Abstract
Increased adipocyte lipolysis links obesity to insulin resistance. The lipid droplet coating-protein Perilipin participates in regulation of lipolysis and is implicated in obesity. In the present study we investigate epigenetic regulation of the PLIN1 gene by correlating PLIN1 CpG methylation to gene expression and lipolysis, and functionally evaluating PLIN1 promoter methylation. PLIN1 CpG methylation in adipocytes and gene expression in white adipose tissue (WAT) was quantified in two cohorts by array. Basal lipolysis in WAT explants and adipocytes was quantified by measuring glycerol release. CpG-methylation of the PLIN1 promoter in adipocytes from obese women was higher as compared to never-obese women. PLIN1 promoter methylation was inversely correlated with PLIN1 mRNA expression and the lipolytic activity. Human mesenchymal stem cells (hMSCs) differentiated in vitro into adipocytes and harboring methylated PLIN1 promoter displayed decreased reporter gene activity as compared to hMSCs harboring unmethylated promoter. Treatment of hMSCs differentiated in vitro into adipocytes with a DNA methyltransferase inhibitor increased levels of PLIN1 mRNA and protein. In conclusion, the PLIN1 gene is epigenetically regulated and promoter methylation is inversely correlated with basal lipolysis in women suggesting that epigenetic regulation of PLIN1 is important for increased adipocyte lipolysis in insulin resistance states.
Introduction
Obesity is associated with adverse metabolic consequences including insulin resistance and development of type 2 diabetes1. One factor linking excess adipose tissue to metabolic disease is increased adipocyte lipolysis resulting in elevated circulating levels of non-esterified fatty acids (NEFA) in the circulation which in turn induce insulin resistance in other organs as reviewed2, 3.
During lipolysis, intracellular triacylglycerides (TAGs) undergo hydrolysis through the action of lipases. The regulation of lipolysis is complex. Hormones exert a tight control on lipolysis. In human the important hormones are catecholamines and heart-derived natriuretic peptides which stimulate, and insulin which inhibits, lipolysis4. Proteins covering lipid droplets in adipocytes also participate in regulation of lipolysis. Perilipin, encoded by the PLIN1 gene, is the most extensively studied lipid droplet protein and inhibits basal lipolysis5. Lipolytic stimuli cause phosphorylation of Perilipin which facilitates hydrolysis of TAG by recruitment of hormone sensitive lipase to the lipid droplet6, 7.
Human adipocyte levels of Perilipin protein are inversely correlated with lipolytic rate8 supporting an important role of Perilipin in lipolytic regulation in vivo. Furthermore, obesity, which is associated with increased basal lipolysis, has repeatedly been shown to be associated with decreased levels of Perilipin protein in adipose tissue8–10. Adipose PLIN1 mRNA has also been reported to be lower in obese as compared to lean subjects, although this has not been confirmed in all studies9. An intronic PLIN1 gene-allele has been associated with higher basal lipolysis, as well as with reduced PLIN1 content in adipocytes8, 10, 11, but the results are preliminary and need to be replicated in additional larger cohorts as discussed12. In addition, the transcription factors PPARG, NFkappaB, and LXRA control PLIN1 gene transcription13–15. Beyond these transcriptional and possible genetic effects, the in vivo control of PLIN1 levels is poorly defined. Adipocyte gene transcription is modulated by epigenetic mechanisms. Recently we reported dysregulated CpG-methylation of lipolytic genes as a major feature of the adipocyte epigenetic signature from obese woman; PLIN1, however, did not display significant differences in DNA-methylation in analysis of the global DNA methylome comparing obese and never-obese women16. Considering the importance of PLIN1 for lipolysis, in the present study we used a candidate gene approach to address epigenetic regulation of the PLIN1 gene in relation to lipolysis. We performed a comprehensive descriptive analysis of CpG methylation in relation to obesity and lipolysis in vivo, made a functional evaluation of CpG-methylation in the PLIN1 promoter, and demonstrate that global demethylation increases levels of PLIN1 mRNA and Perilipin protein.
Results
Clinical data
Clinical data for explorative and validation cohorts are presented in Table 1. Expected differences between obese and never obese women were observed. Thus, the obese women displayed a higher basal lipolysis in white adipose tissue (WAT) explants and in isolated adipocytes, which was accompanied by higher NEFA in the general circulation as compared to never-obese women. There was no significant difference in age between groups.
Table 1.
Clinical characteristics of subjects.
| Explorative cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|
| Never-obese | Obese | P 1 | Never obese | Obese | P 1 | |
| n | 14 | 15 | 38 | 31 | ||
| Age (years) | 45 ± 11 | 46 ± 11 | 0.93 | 40 ± 14 | 42 ± 11 | 0.65 |
| Weight (kg) | 69 ± 7 | 115 ± 11 | 5.1 × 10−6 | 66 ± 8 | 103 ± 20 | 1.0 × 10−12 |
| BMI (kg/m2) | 25.2 ± 2.5 | 41.4 ± 4.5 | 5.1 × 10−6 | 23.5 ± 1.9 | 39.9 ± 6.3 | 1.0 × 10−11 |
| Waist to hip ratio | 0.85 ± 0.06 | 0.98 ± 0.06 | 8.4 × 10−5 | 0.86 ± 0.07 | 0.97 ± 0.05 | 0.05 |
| Systolic blood pressure (mmHg) | 123 ± 19 | 138 ± 22 | 0.052 | 123 ± 17 | 128 ± 12 | 0.10 |
| Diastolic blood pressure (mmHg) | 74 ± 6 | 85 ± 9 | 6.0 × 10−4 | 74 ± 7 | 80 ± 7 | 0.0005 |
| P-Glucose (mmol/L) | 5.1 ± 0.4 | 5.7 ± 1.2 | 0.069 | 4.8 ± 0.5 | 5.1 ± 0.5 | 0.0014 |
| P-Insulin (mU/l) | 4.6 ± 2.3 | 16.0 ± 10.3 | 1.1 × 10−4 | 5.1 ± 2.5 | 13.9 ± 8.6 | 5.8 × 10−8 |
| P-Cholesterol (mmol/l) | 4.7 ± 1.0 | 4.9 ± 0.7 | 0.57 | 4.7 ± 0.9 | 5.0 ± 1.0 | 0.34 |
| P-NEFA (mmol/l) | 0.57 ± 0.17 | 0.83 ± 0.16 | 2.4 × 10−4 | 0.64 ± 0.18 | 0.78 ± 0.27 | 0.02 |
| Basal lipolysis in isolated fat cells (micromoles of glycerol/2 h/107adipocytes)2 | 1.34 ± 1.18 | 3.18 ± 1.47 | 0.0012 | 3.44 ± 2.24 | 8.67 ± 7.34 | 0.0004 |
| Basal lipolysis in adipose tissue (micromoles of glycerol/2 h/107 adipocytes)3 | 1.12 ± 0.64 | 3.43 ± 0.91 | 1.0 × 10−4 | 2.96 ± 1.52 | 5.59 ± 2.77 | 2.3 × 10–5 |
| Average adipocyte volume, picolitres | 442 ± 169 | 994 ± 184 | 5.5 + 10−8 | 471 ± 169 | 907 ± 194 | 1.0 × 10−10 |
1Comparison of never-obese and obese group with unpaired t-test or Kruskal-Wallis test as indicated in the Statistical analysis section. Values are mean ± SD.
2Data available for 14 never-obese and 14 obese women in the explorative cohort, and for 37 never-obese and 31 obese women in the validation cohort.
3Data available for 5 never-obese and 12 obese women in the explorative cohort, and for 32 never-obese and 29 obese women in the validation cohort.
PLIN1 in relation to obesity
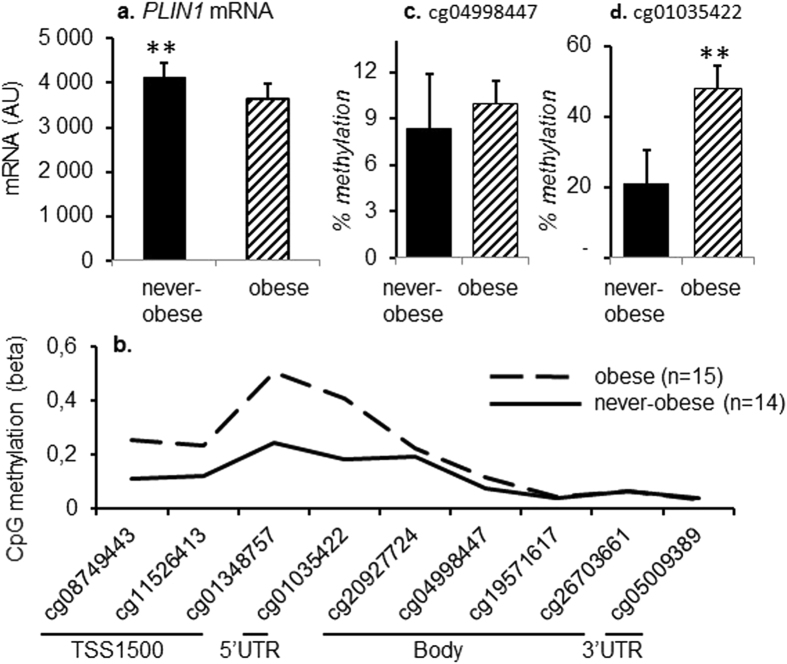
We confirmed that obese women displayed lower expression of PLIN1 mRNA in subcutaneous adipose tissue (P = 0.0058) (Fig. 1a), whereas CpG-methylation of the PLIN1 gene in adipocytes was higher (Fig. 1b), as compared to never-obese women. Differential methylation was most pronounced in the promoter and 5′ region of the gene, and CpG-methylation of adipocyte DNA, quantified as beta-value, was higher in obese as compared to never-obese women of both the explorative and validation cohorts (Table 2). CpG methylation of the PLIN1 gene was not dependent on age in the explorative cohort, whereas a positive correlation was observed in the validation cohort (Table 2). Age did not impact the correlation between CpG-methylation and any other investigated phenotype such as BMI or lipolysis. We validated differentially methylated sites (DMS) by Pyrosequencing in a subset of samples from the explorative cohort (5 obese and 9 never-obese women) and were able to analyze assays for two out of three tested CpG-sites. Results were directionally consistent for cg04998447 (Fig. 1c), and directionally consistent as well as significant for cg01035422 (P 0.0041) (Fig. 1d). The assay for cg08749443 failed in the clinical cohort.
Figure 1.
PLIN1 mRNA levels and CpG-methylation in obese and never-obese women from the explorative cohort. (a) PLIN1 mRNA in WAT of obese (n = 9) and never-obese (n = 9) women. PLIN1 mRNA quantification was performed with microarray. (b) Average methylation of several CpG-sites in the PLIN1 gene assessed by microarray in adipocytes from obese (n = 15) and never-obese (n = 14) women. (c) Methylation of cg04998447 in adipocytes from obese (n = 5) and never-obese (n = 9) women. (d) Methylation of cg01035422 in adipocytes from obese (n = 5) and never-obese (n = 9) women. Quantification of CpG methylation in c. and d. was performed with Pyrosequencing. Values are mean ± SD. **P < 0.01.
Table 2.
DMS in PLIN1 in relation to obesity and age.
| Probe | Position | Gene | Relation to CpG | Obesity status | Age | |||
|---|---|---|---|---|---|---|---|---|
| region | Island | Never-obese | Obese | P 1 | Std beta | P 4 | ||
| Explorative cohort | ||||||||
| cg08749443 | 90224098 | TSS1500 | Open sea | 0.114 ± 0.045 | 0.255 ± 0.113 | 1.1 × 10−4 | −0.119 | 0.54 |
| cg115264132 | 90223712 | TSS1500 | Open sea | 0.122 ± 0.056 | 0.233 ± 0.100 | 2.7 × 10−4 | −0.107 | 0.58 |
| cg013487572 | 90223301 | TSS1500 | Open sea | 0.244 ± 0.095 | 0.505 ± 0.142 | 1.1 × 10−4 | 0.046 | 0.81 |
| cg01035422 | 90222555 | 5′UTR | Open sea | 0.181 ± 0.067 | 0.407 ± 0.122 | 2.5 × 10–5 | −0.045 | 0.82 |
| cg232056603 | 90212699 | Body | S_Shelf | |||||
| cg20927724 | 90209326 | Body | S_Shore | 0.200 ± 0.030 | 0.226 ± 0.047 | 0.012 | 0.044 | 0.92 |
| cg04998447 | 90209223 | Body | Island | 0.078 ± 0.018 | 0.117 ± 0.027 | 1.1 × 10−4 | 0.078 | 0.68 |
| cg19571617 | 90209190 | Body | Island | 0.038 ± 0.009 | 0.046 ± 0.015 | 0.16 | −0.007 | 0.97 |
| cg26703661 | 90208915 | Body | Island | 0.064 ± 0.009 | 0.065 ± 0.021 | 0.62 | 0.08 | 0.68 |
| cg05009389 | 90208810 | 3′UTR | Island | 0.041 ± 0.017 | 0.037 ± 0.008 | 0.68 | 0.052 | 0.79 |
| cg265857243 | 90208739 | 3′UTR | N_Shore | |||||
| Validation cohort | ||||||||
| cg08749443 | 90224098 | TSS1500 | Open sea | 0.254 ± 0.105 | 0.331 ± 0.069 | 0.0008 | 0.318 | 0.0077 |
| cg115264132 | 90223712 | TSS1500 | Open sea | 0.268 ± 0.120 | 0.331 ± 0.084 | 0.0078 | 0.279 | 0.020 |
| cg013487572 | 90223301 | TSS1500 | Open sea | 0.421 ± 0.177 | 0.517 ± 0.091 | 0.004 | 0.384 | 0.0011 |
| cg01035422 | 90222555 | 5′UTR | Open sea | 0.379 ± 0.135 | 0.483 ± 0.078 | 0.0004 | 0.332 | 0.0054 |
| cg232056603 | 90212699 | Body | S_Shelf | |||||
| cg20927724 | 90209326 | Body | S_Shore | 0.212 ± 0.040 | 0.229 ± 0.032 | 0.066 | 0.380 | 0.0013 |
| cg04998447 | 90209223 | Body | Island | 0.089 ± 0.016 | 0.101 ± 0.018 | 0.021 | 0.364 | 0.0021 |
| cg19571617 | 90209190 | Body | Island | 0.053 ± 0.010 | 0.058 ± 0.009 | 0.014 | 0.112 | 0.359 |
| cg26703661 | 90208915 | Body | Island | 0.048 ± 0.012 | 0.044 ± 0.007 | 0.24 | −0.017 | 0.89 |
| cg05009389 | 90208810 | 3′UTR | Island | 0.035 ± 0.009 | 0.032 ± 0.006 | 0.15 | −0.0074 | 0.95 |
| cg265857243 | 90208739 | 3′UTR | N_Shore | |||||
1Comparison of never-obese and obese group with Kruskal-Wallis test. Values are mean ± SD.
2SNP within 10 bps of interrogated CpG-site.
3SNP with MAF >10% in probes.
4Relationship between age and CpG-methylation was assessed by simple regression.
PLIN1 in relation to lipolysis
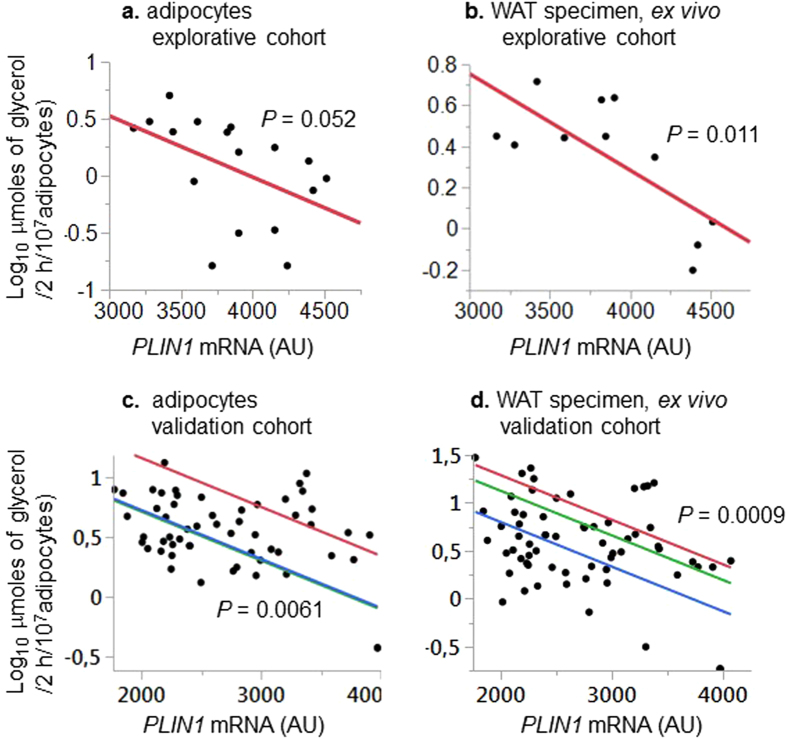
We further studied adipocyte CpG-methylation of PLIN1 in relation to lipolysis in more detail. There were inverse correlations between PLIN1 mRNA and basal lipolysis in isolated adipocytes (Fig. 2a,c) and WAT explants (Fig. 2b,d) in both the explorative and validation cohorts. Methylation of all examined CpG-sites in the PLIN1 gene promoter and 5′ region in adipocytes was inversely related to PLIN1 mRNA, as well as WAT ex vivo and adipocyte basal lipolysis (Table 3). By contrast, only one of five examined CpG-sites in the PLIN1 gene body and 3′ UTR was nominally associated with these phenotypes.
Figure 2.
PLIN1 mRNA in relation to adipose basal lipolysis. (a) Basal lipolysis quantified in isolated subcutaneous adipocytes from women in the explorative cohort (n=17). (b) Basal lipolysis quantified ex vivo in adipose tissue explants obtained by needle aspiration from women in the explorative cohort (n=11). (c) Basal lipolysis quantified in isolated subcutaneous adipocytes from women in the validation cohort (n=56). (d) Basal lipolysis quantified ex vivo in adipose tissue explants obtained by needle aspiration from women in the validation cohort (n=49). See methods section for details about assays for quantifications of lipolysis and PLIN1 mRNA. Results were analyzed by simple (Fig. 2a and b) or multiple (Fig. 2c and d) regression adjusting for array batch.
Table 3.
Relationship between methylation of CpG-sites in the PLIN1 gene, PLIN1 mRNA levels and lipolysis1.
| PLIN1 CpG methylation2 | PLIN1 mRNA3 | Basal lipolysis in adipocytes4 | Basal lipolysis in WAT explants4 | ||||
|---|---|---|---|---|---|---|---|
| Probe | Gene region | Std beta | P | Std beta | P | Std beta | P |
| Detection cohort | |||||||
| cg08749443 | TSS1500 | −0.531 | 0.023 | 0.515 | 0.0051 | 0.437 | 0.079 |
| cg115264135 | TSS1500 | −0.649 | 0.0036 | 0.454 | 0.015 | 0.477 | 0.053 |
| cg013487575 | TSS1500 | −0.612 | 0.007 | 0.543 | 0.0028 | 0.612 | 0.0091 |
| cg01035422 | 5′UTR | −0.592 | 0.0096 | 0.635 | 0.0003 | 0.625 | 0.0073 |
| cg232056606 | Body | ||||||
| cg20927724 | Body | −0.368 | 0.133 | 0.47 | 0.012 | 0.238 | 0.357 |
| cg04998447 | Body | −0.471 | 0.048 | 0.503 | 0.0064 | 0.61 | 0.0093 |
| cg19571617 | Body | −0.415 | 0.087 | 0.342 | 0.074 | 0.427 | 0.087 |
| cg26703661 | Body | −0.045 | 0.859 | 0.146 | 0.457 | 0.249 | 0.335 |
| cg05009389 | 3′UTR | 0.181 | 0.472 | 0.239 | 0.221 | 0.013 | 0.96 |
| cg265857246 | 3′UTR | ||||||
| Validation cohort | |||||||
| cg08749443 | TSS1500 | −0.293 | 0.027 | 0.354 | 0.0024 | 0.432 | 0.0004 |
| cg115264135 | TSS1500 | −0.36 | 0.006 | 0.281 | 0.017 | 0.376 | 0.0022 |
| cg013487575 | TSS1500 | −0.315 | 0.017 | 0.256 | 0.032 | 0.385 | 0.0017 |
| cg01035422 | 5′UTR | −0.318 | 0.016 | 0.317 | 0.0071 | 0.412 | 0.0007 |
| cg232056606 | Body | ||||||
| cg20927724 | Body | −0.134 | 0.32 | 0.132 | 0.27 | 0.286 | 0.022 |
| cg04998447 | Body | −0.153 | 0.26 | 0.285 | 0.012 | 0.386 | 0.0016 |
| cg19571617 | Body | −0.018 | 0.9 | 0.223 | 0.061 | 0.278 | 0.026 |
| cg26703661 | Body | −0.042 | 0.76 | 0.018 | 0.88 | 0.002 | 0.98 |
| cg05009389 | 3′UTR | −0.086 | 0.53 | 0.068 | 0.57 | −0.0062 | 0.96 |
| cg265857246 | 3′UTR | ||||||
1Relationship between degree of CpG-methylation and quantitative phenotypes was assessed by simple regression. 2 PLIN1 CpG methylation was analyzed in DNA extracted from adipocytes using the Infinium Human Methylation 450 (explorative cohort) or EPIC (validation cohort) arrays. 3 PLIN1 mRNA in WAT was quantified by microarray. 4Log10 micromoles of glycerol/2 h/107 adipocytes. 5SNP within 10 bps of interrogated CpG-site. 6SNP with MAF > 10% in probes.
To assess whether CpG-methylation of PLIN1 affects promoter activity, a luciferase reporter gene assay was used. hMSCs were transfected with a plasmid containing the PLIN1 promoter region cloned into a CpG free backbone vector (pCpGL-PLIN). Cells transfected with methylated pCpGL-PLIN plasmid displayed a significant and marked decrease (>60%) of luciferase activity in comparison to those transfected with unmethylated pCpGL-PLIN plasmid (P < 0.001). The methylated and unmethylated control plasmid showed no difference in reporter gene activity (Fig. 3).
Figure 3.
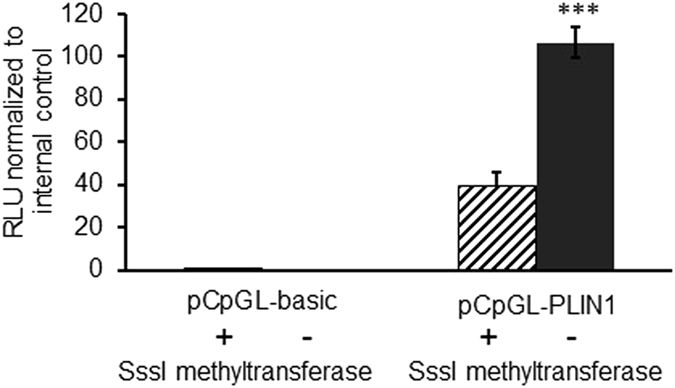
PLIN1 promoter methylation inhibits promoter activity. PLIN1 promoter activity is decreased after (hatched bar) versus without (black bar) DNA methylation by SssI methyltransferase. hMSCs were transfected with methylated and unmethylated pCpGL-PLIN1 plasmid. As negative control, cells were transfected with empty vector, pCpGL-basic. Each sample was prepared in quadruplicates and the experiment was repeated three times. Y axis is the ratio between firefly and renilla luciferase. Renilla luciferase is expressed from a second plasmid as an internal control. RLU = Relative luciferase units. ***P < 0.001.
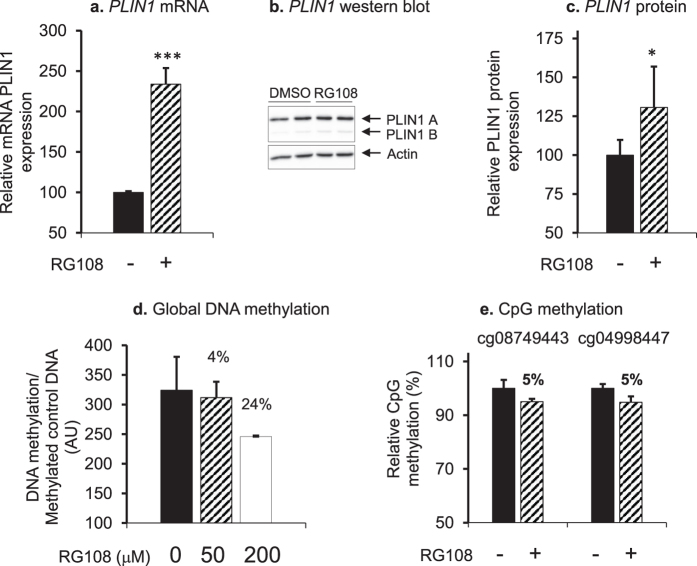
To further evaluate epigenetic regulation of PLIN1, hMSCs differentiated in vitro to adipocytes were treated with 50 or 200 µM of DNA methyltransferase inhibitor RG108 for 24 h. The higher concentration of RG108 (200 µM) was determined to be cytotoxic (results not shown), therefore all subsequent experiments were performed using 50 µM RG108. As a consequence of RG108 treatment, levels of PLIN1 mRNA (Fig. 4a) and Perilipin protein (Fig. 4b,c) were upregulated by about 140% and 30%, respectively. Demethylation activity of RG108 was confirmed by Global DNA methylation Imprint® Methylated DNA Quantification Kit, and showed that treatment of adipocytes with RG108 decreased global DNA methylation by 4% and 24%, respectively (Fig. 4d). To confirm that RG108 demethylated PLIN1, two CpG sites in the promoter were selected for analysis by Pyrosequencing (cg08749443 and cg04998447). The results showed that 50 µM RG108 decreased methylation at both CpGs by 5% (Fig. 4e).
Figure 4.
Global demethylation affects Perilipin levels in adipocytes. (a) PLIN1 mRNA as determined by RT-qPCR and (b,c) Perilipin protein as determined by Western blot were increased after treating hMSCs with DNA methyltransferase inhibitor RG108 (50 µM) (hatched bars) as compared to vehicle (black bars). The experiment was repeated three times. Representative Western blots are shown. These blot pictures were cropped and the full-length blot pictures are presented in Supplementary Fig. S1. Results are presented as relative fold change ± SD vs. vehicle-treated cells. (d) Global DNA methylation in adipocytes was decreased after 24 h treatment with DNA methyltransferase inhibitor RG108 at a concentration of 50 µM (hatched bar) or 200 µM (white bar) compared to non-treated control cells (black bar). The experiment was repeated twice. (e) The methyltransferase inhibitor RG108 decreased methylation of specific CpG-sites in the PLIN1 promoter in adipocytes. Methylation of cg08749443 and cg04998447 was determined by Pyrosequencing after adipocytes were treated with 50 µM RG108 (hatched bar) compared to non-treated control cells (black bar). The analysis was repeated twice, n > 3. ***P < 0.001, **P < 0.01, *P < 0.05.
Discussion
We herein report that PLIN1, a key regulator of basal lipolysis, is subject to functional regulation by epigenetic modifications. It is demonstrated that CpG methylation of the PLIN1 gene promoter inhibits promoter activity in vitro, and that adipocyte promoter methylation is inversely correlated with PLIN1 mRNA levels in clinical cohorts. Furthermore, a positive correlation between PLIN1 CpG methylation and adipose basal lipolysis ex vivo was found. This indicates that epigenetic regulation is important for the regulation of lipolysis in human WAT.
Differential CpG methylation of PLIN1 was not evident in our original published global methylome analysis of obese versus never-obese women. This is most likely due to the use of a stringent threshold to adjust for multiple testing16. However, the validation of DMS in the PLIN1 promoter in the present study using two cohorts with array data, as well as an independent method (Pyrosequencing) clearly establishes the presence of differential methylation of PLIN1 in obesity. Furthermore, there is a positive correlation between PLIN1 CpG-methylation in intact WAT and BMI (see supplementary tables in Rönn T et al.17). The reported relationship is weaker than the one observed here, which might be due to the fact that we study isolated adipocytes and hereby avoid the confounding effects of other cell types in intact WAT. By contrast CpG-methylation of PLIN1 has not been associated with BMI in reported epigenome-wide association studies on whole blood or leukocytes suggesting that the effect is specific to adipocytes or WAT where PLIN1 influences lipolysis18, 19. We observed some correlation between CpG methylation of the PLIN1 promoter and age in the validation, but not in the explorative cohort. This is in agreement with reported finding for WAT that, although the average methylation of CpG-sites covering the genome is positively correlated with age, only a minor proportion of individual CpG-sites display significant correlation between methylation and age17. Specifically, CpG-methylation of PLIN1 did not correlate with age in the large cohorts of men and women studied by Rönn et al.
Increased lipolysis is implicated in insulin resistance, as well as in a number of other conditions including cachexia, hepatosteatosis, and cardiovascular disease2, 20–22. In addition, there is evidence for epigenetic dysregulation in these disease states23–25. Despite the importance of PLIN1 for lipolytic regulation5, the PLIN1 gene locus has not come out as a susceptibility locus in genome-wide association studies for e.g. obesity or insulin resistance26–28. The finding that CpG methylation controls the activity of the PLIN1 promoter thus shed new light on the regulation of adipocyte lipolysis and potentially why lipolysis is altered in metabolic diseases. The degree of global DNA demethylation, 4–24%, reported here is in the same range as has been reported previously when treating non-dividing cells with a global methyltransferase inhibitor29. Future work is needed to define how CpG methylation interacts with other known regulators of lipolysis4. In the present study methylation of all examined CpG-sites in the PLIN1 promoter were positively associated with obesity and lipolysis in the clinical cohorts. Transcriptional regulation of PLIN1 is incompletely defined; the transcription factors PPARG, NFkappaB and LXRA have been shown to regulate PLIN1 transcription13–15. The reported binding motifs for PPARG and LXRA do not contain any CpG-sites, nor the predicted binding site for NFkappaB. As regards the studied CpG-sites in the PLIN1 promoter, cg08749443 overlaps a predicted binding site for Sp1, whereas the other CpG-sites show no overlap with predicted transcription factor binding sites according to AliBaba using the public Transfac database to predict transcription factor binding matrices (http://www.gene-regulation.com/pub/databases.html). The results, thus, do not permit us to draw any conclusion if methylation of some part(s) of the promoter is more important than others.
What might in turn regulate CpG-methylation of the PLIN1 promoter? It is established that external factors such as diet and physical exercise influence the methylome30–32; however, in the few available human intervention studies that have the DNA methylome as one outcome neither physical exercise nor high-fat overfeeding are associated with differential CpG methylation of PLIN1 30, 32. Thus, it remain to establish if and which behavioral factors influence CpG-methylation of the PLIN1 promoter. In addition, it is possible that metabolic factors such as fat mass (obesity) and enlarged fat cells are more important.
There are some limitations with the present study. We only examined women and one adipose region. Perilipin protein in WAT has been reported to be higher in men than in women9. Unfortunately, we do not have isolated adipocytes from men and therefore cannot investigate in clinical cohorts if epigenetic regulation of PLIN1 is gender specific. It is possible that findings in men and fat cells from other depots such as visceral WAT are somewhat different although it is unlikely that such variations are of qualitative nature. Furthermore, investigations of visceral adipose tissue necessitate the use of general surgery procedures which by themselves alter WAT gene expression33.
In conclusion, the PLIN1 gene is epigenetically regulated and promoter methylation is inversely correlated with basal lipolysis in women suggesting that epigenetic regulation of PLIN1 is important for increased adipocyte lipolysis in insulin resistant states such as obesity.
Methods
Subjects
The subjects in this study have been described before16. Briefly, the discovery cohort comprised fifteen obese women (body mass index (BMI) >30 kg/m2) and fourteen never-obese healthy control women (BMI <30 kg/m2) who were recruited in association with planned visits to our surgical units for gastric by-pass surgery because of obesity and through local advertisement for the purpose of studying WAT factors regulating body weight. Clinical data are presented in Table 1. All 14 never-obese women were healthy. Three of the obese women had type 2 diabetes, out of which two were treated with diet and metformin, and one subject with diet alone. CpG methylation of examined CpG-sites did not differ significantly between metformin treated and other women. For six CpG-sites methylation beta-values in the metformin treated women were within the interval defined by the average + SD for the women not treated with metformin; for remaining three CpG-sites the methylation beta-value of 1≥ subject was outside this region, but not in a consistent direction. Overall, this suggests that metformin does not influence methylation of PLIN1. Nine of the obese individuals were treated for hypertension and one patient had stable multiple sclerosis and did not receive any drugs. The women undergoing gastric by-pass surgery participated in a trial on the effect of bariatric surgery (NCT01785134 at www.clinicaltrials.gov) and were investigated before surgery. The study was approved by the regional ethics board in Stockholm and written informed consent was obtained from each subject. The experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
Transcriptome analysis on WAT specimens was conducted for 18 of the above individuals (9 obese, and 9 never-obese). For the remaining subjects included in this study we did not have sufficient amount of WAT for transcriptome analysis.
For validation of PLIN1 results we studied an independent group of women with a wide variation in BMI (n = 69, age 40 ± 12 years, BMI 30.9 + 9.3 kg/m2) who were examined in the same manner as the explorative cohort. Global transcriptome profiles was available on subcutaneous WAT from 57 of these women as reported34.
Clinical examination
Participants were investigated at 8 AM after an overnight fast. All subjects had been weight stable (< ± 2 kg body weight change) during at least 6 months prior to investigation according to self-report Anthropometric measurements were performed followed by venous blood sampling. Plasma and serum were used for analysis of NEFA and other clinical chemistry variables as described35. An abdominal subcutaneous WAT biopsy was obtained by fine needle aspiration as described36.
WAT handling
The adipose tissue was brought to the laboratory, rinsed repeatedly in saline and visual blood vessels and cell debris were removed. Adipose tissue specimen (about two grams) were divided into portions, one of which was subjected to collagenase treatment to obtain isolated adipocytes as previously reported37. The mean weight and volume of these cells were determined as previously described38, 39. 200 µl of packed isolated adipocytes and 300 mg unfractionated WAT pieces were frozen in liquid nitrogen and kept at −70 °C for subsequent DNA (cells) or RNA (tissue) preparation, whereas remaining tissue was used immediately for cell culture experiments.
Lipolysis assay
Basal lipolytic activity was determined in adipose tissue explants as described40. In brief, pieces of adipose tissue (200 or 300 mg) were incubated for 2 h (100 mg/ml) at 37 °C with air as the gas phase in Krebs–Ringer phosphate buffer (pH 7.4) supplemented with glucose (8.6 mmol/l), ascorbic acid (0.1 mg/ml) and bovine serum albumin (20 mg/ml). Glycerol release into the medium was measured using a sensitive bioluminescence method and expressed as amount of glycerol release per 2 h and 107 adipocytes. Adipocytes are the only adipose source of glycerol, which is an end product of lipolysis and only metabolized by adipose tissue to a minimal extent. Methodological experiment revealed that glycerol release in these type of experiments is linear with incubation time for at least 4 h. Cross-sectional and longitudinal studies have demonstrated that basal lipolytic activity is strongly and negatively related to in vivo insulin sensitivity.
Basal lipolysis in isolated adipocytes was investigated using the same protocol as described above in diluted adipocyte suspensions (2% v/v).
Quantification of PLIN1 CpG methylation by array
PLIN1 CpG methylation in the explorative cohort was analyzed in DNA extracted from adipocytes in a previously reported dataset applying the Infinium Human Methylation 450 BeadChip assay (Illumina, San Diego, CA, USA)16. The beta value (β), which represents the ratio of intensities between methylated and unmethylated alleles, was used to quantify methylation at specific CpG loci. The β-values vary from 0 (no methylation) to 1 (100% methylation). Of 11 probes mapping to the PLIN1 gene, we excluded two containing SNPs with MAF >10% within the probes according to Illumina annotation.
CpG-methylation in adipocytes from the validation cohort was analyzed by EPIC arrays. Briefly, 500 ng of genomic DNA was bisulfate converted with EZ-96 DNA Methylation kit (Zymo Research, Irvine, CA, USA) and genome wide DNA methylation analysis was performed using the Infinium Human Methylation EPIC BeadChip (Illumina, San Diego, CA, USA). The laboratory procedures were performed according to the manufacturer’s protocol. For analysis, visualization and extraction of methylation data, the GenomeStudio software version 2011.1 (Illumina Inc.) was used. The analysis reported here was limited to CpG-sites in the PLIN1 gene region that should be confirmed from the explorative cohort. A comprehensive analysis of the full EPIC data is ongoing.
Pyrosequencing assay
Five of the obese and nine of the never-obese women were used for validation of DMS by pyrosequencing; from remaining women we did not have any cells left. Genomic DNA was prepared from adipocytes using the QiAamp DNA Mini kit (Qiagen, Hilden, Germany). The DNA purity and quality was confirmed by A260/280 ratio >1.8 on a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, US). The DNA concentration was measured by Qubit (Life technologies, Stockholm, Sweden).
Adipocyte genomic DNA (200 ng) was bisulfite converted according to the manufacturer’s instructions using the EZ DNA Methylation-Gold™ Kit (Zymo Research, Orange, CA, USA). Primers for PCR amplification and sequencing were designed using the PyroMark Assay Design 2.0 (Qiagen,). The following primers were used; for cg01035422 forward: TGTAAGGTAGGTGTTTTAGGATTTTAATA; biotinylated reverse: TAACCCTATTATCTCTCCCTCTCT and sequencing: GGTGTTTTAGGATTTTAATATTTAT. The nucleotide dispensing order was: TTYGGTTGAT CGTTATTTTA GTTTTATA; for cg04998447 forward: TTTGGGGAGTTGAGGGTT; biotinylated reverse: CCCCAACCTATATCCTCCT and sequencing: AGTTTTGGTTTGGTTTT. The nucleotide dispensing order was: GGGTTTTGYG TTTTTGATTT AT. Finally for cg08749443 biotinylated forward: TTTAGGAGAGTTTAGAGGGAAGATAGAAGT; reverse: AACCTAAATCCCTACTCTCACTTAATAA and sequencing: AAAAATAAAAAAAACAAATAAATAC. The dispensing order was: RAAAAAAAAA AAAAAATAAA ACTCTA. One µl bisulfite converted DNA was amplified using the PyroMark PCR kit (Qiagen) according to the manufacturer’s instructions applying annealing temperature of 58 °C. The entire PCR product was mixed with 4 µM sequencing primer and sepharose beads (GE Healthcare, Danderyd, Sweden) and bisulfite pyrosequenced using the PSQ 96 ID system (Qiagen) with PyroMark Gold Q96 reagents (Qiagen).
Quantification of PLIN1 expression
PLIN1 mRNA in the clinical samples was quantified by microarray using Gene 1.1 ST arrays (Affymetrix, Santa Clara, CA, US) in 20 of the individuals in the explorative cohort, and by Gene 1.0 or 1.1 ST arrays in 57 of the women in the validation cohort; both groups formed parts of larger studies as described16, 34. For remaining subjects we did not have sufficient amount of WAT for transcriptome analysis.
Total RNA and DNA was extracted from cell culture samples using the AllPrep DNA/RNA Kit (Qiagen). Concentration and purity of nucleic acids was measured using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Lafayette, CO). cDNA synthesis was performed using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA) together with random hexamer primers (Invitrogen, Carlsbad, CA). Assessment of PLIN1 mRNA levels was performed using SYBR Green Mix (Bio-Rad Laboratories) and primers: PLIN1 forward 5′-TGGAAACTGAGGAGAACAAG-3′ and reverse 5′-ATGTCACAGCCGAGATGG-3′. Expression was normalized to the internal reference gene 18S forward 5′-TGACTCAACACGGGAAACC-3′ and reverse 5′-TCGCTCCACCAACTAAGAAC-3′ using the ΔΔCt method41.
Construction of reporter vector
PLIN1 promoter was PCR amplified using KAPA HotStart ReadyMix (Kapa Biosystems, Wilmington, MA) from human genomic DNA (Roche, Basel, Switzerland) using primers, forward 5′-TATTGGATCCGTACAGCCCAGCACATTCACAACT-3′ and reverse 5′-TATTAAGCTTGCCCCAGGACCCCAACAC-3′ (Sigma Aldrich, Dorset, UK) and cloned into the pCpGL-basic vector (kindly provided by prof. M. Rehli, Regensburg, Germany) via BamHI and HindIII (Thermo Scientific) sites, which yielded the pCpGL-PLIN vector. The correct insertion of a construct was controlled by sequencing. The cloned genomic sequence covered 1,731 base pairs upstream of the PLIN1 transcription start site, and included all CpG-sites in the promoter region of PLIN1 whose methylation status was assayed by microarray.
In vitro methylation of plasmid DNA
Both plasmids, pCpGL-PLIN and pCpGL-basic (no insert), were methylated using SssI methyltransferase (New England Biolabs, Hitchin, UK) according to the manufacturer’s recommendation. In brief, 10–15 µg of plasmid DNA was incubated with or without SssI methyltransferase (20 U/µl; 2 U/µg DNA) in the presence of 640 µM S-Adenosylmethionine (SAM) (New England Biolabs) for four hours at 37 °C, with another 640 µM SAM being added after the first two hours of incubation. Plasmid DNA was purified using QIAquick PCR purification kit (Qiagen). Methylation of plasmid DNA was controlled by digestion using methylation sensitive restriction enzyme HpaII (Thermo Fisher Scientific) for four hours at 37 °C.
PLIN1 promoter reporter assays
Human mesenchymal stem cells (hMSCs) isolated from adipose tissue and differentiated in vitro to adipocytes were used for the transfection with plasmids42. Day 9 of differentiated cells were transfected using Neon electroporator (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The cell amount per 10 µl electroporation tip was 100.000 together with 500 ng of methylated or unmethylated pCpGL-basic or pCpGL-PLIN plasmids and 10 ng of a plasmid containing Renilla luciferase gene (Promega, Madison, WI). Electroporation conditions were 1400 Volts, 20 ms width, and 2 pulses. Following electroporation the cells were cultured in 48-well plates for 24 hours there after luciferase activities were measured in cell lysates using Dual Luciferase Reporter Assay System (Promega) according manufacturer’s instructions. Each sample was prepared in quadruplicates and the experiment was repeated three times.
Global demethylation assays in vitro
hMSCs differentiated in vitro to adipocytes were treated with 50 µM or 200 µM of DNA methyltransferase inhibitor RG108 (Abcam, Cambridge, UK) at differentiation day 11–12 for 24 h. Medium was collected and the cells were used to isolate RNA/DNA (AllPrep DNA/RNA Kit, Qiagen) and proteins. Isolated DNA was used for measuring global shifts in DNA methylation (Imprint® Methylated DNA Quantification Kit, Sigma Aldrich,) according to the manufacturer’s instructions. Briefly, 60 ng of DNA was diluted in DNA Binding Solution and incubated at 37 °C for 60 mins. After washing, Capture Antibody at dilution 1:1000 was added and incubated at RT for 60 mins. After washing, Detection Antibody was added to each well at dilution 1:1000 and incubated at RT for 30 mins. Thereafter Developing Solution was added and incubated at RT for 1–10 mins. The reaction was stopped by adding Stop Solution and the absorbance was measured at 450 nm (NanoQuant spectrophotometer, Tecan, Männedorf, Switzerland). Cytotoxicity of RG108 was determined in cell culture medium using Cytotoxicity determination kit (Roche Applied Science, Mannheim, Germany) according to the instruction of the manufacturer.
Analysis of protein expression
Approximately 250,000 hMSCs were lysed in 140 μl RIPA buffer (150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0). 15–20 µg of total protein was separated by SDS-PAGE and Western blot was performed according to standard procedures. The membranes were blocked in 3% ECL Advance™ Blocking Agent (GE Healthcare, Buckinghamshire, UK). Primary antibodies against Perilipin (GP29, Progen, Heidelberg, Germany) and β-actin (Sigma Aldrich) were used. Secondary guinea-pig/rabbit-IgG antibodies were conjugated to horseradish peroxidase (Sigma-Aldrich). Protein bands were detected by chemiluminescence using ECL™ Select Western Blotting Detection Kit (GE Healthcare) in Chemidoc XRS system (Bio-Rad Larobarotries) and quantified by Quantity One software (Bio-Rad Laboratories).
Statistical analysis
Statistical analyses were performed in JMP v. 12. Shapiro-Wilks test was used to test whether the distribution of analyzed quantitative variables deviated from normality. Two-group comparisons of normally distributed variables were performed by two-sided Student’s t-test (age, diastolic blood pressure, cholesterol, NEFA, lipolysis measures, adipocyte volume, and PLIN1 mRNA and protein) and otherwise by Kruskal-Wallis test. Five of nine analyzed CpG-sites were not normally distributed; for simplicity all DNA methylation results were analyzed by Kruskal-Wallis test. Pyrosequencing results were due to small sample size analyzed by non-parametric Kruskal-Wallis test. Relationship between degree of CpG-methylation and quantitative phenotypes was assessed by simple regression. Relationship between PLIN1 mRNA and quantitative phenotypes was assessed by multiple regression adjusting for batch. Lipolysis values were log10-transformed before analysis to become normally distributed. Values are mean + SD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This study was supported by the SRP Diabetes program at Karolinska Institutet, CIMED, the Swedish Research Council, Novo Nordic Foundation, the Swedish Diabetes Foundation, and the Stockholm County Council.
Author Contributions
I.D. and P.A. planned the study and recruited patients. L.B., C.Z., A.K., and K.D.W. planned and performed the in vitro studies. P.P. performed the Pyrosequencing. I.D. and I.S. analyzed data. I.D. wrote the draft manuscript. All authors contributed to the interpretation of data and revision of the manuscript draft, and approved the final version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09232-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langenberg C, et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner, P. & Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab25, 255–262, doi:10.1016/j.tem.2014.03.002S1043-2760(14)00044-7 [pii] (2014). [DOI] [PubMed]
- 3.Morigny, P., Houssier, M., Mouisel, E. & Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie125, 259–266, doi:10.1016/j.biochi.2015.10.024S0300-9084(15)00344-2 [pii] (2016). [DOI] [PubMed]
- 4.Fruhbeck, G., Mendez-Gimenez, L., Fernandez-Formoso, J. A., Fernandez, S. & Rodriguez, A. Regulation of adipocyte lipolysis. Nutr Res Rev27, 63–93, doi:10.1017/S095442241400002XS095442241400002X [pii] (2014). [DOI] [PubMed]
- 5.Brasaemle, D. L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res48, 2547–2559, doi:R700014-JLR200 [pii]10.1194/jlr.R700014-JLR200 (2007). [DOI] [PubMed]
- 6.Souza, S. C. et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem277, 8267-8272, doi:10.1074/jbc.M108329200M108329200 [pii] (2002). [DOI] [PubMed]
- 7.Granneman, J. G. et al. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem282, 5726–5735, doi:M610580200 [pii]10.1074/jbc.M610580200 (2007). [DOI] [PubMed]
- 8.Mottagui-Tabar S, et al. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia. 2003;46:789–797. doi: 10.1007/s00125-003-1112-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes Res. 2003;11:930–936. doi: 10.1038/oby.2003.128. [DOI] [PubMed] [Google Scholar]
- 10.Ray, H., Pinteur, C., Frering, V., Beylot, M. & Large, V. Depot-specific differences in perilipin and hormone-sensitive lipase expression in lean and obese. Lipids Health Dis8, 58, doi:10.1186/1476-511X-8-581476−511X-8-58 [pii] (2009). [DOI] [PMC free article] [PubMed]
- 11.Kern PA, Di Gregorio G, Lu T, Rassouli N, Ranganathan G. Perilipin expression in human adipose tissue is elevated with obesity. J Clin Endocrinol Metab. 2004;89:1352–1358. doi: 10.1210/jc.2003-031388. [DOI] [PubMed] [Google Scholar]
- 12.Soenen S, et al. Relationship between perilipin gene polymorphisms and body weight and body composition during weight loss and weight maintenance. Physiol Behav. 2009;96:723–728. doi: 10.1016/j.physbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Dalen KT, et al. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 14.Laurencikiene, J. et al. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J Lipid Res48, 1069–1077, doi:M600471-JLR200 [pii]10.1194/jlr.M600471-JLR200 (2007). [DOI] [PubMed]
- 15.Stenson, B. M. et al. Liver X receptor (LXR) regulates human adipocyte lipolysis. J Biol Chem286, 370–379, doi:10.1074/jbc.M110.179499M110.179499 [pii] (2011). [DOI] [PMC free article] [PubMed]
- 16.Arner, P. et al. The epigenetic signature of subcutaneous fat cells is linked to altered expression of genes implicated in lipid metabolism in obese women. Clin Epigenetics7, 93, doi:10.1186/s13148-015-0126-9126 [pii] (2015). [DOI] [PMC free article] [PubMed]
- 17.Ronn, T. et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum Mol Genet, doi:ddv124 [pii]10.1093/hmg/ddv124 (2015). [DOI] [PubMed]
- 18.Dick, K. J. et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet, doi:S0140-6736(13)62674-4 [pii]10.1016/S0140-6736(13)62674-4 (2014). [DOI] [PubMed]
- 19.Demerath EW, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24:4464–4479. doi: 10.1093/hmg/ddv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agustsson, T. et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res67, 5531–5537, doi:67/11/5531 [pii]10.1158/0008-5472.CAN-06-4585 (2007). [DOI] [PubMed]
- 21.Frayn, K., Bernard, S., Spalding, K. & Arner, P. Adipocyte triglyceride turnover is independently associated with atherogenic dyslipidemia. J Am Heart Assoc1, e003467, doi:10.1161/JAHA.112.003467jah3115 [pii] (2012). [DOI] [PMC free article] [PubMed]
- 22.Wueest, S. et al. Mesenteric Fat Lipolysis Mediates Obesity-Associated Hepatic Steatosis and Insulin Resistance. Diabetes65, 140–148, doi:10.2337/db15-0941db15-0941 [pii] (2016). [DOI] [PubMed]
- 23.Dupre-Aucouturier, S., Castells, J., Freyssenet, D. & Desplanches, D. Trichostatin A, a histone deacetylase inhibitor, modulates unloaded-induced skeletal muscle atrophy. J Appl Physiol (1985)119, 342–351, doi:10.1152/japplphysiol.01031.2014japplphysiol.01031.2014 [pii] (2015). [DOI] [PubMed]
- 24.Kammel, A. et al. Early hypermethylation of hepatic Igfbp2 results in its reduced expression preceding fatty liver in mice. Hum Mol Genet, doi:ddw121 [pii]10.1093/hmg/ddw121 (2016). [DOI] [PMC free article] [PubMed]
- 25.Arner, P. et al. The epigenetic signature of systemic insulin resistance in obese women. Diabetologia, doi:10.1007/s00125-016-4074-510.1007/s00125-016-4074-5 [pii] (2016). [DOI] [PMC free article] [PubMed]
- 26.Scott RA, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature518, 197–206, doi:10.1038/nature14177nature14177 [pii] (2015). [DOI] [PMC free article] [PubMed]
- 28.Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature518, 187–196, doi:10.1038/nature14132nature14132 [pii] (2015). [DOI] [PMC free article] [PubMed]
- 29.Yamagata Y, et al. Rapid turnover of DNA methylation in human cells. Epigenetics. 2012;7:141–145. doi: 10.4161/epi.7.2.18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronn, T. et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet9, e1003572, doi:10.1371/journal.pgen.1003572PGENETICS-D-13-00070 [pii] (2013). [DOI] [PMC free article] [PubMed]
- 31.Boque N, et al. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;57:1473–1478. doi: 10.1002/mnfr.201200686. [DOI] [PubMed] [Google Scholar]
- 32.Gillberg, L. et al. Adipose tissue transcriptomics and epigenomics in low birthweight men and controls: role of high-fat overfeeding. Diabetologia59, 799–812, doi:10.1007/s00125-015-3852-910.1007/s00125-015-3852-9 [pii] (2016). [DOI] [PubMed]
- 33.Mutch, D. M. et al. Needle and surgical biopsy techniques differentially affect adipose tissue gene expression profiles. Am J Clin Nutr89, 51–57, doi:10.3945/ajcn.2008.26802ajcn.2008.26802 [pii] (2009). [DOI] [PubMed]
- 34.Dahlman, I. et al. Numerous Genes in Loci Associated With Body Fat Distribution Are Linked to Adipose Function. Diabetes65, 433–437, doi:10.2337/db15-082865/2/433 [pii] (2016). [DOI] [PubMed]
- 35.Andersson K, Eneroth P, Arner P. Changes in circulating lipid and carbohydrate metabolites following systemic nicotine treatment in healthy men. Int J Obes Relat Metab Disord. 1993;17:675–680. [PubMed] [Google Scholar]
- 36.Kolaczynski JW, et al. A new technique for biopsy of human abdominal fat under local anaesthesia with Lidocaine. Int J Obes Relat Metab Disord. 1994;18:161–166. [PubMed] [Google Scholar]
- 37.Rodbell M, Krishna G. Preparation of isolated fat cells and fat cell “ghosts”; methods for assaying adenylate cyclase activity and levels of cyclic AMP. Methods Enzymol. 1974;31:103–114. doi: 10.1016/0076-6879(74)31010-5. [DOI] [PubMed] [Google Scholar]
- 38.Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–2342. doi: 10.1007/s00125-005-1961-6. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9:110–119. [PubMed] [Google Scholar]
- 40.Girousse, A. et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol11, e1001485, doi:10.1371/journal.pbio.1001485PBIOLOGY-D-12-02729 [pii] (2013). [DOI] [PMC free article] [PubMed]
- 41.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods25, 402–408, doi:10.1006/meth.2001.1262S1046-2023(01)91262-9 [pii] (2001). [DOI] [PubMed]
- 42.Pettersson AM, et al. LXR is a negative regulator of glucose uptake in human adipocytes. Diabetologia. 2013;56:2044–2054. doi: 10.1007/s00125-013-2954-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.