Abstract
Background
Prevalence of smoking among individuals with post-traumatic stress disorder (PTSD) is disproportionately high, and PTSD is associated with especially poor response to smoking cessation treatment.
Objective
The current study examined whether integrating treatments for smoking cessation (varenicline plus smoking cessation counseling; VARCC) and PTSD (prolonged exposure therapy; PE) enhances smoking outcomes among smokers diagnosed with PTSD.
Method
142 adults with nicotine dependence and PTSD were randomized to a treatment program consisting of varenicline, smoking cessation counseling, and PE (VARCC+PE) or to VARCC only. Seven-day point prevalence abstinence (PPA) at post-treatment (3-months post-quit day) and follow-up (6-months post-quit day), verified by serum cotinine levels and exhaled carbon monoxide, was our primary smoking outcome. Psychological outcomes were PTSD and depression severity. Mixed effects models included baseline PTSD severity as a moderator of treatment condition effects.
Results
Overall, VARCC+PE participants did not show greater PPA than VARCC participants. However, treatment effects were moderated by baseline PTSD severity. For participants with moderate and high PTSD severity, VARCC+PE led to significantly higher PPA than VARCC alone (ps<.05). No differences between treatment conditions emerged for participants with low baseline PTSD severity. Participants who received PE showed significantly greater reduction of PTSD and depression symptoms than those who did not receive PE.
Conclusions
Integrating psychological treatment for PTSD and smoking cessation treatment enhances smoking cessation for participants with moderate or severe PTSD symptom severity, but does not enhance smoking cessation for participants with low baseline PTSD severity.
Keywords: nicotine dependence, PTSD, smoking cessation, prolonged exposure, varenicline
Cigarette smoking is the single most preventable cause of death in the U.S. (U.S. Department of Health and Human Services, 2014). The prevalence of smoking among individuals with Posttraumatic Stress Disorder (PTSD) is particularly high, with past-year estimates around 44.6% (e.g., Acierno, Kilpatrick, Resnick, Saunders, & Best, 1996; Stewart, 2014). Smokers with PTSD not only smoke more heavily compared to smokers without PTSD, but also demonstrate greater nicotine dependence (ND) and lower quit rates following smoking cessation interventions (Feldner, Babson, & Zvolensky, 2006; Lasser et al., 2000; Moran, Sampath, Kochunov, & Hong, 2013). PTSD is associated with a wide range of affective disturbances that may contribute to increased nicotine dependence and relapse following attempts to quit. For example, depressive symptoms are highly related to PTSD severity, and have been found to be related to smoking cessation difficulties (Kelly, Jensen, & Sofuoglu, 2015; Leventhal, Zvolensky, & Schmidt, 2011).
The current clinical practice guidelines for treating tobacco dependence (U.S. Public Health Service, 2008) outline several front-line interventions for smoking cessation that were derived from a vast body of research on smoking cessation spanning over 30 years (around 35 meta-analyses), including counseling approaches (particularly those that incorporate problem solving and social support) and a range of pharmacological approaches such as nicotine replacement therapy (NRT), buproprion, and varenicline. Several studies have found that varenicline, a selective α4β2 neuronal nicotinic acetylcholine receptor partial agonist, is well tolerated and more effective in reducing smoking than bupropion, the only other approved non-NRT medication for smoking (Cahill, Stevens, Perera, & Lancaster, 2013; Gonzales et al., 2006; Jorenby et al., 2006; Nides et al., 2006). It is important to note that while varenicline is one of the first-line pharmacological treatments for smoking, sustained smoking cessation rates following this treatment are still relatively low (22–45% abstinence rates at 12-month follow-up in non-clinical samples; Gonzales et al., 2006; Kralikova et al., 2013). The clinical guidelines recommend a combination of counseling and medication as the most effective approach to promote successful quit attempts (U.S. Public Health Service, 2008).
To our knowledge, there are only a handful of studies examining the efficacy of varenicline or other smoking cessation interventions in samples of smokers with PTSD. These studies have yielded mixed smoking outcomes and, as might be expected, no improvement in PTSD (Dennis et al., 2016; Gibson & Zvolensky, 2006; Hertzberg, Moore, Feldman, & Beckham, 2001; McFall et al., 2005). In contrast, there are several highly effective psychosocial treatments for PTSD. Of these, prolonged exposure (PE) has received the greatest empirical evidence for its efficacy (e.g., Foa et al., 1999; Foa et al., 2005), with response rates ranging from 65–80% and medium to large effect sizes in comparison to other treatments (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010).
In order to target both smoking quit rates and PTSD symptoms, several studies have examined the outcome of integrating treatments for PTSD and smoking cessation. One large (N=943) multi-site study examined the impact of integrating smoking cessation treatment into routine PTSD treatment in PTSD Veteran Affairs (VA) specialty clinics, compared to a standard smoking cessation treatment consisting of smoking cessation counseling and pharmacological smoking cessation interventions (McFall et al., 2010). Study results indicated superior, albeit low (8.9%), abstinence rates for those receiving integrated care versus those receiving smoking cessation only (4.5%). Notably, no standardized treatment protocol for PTSD was delivered in this study, and while both groups improved slightly (10%) on PTSD severity, no group differences in psychiatric symptom improvement emerged. Another VA study (n=178) examined the integration of motivational interviewing techniques for smoking cessation into a 12-week PTSD telehealth self-monitoring procedure (with no active PTSD treatment) compared to PTSD self-monitoring telehealth procedures alone, using a randomized controlled design (Battaglia et al., 2016). No differences between the groups emerged on smoking outcomes, but participants who received self-monitoring and smoking cessation showed greater improvement in PTSD and depression symptoms. In civilians, another study examined an 8-week group-based protocol that incorporated trauma management treatment (i.e., interoceptive and in vivo exposures to trauma-related internal and external triggers) with comprehensive smoking cessation versus smoking cessation only, in smokers exposed to the World Trade Center disaster (Gonzalez et al., 2016). Study results indicated no significant differences between groups on smoking outcomes; however, contrary to expectations, the group who received PTSD treatment did not show superior PTSD outcomes.
Only two other small, open clinical studies have evaluated concurrent or integrated treatments for smoking cessation with empirically-supported protocol treatments for PTSD. The first study (N=6) examined the outcome of 12 biweekly sessions of individual cognitive processing therapy (CPT) for PTSD combined with the nicotine patch; the four participants who completed the program showed substantial reductions in PTSD by the end of the therapy but all were smoking two months later (Feldner, Smith, Monson, & Zvolensky, 2013). The second study (N=19) examined the outcome of nine individual weekly sessions of Acceptance and Commitment Therapy (ACT) modified for individuals with PTSD and ND, combined with 8 weeks of the nicotine patch (Kelly et al., 2015). This study had no control group. Abstinence rates for the study completers (N=14) were 50% at post-treatment and 20% at 3-month follow-up. PTSD symptoms significantly decreased through the 3-month follow-up.
Hypothesizing that the distress associated with co-morbid psychiatric disorders may impede successful quit attempts, researchers have been investigating certain psychological disturbances that may impede the success of smoking cessation treatments. For example, two studies have found that for smokers with co-morbid depression, the combination of cognitive behavioral interventions for depressive symptoms (e.g., behavioral activation or cognitive techniques) and varenicline or other smoking cessation interventions, yielded both superior reductions in depression and superior smoking outcomes, compared to smoking cessation interventions alone (Frye, 2015; Hartmann-Boyce, Stead, Cahill, & Lancaster, 2014). These findings are consistent with results showing the role of depressive symptoms in maintaining smoking (Leventhal et al., 2011). Thus, it is possible that these affective symptoms, which are highly comorbid with PTSD (e.g., Gadermann, Alonso, Vilagut, Zaslavsky, & Kessler, 2012; Rytwinski, Scur, Feeny, & Youngstrom, 2013), may partially account for poor smoking cessation outcomes in individuals with comorbid PTSD and nicotine dependence. If so, integrating treatment for PTSD (which has also been shown to reduce depression; e.g., Foa et al., 2005; Rothbaum, Astin, & Marsteller, 2005) with smoking cessation treatment may enhance the success of treatment in smokers with PTSD, as well as ameliorate their distress related to PTSD symptoms. Indeed, recent studies have found that the belief that smoking will reduce unpleasant affective symptoms seems to underlie the observed relationship between smoking and PTSD (Mahaffey et al., 2016). However, the severity level of PTSD and its impact on the success of integrated PTSD-smoking cessation treatments has not yet been examined.
The current paper reports on results from a large-scale, randomized controlled trial (RCT) that evaluated whether integrating a recommended evidence-based intervention for smoking cessation (a combination of varenicline and smoking cessation counseling [VARCC]) with a recommended treatment for PTSD (PE) results in higher rates of smoking cessation than VARCC alone, in smokers with PTSD. We posited that by reducing PTSD symptoms, PE would ameliorate the psychological disturbances (e.g., depression) that interfere with successful smoking cessation and thus would enhance the likelihood of a successful quit attempt. Specifically, we suggested that the integration of PE with varenicline would diminish several barriers to successful quit attempts, such as depressive and anxiety symptoms. Hence, we hypothesized that 1) overall, smokers with comorbid PTSD receiving integrated VARCC and PE would exhibit superior smoking outcomes compared to those receiving VARCC only. Further, because those with higher PTSD symptom severity tend to have higher depressive symptoms and anxiety, these psychological symptoms are more likely to undermine a quit attempt in individuals with higher PTSD symptom severity (compared to those with milder PTSD severity). It follows that individuals with higher PTSD symptom severity will obtain greater benefit from the integrated treatment than those with lower PTSD symptom severity. Accordingly, we further hypothesized that 2) the effects of integrating VARCC and PE would be moderated by baseline PTSD severity, such that those with higher PTSD severity would benefit more from adding PE to VARCC on smoking outcomes than those with lower PTSD severity. Finally, based on the extensive literature supporting the efficacy of PE (Powers et al., 2010), we hypothesized that 3) participants receiving PE would show greater reductions in PTSD and depression symptoms compared to those receiving VARCC only.
Methods
Participants
Participants were 142 male and female cigarette smokers with chronic PTSD who sought treatment to ameliorate their PTSD symptoms and to quit smoking. Participants were recruited through advertisements and referrals to Center for the Treatment and Study of Anxiety (CTSA). Inclusion criteria were (1) smoking equivalent to 10 or more cigarettes per day during past year, (2) current diagnosis of chronic PTSD according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000) and (3) clinically significant trauma-related symptoms, as indicated by a score of at least 15 on the PTSD Symptom Severity Interview (PSS-I; Foa, Riggs, Dancu, & Rothbaum, 1993). The cut-off score of 15 or higher for the PSS-I was chosen to be consistent with previous PE outcome studies (e.g, Foa et al., 2013) and corresponds to mild PTSD. Individuals taking psychotropic medications at intake had to be stabilized on that medication regimen (i.e., reporting no changes in medication type or dosage) for the past 3 months. Exclusion criteria were: (1) history of drug or alcohol abuse or dependence in past 3 months, (2) unwillingness to stop smoking marijuana during the first 13 weeks of the study, (3) unwillingness to refrain from using NRT over course of the study, (4) current intimate relationship with a physically or sexually abusive partner, (5) current suicidal ideation with intent and/or plan, (6) history of psychosis or bipolar disorder, (7) significant cardiovascular disease or uncontrolled hypertension in past 6 months, and (8) pregnancy or nursing.
Procedure
Participant enrollment began on January 22, 2009 and ended on June 9, 2014. Data collection was completed on December 15, 2014. The University of Pennsylvania institutional review board approved the study protocol. After providing written informed consent, participants completed an intake evaluation comprised a psychiatric diagnostic interview, physical examination, and laboratory assessments to determine eligibility. Eligible participants completed a baseline assessment and were block randomized (in groups of 4–6 participants) using a randomization generator built into the study database by the study research assistant, to a treatment program consisting of varenicline, smoking cessation counseling, and PE (VARCC+PE) or to a treatment program of varenicline and smoking cessation counseling only (VARCC). At the baseline visit, medication was initiated and a smoking quit date was set for approximately one week later. The study nurse provided a brief overview of the study treatment conditions, prepared the participant for the quit date, reviewed instructions on dispensed medication, and emphasized the importance of medication compliance and smoking abstinence.
Participants were informed of their treatment condition at the end of the baseline session and were scheduled to meet with their therapist on their quit date. Regardless of whether participants succeeded in quitting on their designated quit date, they continued with treatment per the descriptions below and worked with their therapist to improve quit attempts. The study nurse met with the participants at each treatment visit to dispense medication, and to monitor health status, medication compliance, and side effects. Doctoral level psychologists at CTSA conducted the treatment over 12 weekly visits. Given the FDA black box warning for varenicline regarding potential neuropsychiatric side effects, changes in mood and suicidal thinking were assessed at every appointment by both the study nurse and therapists. All therapists were certified in PE (i.e., completed an intensive 4-day PE workshop followed by case consultation on at least 2 PE training cases), completed a 2-day training with one of the co-authors (PG) on smoking cessation counseling, and received weekly group supervision that included review of video-recorded sessions. Evaluators, blind to treatment assignment, conducted assessments at baseline, post-treatment (3-months post-quit day), and 3 months after treatment completion (6-months post-quit day). Self-report measures were also completed at these assessment points. Participants were compensated for each assessment ($50 for baseline and post-treatment; $100 for follow-up) and treatment visits ($15). Participants randomized to VARCC-only were offered PE free of cost after their study participation was completed.
Treatments
Varenicline
Varenicline (Chantix) is an FDA-approved selective α4β2 neuronal nicotinic acetylcholine receptor (nAChR) partial agonist. The FDA-approved dosing regimen was employed as follows: Medication dose was titrated during week 0 with 0.5 mg per day for 3 days, followed by 0.5 mg twice daily for the next 4 days. On day 8, the medication was increased to 1.0 mg twice daily and maintained at this dose through day 84. Participants began the medication for 1 week prior to the target quit date and continued for a total of 12 weeks.
Medication Adherence
Medication adherence was calculated as the percent of the prescribed number of pills taken and verified by pill count and Timeline Follow Back Interview (TLFB; see description below). Medication adherence was excellent across conditions. The mean adherence rate (total amount taken/total amount prescribed) across 12 treatment weeks was 95%, with no significant difference across conditions (VARCC+PE: 94.5%; VARCC: 95.1%), t(22)=.66, p=0.52).
Smoking Cessation Counseling
Smoking cessation counseling was conducted weekly for participants in both conditions for 20–30 minutes for 12 visits and followed the medical management model used in Project COMBINE (Anton et al., 2006). Motivational interviewing was a core technique employed across all sessions. Smoking cessation interventions also included: monitoring progress (i.e., change in smoking status and medication effects), provision of support and encouragement, reinforcing the importance of abstinence, reviewing medication adherence and side effects, monitoring the level of psychological distress and the urge to smoke, and problem solving as needed. Therapists routinely reminded the participants to review and consult the “Clearing the Air” educational booklet for homework, tips on monitoring smoking urges, stimulus control, and alternative activities to engage in when experiencing the urge to smoke.
Prolonged Exposure (PE) Integrated with Smoking Cessation Counseling and Varenicline (VARCC+PE)
The 12 integrated VARCC+PE weekly sessions were 110–120 minutes long, consisting of 20–30 minutes of smoking cessation counseling (described above) followed by 90 minutes of PE. PE includes two main components: 1) imaginal exposure consisting of revisiting and recounting aloud the most upsetting traumatic memory, followed by processing of the revisiting experience; and 2) gradual in vivo exposure to safe but avoided trauma reminders. Participants were assigned homework weekly, which consisted of repeated listening to a recording of the trauma recounting made in each session, and repeated in-vivo exposure to safe situations avoided because of trauma-related distress (Foa, Hembree, & Rothbaum, 2007).
Treatment Fidelity
Fifteen percent of all therapy sessions were randomly selected, reviewed, and rated for treatment fidelity using the PE Therapist Adherence and Competency Rating Scale for PE, and the Adherence Competence Rating Scale for medication management counseling (used in other tobacco dependence treatment studies, e.g. Lerman et al., 2015). PE fidelity was 95%. The fidelity of smoking cessation counseling was 94%.
Measures
Smoking Abstinence
Smoking status was assessed using the TLFB and was verified by serum cotinine (COT ≤ 15ng/ml; Benowitz et al., 2002) and exhaled carbon monoxide (CO < 10; SRNT Subcommittee on Biochemical Verification, 2002) at post-treatment (3-months post-quit day) and follow-up (6-months post-quit day). The TLFB employs a calendar method to evaluate when and how many cigarettes the participant smoked over the past 30 days. Seven-day point prevalence abstinence (PPA) was defined as self-reported abstinence for 7 days prior to the assessment, serum cotinine level of <15ng/ml, and CO < 10 ppm. Consistent with recent recommendations, if neither CO nor cotinine levels were obtained due to participant absence or equipment malfunction to verify abstinence at an assessment, abstinence at that assessment was considered missing data.
Psychiatric Symptoms
The Structured Clinical Interview for DSM-IV (SCID) was used to confirm presence of PTSD, the absence of psychotic disorders and other Axis I substance disorder diagnoses, and to evaluate the presence of other Axis I disorders. The SCID is a semi-structured interview that yields current and lifetime DSM-IV Axis I diagnoses. The SCID has strong psychometrics, with joint inter-rater reliability coefficients for different disorders ranging from 0.60 to 0.83 (Lobbestael, Leurgans, & Arntz, 2011). PTSD symptom severity was assessed using the PTSD Symptom Scale, Interview Version (PSS-I), a 17-item clinical interview that evaluates DSM-IV PTSD symptoms on a frequency/severity scale (Foa, Riggs, Dancu, & Rothbaum, 1993). The PSS-I has a range of scores from 0 to 51, with higher scores reflecting more severe PTSD symptoms. The PSS-I has excellent internal consistency (α=.90), very good one-month test-retest reliability (r=.80), good inter-rater reliability (ICC=.073), and good convergent validity with the SCID-IV PTSD diagnosis (κ=.75) (Powers, Gillihan, Rosenfield, Jerud, & Foa, 2012). In the current study, the Cronbach’s α for the PSS-I was .66–.93 across the three assessment points. Depression severity was assessed using the 17-item version of Hamilton Depression Scale (HAM-D), scores ranging from 0 to 50, with higher scores reflecting more severe depression symptoms (Hamilton, 1960). The HAM-D has strong psychometric properties, including internal consistency (α=.79), inter-rater reliability (r=.94), and test-retest reliability (rs=.64–.98; Traković et al., 2011), as well as good convergent validity with other measures of depression (Bagby, Ryder, Schuller, & Marshall, 2004). In the current study, Cronbach’s α for the HAM-D was .75–.88 across the three assessment points.
Statistical Analyses
PPA was analyzed using GLMM (mixed effect model [MEM] with a logistic link function) because it was a dichotomous outcome. Our GLMM analyses were intent-to-treat analyses that included all subjects with at least one assessment, thereby increasing power and generalizability. Thus, these analyses align with the recommendations made by the Society for Research on Nicotine and Tobacco for analysis of smoking outcomes (Hall et al., 2001).
The analyses of PPA were coded to implement the GLMM/MEM equivalent of a 2 × 2 repeated-measures ANCOVA, with treatment condition (VARCC+PE vs. VARCC) and Time (post-treatment and follow-up) as the independent variables in the ANCOVA. We hypothesized 1) superior overall PPA in VARCC+PE compared to VARCC, and 2) the benefit of VARCC+PE over VARCC would be greater for participants with higher baseline PTSD severity. To test the latter hypothesis, we added the baseline PSS-I x Treatment interaction to the ANCOVA model. To control for other variables that might be related to baseline PSS-I and hence might be responsible for its effects, we included the following variables as covariates in all models: sex, age, education, minority ethnicity (non-Caucasian vs. Caucasian), cohabitation status (married/living together vs. living alone), employment (employed vs. unemployed), baseline cotinine level, and number of treatment sessions attended. Since these variables were included to control for third variables that might confound the relation between PSS-I and outcomes, we also included the interactions between them and treatment condition to control for their effects on the baseline PSS-I x Treatment interaction.
Analyses of the psychological outcomes (PSS-I and HAM-D) (hypothesis 3) were performed using a similar MEM design except the ANCOVA model was 2 × 3 (Treatment x Time, Time being coded as baseline, post-treatment, and follow-up) instead of 2 × 2. We expected a Treatment x Time interaction, with greater improvement from pre-treatment to post-treatment and to follow-up for those in VARCC+PE vs. VARCC.
Approximate effect sizes for t-statistics were calculated using the t to d conversion (Borenstein, 2009).
Results
Participant Characteristics
Baseline participant demographics are presented in Table 1. Age ranged from 18–75 years (M=42.7 years); 60.6% were male; 73.9% were Black/African American. Average baseline score on the PSS-I was 28.34 (SD=6.60), indicating moderately severe PTSD. Average baseline score on the HAM-D was 15.81 (SD=6.53), indicating mild depression. As expected, baseline PSS-I scores were highly related to baseline HAM-D scores, r=.63, p<.001. Mean cigarette use per day over the preceding week was 17.77 cigarettes (SD=9.43), and mean scores on the Fagerstrom Test of Nicotine Dependence were 5.93 (SD=1.99) indicating moderate levels of nicotine dependence. 55.6% of the sample (n=79) met criteria for current comorbid major depressive disorder; 34.51% (n=49) met criteria for a co-morbid anxiety disorder; 34.5% (n=49) met criteria for a current alcohol use disorder; and 43% (n=61) met criteria for a current other substance use disorder. In addition, most participants (97.9%) endorsed experiencing more than one traumatic event. These included: serious accidents (n=74), natural disasters (n=17), non-sexual assault by someone known to them (n=65), non-sexual assault by stranger (n=63), sexual assault by someone known to them (n=42), sexual assault by stranger (n=31), combat trauma (n=12), childhood sexual abuse (n=47), imprisonment (n=33), torture (n=12), life-threatening illness (n=17), and other traumatic events (n=55).
Table 1.
Demographic Characteristics.
| Treatment Condition | VARCC (n = 70) | VARCC+PE (n = 72) | Total Sample (n = 142) |
|---|---|---|---|
| Age (mean, SD) | 42.6 (10.2) | 42.8 (9.7) | 42.7 (9.9) |
| Gender (n, %) | |||
| Female | 30 (42.9) | 26 (36.1) | 56 (39.4) |
| Male | 40 (57.1) | 46 (63.9) | 86 (60.6) |
| Race/Ethnicity (n, %) | |||
| Black/African-American | 49 (70.0) | 56 (77.8) | 105 (73.9) |
| White/Caucasian | 17 (24.3) | 15 (20.8) | 32 (22.5) |
| Hispanic/Latino | 7 (10.0) | 3 (4.2) | 10 (7.0) |
| Native American | 2 (2.9) | 1 (1.4) | 3 (2.1) |
| Household Income/yr (n, %) | |||
| <$20,000 | 43 (61.4) | 46 (63.9) | 89 (62.8) |
| $20,000–$35,000 | 14 (20.0) | 17 (23.6) | 31 (21.8) |
| $35,001 – $50,000 | 6 (8.6) | 4 (5.6) | 10 (7.0) |
| $50,001–$75,000 | 3 (4.3) | 4 (5.6) | 7 (4.9) |
| >$75,000 | 2 (2.9) | 1 (1.4) | 3 (2.2) |
| Unknown | 2 (2.9) | 0 (0.0) | 2 (1.4) |
| Employment (n, %) | |||
| Full-time | 14 (20.0) | 10 (13.9) | 24 (16.9) |
| Part-time | 16 (22.9) | 15 (20.8) | 31 (21.8) |
| Retired or Unemployed | 38 (54.3) | 47 (65.3) | 85 (59.9) |
| Unknown | 2 (2.9) | 0 (0.0) | 2 (1.4) |
| Smoking indices (mean, SD) | |||
| Serum cotinine | 220.4 (122.3) | 221.7 (137.2) | 221.0 (129.5) |
| Carbon monoxide | 14.0 (5.6) | 13.7 (6.5) | 13.9 (6.1) |
| Average cigarettes/day | 18.1 (9.6) | 17.4 (9.3) | 17.8 (9.4) |
| Psychological indices (mean, SD) | |||
| PSS-I | 28.9 (7.0) | 27.8 (6.2) | 28.3 (6.6) |
| HAM-D | 16.1 (6.6) | 15.6 (6.5) | 15.8 (6.5) |
Note: VARCC, Varenicline and smoking cessation counseling; PE, Prolonged Exposure; PSS-I, PTSD Symptom Severity Inventory; HAM-D, Hamilton Depression Scale.
Figure 1 displays the CONSORT diagram. Fourteen participants had missing data at post-treatment. Seventeen were missing data at follow-up. Seventy-two participants were assigned to VARCC+PE and 70 to VARCC treatment. ANOVAs and Chi-square tests showed no differences between conditions on any of the baseline demographic or study variables (ps>.300), nor on the proportion of missing data at post-treatment or follow-up (ps>.780). Participants in VARCC+PE completed slightly, but not significantly (p=.124), fewer treatment sessions (M=8.3, SD=3.50) than those in VARCC (M=9.2, SD=3.71). Table 2 includes estimated model percentages/means for all outcomes at all three assessments. During the course of the study, there were 12 serious adverse events: 3 involved suicidal ideation (2 requiring hospitalization); 2 involved hospitalization secondary to drug or alcohol relapse; and the remaining serious adverse events involved hospitalizations secondary to unrelated medical problems (e.g., fractured ankle, renal disease, pneumonia, COPD).
Figure 1.
CONSORT flow diagram. SUD = substance use disorder. VARCC = Varenicline and smoking cessation counseling. PE = prolonged exposure therapy.
Table 2.
Model Estimated Means for Treatment Outcomes Over Course of Study.
| Baseline | Post-Treatment | Follow-up | |
|---|---|---|---|
|
|
|||
| 7-day PPA | |||
| VARCC+PE | 0.0% | 20.0% | 9.8% |
| VARCC | 0.0% | 6.0% | 1.8% |
|
| |||
| PSS-I | |||
| VARCC+PE | 27.5 | 11.5 | 10.8 |
| VARCC | 28.9 | 19.3 | 16.8 |
|
| |||
| HAM-D | |||
| VARCC+PE | 15.5 | 6.8 | 7.3 |
| VARCC | 16.1 | 12.3 | 11.8 |
Note: VARCC, Varenicline and smoking cessation counseling; PE, Prolonged Exposure; PSS-I, PTSD Symptom Severity Inventory; HAM-D, Hamilton Depression Scale; 7-day PPA = point prevalence abstinence in the past 7 days. *= significant difference between groups at p<.01. The treatment groups had the following sample sizes: VARCC+PE = 72, VARCC = 70. Post-treatment is 3-months post-quit day. Follow-up is 6-months post-quit day.
The Effect of Treatment on Smoking Outcomes
Our analysis for hypothesis 1 (lower PPA in VARCC+PE than in VARCC alone) showed that the Treatment x Time interaction was not significant, indicating that the Treatment main effect did not differ between post-treatment and follow-up (see Table 3 for results of this analysis). The analysis indicated that, although PPA was somewhat higher in VARCC+PE than in VARCC (20% vs. 6%), this difference was not significant for the average participant, b=1.58, 95% CI: [3.43, −0.26], t(193)=1.69, p=.092, d=.24. However, results supported our second hypothesis that baseline PTSD severity would moderate the effect of treatment on smoking outcomes. Specifically, the Treatment x baseline PSS-I interaction was significant, b=.15, 95% CI: [0.29, 0.01], t(176)=2.11, p=.036, d=.32. This interaction indicated that the benefit of VARCC+PE over VARCC increased as baseline PSS-I increased (see Figure 2). For example, although the abstinence rate for the average participant was 20% in VARCC+PE compared to 6% for those in VARCC, for participants who were 0.5 SD above the mean PSS-I at baseline (baseline PSS-I=32), abstinence rates for those in VARCC+PE were 22.8%, about 4 times higher than the 4.8% abstinence rate for those in VARCC-only.
Table 3.
Regression Coefficients for Treatment Outcomes.
| Abstinence (PPA) | PTSD Symptomsab | Depression Symptomsab | |
|---|---|---|---|
| Treatment Conditiona | 1.58+ | −4.26* | −3.52* |
| Time (Post-treatment vs. Follow-up)a | −1.05* | 77.32***a | 66.61***a |
| Treatment x Timea | .43 | 11.56***a | 9.88***a |
| Baseline PSSIb | −.03 | ||
| Treatment x Baseline PSS-Ib | .15* | ||
| Number of Sessions Attended | .34** | −.11 | −.20 |
| Gender (0=female, 1=male) | −.26 | .38 | −.30 |
| Cohabiting (0=no, 1=yes) | −.59 | 1.88 | .10 |
| Employed (0=no, 1=yes) | .70** | −.99 | 2.80+ |
| Minority (0=Caucasian, 1=not Caucasian) | −.33 | −.62 | −.94+ |
| Education Level | .45+ | .93 | .85 |
| Age | .60** | −.69 | .23 |
| Baseline Cotinine | .01 | .24 | .38 |
| Treatment x Number of Sessions Attended | −.33 | .60+ | .37 |
| Treatment x Gender | .50 | 1.75 | 1.68+ |
| Treatment x Cohabitating | 1.49 | 2.23 | .29 |
| Treatment x Employed | −.51 | −.57 | −.73 |
| Treatment x Minority | −.38 | .92 | .01 |
| Treatment x Education Level | −.42 | −.03 | −1.60 |
| Treatment x Age | −.65 | −.13 | 1.07 |
| Treatment x Baseline Cotinine | −.57 | .53 | .81 |
Significance codes:
≤0.001;
≤0.01;
≤ 0.05;
≤ .099.
All predictors were centered at their means. Thus, results shown are for the average participant, across conditions and Time. Coding for the dichotomous variables was the coding before centering at their means. Abstinence (PPA) was coded 1=abstinent, 0=not abstinent.
The analyses of PTSD symptoms and depression symptoms were ANCOVAs with Time having 3 levels. Thus, for these Time effects, there is no single regression coefficient for the effects in the ANCOVA. For these Time effects, we report the F statistic. For all other effects in these analyses of PTSD and depression symptoms, we report regression coefficients.
Our primary analyses for PTSD symptoms and depression symptoms did not include the moderating effects of baseline PSS-I.
Figure 2.
Verified 7-Day Point Prevalence Abstinence at Post-Treatment as a Function of Baseline PSS-I.
Note: VARCC = Varenicline and smoking cessation counseling; PE = Prolonged Exposure; PSS-I = PTSD Symptom Severity Inventory; 7-day PPA = point prevalence abstinence in the past 7 days.
Since our results indicated that VARCC+PE was more beneficial for participants with higher levels of baseline PSS-I, we determined the “region of significance” for the advantage of VARCC+PE over VARCC. That is, we empirically determined the levels of baseline PSS-I for which VARCC+PE significantly outperformed VARCC for PPA. To do so, we employed the Aiken and West (1991) centering technique, which uses the entire sample to estimate treatment condition differences in smoking outcomes at each level of baseline PSS-I. This analysis demonstrated that, for participants with baseline PSS-I above 31, those in VARCC+PE showed significantly higher abstinence than VARCC. Conversely, participants with lower levels of baseline PSS-I severity did not show significantly different outcomes between the two conditions. In addition, although Figure 2 does show a slight reversal of the benefit of VARCC+PE at very low levels of PSS-I, the Aiken and West (1991) analysis of estimated treatment condition differences showed that, even for participants with baseline PSS-I=15 (our inclusion criterion), VARCC was not significantly superior to VARCC+PE (difference=8.4%, p>.50), possibly due to our low power to detect small effect sizes.
Finally, our GLMM ANCOVA also showed that PPA decayed somewhat between the post-treatment assessment and the follow-up assessment, b=−1.05, 95% CI: [−191, −.19], t(187)=−2.41, p=.017, d=.35.
The Effect of Treatment on PTSD and Depressive Symptoms
While our analyses of PPA included only the assessments after the quit week, the analyses of the psychological outcomes (PSS-I and HAM-D) included assessments at pre-treatment as well as at post-treatment and at follow-up. We expected greater improvement over time in VARCC+PE vs. VARCC on both psychological outcomes. As noted in the Data Analysis section, these data were analyzed using a 2 × 3 ANCOVA (2 treatments x 3 time points), implemented with MEM to retain all subjects. To be consistent with the PPA analysis, we included the same covariates as in the GLMM analysis (see Table 3 for all the predictors in the models). The Treatment x Time interaction was significant for both PTSD and depressive symptoms, F(2,143)=11.56, p<.001 for PSS-I; F(2,113)=9.88, p<.001 for HAM-D, indicating greater improvement over time in VARCC+PE than in VARCC alone (see Table 2 for the means at each assessment). Contrasts indicated that participants receiving VARCC+PE, compared to those receiving VARCC, had significantly lower PSS-I and HAM-D at both post-treatment and follow-up (ps<.05).
In exploratory analyses, we investigated whether baseline PSS-I moderated the effect of treatment on the change in PSS-I and HAM-D over time. We added the baseline PSS-I x Treatment x Time interaction (and its subcomponents) to the 2 × 3 ANCOVA for PSS-I and HAM-D above. The baseline PSS-I x Treatment x Time interaction was significant for HAM-D, F(2,114)=3.47, p<.034, but not for PSS-I (p>.85). For HAM-D, the benefit of VARCC+PE compared to VARCC was greater for participants with higher baseline PSS-I. However, this triple interaction also meant that, for participants with low levels of baseline PSS-I (e.g., PSS-I=20), improvement in HAM-D over time was not greater in VARCC+PE than in VARCC alone (p>.50).
Finally, since gender and the gender x treatment condition interaction were included as covariates in all the analyses, we can report that neither the gender main effect nor the gender x treatment interaction was a significant predictor of any of the outcomes (Table 3).
Post-Hoc Analyses: Changes in PSS-I and HAM-D as Mediators of Treatment Effects on Smoking Outcomes
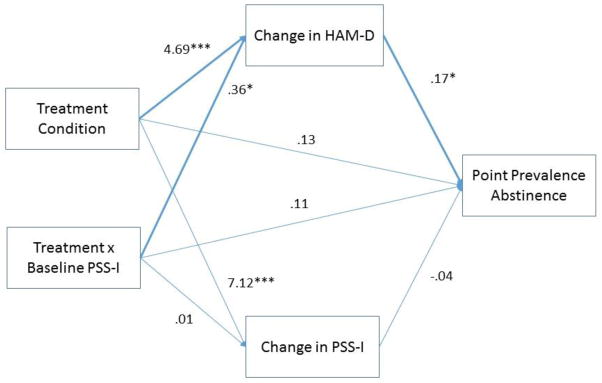
Given the findings of higher baseline PSS-I being a moderator of abstinence and depression symptoms, we decided to investigate whether the effects of Treatment Condition and the Treatment x baseline PSS-I interaction on PPA were mediated by changes in PTSD severity and depressive symptoms. Changes in PSS-I and HAM-D were both added as simultaneous predictors to the GLMM analyzing abstinence for hypotheses 1 and 2 (see Figure 3 for an illustration of the mediation model). The change in each mediator was calculated as the average of the change from baseline to post-treatment and the change from baseline to follow-up. Lower numbers indicated greater decreases in the mediator. The regression coefficients for changes in PSS-I and HAM-D in these analyses provided the “b” paths in the mediation analyses (see Figure 3). The “a” paths in the mediation analysis were calculated as the effect of Treatment Condition and the Treatment x baseline PSS-I interaction on the mediators. The significance of each mediated pathway was computed using RMediation (MacKinnon & Tofighi, 2013).
Figure 3.
HAM-D and PSS-I as Mediators of Abstinence.
Mediation analyses indicated that greater changes (decreases) in HAM-D were related to greater abstinence, b=.17, p=.044 (Figure 3), but changes in PSS-I were not related to abstinence. Since Treatment Condition and the Treatment x baseline PSS-I interaction were both significantly related to changes in HAM-D, b=4.69, p<.001 and b=2.37, p=.029, respectively (Figure 3), RMediation showed that the mediated pathway from Treatment Condition to changes in HAM-D to abstinence, and from Treatment x baseline PSS-I to changes in HAM-D to abstinence, were both significant, a*b=.797, 95% CI [.057, 1.72], and a*b=.061, 95% CI [.001, .158].
Discussion
The present study is the first large scale RCT to examine whether integrating evidence-based treatments for PTSD and smoking cessation have a greater impact on smoking and PTSD outcomes than smoking cessation alone, among smokers with PTSD. Accordingly, the study was designed to answer a very important clinical question: can we improve smoking cessation outcomes for smokers with PTSD (which are typically very poor) by treating their PTSD symptoms with an efficacious treatment for PTSD (PE) in combination with standard smoking cessation treatment (VARCC)? To this end, we examined two hypotheses regarding smoking outcomes: first, participants receiving the integrated treatment would report better smoking outcomes at post-treatment and at follow-up than would participants receiving smoking cessation alone. Second, the effects of integrated treatment on smoking outcomes would be moderated by baseline PTSD severity, such that individuals with high PTSD severity would show greater benefit from receiving the integrated treatment that included PE than individuals with low PTSD severity.
Contrary to our first hypothesis, the integrated treatment did not lead to lower PPA than smoking cessation alone for the average participant. However, consistent with our second hypothesis, individual baseline PTSD severity influenced the benefit of integrating PE with smoking cessation treatment. Specifically, VARCC+PE resulted in superior smoking outcomes than VARCC for individuals with higher baseline PTSD severity, but not for those with lower baseline PTSD severity. In fact, only about 5% of patients with a baseline PSS-I of 32 or above who received VARCC alone were abstinent at post-treatment, whereas almost 23% of those given VARCC+PE were abstinent at post-treatment. This represents more than a 450% increase in abstinence rates for those with higher baseline PTSD who received treatment for PTSD.
Several possible explanations for our findings regarding the second hypothesis can be entertained. Perhaps the most compelling explanation is that individuals with high PTSD severity also have high levels of negative affect, which interfere with smoking cessation (Kelly et al., 2015; Leventhal et al., 2011). Consequently, it is unlikely that a standard smoking cessation treatment alone will be successful for individuals with high PTSD severity. In contrast, when PE is integrated with smoking cessation treatment, this integrated therapy substantially reduces PTSD symptoms and the psychological problems associated with PTSD, such as negative affect or depression (Foa et al., 2005; Frye, 2015; Hartmann-Boyce et al., 2014; Rothbaum et al., 2005). It follows that the integrated therapy would reduce these psychological barriers to smoking cessation and thereby allows individuals with high PTSD severity to successfully quit smoking. Indeed, the results of the current study supported our third hypothesis that the integrated treatment would ameliorate psychological problems: VARCC+PE was associated with significantly greater reductions in PTSD and depressive symptoms than VARCC alone. Furthermore, our post-hoc moderation analyses revealed that those with higher levels of PTSD at baseline showed the greatest improvements in depression when receiving PE.
Post-hoc mediation analysis were consistent with the idea that decreases in depression symptoms, but not PTSD, partially mediated the superior abstinence rates observed in patients with higher PTSD who received PE. In particular, participants with higher baseline PTSD had greater reductions in depression symptoms in VARCC+PE than in VARCC alone, and those reductions in depression symptoms were related to improvements in abstinence. These results, along with the fact that higher baseline PTSD symptoms were correlated with higher baseline depression symptoms, are consistent with our proposed explanation for the mechanism by which VARCC+PE leads to improved PPA for individuals with high baseline PTSD severity. Specifically, individuals with high baseline levels of PTSD also have high levels of depression, which, in turn, impedes smoking cessation, and PE serves to reduce these barriers and to improve smoking outcomes in these individuals (Mahaffey et al., 2016). On the other hand, for individuals with low PTSD severity, depression is less likely to be prominent and thus may not comprise a significant barrier to a successful quit attempt. Further, in the present study, VARCC+PE did not seem to improve depression symptoms more than VARCC for individuals with low baseline PTSD severity. Thus, for these individuals, both the benefit of reducing PTSD (and co-occurring depressive symptoms) and the magnitude of the reduction in depression symptoms via PE might be limited for improving PPA. Future studies should include measures of these proposed mediators at frequent intervals throughout treatment to better understand the mechanisms underlying the effects of PE on individuals with higher baseline PTSD. This includes exploration into other possible mediators of smoking cessation (e.g., anxiety sensitivity; Smits et al., 2015). Since abstinence rates among individuals with lower baseline PTSD were relatively low in both the VARCC-only and VARCC+PE conditions, additional research is needed to better understand barriers to smoking cessation in this group of individuals with the ultimate goal of developing more effective treatments.
It should also be noted that there was an indirect (mediated) effect of the treatment condition main effect on PPA through depression symptoms, even though the effect of treatment condition on PPA was non-significant for the average participant. It is important to note that an independent variable (treatment condition) can have an indirect effect on an outcome (abstinence) through a mediator (depression symptoms), even though its overall effect on the outcome is not significant. This can result from many factors, such as other indirect or direct effects that counter the particular indirect (mediated) effect under investigation. In our case, many factors may impact abstinence for an individual with moderate PTSD severity, and while depression symptom severity has some effect on abstinence, it may not be a very important factor for those with moderate severity and may not account for a great deal of the variability in smoking cessation success for these individuals. However, for individuals with greater PTSD severity (and hence greater depression symptoms), depression symptom severity may be a more important factor in determining smoking cessation success.
The current study has several strengths. It employed gold standard measures of both PTSD and smoking severity, including biological measures of smoking (CO and cotinine). The study also utilized two treatments that have considerable evidence for their effectiveness. Further, both treatments were closely monitored and showed excellent adherence, and the sample size was large enough to interpret the present results with confidence. The sample examined in this study also consisted of a high proportion of minority participants, although there was less diversity in socioeconomic status and employment status. Future studies should aim to have greater variability in these demographic variables. In addition, it is important to note that the retention rate for post-treatment and follow-up assessments in both conditions was considerably higher than that reported in previous studies of comorbid smoking and PTSD (e.g., Gonzalez et al., 2016; McFall et al., 2010). Possible reasons for this high retention rate include significant and frequent contact with various study staff in both conditions, and appreciable participant payments for completion of each assessment and treatment session. However, these features are not particularly unique to this study and exist in many other studies examining such comorbid samples (e.g. Foa et al., 2013); therefore it is unclear what particular factors drove the high retention rate in the current study. It would be beneficial to more closely examine factors that were involved in the high retention in future studies in order to reduce the typically high dropout rates of participants in such treatment trials.
Several limitations should be noted: since our primary smoking outcome – PPA verified by CO/cotinine – was only assessed at two time-points (post-treatment and follow-up), we were unable to examine the causal interplay between smoking and psychological outcomes. Second, though the follow-up was 6-months post-quit day, it was only 3-months after the end of active treatment; longer follow-up periods are needed to evaluate the effects of integrated treatment on relapse. Third, medication adherence for varenicline was measured using pill count by the study nurse, and not with more advanced methods of medication adherence such as electronic, timestamped pill dispensers, which provide more valid indicators of medication adherence. Fourth, it is important to note that participants in the VARCC+PE group received weekly sessions that were 110–120 minutes long compared to those in the VARCC-only group who received weekly sessions that were only 20–30 minutes long. While the smoking cessation portion of interventions were of equal length across groups, the greater overall contact time with a therapist might have contributed to the better smoking outcomes observed for individuals with higher PTSD severity who received VARCC+PE; future studies should keep therapeutic contact time similar between groups. Fifth, since the current study employed PE experts in an academic setting to deliver the treatment, future research should replicate the current study in community-based clinics to determine the effectiveness of this combined treatment in non-academic settings. Finally, since the mediators in the mediation analysis were assessed at the same time point as the outcome, causality cannot be inferred.
Public Health significance.
The current study indicated that adding prolonged exposure therapy for PTSD to smoking cessation treatment for smokers with moderate to severe posttraumatic stress disorder, but not for smokers with low PTSD severity, significantly improved smoking abstinence up to three months following the end of treatment. These results serve as guidelines for therapists who treat individuals with comorbid nicotine dependence and PTSD.
Acknowledgments
This work was funded by National Institute on Drug Abuse grant awarded to Dr. Edna B. Foa (R01-DA023507-01A1). Dr. Foa developed Prolonged Exposure, a therapy used in this study. She receives royalties from the sale of Reclaiming your Life from a Traumatic Experience Workbook and Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide by Oxford University Press. Dr. Foa also receives payment for training workshops she conducts on Prolonged Exposure therapy. Drs. Asnaani, Rosenfield, Zandberg, Gariti, and Ms. Imms report no competing interests.
References
- Acierno RA, Kilpatrick DG, Resnick HS, Saunders BE, Best CL. Violent assault, posttraumatic stress disorder, and depression: Risk factors for cigarette use among adult women. Behavior Modification. 1996;20(4):363–384. doi: 10.1177/01454455960204001. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, … Longabaugh R. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: Has the gold standard become a lead weight? The American Journal of Psychiatry. 2004;161(12):2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Peterson J, Whitfield E, Min S, Benson SL, Maddox TM, Prochazka AV. Integrating motivational interviewing into a home telehealth program for veterans with posttraumatic stress disorder who smoke: A randomized controlled trial. Journal of Clinical Psychology. 2016;72(3):194–206. doi: 10.1002/jclp.22252. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Peyton J, Ahijeyych K, Jarvis MJ, Hall S, Lehouezec J, … Velicer W. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Borenstein M. Effect sizes for continuous data. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta analysis. New York: Russell Sage Foundation; 2009. pp. 221–237. [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: An overview and network meta-analysis. The Cochrane Database of Systematic Reviews. 2013;5(5) doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Kimbrel NA, Dedert EA, Beckham JC, Dennis MF, Calhoun PS. Supplemental nicotine preloading for smoking cessation in posttraumatic stress disorder: Results from a randomized controlled trial. Addictive Behaviors. 2016;59:24–29. doi: 10.1016/j.addbeh.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clinical Psychology Review. 2006;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Smith RC, Monson CM, Zvolensky MJ. Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent, multiple-baseline design. Behavior Therapy. 2013;44(3):514–528. doi: 10.1016/j.beth.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. Journal of Consulting and Clinical Psychology. 1999;67(2):194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences: Therapist guide. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr, Oslin D, … Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA. 2013;310(5):488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Frye M. Varenicline for bipolar depression with comorbid nicotine dependence. Bipolar Disorders. 2015;17:38–39. [Google Scholar]
- Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depression and Anxiety. 2012;29(9):797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LE, Zvolensky MJ. A comparison of self-guided smoking cessation outcomes between those with and without PTSD. Poster presented at 22nd International Society for Traumatic Stress Studies; Los Angeles, CA. 2006. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB … Varenicline Phase 3 Study Group. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Friedberg F, Li X, Zvolensky MJ, Bromet EJ, Mahaffey BL, … Kotov R. Trauma-focused smoking cessation for smokers exposed to the World Trade Center Disaster: A randomized clinical trial. Nicotine and Tobacco Research. 2016:ntw384. doi: 10.1093/ntr/ntw384. [DOI] [PubMed] [Google Scholar]
- Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine and Tobacco Research. 2001;3(3):193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurological and Neurosurgical Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2013 reviews. Addiction. 2014;109(9):1414–1425. doi: 10.1111/add.12633. [DOI] [PubMed] [Google Scholar]
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic posttraumatic stress disorder. Journal of Clinical Psychopharmacology. 2001;21(1):94–98. doi: 10.1097/00004714-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE … Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Jensen KP, Sofuoglu M. Co-occurring tobacco use and posttraumatic stress disorder: Smoking cessation treatment implications. The American Journal on Addictions. 2015;24(8):695–704. doi: 10.1111/ajad.12304. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Sido H, Forsyth JP, Ziedonis DM, Kalman D, Cooney JL. Acceptance and commitment therapy smoking cessation treatment for veterans with posttraumatic stress disorder: A pilot study. Journal of Dual Diagnosis. 2015;11(1):50–55. doi: 10.1080/15504263.2014.992201. [DOI] [PubMed] [Google Scholar]
- Kralikova E, Kmetova A, Stepankova L, Zvolska K, Davis R, West R. Fifty-two week continuous abstinence rates of smokers being treated with varenicline versus nicotine replacement therapy. Addiction. 2013;108(8):1497–1502. doi: 10.1111/add.12219. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, … Tyndale RF. A randomized placebo-controlled trial to test a genetically-informed biomarker for personalizing treatment for tobacco dependence. The Lancet Respiratory Medicine. 2015;3(2):131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ, Schmidt NB. Smoking-related correlates of depressive symptom dimensions in treatment-seeking smokers. Nicotine & Tobacco Research. 2011;13(8):668–676. doi: 10.1093/ntr/ntr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV axis I disorders (SCID I) and axis II disorders (SCID II) Clinical Psychology and Psychotherapy. 2011;18(1):75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Tofighi D. Statistical mediation analysis. In: Schinka JA, Velicer WF, Weiner IB, Schinka JA, Velicer WF, Weiner IB, editors. Handbook of psychology: Research methods in psychology. 2. Vol. 2. Hoboken, NJ, US: John Wiley & Sons Inc; 2013. pp. 717–735. [Google Scholar]
- Mahaffey BL, Gonzalez A, Farris SG, Zvolensky MJ, Bromet EJ, Luft BJ, Kotov R. Smoking to regulate negative affect: Disentangling the relationship between posttraumatic stress and emotional disorder symptoms, nicotine dependence, and cessation-related problems. Nicotine & Tobacco Research. 2016;18(6):1471–1478. doi: 10.1093/ntr/ntv175. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte CA, Chow B, Bailey S, Baker DG, … Smith MW. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. JAMA. 2010;304(22):2485–2493. doi: 10.1001/jama.2010.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, … Steele B. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162(7):1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Kochunov P, Hong LE. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophrenia Bulletin. 2013;39(6):1373–1381. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: Results from a 7-week, randomized, placebo-and-bupropion-controlled trial with 1-year follow-up. Archives of Internal Medicine. 2006;166(15):1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Powers MB, Gillihan SJ, Rosenfield D, Jerud AB, Foa EB. Reliability and validity of the PDS and PSS-I among participants with PTSD and alcohol dependence. Journal of Anxiety Disorders. 2012;26(5):617–623. doi: 10.1016/j.janxdis.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30(6):635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Astin MC, Marsteller F. Prolonged exposure versus eye movement desensitization and reprocessing (EMDR) for PTSD rape victims. Journal of Traumatic Stress. 2005;18(6):607–616. doi: 10.1002/jts.20069. [DOI] [PubMed] [Google Scholar]
- Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. Journal of Traumatic Stress. 2013;26(3):299–309. doi: 10.1002/jts.21814. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stewart TC. Doctoral dissertation. Yeshiva University; 2014. Nicotine and PTSD symptom duration. [Google Scholar]
- Smits JA, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, … Brown RA. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosomatic Medicine. 2015;78(3):354–364. doi: 10.1097/PSY.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J. Reliability of the Hamilton rating scale for depression: A meta-analysis over a period of 49 years. Psychiatry Research. 2011;189(1):1–9. doi: 10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- U.S. Public Health Service. A clinical practice guideline for treating tobacco use and dependence 2008 update: A U.S. Public Health Service Report. American Journal of Preventative Medicine. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]