiated for 99% of infants, most in the first 24 hours after birth.
Timing of enrollment
Study enrollment occurred from birth through day 3 of life for 5% of infants, while 12% were enrolled between days 4–7, and 83% were enrolled between days 8–60 (Table 1). The timing of enrollment was similar for infants born at home (5%, 13%, 82%), and in hospital (4%, 11%, 85%). Median admission weight was 2890 grams. A larger proportion of infants enrolled between 0–3 days were preterm (37%) compared to those enrolled later (4–7 days: 17%; 8–28: 18%; 29–60 days: 11%; p<0.001).
Infant clinical signs at enrollment
At least two clinical signs were reported for 79% of infants at the first episode of hospital admission (Table 2). Overall, fever was the most frequently reported sign (55%), followed by poor feeding/poor sucking (34%), tachypnea (31%), decreased activity or lethargy (22%), and chest wall retractions (21%). Of infants enrolled between birth and 3 days, 95% presented with two or more signs compared to 75–80% of those enrolled at later ages. The proportion of infants reporting each clinical sign was significantly different by age of enrollment for most signs. The percent with fever increased with increasing age (p<0.001) from 13% of those enrolled between 0–3 days to 64% of those enrolled between 29–60 days. Poor feeding/poor sucking decreased with increasing age (from 79% at 0–3 days to 23% at 29–60 days), as did decreased activity/lethargy (from 66% to 14%; p<0.001 for each). The percent of infants with tachypnea varied but was highest among those enrolled at 29–60 days of life, as were chest wall retractions. While no infants enrolled 0–3 days presented with a cough, the percent increased with increasing age to 29% among those enrolled at 29–60 days. Jaundice and hypothermia were reported more frequently among infants enrolled between 0–3 days than among those enrolled later. Pustules were most frequently reported among those enrolled between 4–7 days (45%). Umbilical discharge/infection was reported most commonly among infants enrolled between 4 and 28 days (12%).
Table 2.
Infant clinical signs noted by study physician at enrollment/first episode admission by age at enrollment
| Signs at sepsis work-up | No. of infants with sign by age at enrollment n (% of N enrolled)
|
||||
|---|---|---|---|---|---|
| 0–3 days (N=38) | 4–7 days (N=103) | 8–28 days (N=376) | 29–60 days (N=325) | Overall (N=842) | |
|
| |||||
| Poor feeding/poor sucking** | 30 (79%) | 47 (46%) | 134 (36%) | 76 (23%) | 287 (34%) |
|
| |||||
| Fever** | 5 (13%) | 28 (27%) | 219 (58%) | 208 (64%) | 460 (55%) |
|
| |||||
| Cyanosis | 2 (5%) | 0 (0%) | 7 (2%) | 3 (1%) | 12 (1%) |
|
| |||||
| Abdominal distension** | 4 (11%) | 9 (9%) | 60 (16%) | 15 (5%) | 88 (10%) |
|
| |||||
| Seizures | 4 (11%) | 5 (5%) | 10 (3%) | 11 (3%) | 30 (4%) |
|
| |||||
| Bleeding** | 3 (8%) | 1 (1%) | 2 (1%) | 2 (1%) | 8 (1%) |
|
| |||||
| Tachypnea** | 10 (26%) | 8 (8%) | 97 (26%) | 143 (44%) | 258 (31%) |
|
| |||||
| Shock** | 3 (8%) | 0 (0%) | 6 (2%) | 0 (0%) | 9 (1%) |
|
| |||||
| Decreased activity or lethargy** | 25 (66%) | 28 (27%) | 85 (23%) | 44 (14%) | 182 (22%) |
|
| |||||
| Hypothermia** | 10 (26%) | 7 (7%) | 20 (5%) | 4 (1%) | 41 (5%) |
|
| |||||
| Diarrhea* | 0 (0%) | 1 (1%) | 15 (4%) | 26 (8%) | 42 (5%) |
|
| |||||
| Apneic spells | 2 (5%) | 1 (1%) | 4 (1%) | 3 (1%) | 10 (1%) |
|
| |||||
| Jaundice** | 9 (24%) | 19 (18%) | 8 (2%) | 1 (<1%) | 37 (4%) |
|
| |||||
| Chest wall retractions** | 9 (24%) | 5 (5%) | 72 (19%) | 95 (29%) | 181 (21%) |
|
| |||||
| Sclerema | 0 (0%) | 2 (2%) | 4 (1%) | 0 (0%) | 6 (1%) |
|
| |||||
| Cough** | 0 (0%) | 2 (2%) | 52 (14%) | 95 (29%) | 149 (18%) |
|
| |||||
| Pustules** 1/ | 1 (3%) | 46 (45%) | 57 (15%) | 4 (1%) | 108 (13%) |
|
| |||||
| Abscess** | 0 (0%) | 0 (0%) | 28 (7%) | 7 (2%) | 35 (4%) |
|
| |||||
| Cellulitis | 0 (0%) | 2 (2%) | 6 (2%) | 7 (2%) | 15 (2%) |
|
| |||||
| Umbilical discharge/infection** 2/ | 1 (3%) | 14 (14%) | 42 (11%) | 8 (2%) | 65 (8%) |
|
| |||||
| Vomiting | 2 (5%) | 0 (0%) | 5 (1%) | 7 (2%) | 14 (2%) |
|
| |||||
| Dehydration | 1 (3%) | 8 (8%) | 12 (3%) | 10 (3%) | 31 (4%) |
|
| |||||
| Oral thrush† | 0 (0%) | 0 (0%) | 13 (3%) | 2 (1%) | 15 (2%) |
|
| |||||
| Other | 9 (24%) | 15 (15%) | 61 (16%) | 49 (15%) | 134 (16%) |
|
| |||||
| Number of signs reported per infant† | |||||
| 1 | 2 (5%) | 27 (26%) | 75 (20%) | 70 (22%) | 174 (21%) |
| 2 | 13 (34%) | 34 (33%) | 129 (34%) | 106 (33%) | 282 (33%) |
| 3 | 9 (24%) | 24 (23%) | 76 (20%) | 95 (29%) | 204 (24%) |
| 4+ | 14 (37%) | 18 (17%) | 96 (26%) | 54 (17%) | 182 (22%) |
Includes mention of “pustules”, “boils”, and “pyoderma”.
Includes mention of “umbilical discharge”, “omphalitis”, and “umbilical sepsis”.
p ≤ 0.001,
p ≤ 0.01,
p ≤ 0.05 for a difference in the proportions by age at enrollment, by chi-square test.
Clinical findings
Among the 842 infants enrolled in the study for suspected sepsis, 23 (3%) were later brought back to the study hospital for a second episode of suspected sepsis. All infants had blood cultures taken at each hospital admission. Overall, 84 infants (10% of enrolled infants) had culture proven sepsis: 81 with a positive blood culture on first admission and 3 with a negative blood culture on first admission but positive on second admission. Thus, overall 81/842 (9.6%) infants on first episode and 4/23 (17.4%) infants on second episode had culture proven sepsis. Five of the 84 cases of culture proven sepsis (6%) were early onset and 79 (94%) were late onset.
CSF cultures were taken for 17 infants on first episode of which 3 were positive, 17.6% of those cultured or 0.4% of those enrolled. All 3 infants with a positive CSF also had a positive blood culture. None of the 23 infants with a second episode of suspected sepsis had CSF cultures taken at the second admission. A chest x-ray positive for pneumonia was reported for 301/842 (36%) infants on first episode and for 12/23 (52%) infants on second episode. Of the 301 infants with a chest x-ray positive for pneumonia on first episode, 26 (9%) had a positive blood culture; 2/12 (17%) with pneumonia on second episode had a positive blood culture.
Table 3 shows findings at first episode for infants classified into mutually exclusive categories. Overall, the largest group of infants (51%) had blood culture negative clinical infections. The next largest group had pneumonia alone (33%), while 6% of infants had culture proven sepsis alone, and 3% had culture proven sepsis with pneumonia and/or meningitis. The distribution of diagnoses differed significantly by age at enrollment (p<0.01). More than half of infants enrolled in the first week of life had clinical infections (0–3 days: 61%, 4–7 days: 64%) while the percent declined somewhat among those enrolled later (8–28 days: 52%, 29–60 days: 45%). The percent of infants with culture proven sepsis (alone or with pneumonia and/or meningitis) was high in the first week (13% at 0–3 days and 17% at 4–7 days) but declined steadily after the first week: 10% during 8–28 days to 7% of those enrolled between 29–60 days. Correspondingly, the percent of infants with pneumonia alone was highest among those enrolled at older ages (8–28 days: 32%, 29–60 days: 43%).
Table 3.
Diagnosis of neonates enrolled for suspected sepsis1/
| Age at enrollment (days)
|
Overall | ||||
|---|---|---|---|---|---|
| 0–3 | 4–7 | 8–28 | 29–60 | ||
|
| |||||
| Positive BC alone | 5 (13%) | 12 (12%) | 27 (7%) | 9 (3%) | 53 (6%) |
|
| |||||
| Pneumonia alone2/ | 8 (21%) | 7 (7%) | 120 (32%) | 140 (43%) | 275 (33%) |
|
| |||||
| Meningitis alone | 0 | 0 | 0 | 0 | 0 |
|
| |||||
| Positive BC + pneumonia | 0 | 3 (3%) | 9 (2%) | 13 (4%) | 25 (3%) |
|
| |||||
| Positive BC + meningitis | 0 | 2 (2%) | 0 | 0 | 2 (<1%) |
|
| |||||
| Positive BC + meningitis + pneumonia | 0 | 0 | 1 (<1%) | 0 | 1 (<1%) |
|
| |||||
| Clinical infection3/ | 23 (61%) | 66 (64%) | 195 (52%) | 145 (45%) | 429 (51%) |
|
| |||||
| Other4/ | |||||
| AB treatment <5 days, no AB given at discharge | 2 (5%) | 11 (11%) | 23 (6%) | 17 (5%) | 53 (6%) |
| No antibiotics given | 0 | 2 (2%) | 1 (<1%) | 1 (<1%) | 4 (<1%) |
|
| |||||
| TOTAL | 38 | 103 | 376 | 325 | 842 |
Infants were classified in mutually exclusive categories based on findings at first episode enrollment. Number (column percent) is shown.
Pneumonia diagnosed by chest x-ray.
Blood culture negative, CSF negative or not tested, no pneumonia, but treated with antibiotics for 5 or more days while in the hospital or <5 days and antibiotics given at discharge or infant died within 5 days of enrollment.
Blood culture negative, CSF negative or not tested, no pneumonia, treated with antibiotics <5 days, or no antibiotics given, and survived.
Pathogens
Overall, gram negative organisms were identified on 58 (69%) blood cultures, gram positives on 22 (26%), mixed infections on 3 (3.6%), and fungi (Candida sp.) was identified on one culture (Table 4). The most frequent pathogen was Klebsiella which was found on 39 cultures (46%; 37 as a single organism and 2 mixed infections, one with E. coli and one with Streptococcus sp.), followed by S. aureus (26%; 21 single organism and 1 mixed infection with E.coli) and E. coli (15%; 11 single organism and 2 mixed infections previously noted). Group B Streptococcus was not isolated on any culture, although Streptococcus sp. (undifferentiated) was reported on two cultures, one positive for the single organism and the other positive for both Klebsiella and Streptococcus sp. No significant differences were detected in the distribution of organisms by age of enrollment.
Table 4.
Distribution of pathogens associated with early and late-onset culture proven sepsis1/
| Pathogen | Age at Enrollment | Overall | Deaths (n) | |||
|---|---|---|---|---|---|---|
| 0–3 days | 4–7 days | 8–28 days | 29–60 days | |||
| Gram negative organisms | 5 | 14 | 25 | 14 | 58 | 5 |
| Klebsiella2/ | 2 | 9 | 18 | 8 | 37 | 2 |
| E. coli 3/ | 1 | 2 | 5 | 3 | 11 | 0 |
| Other Gram negatives4/ | 2 | 3 | 2 | 3 | 10 | 3 |
| Gram positive organisms | 0 | 2 | 10 | 10 | 22 | 1 |
| Staphylococcus aureus | 0 | 2 | 9 | 10 | 21 | 1 |
| Other Gram positives5/ | 0 | 0 | 1 | 0 | 1 | 0 |
| Fungi | 0 | 1 | 0 | 0 | 1 | 0 |
| Candida | 0 | 1 | 0 | 0 | 1 | 0 |
| Mixed infections6/ | 0 | 0 | 2 | 1 | 3 | 0 |
| Total # infants with a +BC | 5 | 17 | 37 | 257/ | 84 | 6 |
Number of infants with a positive blood culture and infant deaths are shown by pathogen type. Most infants were admitted to the hospital for suspected sepsis only once during the 60 day study period; 23 infants were admitted twice. Pathogens are shown for 81 infants with a positive blood culture on first admission (80 were enrolled only once; 1 infant was enrolled twice with a positive blood culture on first admission but not on second admission), and for three infants with negative blood cultures on first admission but a positive blood culture on the second admission.
Includes Klebsiella sp. (22), K. pneumoniae (13), K. ornithinolytica (1), K. oxytoca (1). One infant with Klebsiella sp. on blood culture on day 12 of life also had a CSF culture positive for aerobic Gram negative rods and died the day after admission.
One infant with E. coli on blood culture on day 4 of life also had a CSF culture positive for Pseudomonas aeruginosa.
Other Gram negatives includes Aeromonas hydrophila (2), Citrobacter freundii (1), Enterobacter cloacae (1), Flavobacterium oryzihabitans (2), Pasteurella pneumotropica (1), Proteus vulgaris (1), Providencia sp. (1), and Stenotrophomonas maltophila (1). The infant with Citrobacter freundii on blood culture on day 4 of life also had a CSF culture positive for aerobic Gram negative rods and died 3 days after admission.
Other Gram positives includes Streptococcus sp. [including enterococcus] (1).
Includes Klebsiella pneumoniae + E.coli (1), Klebsiella sp. + Streptococcus sp. (1), E. coli + S. aureus (1).
Includes 3 infants with a blood culture positive for S. aureus on second admission (negative blood culture at first admission).
Pathogens found on blood in the first 7 days of life are shown separately for home and hospital born infants in Table 4a. Differences in the proportion of gram negative infections were not found by place of birth. All 5 infants with a positive blood culture born at home or in hospital and enrolled at 0–3 days had a gram negative infection. Nine of 10 (90%) infants enrolled between 4–7 days who were born at home had gram negative infections compared to 5/7 (71%) born in hospital.
Table 4a.
Distribution of pathogens associated with culture proven sepsis occurring in the first 7 days of life by place of delivery1/
| Pathogen | Age at Enrollment and Place of Delivery | |||
|---|---|---|---|---|
| 0–3 days | 4–7 days | |||
| Home | Hospital | Home | Hospital | |
| Gram negative organisms | 2 | 3 | 9 | 5 |
| Klebsiella2/ | 1 | 1 | 6 | 3 |
| E. coli | 0 | 1 | 1 | 1 |
| Other Gram negatives3/ | 1 | 1 | 2 | 1 |
| Gram positive organisms | 0 | 0 | 1 | 1 |
| Staphylococcus aureus | 0 | 0 | 1 | 1 |
| Other Gram positives | 0 | 0 | 0 | 0 |
| Fungi | 0 | 0 | 0 | 1 |
| Candida | 0 | 0 | 0 | 1 |
| Mixed infections | 0 | 0 | 0 | 0 |
| Total # infants with a +BC | 2 | 3 | 10 | 7 |
Number of infants with a positive blood culture. Hospital delivery includes infants delivered at non-study hospitals: one enrolled 0–3 days (Balakati PHC, BBS), three enrolled 4–7 days (Balakati PHC and Balipatna CHC, BBS; Rourkela Government Hospital).
Includes Klebsiella sp. (8), K. pneumoniae (2), K. oxytoca (1).
Other Gram negatives includes 0–3 days, home born: Aeromonas hydrophila; 0–3 days, hospital born: Proteus vulgaris; 4–7 days, home born: Aeromonas hydrophila, Flavobacterium oryzihabitans; 4–7 days, hospital born: Citrobacter freundii.
Gram negative species were found on all 3 positive CSF cultures. One infant had Pseudomonas aeruginosa on CSF and E. coli on blood culture and survived. Aerobic gram negative rods were reported on CSF for 2 infants. One of these infants had Citrobacter freundii on blood culture and the other had Klebsiella sp. on blood culture; both infants died.
Antibiotic susceptibility
Antibiotic sensitivities were examined for the gram positive and gram negative organisms most frequently cultured from blood. Among S. aureus isolates tested, a majority were sensitive to Cef 1 (89%), Cef 2 (90%), Cef 3 (80%), ciprofloxacin (70%), amikacin (86%), gentamicin (95%), and vancomycin (73%) (Table 5). Thirteen of 22 isolates (59%) were sensitive to oxacillin, 8 (36%) were intermediate, and 1 (5%) was resistant. Six of 22 S.aureus isolates (27%) were intermediate to vancomycin but none were resistant; all 6 were also intermediate to oxacillin. Twelve (55%) S. aureus isolates were intermediate to ampicillin and 8 (36%) were resistant. All 13 S.aureus isolates tested were resistant to penicillin.
Table 5.
Antibiotic susceptibility of major organisms found on blood culture in community-acquired infection1/
| Selected gram positive organisms (CoNS excluded) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Organism | Cef 12/ | Cef 23/ | Cef 34/ | Ciprofloxacin | Amikacin | Ampicillin | Gentamicin | Oxacillin5/ | Penicillin | Vancomycin |
|
| ||||||||||
| S. aureus (N=22) | ||||||||||
| Total tested | 19 | 20 | 20 | 20 | 22 | 22 | 21 | 22 | 13 | 22 |
| Sensitive | 17 (89%) | 18 (90%) | 16 (80%) | 14 (70%) | 19 (86%) | 2 (9%) | 20 (95%) | 13 (59%) | 0 | 16 (73%) |
| Intermediate | 0 | 1 (5%) | 3 (15%) | 4 (20%) | 3 (14%) | 12 (55%) | 1 (5%) | 8 (36%) | 0 | 6 (27%) |
| Resistant | 2 (11%) | 1 (5%) | 1 (5%) | 2 (10%) | 0 | 8 (36%) | 0 | 1 (5%) | 13 (100%) | 0 |
| Selected gram negative organisms | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Organism | Cef 12/ | Cef 23/ | Cef 34/ | Ciprofloxacin | Amikacin | Ampicillin | Gentamicin | Imipenem |
|
| ||||||||
| Klebsiella sp. (N=39) | ||||||||
| Total tested | 18 | 6 | 39 | 30 | 38 | 36 | 36 | 21 |
| Sensitive | 10 (56%) | 3 (50%) | 19 (49%) | 22 (73%) | 32 (84%) | 6 (17%) | 31 (86%) | 21 (100%) |
| Intermediate | 4 (22%) | 1 (16%) | 7 (18%) | 4 (13%) | 4 (11%) | 4 (11%) | 0 | 0 |
| Resistant | 4 (22%) | 2 (33%) | 13 (33%) | 4 (13%) | 2 (5%) | 26 (72%) | 5 (14%) | 0 |
|
| ||||||||
| E. coli (N=13) | ||||||||
| Total tested | 8 | 1 | 13 | 13 | 12 | 13 | 13 | 8 |
| Sensitive | 3 (38%) | 1 (100%) | 3 (23%) | 9 (69%) | 9 (75%) | 4 (31%) | 10 (77%) | 8 (100%) |
| Intermediate | 1 (13%) | 0 | 2 (15%) | 1 (8%) | 2 (17%) | 0 | 1 (8%) | 0 |
| Resistant | 4 (50%) | 0 | 8 (62%) | 3 (23%) | 1 (8%) | 9 (69%) | 2 (15%) | 0 |
N (percent of tested) shown. Includes organisms found on first and second episodes.
Cef 1 = cephalexin, cephalothin, cefazolin.
Cef 2 = cefamandole, cefmetazole, cefonicid, cefaclor, cefotetan, cefoxitin, cefuroxime
Cef 3 = cefixime, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, cefoperazone.
Includes cloxacillin.
The majority of Klebsiella isolates tested were sensitive to amikacin (84%), gentamicin (86%), ciprofloxacin (73%), imipenem (100%), and Cef 1 (56%). Three of six isolates tested (50%) were sensitive to Cef 2 and 19/39 (49%) isolates were sensitive to Cef 3. Most Klebsiella isolates were resistant or intermediate to ampicillin (30/36 tested, 83%), 13/39 (33%) were resistant to Cef 3, and 4/18 (22%) were resistant to Cef 1.
All 8 E.coli isolates tested were sensitive to imipenem and the majority tested were sensitive to amikacin (75%) and gentamicin (77%). Among 13 E.coli isolates, 9 (69%) were resistant to ampicillin and 8 (62%) were resistant to Cef 3; one isolate tested showed resistance (100%) against Cef 2, 4 of 8 tested (50%) were also resistant to Cef 1.
Mortality
Overall, 793 (94%) infants completed the study, 12 (1%) withdrew, 2 (<1%) were transferred, and 35 (4%) died during the 60-day study period (Figure 2). The percent of infants who died was somewhat higher at BBS (22/384=6%) than at RKL (13/458=3%), p=0.04. Median age at death was 14 days (25th–75th percentile: 7 – 32). Over 50% of infant deaths (20/35) were attributed to sepsis, meningitis, and/or pneumonia.
Infant deaths are shown by final culture status and age at enrollment in Table 6. Of 83 infants with a positive blood culture at last admission, 6 (7.2%) died compared to 29/759 (3.8%) with negative cultures, p=0.15. Although small in number, deaths were proportionately high in infants diagnosed with meningitis as well as culture positive sepsis (2/3, 67%). Of the 6 deaths in blood-culture positive cases, five were infants with gram negative infections while one infant had S.aureus infection. However, there was no E. coli sepsis-related deaths reported.
Table 6.
Deaths by final culture status and age at enrollment1/
| Age at Enrollment | ||||
|---|---|---|---|---|
|
| ||||
| 0–3 days | 4–28 days | 29–60 days | Overall | |
|
| ||||
| Positive BC alone | 2/4 (50%) | 1/39 (3%) | 0/10 (0%) | 3/53 (6%) |
|
| ||||
| Pneumonia alone2/ | 2/8 (25%) | 7/120 (6%) | 2/149 (1%) | 11/277 (4%) |
|
| ||||
| Positive BC + pneumonia | na | 1/12 (8%) | 0/15 (0%) | 1/27 (4%) |
|
| ||||
| Positive BC + meningitis | na | 1/2 (50%) | na | 1/2 (50%) |
|
| ||||
| Positive BC + meningitis + pneumonia | na | 1/1 (100%) | na | 1/1 (100%) |
|
| ||||
| Clinical infection3/ | 4/22 (18%) | 11/251 (4%) | 3/151 (2%) | 18/424 (4%) |
|
| ||||
| Other4/ | ||||
| AB treatment <5 days, no AB given at discharge | 0/2 (0%) | 0/34 (0%) | 0/17 (0%) | 0/53 (0%) |
| No antibiotics given | na | 0/3 (0%) | 0/2 (0%) | 0/5 (0%) |
| TOTAL | 8/36 (22%) | 22/462 (5%) | 5/344 (1%) | 35/842 (4%) |
Groups are mutually exclusive. 23 infants were classified based on findings at a second episode admission. Therefore, numbers in each group do not always match with numbers in Table 3.
Pneumonia diagnosed by chest x-ray.
Blood culture negative, CSF negative or not tested, no pneumonia, but treated with antibiotics for 5 or more days while in the hospital or <5 days and antibiotics given at discharge or infant died within 5 days of enrollment.
Blood culture negative, CSF negative or not tested, no pneumonia, treated with antibiotics <5 days, or no antibiotics given, and survived.
Death rates were relatively high in infants enrolled between birth and 3 days of life compared to those enrolled between 4–60 days, regardless of culture status (0–3 days: 22%, 4–28 days: 5%, 29–60 days: 1%, p<0.001). Among infants with a positive blood culture, 2/4 (50%) enrolled between birth and 3 days died compared to 4/54 (7%) enrolled 4–28 days, while no deaths were reported in blood culture positive infants enrolled between 29–60 days, p<0.01. Similarly, among those with pneumonia alone, the proportion who died decreased rapidly after 0–3 days from 25% to 6% to 1%, p<0.01. The proportion of infants with clinical infection who died also declined with age at enrollment, p<0.01. Apart from the 35 deaths in the 842 infants enrolled for clinical sepsis, our population-based demographic surveillance recorded an additional 388 deaths during the three-year period, of which 158 were during day 0–1 (data not shown). These infants could not be reached before death.
DISCUSSION
This is the first report of a population-based surveillance of neonatal infection from the Indian sub-continent conducted in the community setting where Bactec blood culture was done in all admitted infants. In this large study, we had the opportunity to conduct a rigorous surveillance of sickness in the first two months of life in 12,622 infants over a three year period. The signs and symptoms of sickness presented by these infants are similar to those reported in other studies in hospital settings in India and community-based studies in Pakistan, Bangladesh, and multiple African countries.6 Since our study pediatricians admitted just under half of the 1,690 infants identified by the AWWs to have possible infection, and we admitted several infants with second episodes, we are confident that our trained AWWs rigorously monitored and identified cases with a high degree of accuracy at the village level. The specificity of diagnosis is also reflected in not admitting half of the cases that came to hospital for treatment and identification of more than two clinical signs in about 80% of enrolled infants by study pediatricians. Our total of 842 infants with clinical sepsis represents 67 cases/1000 live births in the community surveillance population. Culture-confirmed sepsis was diagnosed in 84 infants, a rate of 6.7 cases per 1000 live births, of which 4.2/1000 live births were exclusively bacteremic Interestingly, we identified only three cases of meningitis of which two died in spite of prompt hospital treatment. All three cases had concomitant blood culture positivity, apart from bacterial growth in CSF. While it is difficult to comment on the virulence of co-infecting organisms, known pathogens such as Klebsiella pneumoniae, and Citrobacteri freundii appeared to have a causal link with death compared to E. coli when overall mortality data in our population are examined in conjunction with infecting bacterial species.
One of our aims in conducting this village level surveillance was to disaggregate and quantify pneumonia, clinical sepsis, and blood culture proven sepsis – the three elements often combined and treated as one disease entity due to lack of clarity on individual components at the community level. In our enrolled population, about half had clinical sepsis as judged by expert clinicians where clinical signs were the only identifying features. However, we were able to conclusively record chest x-ray positive pneumonia in 33% that were culture negative and an additional 25% of pneumonia cases were culture positive. Although pneumonia causing organisms are often not grown from blood, our study demonstrates that it is possible to discern between the two and quantify both conditions so that this information can be used while developing guidelines for empiric treatment of these conditions. Due to our moderately large sample size, we have been able to capture infants in each unique category and those with overlaps. However, for the purpose of this report, we have not examined the extended list of clinical signs in the context of various risk factors, exposures, and type of infection. Multivariate models fitting these parameters could ultimately help in improving the algorithm or prediction rules to diagnose and manage neonatal sepsis in the village level with lack of access for evaluation by medical professionals.
One striking finding in our study is the timing of presentation of neonatal sepsis in the community setting, with the smallest numbers during the first 3 days of life. We recorded 38, 103, 376, and 325 hospital admissions with a diagnosis of clinical sepsis during 0–3, 4–7, 8–28, and 29–60 days of life, respectively. An increase was also observed in the number of infants with culture positive sepsis; 5, 17, 37, and 22 during the four time periods respectively. Thus, 5 of 84 infants (6%) with culture positive sepsis had early onset while 79 (94%) had late onset sepsis. In a study conducted during the contemporary period in two tertiary care settings in India (cities of Delhi and Chandigarh) severe infection-associated admissions were 7–24% during 0–6 days of life and increased to 51–59% during 7–59 days of life.9 While such hospital-based information has not been examined further with regard to restricting empiric antibiotic therapy in the first three days of life, our study for the first time suggests that early onset neonatal sepsis may not be as much of a problem in India as late onset sepsis, thanks to advances made in maternal health, clean cord cutting, and almost complete eradication of neonatal tetanus.
Another important aspect of our results is the not-so-high culture positivity. In a review of hospital-based neonatal infections in developing countries, Zaidi et al. reported infection rates ranging between 6.8 and 38 per 1000 live births and culture positivity to be between 1.7 and 33 per 1000 live births. The incidence of culture positivity in South Asia was 15/1000.10 The rate of culture proven sepsis in our population was in the lower range reported by Zaidi (6.7/1000) but the rate of clinical infection higher (67/1000). These results indicate that the underlying cause of symptomatic young infants may not always be of bacterial origin. We can say this with some degree of confidence, because none of our mothers received antibiotics during the last month of pregnancy and all blood draws were done before babies were started on antibiotics. We also used Bactec-Peds Plus blood culture bottles that assure highly efficient recovery of organisms from blood. Although Klebsiella and E. coli have been reported as major Gram negative organisms in previous studies, it is worth noting that among the culture positive cases in our study none of the young infants with E. coli sepsis died (0/11), whereas two of 37 with Klebsiella and three of 10 with other Gram negative infection died. Although intravenous therapy was started immediately after blood draw and antibiotics were often changed based on culture and sensitivity results, these Klebsiella and other non-E. coli-related deaths have clinical significance from a prognostic standpoint and may provide directions when preventative modalities are envisioned to address such bacterial infections.
In a review of blood culture results of neonatal sepsis in the first 90 days of life from 63 centers in Latin America, Middle East, South Asia, East Asia and Pacific, and Africa, six centers were from India, but all six were intensive care units or tertiary care hospitals.6 Although India-specific information was not available in this report, the South Asian region appeared to have 9.8% S. aureus, 0.8% Group B Sterptococcus, 23% Klebsiella, and 12% E. coli during the first 28 days of life. While the exact percent distribution in each geographic region was not described, S. aureus, Group B Streptococcus, Klebsiella, and E. coli were 13.7%, 9.7%, 5.6%, and 9.3% respectively of all organisms during the 7–59 day period.6 It was not clear why Group B Streptococcus, an organism typically considered to be maternally acquired would manifest in late sepsis up to 59 days. It was also very important to note in this compilation that 57.4% of all infections during days 0–3 were Gram negative in nature. In our study, all five infants with culture proven infections on days 0–3 had gram negative infections. Such Gram negatives in early onset sepsis have also been reported in other hospital-based studies in the developing world setting.10 When we compared pathogens cultured during 0–3 and 4–7 days of life in home-born and hospital born infants, no discernible difference could be noted by place of birth. Although interesting and important from a pathogenesis point of view, the source of these infections remains undetermined at this time.
A more recent report in 2015 by Hamer and colleagues11 described the etiology of bacteremia and antimicrobial resistance of the infecting organisms in 0–59 day old infants in six countries using data collected in a period similar to ours (starting around 2004). This multi-country study enrolled 1,437 infants in South Africa, Ghana, Bolivia, India, Pakistan, and Bangladesh and performed blood culture in 782 infants. Two tertiary care centers in India contributed data on 34 bacterial isolates for this report. S.aureus predominated, followed by Acinetobacter spp., Enterobacter spp., E.coli, and Klebsiella and this report described an overall very high resistance to ampicillin and amoxicillin (97%), 1st generation cephalosporins (Cef 1, 82%), 2nd generation cephalosporins (Cef 2, 52%), and 3rd generation cephalosporins (Cef 3, 61%), followed by gentamicin (44%) and amikacin (38%). In our current community-based study of 1,690 infants, and 842 blood cultures, we observed somewhat different antimicrobial resistance patterns in 84 bacterial isolates. S.aureus exhibited 100% resistance to penicillin, but 36% to ampicillin and resistance to Cef 1, 2, and 3 ranged between 5–11%. However, 22% of Klebsiella isolates were resistant to Cef 1, and 33% to both Cef 2 and 3. More E. coli isolates exhibited resistance to Cef 1 (50%) and Cef 3 (62%) with none resistant to Cef 2. One significant finding in our study was a very high sensitivity of S.aureus to gentamicin (95%) as well as high sensitivity of Klebsiella (86%) and E. coli (77%). No resistance was seen to imipenem or to vancomycin, although 6 of 22 (27%) S.aureus isolates tested were intermediate to vancomycin. Although these resistance patterns may appear to be lower than those reported in the hospitals, in fact, these rates (about one third of the isolates) are quite alarming. A majority of these infants (65%) were born at home, were never exposed to a hospital environment until getting sick, never received injectable or oral antibiotics, nor were their mothers exposed to antibiotics during the last month of pregnancy. In addition, many of the same organisms tested earlier by our group in a separate study found an equally high number of the Gram negatives to be extended spectrum beta-lactamase (ESBL) producers and harbor multidrug resistance.12 Such phenomena are recipes for treatment failure that we have observed in a small number of cases in this study in spite of extremely prompt treatment.
It is intriguing to note a very high level of sensitivity to gentamicin and Cef 2. Similarly, most isolates were uniformly sensitive to amikacin. During our study initiation, although ampicillin and gentamicin were theoretically recommended as first line drugs, they were seldom used by clinicians; only 381, or 45% received this therapy and 90 (11%) did not receive ampicillin or gentamicin but got at least one other antibiotic as first line drugs in our study population. After the advent of cephalosporins, especially the third generation broad spectrum cephalosporins, the use of gentamicin has gone down drastically in India, and in fact, gentamicin, ampicillin, or amikacin were not readily available in the hospital formulary or drug stores in the districts where we conducted the study. Removal of an antibiotic from the therapeutic regimen may lead to the reversal of microbial resistance into susceptible phenotypes and such phenomena have been described in the literature with serious public health ramifications. For example, resistance to inexpensive chloroquine in multiple African countries had led to the use of sulfadoxine – pyrimethamine, and artemisinin until resistance to these new lines of antimalarial drugs emerged. However, chloroquine once again became highly efficacious in Malawi, 12 years after it was withdrawn from use because of rates of treatment failures in more than 50% of cases.13 Such antibiotic withdrawal has been suggested as one of the possible mechanisms for overcoming antimicrobial resistance14 and should be considered when regimens are recommended for community level treatment of neonatal sepsis with empiric antibiotics.
Although we used a very tight community surveillance, we were unable to verify the cause of death in the additional 388 infants in our cohort of 12,622 infants who died and were not enrolled. In many of these deaths, especially in the 158 deaths on day 0, we cannot rule out early onset sepsis. Infection vertically transmitted from the mother or acquired during delivery and perinatally in the home environment or in the hospitals can theoretically cause such early sepsis in the newborns. However, any such infection would typically take about 48 hours to manifest and will not produce frank sepsis and death in less than 24 hours unless there is history of extremely prolonged rupture of membranes. Only 5% of our babies were delivered more than 24 hours after rupture of membranes. Although many non-sepsis related indications were recorded in the system pointing towards birth asphyxia (e.g. baby did not cry at birth), there is a need to improve case detection in the first 12–24 hours of neonatal life. As we strive to get answers to these fundamental questions, many other health system-related changes have taken place in India. For example, introduction of a new cadre of village level lady workers called ASHA (Accredited Self Help Activist), and promotion of hospital delivery with financial incentive to the mother has resulted in a sharp drop in home deliveries. The same districts where we conducted this study are now recording close to 100% facility births although there is overcrowding of the delivery rooms and limited space for mothers and babies in the postnatal wards. Some primary health care centers and hospitals are routinely using injectable antibiotics in mothers undergoing normal vaginal deliveries. Have these interventions improved or deteriorated the problem of neonatal sepsis in the Indian community? Are there new organisms, or change in their antimicrobial resistance patterns? Are there any antimicrobial stewardship activities in India? This paper describes results of the surveillance conducted several years ago in rural India. Lacking health system-based ongoing surveillance of infections and tracking of antimicrobial resistance, information provided in the current paper will help policy makers and practitioners stay vigilant for any changes and institute appropriate practice modalities. Our largest group (51%) being blood culture negative clinical infections and one third of our cohort showing signs of respiratory involvement raise intuitive questions on their etiology. Could those be due viral or mycoplasma infections? Results of new studies with inclusion of larger populations in south Asia,15,16 with an emphasis on reaching all deliveries in the first 24 hours and conducting an even more comprehensive microbiology utilizing traditional and molecular tools will soon provide critical answers to some of these questions and enable us design evidence-based interventions at the community level to prevent and treat neonatal sepsis in India and other south Asian countries.
Acknowledgments
Funding: This study was funded by NIH grant U01 HD 40574 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, USA.
This study is dedicated to late Dayananda Das, former director of Jana Sikhshan Sansthan, Rourkela for his sincere commitment, superlative management skills, flawless execution, and successful completion of the study protocol in 223 villages of Odisha.
We are thankful to the Government of Odisha, Department of Health and Family Welfare, the Indian Council of Medical Research and the Ministry of Health and Family Welfare Government of India for their review and providing timely approvals for this study. Ms. Meena Gupta, Principal Secretary of Health and Family Welfare, Government of Odisha deserves our earnest gratitude for her proactive assistance in enabling the first study of this nature being conducted at a government facility in Odisha and establishing the first neonatal special care unit in Bhubaneswar city, and also for coordinating with the Department of Woman and Child Development for involving the Anganwadi workers in the surveillance. Keshab Pradhan and Kallola Mishra conducted data entry and overall management in the hospital. We appreciate the 223 courageous Anganwadi workers who embarked on a medical project of this nature and performed at a level comparable to any other paramedical personnel in the government system. This study was funded by NIH grant U01 HD 40574 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, USA.
Footnotes
AUTHORS CONTRIBUTIONS
PP conceived and designed the study and wrote the first draft of the paper. PP, SP, JM, IG, NB, JJ, and RC wrote the protocol and manual of operations. RS, LP, AM, and SM were responsible for clinical diagnosis and case management. PM, DC, and NS were in-charge of lab management. NH and SK conducted data analyses. All authors contributed during the development of study forms and multiple aspects of clinical, laboratory, and data management, and contributed during revision and preparation of the final version of the manuscript.
Conflict of Interests: The authors declare no conflict of interest.
Contributor Information
Pinaki Panigrahi, Department of Epidemiology and Pediatrics, Center for Global Health and Development, College of Public Health, University of Nebraska Medical Center, Omaha, NE, USA 68198-4385, Tel- 402-559-4960 Fax- 402-559-2330.
Dinesh S. Chandel, Department of Environmental, Agricultural, & Occupational Health, Center for Global Health and Development, College of Public Health, University of Nebraska Medical Center, Omaha, NE, USA 68198-7696.
Nellie I. Hansen, Biostatistics & Epidemiology Division, RTI International, Research Triangle Park, NC, USA 27709.
Nidhi Sharma, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India 110029.
Sarah Kandefer, Social Policy, Health, & Economics Research Division, RTI International, Atlanta, GA 30329.
Sailajanandan Parida, Department of Pediatrics, SCB Medical College, Cuttack, Odisha, India 753007.
Radhanath Satpathy, Asian Institute of Public Health, Bhubaneswar, Odisha, India 751002.
Lingaraj Pradhan, Department of Pediatrics, Capital Hospital, Bhubaneswar, Odisha, India 751001.
Arjit Mohapatra, Asian Institute of Public Health, Bhubaneswar, Odisha, India 751002.
Subhranshu S. Mohapatra, Asian Institute of Public Health, Bhubaneswar, Odisha, India 751002.
Pravas R. Misra, Asian Institute of Public Health, Bhubaneswar, Odisha, India 751002.
Nandita Banaji, Department of Microbiology, Indira Gandhi Medical College & Research Institute, Puducherry, India 605009.
Judith A. Johnson, Emerging Pathogens Institute, University of Florida, Gainesville FL 32611.
John Glenn Morris, Jr., Epidemiology, Pediatrics, & Human Development, Michigan State University College of Human Medicine, East Lansing MI 48824.
Ira H. Gewolb, Department of Pediatrics/Neonatology, Sparrow Hospital - Neonatology, 1215 E Michigan Ave, Lansing MI 48912, Tel- 517-364-2591 Fax- 517-364-3994.
Rama Chaudhry, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India 110029.
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Waters D, Jawad I, Ahmad A, Lukšic I, Nair H, Zgaga L, et al. Aetiology of community-acquired neonatal sepsis in low and middle income countries. J Glob Health. 2011;1:154–170. [PMC free article] [PubMed] [Google Scholar]
- 3.Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:731–741. doi: 10.1016/S1473-3099(14)70804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laxminarayan R, Chaudhury RR. Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med. 2016;13(3):e1001974. doi: 10.1371/journal.pmed.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.African Neonatal Sepsis Trial (AFRINEST) group. Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet. 2015;385:1767–76. doi: 10.1016/S0140-6736(14)62284-4. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi AK, Thaver D, Ali SA, Khan TA. Pathogens Associated with Sepsis in Newborns and Young Infants in Developing Countries. Pediatr Infect Dis J. 2009;28:S10–S18. doi: 10.1097/INF.0b013e3181958769. [DOI] [PubMed] [Google Scholar]
- 7.The WHO Young Infants Study Group. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. Pediatr Infect Dis J. 1999;18(suppl 10):S17–S22. doi: 10.1097/00006454-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards (NCCLS) Development of in vitro susceptibility testing criteria and quality control parameters: approved guideline [M23-A2] 2. Wayne, PA: NCCLS; 2001. [Google Scholar]
- 9.The Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;371:135– 142. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 10.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 11.Hamer DH, Darmstadt GL, Carlin JB, Zaidi AK, Yeboah-Antwi K, Saha SK, et al. Etiology of Bacteremia in Young Infants in Six Countries. Pediatr Infect Dis J. 2015;34(1):e1–e8. doi: 10.1097/INF.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandel DS, Johnson JA, Chaudhry R, Sharma N, Shinkre N, Parida S, et al. Extended-spectrum β-Lactamase producing Gram-negative bacteria causing Neonatal sepsis in India in Rural and Urban settings. J Med Microbiol. 2011;60(4):500–507. doi: 10.1099/jmm.0.027375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of chloroquine antimalarial efficacy in Malawi. N Eng J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 14.Stokes DJ, Kelly AF, Gould SW, Cassar CA, Fielder MD. The withdrawal of antimicrobial treatment as a mechanism for defeating resistant microorganisms. FEMS Immunol Med Microbiol. 2008;53:300–305. doi: 10.1111/j.1574-695X.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 15.Islam MS, Baqui AH, Zaidi AK, Bhutta ZA, Panigrahi P, Bose A, et al. Infection Surveillance Protocol for a Multicountry Population-based Study in South Asia to Determine the Incidence, Etiology and Risk Factors for Infections Among Young Infants of 0 to 59 Days Old. Pediatr Infect Dis J. 2016;35(Suppl 1):S9–S15. doi: 10.1097/INF.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 16.Satpathy R, Nanda P, Nanda NC, Bal HB, Mohanty R, Mishra A, et al. Challenges in Implementation of the ANISA Protocol at the Odisha Site in India. Pediatr Infect Dis J. 2016;35(Suppl 1):S74–S78. doi: 10.1097/INF.0000000000001112. [DOI] [PubMed] [Google Scholar]