Abstract
Background
The Duffy Antigen Receptor for Chemokines (DARC) is an atypical receptor that regulates pro-inflammatory cytokines. However, the role of DARC in asthma pathophysiology is unknown.
Objective
To determine the role of DARC in allergic airways disease in mice, and the association between DARC single nucleotide polymorphisms (SNPs) and clinical outcomes in patients with asthma.
Methods
Mice with targeted disruption of the Darc gene (DarcΔE2) or WT mice were challenged over three weeks with house dust mite (HDM) antigen. Allergic airways disease was assessed 24 hours and 7 days following the final challenge. Additionally, associations between DARC SNPs and clinical outcomes were analyzed in a cohort of poorly-controlled asthmatics.
Results
Total airway inflammation following HDM did not differ between DarcΔE2 and WT mice. At 24 hours, DarcΔE2 mice had increased airway hyperresponsiveness; however, at 7 days airway hyperresponsiveness had completely resolved in DarcΔE2 but persisted in WT mice. In poorly-controlled asthmatics, DARC SNPs were associated with worse asthma control at randomization and subsequent increased risk of healthcare utilization (odds ratio 3.13(1.37–7.27), p=0.0062).
Conclusions and Clinical Relevance
Our animal model and human patient data suggest a novel role for DARC in the temporal regulation in asthma pathophysiology and symptoms.
Keywords: Duffy Antigen Receptor for Chemokines, Airway Hyperresponsiveness, Asthma
Introduction
The chronic inflammatory environment in asthma is characterized by chemokines interacting with G-protein-coupled receptors on target leukocytes (1). However, four atypical chemokine receptors have been described which lack the structural components to induce downstream signaling (2) and are thought to regulate inflammatory responses by acting as chemokine decoys or scavengers. One such atypical receptor is the Duffy Antigen Receptor for Chemokines (DARC) which strongly binds many inflammatory chemokines of both the CXC and CC subfamilies (3). Highlighting the importance of DARC to cytokine homeostasis, a single nucleotide polymorphism (SNP) in DARC accounts for 20% of the variability in serum CCL2 in humans (4). Despite the substantial role of DARC as a promiscuous regulator of inflammatory chemokines, its role in asthma is largely unknown.
A role for DARC in asthma pathophysiology was suggested by the association of a SNP in DARC with asthma prevalence in African descendants (5). Although any causal link has yet to be determined, DARC is expressed on several cells relevant to asthma such as erythrocytes, lung endothelial cells, alveolar epithelial cells (6) and airway smooth muscle (ASM) cells (7). Erythrocyte DARC internalizes circulating cytokines which prevents the interaction between cytokines and leukocytes (8). In contrast, endothelial DARC promotes chemokine transcytosis and subsequent leukocyte migration into organs (9). Although the function of DARC in airway epithelial cells is presently unclear, Darc expression is upregulated in airway epithelium in response to acute airway inflammation (10). Global ablation of Darc in mice reduced airway neutrophilia during acute lung injury (11–13); however, whether DARC influences the severity of asthma, and by what mechanisms, has yet to be determined.
Due to the role of DARC in pro-inflammatory chemokine regulation and the contribution of other decoy receptors to resolution of inflammation (14), we hypothesized that a loss of Darc function would exaggerate severe allergic airways disease and prolong resolution of pathophysiology. Therefore we determined, in a mouse model of allergic airways disease, the effect of genetic deletion of DARC on the severity and resolution of asthma pathophysiology. In order to establish the clinical translatability of our findings to human asthma we determined whether DARC single nucleotide polymorphisms (SNPs) were associated with clinical outcomes in a clinical trial of patients with severe asthma.
Materials and Methods
Animals and animal study design
Mice in which a 90bp region of the Darc gene locus had been eliminated from exon II and bred on a C57Bl\6J background (Darc ΔE2 mice) (15) were maintained as a breeding colony under pathogen-free conditions. Deletion of the 90kb region leads to loss of normal DARC mRNA expression and a substantially reduced affinity of erythrocytes to CXCL8 and CCL2 (15). Wild-type C57Bl\6J mice (WT) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were studied at eight weeks of age and all experiments were approved by the Institutional Animal Care and Use Committee at the University of Vermont (#11-029).
House dust mite (HDM) antigen administration
Mice were administered 50ug of intranasal HDM (Greer Laboratories, Lenoir, NC, USA) in sterile PBS (1 mg protein/mL) or PBS alone on 15 occasions over three weeks (Figure 1A). Mice were evaluated 24 hours or 7 days following the final instillation. Airway mechanics were assessed by the forced oscillation technique as Newtonian resistance, a measure of airway resistance, tissue damping, a measure of tissue resistance, and tissue elastance, a measure of lung stiffness (13). Lungs were lavaged with 1.0ml of PBS and centrifuged at 1200xg for 5 minutes to isolate cells. The pellet was re-suspended for total and differential cell counts and the supernatant used for quantification of cytokines. Lung samples were taken for protein lysates, RNA extraction and analysis of collagen content. Additional methodological details are provided in the Online Supplement.
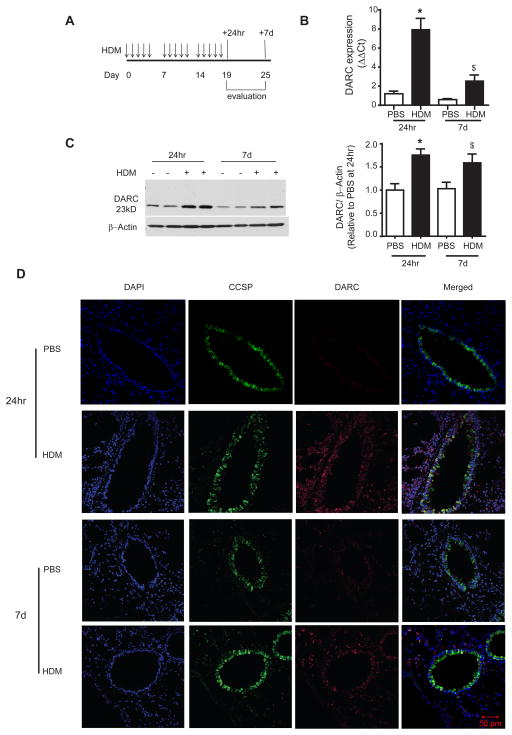
Figure 1.
Increase in DARC expression in alveolar epithelial cells in wild type mice 24 hours and 7 days following house dust mite (HDM) challenge. (A) Mice were challenged with 15 intranasal instillations of HDM over three weeks and evaluated at 24 hours and 7 days following the final instillation. DARC (B) mRNA and (C) protein expression was increased in lung homogenates at both 24 hours and 7 days. Lung tissue sections were stained using an antibody for DARC (red) and Club Cell Secretory Protein (CCSP, green), as a marker of airway epithelial cell co-localization. Data are presented as mean ± SEM of 8–9 mice/group. ANOVA with Tukey post-hoc comparisons: * p < 0.05 and $ p ≤ 0.08 vs respective PBS.
Human genomic analyses
Eleven DARC SNPs (5–15% frequency) were identified in the CEU cohort of the 1000 Genomes dataset (16) available at www.1000genomes.org (10 of which were present in our sample). A twelfth SNP, rs12075, was included due to its association with circulating pro-inflammatory cytokines (4). Tag SNPs were interrogated by TaqMan genotyping in 169 poorly controlled White asthmatic patients who participated in the LODO ALA-ACRC clinical trial (17). See Online Supplement for a description of the study population, prevalence of the analyzed SNPs in the current population (Table E1) and schematic of the study design (Figure E1). Clinical severity was assessed as contact with healthcare providers due to asthma (total contacts), unscheduled visits to asthma clinics and asthma control (ACQ (18) and Asthma Symptom Utility Index, ASUI (19)). The study was approved by the Western Institutional Review Board and local IRB approval was obtained from all individual sites. Written informed consent was obtained from all patients.
Statistical analysis
Animal data were evaluated using two- or three-way ANOVA (AHR data) with Tukey post-hoc comparisons or Kruskal-Wallis with Dunn post-hoc comparisons. Data are presented as mean ± SEM or median ± IQR and p values < 0.05 were regarded as statistically significant (JMP® Pro 10, SAS Institute Inc., Cary, NC, USA). Associations between DARC SNPs and continuous clinical outcomes were analyzed by general linear modeling assuming an additive model, controlling for age, BMI, and gender (SPSS release 22.0.0.1). Reported means are inverse Box-Cox transformed (95% confidence intervals). Associations between SNPs and incident phenotypes were analyzed by Fisher’s Exact test assuming a dominant model, coding individuals who experienced one or more incidents as 1. Analyses were stratified by race, and where required, by treatment. The Bonferroni corrected p-value for significance was 0.0045–0.0063 depending on the number of SNPs included in each analysis.
Results
Increased DARC expression in lung epithelium in mice with allergic airways disease
Darc mRNA (Figure 1B) and protein expression (Figure 1C) in lung homogenates was significantly increased at 24 hours following HDM challenge. The increased levels of both tended to persist at 7 days (p = 0.08 and 0.06, respectively). Although the increase in mRNA due to HDM challenge was reduced at 7 days compared to 24 hours (p = 0.001) there was no difference in protein expression between time points (p = 0.52). Immunofluorescence staining indicated that the increase in DARC was partly explained by increased expression in the airway epithelium, as evidenced by co-localization of DARC and Club Cell Secretory Protein (CCSP, Figure 1D).
DARC promotes the resolution of HDM-induced neutrophilic airway inflammation
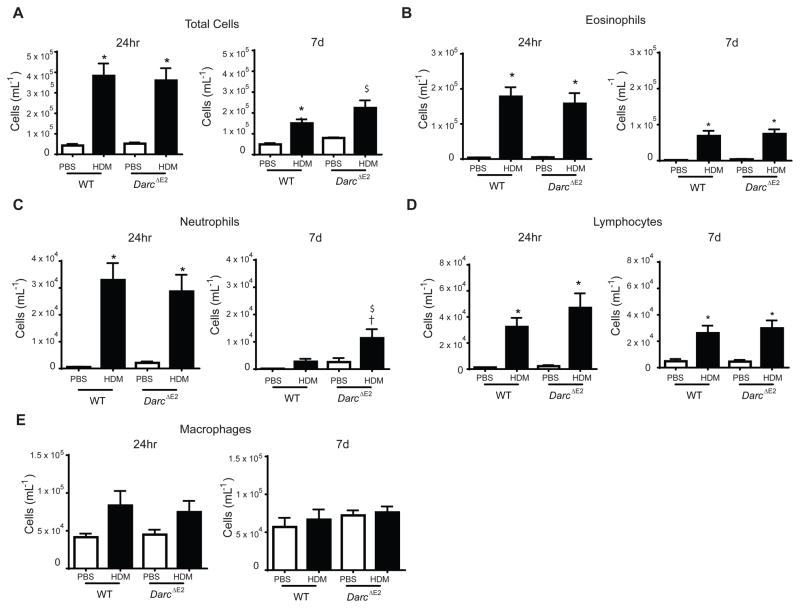
The increase in total leukocytes in BALF following HDM challenge did not differ between WT and DarcΔE2 mice at either 24 hours or 7 days (Figure 2A). Similarly, the increase in individual cell differentials following HDM challenge at either time-point did not differ between WT and DarcΔE2. The exception was neutrophil levels at 7 days which were increased in Darc ΔE2 compared to WT mice suggesting that DARC contributes to the resolution of neutrophilic inflammation.
Figure 2.
Airway inflammation measured in bronchoalveolar lavage from WT and DarcΔE2 evaluated 24 hours and 7 days following house dust mite challenge. Shown are (A) total cell counts, (B) eosinophils, (C) neutrophils, (D) lymphocytes and (E) macrophages. Data are presented as mean ± SEM of 7–10/group except for DarcΔE2 PBS at 7days (n = 4). ANOVA with Tukey post-hoc comparisons: * p < 0.05 and $ p = 0.06 vs respective PBS, † p < 0.05 WT HDM vs DarcΔE2 HDM.
To determine the effect of Darc ablation on the immune phenotype we measured gene expression of Th1, Th2 and Th17 associated cytokines in lung samples (Figure E2). Il-4 and Il-13 were increased by HDM but were not different between Darc ΔE2and WT mice. Similarly, Il-17A did not differ between Darc ΔE2and WT mice. The Th1-associated cytokine interferon-γ did was not altered by HDM in either group. These findings suggest a Th2 immune phenotype that was unaltered by DARC.
To determine the effect of DARC on chemokines levels during allergic airways disease we measured chemokines in BAL which encompassed high (CCL11, CXCL1, CCL2, CCL5,), moderate (CXCL10, CXCL9) and low (CCL3, CCL4) DARC affinity (3, 20). Although HDM increased BAL levels of CXCL10, CCL2 and CXCL9 at 24 hours these did not differ between WT and DarcΔE2 mice (Figure E3A). In contrast, there were trends for increased CXCL1, CXCL2 and CCL5 at 24 hours in DarcΔE2 mice; however, increased levels were also observed for CXCL1, CXCL2 and CCL2 in DarcΔE2 PBS mice relative to WT mice (~2 fold). Indeed, the significant genotype factor but non-significant interaction factor suggests that the increase in cytokines in DarcΔE2 mice was a consequence of increased baseline levels rather than an exaggerated response to HDM. These differences in cytokine levels were no longer apparent at 7 days, except a small increase in CXCL10 in HDM-challenged DarcΔE2 mice (Figure E3B).
DARC regulates the severity and resolution of HDM-induced airway hyperresponsiveness
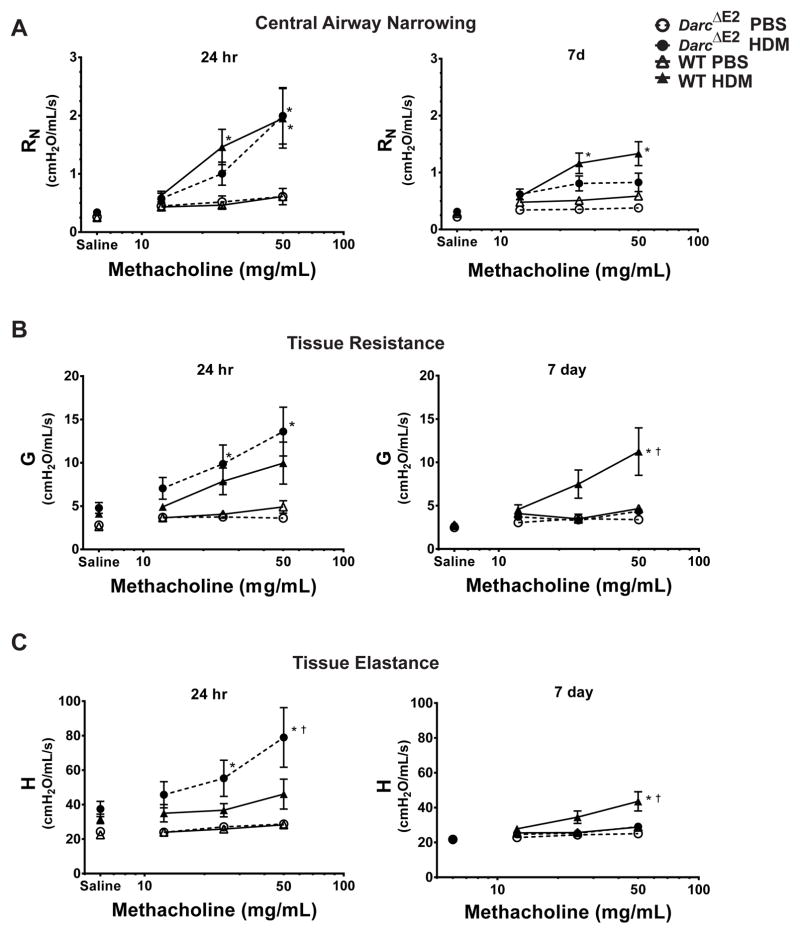
We next determined the role of DARC in the severity and resolution of AHR (Figure 3). Baseline respiratory mechanics after vehicle control (saline) were similar between WT and DarcΔE2 mice. At 24 hours following HDM, AHR measured by central airway resistance and tissue resistance did not differ between Darc ΔE2 and WT mice (Figure 3A and B). However, AHR as measured by tissue elastance was increased in HDM-challenged Darc ΔE2 mice compared to WT mice (Figure 3C). At 7 days, AHR measured by central airway resistance tended to be reduced in HDM-challenged Darc ΔE2 mice compared to WT mice, although this did not reach statistical significance (Figure 3A). However, AHR measured by both tissue resistance and tissue elastance was significantly reduced in Darc ΔE2 compared to WT mice (Figure 3B and C). Taken together, the presence of DARC reduced AHR during peak inflammation but maintained AHR despite resolution of inflammation.
Figure 3.
DARC regulates the severity and resolution of airway hyperresponsiveness. Respiratory mechanics were measured by the forced oscillation technique following administration of nebulized PBS control and three doses of methacholine. Respiratory impedance was partitioned into measures of (A) Newtonian resistance (Rn), a measure of central airway resistance, (B) tissue dampening (G), a measure of tissue resistance, and (C) tissue elastance (H), a measure of the stiffness of the lung. The response to methacholine at each dose was quantified as the average of the three peak measurements for each parameter. Data are presented as mean ± SEM of 7–10/group except for Darc ΔE2 PBS at 7days (n = 4). ANOVA with Tukey post-hoc comparisons: * p < 0.05 vs genotype PBS, † p < 0.05 WT HDM vs Darc ΔE2 HDM.
DARC does not alter HDM-induced airway remodeling
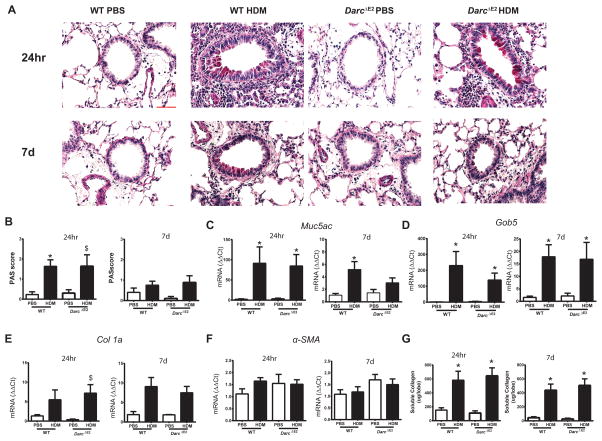
Mucous hyperplasia following HDM challenge was similar between WT and Darc ΔE2 mice measured histologically (Figure 4A and B) and by evaluation of Muc5ac and Gob5 mRNA (Figure 4C and D). The increase in soluble collagen and Collagen 1 mRNA following HDM challenge also did not differ between WT and DarcΔE2 mice at either time-point (Figure 4E and G). There was no effect of HDM on airway smooth muscle, as measured by α-smooth muscle actin mRNA, in either WT or DarcΔE2 mice (Figure 4F). Taken together these findings suggest that DARC does not alter airway remodeling in allergic airways disease.
Figure 4.
Airway remodeling in WT and Darc ΔE2 evaluated 24 hours and 7 days following house dust mite challenge. (A) Periodic Acid-Schiff staining of airway mucus (magnification: 40x, red scale bar = 50μm) and (B) quantification of staining intensity determined by two blinded investigators. Quantification of mRNA levels of (C) Muc5ac, (D) Gob5, (E) Collagen 1 and (F) α-smooth muscle actin, α–SMA (G) in lung tissue homogenates by q-PCR. Results are presented as fold change compared to PBS controls. (G) Soluble collagen measured by the Sircol assay. Data are presented as mean ± SEM of 7–10/group except for DarcΔE2 PBS at 7days (n = 4). Collagen 1A, Muc5AC, Gob5 and α-smooth muscle actin mRNA data were log transformed for statistical analyses. ANOVA with Tukey post-hoc comparisons: * p < 0.05 vs respective PBS, $ p ≤ 0.08 vs respective PBS.
DARC SNPs are associated with symptoms and healthcare utilization in patients with asthma
The potential clinical relevance of our in vivo animal findings were explored by analyzing the associations between twelve common (5–15% frequency) DARC SNPs and clinical outcomes in patients with asthma, stratified by race (Table 1 and E2). In poorly controlled White asthmatics, rs12042349 was associated with worse asthma control at randomization as measured by ACQ (major homozygote (MM) = 3.14 (2.99–3.30), heterozygote (mM) = 3.75 (3.36–4.22), p=0.0023) with a trend towards an association with ASUI (MM=0.75 (0.72–0.77), Mm=0.67 (0.57–0.71), p=0.0062). After 24 weeks of treatment, Whites with at least one copy of rs35333710 tended to have an increased risk of requiring contact with a healthcare provider for asthma (odds ratio 3.13 (1.37–7.27), p=0.0062). When the association with rs35333710 was stratified by treatment, the odds ratios for requiring contact with a healthcare provider for asthma were 4.67(0.93–23.37), p=0.061, 2.34(0.58–9.50), p=0.34, and 2.71(0.64–11.53), p=0.20 for placebo, montelukast, and theophylline treatments, respectively.
Table 1.
Associations of single nucleotide polymorphisms in DARC with clinical outcomes in White asthmatics
| LODO (n = 169) | |||
|---|---|---|---|
| SNP | ASUI randomization (V2) # | ACQ randomization (V2) # | Sum Total contacts at 24 wks (V6) |
| rs41313908 | 0.93 | 0.068 | 1.0 |
| rs35333710 | 0.92 | 0.69 | 0.0062 |
| rs140772227 | 0.79 | 0.97 | 0.55 |
| rs3027016 | 0.23 | 0.11 | 0.59 |
| rs34599082 | 0.31 | 0.73 | 0.41 |
| rs12075 | 0.42 | 0.037 | 0.029 |
| rs36007769 | 0.53 | 0.74 | 0.27 |
| rs12042349 | 0.0062 | 0.0023* | 0.42 |
| rs111444457 | NA | NA | NA |
| rs139415758 | 0.77 | 0.31 | 1.0 |
| rs2073090 | 0.0050 | 0.044 | 0.45 |
Corrected p-value below requirement for significance (0.0045–0.0063 depending on number of SNPs analyzed).
Data non-normally distributed and Box-Cox transformed.
Contacts = contact, whether in person or other means, with healthcare providers due to asthma, ACQ = Asthma control questionnaire, LODO = Low Dose theophylline vs montelukast study (17)
Discussion
By combining an animal model and human genetic data we herein report the novel role for the Duffy Antigen Receptor for Chemokines (DARC) in asthma pathophysiology. Our animal model highlights the temporally complex interaction between DARC and asthma; DARC reduced AHR and cytokine levels during the height of airway inflammation but promoted the persistence of AHR during the resolution phase. Consistent with the effect of DARC in the animal model, DARC SNPs were associated with worse asthma control and healthcare utilization in poorly-controlled asthmatics. Interestingly, these associations were only evident in patients on ICS and/or LABA, highlighting the novel potential effect of DARC genotype on treatment response.
Our human data reveals a complex and potentially important role for genetic alterations of DARC in the manifestation of asthma symptoms. In White poorly controlled asthmatics, ~75% of which were prescribed ICS and LABA, rs12042349 was related to increased asthma symptoms at randomization. Importantly, the magnitude of the increase in ACQ associated with having one copy of rs12042349 was greater than the minimal clinically important difference (21). Similarly, increased asthma symptoms as assessed by total contacts with healthcare providers due to asthma was associated with rs35333710 in those patients continuing on their pre-study treatment (ICS or ICS+LABA). In contrast, there was no association in patients on additional montelukast or theophylline. Taken together, these findings may reveal a novel interaction between select DARC SNPs and the efficacy of ICS and/or LABA in controlling asthma symptoms. However, further research is required to verify this interaction and determine whether non-ICS treatments confer particular benefits in patients with specific DARC SNPs.
The present findings strengthen the role of DARC as a promiscuous, but specific, scavenger of inflammatory chemokines. Our findings of increased DARC mRNA and protein expression during allergic airways disease are consistent with the increased DARC gene expression in bronchial epithelial cells of steroid-naïve asthmatics compared to non-asthmatics (Gene Expression Omnibus repository number GSE23611 www.ncbi.nlm.nih.gov/projects/geo (22)). This suggests that DARC upregulation may be a protective mechanism to maintain chemokine homeostasis during allergic airways disease. Consistent with findings in models of acute lung injury (10, 13, 23, 24), loss of DARC activity in our murine model was associated with increased levels of CXCL1, CXCL2, CCL2 and CL5 at 24 hours; all known to have high affinity for DARC in humans (3) and mice (20). This suggests that loss of DARC function likely alters chemokine homeostasis which promotes an exaggerated chemokine response during allergen challenge.
Surprisingly, the effect of DARC on pro-inflammatory cytokines did not translate into differences in overall airway inflammation. However, this lack of effect of DARC status on total inflammation is consistent with findings in patients with acute lung injury (23) and endotoxemia (25). There are conflicting reports from animal models as to the effect of DARC on airway inflammation following acute lung injury with both increased (15, 24) and decreased (10, 11) airway neutrophilia. Subtle differences in time points exist between these studies and conflicting findings may reflect a role for DARC in the resolution of airway neutrophilia, as reported in the present study. Our findings suggest a complex temporal interaction between DARC, chemokines and neutrophil recruitment. At 24 hours, chemokines associated with neutrophil recruitment were increased in BAL from mice lacking DARC and we speculate that this translated into the delayed resolution of airway neutrophils at 7 days. It is likely that in WT mice, airway epithelial DARC functions to sequester circulating chemokines thereby reducing the signal for neutrophil migration. Without this function of DARC, airway epithelial cells are unable to act as a “chemokine sink” and thus neutrophil migration into the airways is extended ie delayed resolution. Given DARC’s role in regulating airway neutrophilia, and it may be that DARC plays a more substantial role in allergic airways disease characterized by exaggerated neutrophilia, such as that seen with transfer of TH17 cells (26). These neutrophilic models are refractory to corticosteroids and may contribute further mechanistic insight into the association between DARC SNPs and asthma symptoms in asthmatics who were poorly controlled despite treatment. Alternatively, loss of DARC function may simply equate to reduced competition binding which allows increased interaction of cytokines with other receptors. CXCR2 binds CXCL1 and CXCL2, mediating the activation and migration of neutrophils into the airways (27). Despite no change in CXCR2 mRNA in DarcΔE2 lung homogenates in the present study (data not shown), it is possible that reduced receptor competition led to increased chemokine binding with CXCR2 and thus delayed resolution of neutrophilia.
Our animal model provides important clues as to the mechanisms underlying the association between DARC SNPs and asthma symptoms in humans. Firstly, DARC is unlikely to affect asthma severity through effects on airway inflammation or immune phenotype. Indeed, the effect on neutrophils was rather small and conflicts with previous findings suggesting that neutrophils promote AHR (28, 29). Although recent in vitro findings suggest that loss of DARC promotes CXCL1-induced ASM proliferation (7) we did not detect an effect of DARC on α-smooth muscle actin mRNA or any other markers of airway remodeling. Although we cannot rule out that the relatively short exposure period (three weeks) may be insufficient to detect an effect of DARC on airway remodeling, we were able to detect an effect of AHR at this time-point. Furthermore, a lack of effect on airway remodeling is consistent with findings in other diseases associated with structural remodeling (30, 31). In contrast, the loss of DARC function led to more severe AHR during peak allergen responses suggesting DARC may contribute to worse bronchoconstriction following allergen exposure. This is consistent with increased symptoms, assessed by questionnaire and healthcare utilisation due to asthma, in patients with DARC SNPs. Although the exact mechanisms by which DARC alters AHR remain unknown, it is possible that they are mediated by increased chemokine levels. For example, CXCL1 has been shown to induce airway smooth muscle contraction (32) and promote mast cell recruitment to airway smooth muscle (33), both of which could increase the extent of bronchoconstriction. However, further research is required to determine whether the loss of DARC alters airway smooth muscle function or contributes to AHR and asthma symptoms via other mechanisms.
The present study uniquely combines a murine model of allergic airways disease and a long-term clinical trial of severe asthmatics to examine the potential role of DARC in the temporal manifestation of asthma. Nonetheless, the present study does have some limitations. Firstly, the LODO population was predominantly White and therefore underpowered to determine associations between clinical outcomes and DARC SNPs in Blacks. Therefore findings cannot be generalized to Blacks in which DARC is known to be associated with asthma prevalence (5) and subsequent analyzes specifically in this population are needed to determine the relevance of DARC SNPs on asthma symptoms in Blacks. As such, we did not include the predominant DARC SNP in Blacks, rs2814778, as its frequency in our population was 0–0.5% in Whites and 70–80% in Blacks. However, rs2814778 completely ablates erythrocyte DARC with no effect on DARC on other cells, such as endothelial and epithelial cells. Therefore our mouse model, in which Darc was ablated in all cell types, is less applicable to the effect of rs2814778 in Blacks. In contrast, rs12075 in European descendants causes a 50% minor allele frequency for each of the two principal antigens, Fya and Fyb. This SNP alters the level of DARC activity on erythrocytes and non-erythrocyte cells uniformly to produce three phenotypes of DARC activity; DARC which binds chemokines with “high activity”, “moderate activity” (heterozygous) or “low activity”. Thus the global ablation of Darc in mice would seem to be most analogous to the “low activity” phenotype in Whites. While the effects of other DARC SNPs on DARC function are unknown, the present finding that DARC contributes to asthma severity is most likely only applicable to White patients with asthma.
Through a combination of animal and human data we suggest that DARC plays a role in regulating the severity and resolution of asthma pathophysiology. Our findings in mice suggest that DARC alters the severity and resolution of AHR. These findings are complimented by our human analyses in which select DARC SNPs are associated with worse asthma control and symptoms. Interestingly, our data unveils an important gene-treatment interaction in which select DARC SNPs are associated to worse clinical outcomes only in patients prescribed ICS/LABA. Despite the underlying mechanisms remaining unclear, our study suggests that assessment of DARC status may provide the ability to discriminate those uncontrolled patients whom may gain the most benefit from alternative treatment strategies.
Supplementary Material
Acknowledgments
This research was supported by NIH-COBRE P30 GM103532 and funding from the American Lung Association Airways Clinical Research Centers. DGC was supported by a CJ Martin Fellowship from the National Medical Research Council of Australia (NHMRC-ECF 1053790).
Footnotes
None of the authors have any conflict of interests to disclose.
References
- 1.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. The EMBO journal. 1999;18(3):501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature reviews Immunology. 2006;6(12):907–18. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 3.Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochemical and biophysical research communications. 2004;321(2):306–12. doi: 10.1016/j.bbrc.2004.06.146. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, Durda P, et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood. 2010;115(26):5289–99. doi: 10.1182/blood-2009-05-221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergara C, Tsai YJ, Grant AV, Rafaels N, Gao L, Hand T, et al. Gene encoding Duffy antigen/receptor for chemokines is associated with asthma and IgE in three populations. American journal of respiratory and critical care medicine. 2008;178(10):1017–22. doi: 10.1164/rccm.200801-182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri A, Nielsen S, Elkjaer ML, Zbrzezna V, Fang F, Pogo AO. Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood. 1997;89(2):701–12. [PubMed] [Google Scholar]
- 7.Al-Alwan LA, Chang Y, Rousseau S, Martin JG, Eidelman DH, Hamid Q. CXCL1 Inhibits Airway Smooth Muscle Cell Migration through the Decoy Receptor Duffy Antigen Receptor for Chemokines. Journal of immunology (Baltimore, Md : 1950) 2014 doi: 10.4049/jimmunol.1302860. [DOI] [PubMed] [Google Scholar]
- 8.Rot A. Contribution of Duffy antigen to chemokine function. Cytokine & growth factor reviews. 2005;16(6):687–94. doi: 10.1016/j.cytogfr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nature immunology. 2009;10(1):101–8. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Wurfel MM, Matute-Bello G, Frevert CW, Rosengart MR, Ranganathan M, et al. The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. Journal of immunology (Baltimore, Md : 1950) 2006;177(11):8086–94. doi: 10.4049/jimmunol.177.11.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Molecular and cellular biology. 2000;20(9):3097–101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, et al. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. Journal of immunology (Baltimore, Md : 1950) 2003;170(10):5244–51. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarbock A, Bishop J, Muller H, Schmolke M, Buschmann K, Van Aken H, et al. Chemokine homeostasis vs. chemokine presentation during severe acute lung injury: the other side of the Duffy antigen receptor for chemokines. American journal of physiology Lung cellular and molecular physiology. 2010;298(3):L462–71. doi: 10.1152/ajplung.00224.2009. [DOI] [PubMed] [Google Scholar]
- 14.McKimmie CS, Fraser AR, Hansell C, Gutierrez L, Philipsen S, Connell L, et al. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. Journal of immunology (Baltimore, Md : 1950) 2008;181(11):8171–81. doi: 10.4049/jimmunol.181.11.8170-a. [DOI] [PubMed] [Google Scholar]
- 15.Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, et al. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96(5):1681–4. [PubMed] [Google Scholar]
- 16.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers ALAACR. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. American journal of respiratory and critical care medicine. 2007;175(3):235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. The European respiratory journal. 1999;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 19.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 20.Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N, et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. International immunology. 2003;15(10):1219–27. doi: 10.1093/intimm/dxg121. [DOI] [PubMed] [Google Scholar]
- 21.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. The Journal of allergy and clinical immunology. 2012;129(3 Suppl):S24–33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, et al. Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways. Journal of immunology (Baltimore, Md : 1950) 2011;186(3):1861–9. doi: 10.4049/jimmunol.1002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kangelaris KN, Sapru A, Calfee CS, Liu KD, Pawlikowska L, Witte JS, et al. The association between a Darc gene polymorphism and clinical outcomes in African American patients with acute lung injury. Chest. 2012;141(5):1160–9. doi: 10.1378/chest.11-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reutershan J, Harry B, Chang D, Bagby GJ, Ley K. DARC on RBC limits lung injury by balancing compartmental distribution of CXC chemokines. European journal of immunology. 2009;39(6):1597–607. doi: 10.1002/eji.200839089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma-Stohlawetz P, et al. Duffy antigen modifies the chemokine response in human endotoxemia. Critical care medicine. 2008;36(1):159–65. doi: 10.1097/01.CCM.0000297875.55969.DB. [DOI] [PubMed] [Google Scholar]
- 26.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. Journal of immunology (Baltimore, Md : 1950) 2008;181(6):4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. American journal of physiology Lung cellular and molecular physiology. 2005;289(2):L322–8. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGovern TK, Chen M, Allard B, Larsson K, Martin JG, Adner M. Neutrophilic oxidative stress mediates organic dust-induced pulmonary inflammation and airway hyperresponsiveness. American journal of physiology Lung cellular and molecular physiology. 2016;310(2):L155–65. doi: 10.1152/ajplung.00172.2015. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. American journal of respiratory and critical care medicine. 2009;180(8):720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan W, Liu Q, Lionakis MS, Marino AP, Anderson SA, Swamydas M, et al. Atypical chemokine receptor 1 deficiency reduces atherogenesis in ApoE-knockout mice. Cardiovascular research. 2015;106(3):478–87. doi: 10.1093/cvr/cvv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lettow I, Berres ML, Schmitz P, Muller T, Berg T, Neumann UP, et al. A Duffy antigen receptor for chemokines (DARC) polymorphism that determines pro-fibrotic chemokine serum concentrations is not directly associated with severity of hepatitis C infection. Human immunology. 2011;72(3):273–7. doi: 10.1016/j.humimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. American journal of physiology Cell physiology. 2006;291(5):C957–65. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 33.Alkhouri H, Moir LM, Armour CL, Hughes JM. CXCL1 is a negative regulator of mast cell chemotaxis to airway smooth muscle cell products in vitro. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(3):381–92. doi: 10.1111/cea.12224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.