Abstract
The cardiac L-type calcium channel is a multi-subunit complex that requires co-assembling of the pore-forming subunit CaV1.2 with auxiliary subunits CaVα2δ and CaVβ. Its traffic has been shown to be controlled by these subunits and by the activation of various G-protein coupled receptors (GPCR). Here, we explore the consequences of the prolonged activation of angiotensin receptor type 1 (AT1R) over CaV1.2 channel trafficking. Bioluminescence Resonance Energy Transfer (BRET) assay between β-arrestin and L-type channels in angiotensin II-stimulated cells was used to assess the functional consequence of AT1R activation, while immunofluorescence of adult rat cardiomyocytes revealed the effects of GPCR activation on CaV1.2 trafficking. Angiotensin II exposure results in β-arrestin1 recruitment to the channel complex and an apparent loss of CaV1.2 immunostaining at the T-tubules. Accordingly, angiotensin II stimulation causes a decrease in L-type current, Ca2+ transients and myocyte contractility, together with a faster repolarization phase of action potentials. Our results demonstrate that prolonged AT1R activation induces β-arrestin1 recruitment and the subsequent internalization of CaV1.2 channels with a half-dose of AngII on the order of 100 nM, suggesting that this effect depends on local renin-angiotensin system. This novel AT1R-dependent CaV1.2-trafficking modulation likely contributes to angiotensin II-mediated cardiac remodeling.
Introduction
In mammals, the renin-angiotensin system (RAS) is one of the key factors in the regulation of blood pressure, electrolyte balance and cardiac function. Renin catalyzes the conversion of the 14 amino acid propeptide angiotensinogen into angiotensin I (AngI), which in turn is cleaved by the angiotensin-converting enzyme (ACE) to produce angiotensin II (AngII)1. AngII participates in a series of intracellular signaling cascades via activation of its receptors AT1 and AT2, which belong to the superfamily of seven-transmembrane domain G-protein coupled receptors (GPCR)2.
Most of the classical actions associated with AngII are dependent on the activation of the AT1 receptor (AT1R)3. Binding of AngII results in rapid phosphorylation of the receptor that facilitates β-arrestin recruitment induced desensitization and internalization of AT1R4, 5. Thus, activated AT1R is phosphorylated by GPCR kinases (GRKs) and subsequently β-arrestin is recruited to the receptor. This receptor/β-arrestin interaction leads to receptor desensitization, ending the G protein-dependent signaling by interfering with the coupling between the G protein and its receptor. Furthermore, β-arrestin can also bind to components of the clathrin coat, causing internalization of activated receptors and target to degradation pathways or recycled back to the cell surface6, 7.
Several recent studies have demonstrated the existence of macromolecular complexes involving GPCRs and various ion channels. For example, the dopamine D1 receptor regulates the NMDA receptor through a direct interaction6. AT1R co-immunoprecipitates with the non-selective cation channel TRPV47 and with KV4.3, which is responsible for the fast transient outward current (Ito) in cardiac myocytes8. Likewise, it has been shown that voltage dependent calcium channels (VDCC) interact with various GPCRs. For instance, N-type Ca2+ channels interact with ORL19, GABAb10 and the dopamine D111 and D212 receptors. Interestingly, most of these interactions lead to endocytosis of the channel following activation of the respective receptor8, 9, 11, 13.
In general, VDCCs are protein complexes involving at least three subunits: a CaVα1 subunit that forms the channel pore, and CaVα2δ and CaVβ accessory subunits, responsible for trafficking of the channel complex14 to the plasma membrane. In cardiomyocytes, LTCC clusters, with specific subcellular localization, have been associated with specific functions. Thus, LTCC localized at the T-tubules are mainly responsible for excitation-contraction coupling by its association with ryanodine receptor 2 (RyR2) channels, while LTCC localized at the sarcolemma are implicated in signaling to the nucleus and regulation of gene transcription15.
Several studies have convincingly shown that AngII modulates L-type currents. However, this modulation appears to depend on the cell type and on the species studied. For example, AngII induces an increase of L-type currents in smooth muscle cells16 by a PI3Kdependent mechanism17, 18, while in neurons19, 20 and in glomerular cells, a decrease in these currents has been observed via a Pertussis toxin sensitive G-protein pathway21.
In cardiomyocytes, the effect on this current after AngII application has been shown to be dependent on the species and the patch-clamp configuration used. In hamster22 and guinea pig19 the L-type current is increased, while in rabbit23 and cat24 no change is observed in cells studied with conventional whole-cell (WC) recordings, in contrast, perforated patch experiments show a potentiation of the L-type current. Finally, in rat25 and human26 a decrease of the L-type current has been reported using the conventional WC configuration, while the use of perforated patch configuration in newborn rat cardiomyocytes shows that the degree of L-type current inhibition depend on the speed of inactivation of L-type currents27. This evidence together with a growing number of reports, establishes a clear link between VDCC and GPCRs, suggesting the existence of a functional association between L-type calcium channels and AT1R.
In this work, we demonstrate that AngII stimulation of AT1R causes β-arrestin1 (but not β-arrestin2) recruitment of the L-type calcium channel complex. From a functional stand point, AT1R activation induces internalization of the T-tubule CaV1.2 population after prolonged exposure to AngII, leading to ~60% reduction of endogenous L-type calcium currents, diminished calcium transient amplitude, and reduced action potential duration.
Methods
Constructs
cDNA encoding the angiotensin receptor 1, AT1R (NM_000685), was obtained from the University of Missouri-Rolla cDNA resource center. cDNAs for calcium channel subunits (GenBank™ accession numbers: X15539 (CaV1.2) and AF286488 (CaVα2δ1) were kindly provided by Dr. T. Snutch. CaVβ2b channel subunit (AF423193) was cloned as described in Moreno et al.28. The HA epitope inserted into the extracellular S5-H5 loop of domain II of CaV1.2 for the HA-tagged CaV1.2 construct is described previously29. CaV1.2-YFP and CaVβ1b-YFP were PCR amplified and subcloned into the N1-YFP vector (Clontech) between the NheI and BamHI or EcoRI and BamHI restriction sites respectively. β-arrestin-Rluc constructs were kindly provided by Dr. R. Ramachandran and AT1R-YFP construct was kindly provided by Dr. LM Luttrell.
AD-293 culture and transfection
Tissue culture of AD-293 cells (Agilent) was performed as recommended by the distributor. Transfection solutions for individual culture dishes (35 mm diameter) contained a mixture of cDNA expression vectors (1 μg for each L-type calcium channel subunits (CaVα, CaVβ and CaVα2δ subunits), 1 μg of AT1R and 100 ng of β-arrestin-RLuc) and were transfected into cells by the calcium phosphate method27. Experiments were conducted at room temperature 2–3 days after transfection.
Isolation of cardiomyocytes
Rats were bred in the Animal Breeding Facility from the Facultad de Medicina, Universidad de Chile (Santiago, Chile). All studies were done in accordance and with the approval of the Universidad de Chile Institutional Bioethical Committee. Male Sprague Dawley rats (150–200 gr) were anaesthetized with ketamine plus xylazine and euthanized by isoflurane overdose before heart excision. Ventricular myocytes were isolated by enzymatic digestion in a Langendorff perfusion apparatus. Briefly, the hearts were removed, mounted on a Langendorff apparatus and perfused with modified Tyrode solution containing (mM): 133.5 NaCl, 1.2 NaH2PO4, 4 KCl, 1.8 CaCl2, 1.2 MgSO4, 10 HEPES and 5.5 glucose (pH 7.4 with NaOH) to wash out residual blood in the coronary vessels, Ca2+ was replaced with 5 mM EGTA after 3 min wash. Digestion was done by perfusion of the heart with modified Tyrode solution containing 25 µM Ca2+, 0.45 mg/ml collagenase type II (Worthington) and 0.12 mg/ml protease IV (Sigma-Aldrich) at 37 °C for 7 min, after which the Ca2+ concentration was increased to 50 µM for 7 min and further to 400 µM for 5 min. Subsequently, the heart was removed from the Langendorff apparatus and the dissociated cardiomyocytes were suspended in 400 µM Ca2+-Tyrode solution containing 1 mg/ml BSA, 30 mM taurine and 5 mM pyruvate at room temperature, and the Ca2+ concentration was gradually increased to 1.8 mM. Adult rat cardiomyocytes were cultured on laminin-coated coverslips (10 µg/ml) for 2 hr prior to AngII stimulation.
Bioluminescence resonance energy transfer (BRET)
AD-293 cells were co-transfected with CaV1.2-YFP and β-arrestin-Rluc fusion proteins (+CaVβ and CaVα2δ) or CaVβ-YFP and β-arrestin-Rluc fusion proteins (+CaV1.2 and CaVα2δ). After 48 hours, cells were seeded into a 96-well microplate and coelenterazine H was added 10 minutes before AngII. CaV1.2 recruitment of β-arrestin was detected every 25 seconds as the ratio of the light intensity measured at 535 ± 20 over 475 ± 20 nm using a Tecan fluorometer. BRET signal of cells expressing YFP and Rluc was compared with cells overexpressing only Rluc as control. Every BRET experiment was performed at a controlled temperature of 23 °C.
Antibody feeding assays
AD-293 cells over-expressing hemagglutinin-epitope (HA-epitope) tagged CaV1.2 (+CaVβ and CaVα2δ1) and AT1R were incubated with anti-HA antibody (Roche) for 30 min at 37 °C. Subsequently, cells were stimulated with AngII (1 µM), washed with Hank’s solution and fixed with 4% paraformaldehyde. Surface-remaining CaV1.2 channels were labeled with a secondary antibody (1:1000) conjugated to Alexa Fluor 488 (green, Invitrogen) in PBS. Then, cells were permeabilized with 0.5% triton in PBS (+1% BSA) and total CaV1.2 channels were labeled with a secondary antibody (1:1000) conjugated to Alexa Fluor 594 (red, Invitrogen) in PBS. For image analysis, images were collected on an inverted microscope (Olympus IX-81, UPLFLN 40XO 40x/1.3 oil-immersion objective) and acquired using CellR software (Olympus). All image analyses were performed with ImageJ.
To analyze HA-epitope-tagged CaV1.2 internalization, the green signal was converted to a binary image by setting the threshold function to the onset of the frequency of staining intensities histogram. With this procedure, any green pixel (independent of its original intensity) is set to maximal intensity. To obtain the percentage of internalized channels, the resultant binary image was subtracted from the red signal. This new red-signal image represents the number of internalized channels and the integrated intensities were normalized by dividing them with the integrated intensity of the red signal (that represents the total amount of channels, see Supplementary Figure 1).
Immunofluorescence
Adult rat cardiomyocytes were seeded on laminin pretreated coverslips and then incubated with AngII (1 µM) in the presence or absence of Losartan (100 nM) (Sigma). Subsequently, cardiomyocytes were washed with PBS and fixed with 4% paraformaldehyde. Cardiomyocytes were then permeabilized with 0.02% Triton X-100 in blocking buffer (3% BSA, 5% goat serum in PBS) and incubated for 16 hours with primary antibodies (Anti-CaV1.2, Alomone #ACC-003, rabbit polyclonal, Anti- βarrestin1/2, Santa Cruz #SC-53781, mouse monoclonal, Anti-αActinin, Abcam #AB-9465, mouse monoclonal) before addition of fluorescently labeled secondary antibodies and visualization by confocal microscopy. Intracellular signal percentage was calculated by integrating the cardiomyocyte surface fluorescence (defined by hand for each image) and subtracting it from the total integrated fluorescence. This ensuing signal was then normalized to the total integrated fluorescence.
Colocalization analysis
Intensity Correlation Analysis, performed as described previously29, is based on the principle that when two proteins colocalize their staining intensities should vary synchronously30. Briefly, the Product of the Differences from the Mean (PDM) of each pixel was calculated and the Intensity Correlation Quotient (ICQ) was defined as the ratio of the number of positive PDM pixels to the total pixel numbers. The ICQ values are distributed between −0.5 and +0.5 by subtracting 0.5 from this ratio and a dependent staining is considered when the ICQ value range between 0 < ICQ ≤ + 0.5.
Calcium Imaging
Plated cardiomyocytes were mounted in a perfusion chamber on the stage of an inverted microscope (Olympus IX-81, UPLFLN 40XO 40 x/1.3 oil-immersion objective). Fluorescence was collected using a CCD-based imaging system (Olympus DSU) running CellR software (Olympus). Cardiomyocytes were incubated with Fluo-4 (Molecular Probes, 1 µM) as described in ref. 28 and thoroughly washed with external solution (mM): 100 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 90 sorbitol, 5 glucose and 10 HEPES (pH 7.4 adjusted with Tris-Base). Fluo-4 loaded cardiomyocytes were excited at 480 nm and the fluorescence emission at 510 nm was collected and recorded at 12 Hz. For each experiment, signals were recorded and the background intensity subtracted using equivalent regions of interest (ROI) outside the cardiomyocytes. Fluo-4 results are expressed as normalized fluorescence (F/F0).
Electrophysiology and data analysis
Ca2+ currents were recorded by conventional whole-cell patch clamp31. Borosilicate glass pipettes were pulled to 2–4 MΩ resistance and filled with internal solution containing (mM): 108 CsCl, 4 MgCl2, 2 CaCl2, 10 EGTA 10 HEPES, 5 MgATP and 0.6 LiGTP (pH 7.2 adjusted with CsOH). The bath solution contained (mM): 5 CaCl2, 1 MgCl2, 10 HEPES, 20 TEA-Cl, 10 glucose and 100 NMDG-Cl (pH 7.4 adjusted with Tris). Data were acquired at room temperature using an Axopatch 200B amplifier and pClamp 10 software (Axon Instruments), low pass-filtered at 5 kHz, and digitized at 10 kHz. Series resistance was compensated to 85%. Data analysis, currents fitting and offline leak subtraction were performed in Clampfit 10 (Axon Instruments), and SigmaPlot 11 (Jandel Scientific). Current-voltage (IV) plots were fitted using a modified Boltzmann equation:
| 1 |
where E rev is the reversal potential, Gmax is the maximum slope conductance, k is the slope factor, and V a the half-activation potential.
For action potential (AP) recordings, current-clamp mode was used and APs were elicited by 1 min trains of short (2 ms) depolarizing current injections at a frequency of 1 Hz, the average of the last 30 APs was used for analysis. Intracellular solution for these recordings was (mM): 120 KCl, 1.5 MgCl2, 0.01 CaCl2, 0.5 EGTA, 10 HEPES, 5 MgATP and 0.6 LiGTP (pH 7.2 adjusted with KOH). Extracellular solution was: 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 Glucose and 10 HEPES (pH 7.4 adjusted with NaOH).
Measurement of myocyte shortening
Contractile properties of isolated ventricular myocytes were determined by measuring the fractional shortening of single cell cardiomyocyte at room temperature and superfused with external solution (mM): 100 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 90 sorbitol, 5 glucose and 10 HEPES (pH 7.4 adjusted with Tris-Base). Myocytes were field-stimulated with 2 ms pulses at 1 Hz. Cardiomyocyte shortening and relengthening was measured on the stage of an inverted microscope (Olympus IX-81, UPLFLN 40XO 40 x/1.3 oil-immersion objective) using a CCD-based imaging system in which the myocyte motion was collected and recorded at 12 Hz and analyzed with ImageJ.
Statistics
Data are presented as mean ± sem (n). Statistical analysis of the data was performed with SigmaPlot 11 (Jandel Scientific) using unpaired Student’s t-test, and was considered significant at P < 0.05. One-way ANOVA test was performed for samples exposed to multiple treatments and was considered significant at P < 0.05.
Results
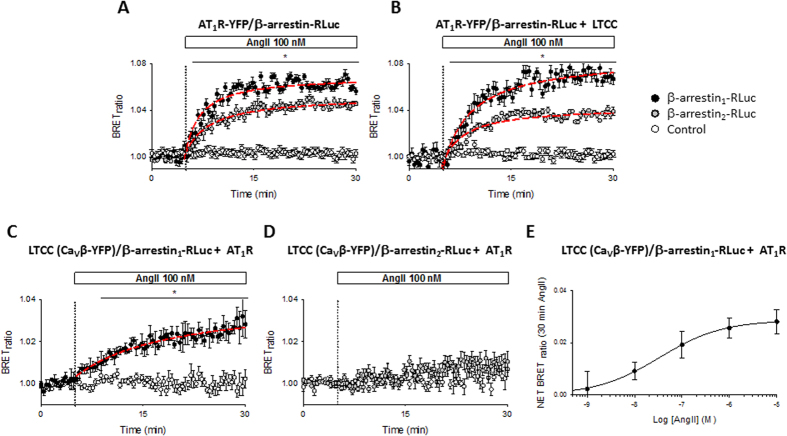
In live cells, β-arrestin recruitment to activated AT1R can be measured by the bioluminescence energy transfer (BRET) assay32. AngII stimulated AD-293 cells overexpressing β-arrestin1/Rluc or β-arrestin2/Rluc together with AT1R/YFP display an increased BRET signal within the first minutes of stimulation (Fig. 1A). Notably, overexpression of L-type calcium channels (CaV1.2, CaVβ and CaVα2δ) in the same cells did not change the BRET signal (Fig. 1B). Given the fact that BRET can only occur between particles located within ~100 Å from each other, this approach enables the study of the AngII-mediated interaction between β-arrestin and CaV1.2 channels in live cells. As shown in Supplementary Figure 2, a significant increase in BRET signal is observed upon AngII treatment only in those AD-293 cells expressing β-arrestin1/Rluc, AT1R and a YFP-tagged L-type calcium channel (CaV1.2/YFP, CaVβ and CaVα2δ), confirming the close proximity between CaV1.2 complexes and activated AT1R. However, C-terminal fusion of YFP to the CaV1.2 α subunit resulted in a relatively small BRET signal. Therefore, the experiment was modified to enhance the BRET signal by fusing YFP to the C-terminus of the CaVβ subunit (Fig. 1C and D), as CaVβ subunits has been shown to have physiological functions in the absence of CaVα subunits33. Besides the usual negative control (cells without the YFP construct), for these experiments we used cells expressing all the constructs, except CaV1.2 (β-arrestin1/Rluc, AT1R, CaVβ/YFP and CaVα2δ). Under this configuration, we detected significant BRET signal changes upon AngII stimulation in those cells expressing β-arrestin1/Rluc, AT1R and the YFP-tagged LTCC channel subunits (CaV1.2, CaVβ/YFP and CaVα2δ). Those cells lacking in CaV1.2 (Fig. 1C and D) or AT1R (not shown) revealed no detectable changes in BRET signal. Interestingly, we only observed an increase in BRET signal upon β-arrestin1 (Fig. 1C) but not with β-arrestin2 overexpression (Fig. 1D), even though AT1R activation is able to recruit both β-arrestin isoforms (Fig. 1A and B)34. Importantly, under our experimental conditions AngII increased the net BRET signal in a concentration-dependent manner with an apparent EC50 of 130 ± 50 nM (Fig. 1E, n = 3).
Figure 1.
BRET assay between AT1R or CaV1.2 channel and β-arrestins upon AngII stimulation. Time course of BRET signal ratio from AD-293 cells transfected with β-arrestin1-RLuc (black circles) or β-arrestin2-RLuc (grey circles) plus AT1R-YFP alone (A) or AT1R-YFP plus the L-type calcium channel (CaV1.2, CaVβ and CaVα2δ) (B). For negative controls (empty circles), AD-293 cells were transfected without the β-arrestins. Time course of BRET signal ratio from AD-293 cells transfected with β-arrestin1-RLuc (C) or β-arrestin2-RLuc (D) plus AT1R and a YFP-tagged L-type calcium channel (CaV1.2, CaVβ/YFP and CaVα2δ). For negative controls (empty circles), AD-293 cells were transfected without the CaV1.2 subunit. Red lines correspond to the best fit to a single rectangular hyperbola. The BRET records are averages of at least five independent experiments (n = 5–8). (E) Concentration-response curve of AngII-induced increase in net BRET signal in AD-293 cells transfected with β-arrestin1-RLuc plus AT1R and a YFP-tagged L-type calcium channel (CaV1.2, CaVβ/YFP and CaVα2δ), the line correspond to the best fit to Hill equation, n = 3. Mean values ± sem are shown.
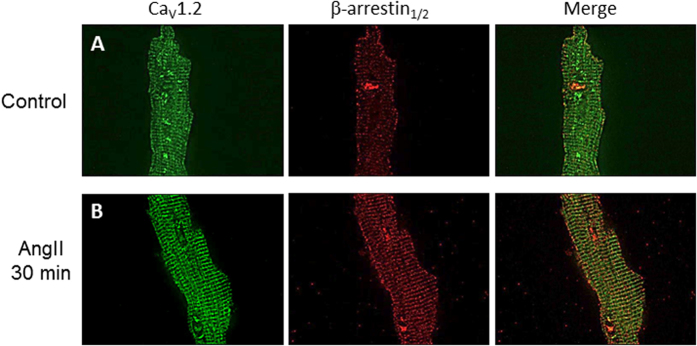
To confirm β-arrestin recruitment to the L-type channel macrocomplex in native cells, adult rat cardiomyocytes were isolated and stimulated with AngII. As expected for CaV1.2 immunostaining, L-type calcium channels were observed in a regularly spaced array consistent with the arrangement pattern of T-tubules, as well as surface sarcolemmal staining (Fig. 2A and Supplementary Figure 3) which highlights different populations of LTCC in cardiomyocytes35. Moreover, immunostaining with a β-arrestin1/2 antibody revealed that in the absence of agonist, these proteins are distributed along the T-tubules and the plasmalemma with a small cluster found into the nucleus (Fig. 2A). A higher amount of β-arrestin1/2 into the T-tubule network was readily observed upon addition of AngII (30 min, 1 µM, Fig. 2B). To estimate the degree of colocalization of both proteins at the plasma membrane we submitted our data to intensity correlation analysis30, a methodology that assumes that if two proteins colocalize, their fluorescence intensities should vary synchronically above the mean fluorescence (see methods). Intensity correlation analysis revealed an increase in the mean Intensity Correlation Quotient (ICQ) values for CaV1.2 and β-arrestin1/2 upon AngII stimulation (Control: 0.18 ± 0.05, n = 6; AngII: 0.29 ± 0.03, n = 8; p < 0.01), which suggests that β-arrestin is mobilized to the site of L-type calcium channel and thus, showing enhanced colocalization in acutely isolated rat cardiomyocytes.
Figure 2.
CaV1.2 and β-arrestin1/2 immunolocalization in adult cardiomyocytes. Representative confocal images of CaV1.2 (left) or β-arrestin (middle) immunofluorescences in cardiomyocytes control (A) or treated with AngII (1 µM) for 30 min (B), the overlay of individual images is shown on the right panel. All fluorescence images were collected at the same gain setting of the microscope. The images are representatives of 6 independent experiments.
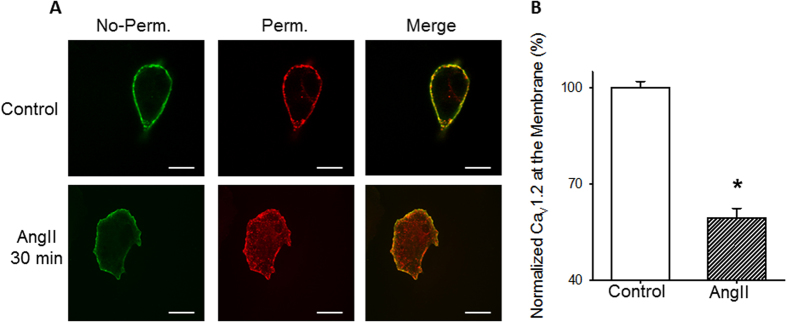
Binding of β-arrestin to AT1R results not only in the desensitization of the heteromeric G-protein response, but also in the recruitment of various components of the endocytic machinery which promotes internalization of the activated receptors from the cell surface36. To explore if binding of β-arrestin resulted in CaV1.2 internalization, we performed antibody feeding experiments (see methods) in AD-293 cells overexpressing an extracellularly tagged CaV1.2 subunit (HA-CaV1.2) and AT1R (Fig. 3). With this protocol, the channels that remained at the membrane were immunolabeled in no-permeabilized cells with a 488-green antibody, and the total of those channels initially localized at the cell surface (internalized plus those that remain at the membrane) immunolabeled with a 594-red antibody after permeabilized the cells with 0.5% triton, allowing for direct observation and relative quantification of internalized channels. Our results (Fig. 3) demonstrate a robust loss of CaV1.2 signal at the plasma membrane confirming that activation of AT1R indeed promotes the internalization of HA-tagged CaV1.2 channels.
Figure 3.
CaV1.2 internalization upon AngII exposure in overexpression system. (A) Confocal images of the distribution of HA-epitope-tagged CaV1.2 constructs in AD-293 cells over-expressing CaV1.2 (plus CaVβ and CaVα2δ) and AT1R before (top) or after (bottom) treatment with 1 µM AngII (30 min). (B) Summary bar graph showing the percentage of channels localized at the plasma membrane, normalized to control condition. n = 6. *p < 0.01 compared with control cells. No-perm, cells before permeabilization, representing the CaV1.2 channels that remain at the plasma membrane; Perm, cell permeabilized that represent the total of channels localized at the cell surface at the beginning of the experiment.
Likewise, immunostaining of CaV1.2 in rat cardiomyocytes treated with AngII, at different time points, revealed a gradual removal of CaV1.2 channel from the T-tubules (Supplementary Figure 3 and Fig. 4). This was significant in rat cardiomyocytes treated with AngII (1 µM) for 1 hr (Fig. 4B and D) where predominantly surface sarcolemmal staining is observed. In contrast, rat cardiomyocytes simultaneously treated with a specific AT1R inhibitor (Losartan, 100 nM, 1 hr) and AngII were undistinguishable from unstimulated cells (99.8 ± 5.5%, n = 4, Fig. 4C). These results suggest that only the LTCC population localized at the T-tubules is susceptible to internalization after prolonged AT1R activation.
Figure 4.
CaV1.2 internalization in rat cardiomyocytes treated with AngII. Representative confocal images of CaV1.2 immunofluorescence in rat cardiomyocytes, control (A) or treated with AngII (1 µM) for 1 hr without (B) or with (C) losartan (100 nM). All fluorescence images were collected at the same gain setting of the microscope, nucleus stained with DAPI. (D) Intracellular CaV1.2 immunofluorescence staining intensity measurements normalized to control cardiomyocytes (n = 6–10 for each condition) from cardiomyocytes treated with AngII (1 µM) for different time points. *p < 0.01 with respect to control.
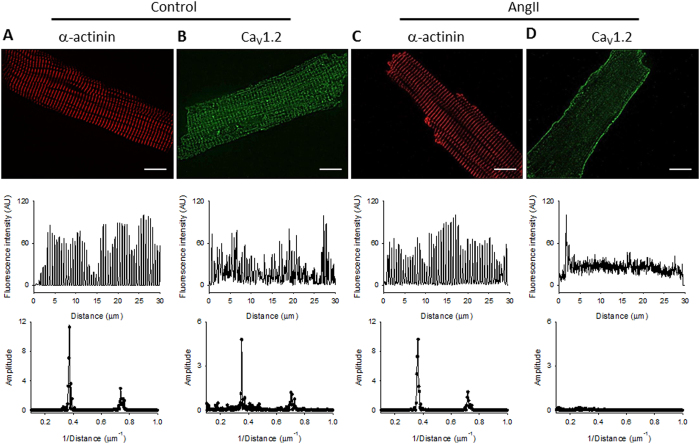
Control experiments involving α-actinin staining ruled out cardiomyocyte detubulation as an explanation of CaV1.2 immunostaining loss after AngII treatment. In brief, an α-actinin antibody was used (Fig. 5) and the spacing between individual T-tubules was assessed by the power spectrum of each image after a fast Fourier transform of the signal. Thus, the power spike as a function of the spatial frequency describes the average distance between individual Z-discs in the cell (Control: 2.71 ± 0.06 μm; AngII: 2.56 ± 0.11 μm, n = 5; NS), demonstrating that α-actinin immunostaining was independent of AngII treatment in our experimental conditions (Fig. 5A and C). Likewise, the average distance between CaV1.2 immunostaining bands obtained in control cardiomyocytes (2.77 ± 0.03 μm, n = 5; Fig. 5B), or in CaV1.2 immunostaining treated with AngII and losartan (2.67 ± 0.10 μm, n = 4; Supplementary Figure 5) were identical, in contrast, the fast Fourier transformation of CaV1.2 immunostaining from cardiomyocytes treated with AngII lacks in a peak (Fig. 5D), confirming that normal spacing of L-type channels is lost in these cardiomyocytes.
Figure 5.
T-tubule integrity in rat cardiomyocytes treated with AngII. Top: representative confocal images of rat ventricular cells stained with an anti-α-actinin antibody (A,C) or an anti-CaV1.2 antibody (B,D). Middle: Fluorescence intensity profiles, in arbitrary units, along the longitudinal axis of each cardiomyocytes. Bottom: graphs of the fast Fourier transformation of the fluorescence profile. (A,B) control cardiomyocytes; (C,D) cardiomyocytes treated with AngII (1 µM) for 1 hr.
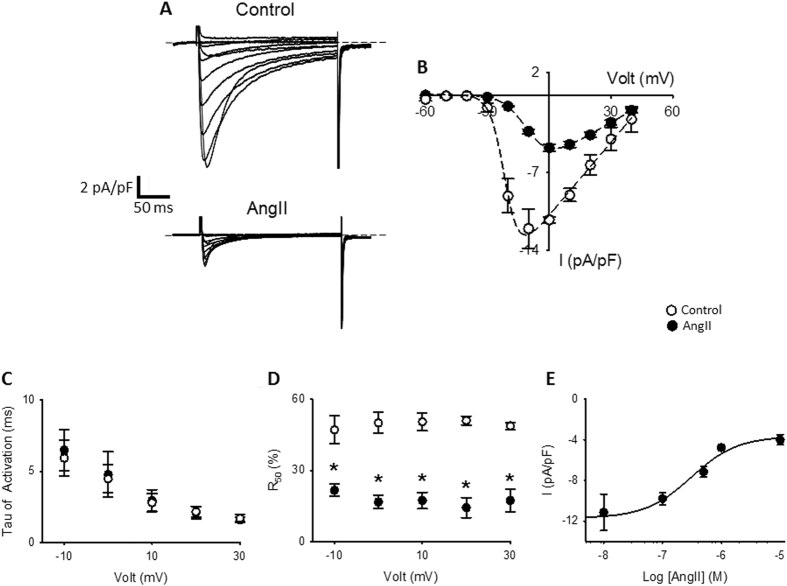
To test if the AT1R activation has functional consequences on CaV1.2, we explored the effect of AngII on endogenous L-type calcium currents in adult rat cardiomyocytes. As seen in Fig. 6A, AngII treatment (1 hr, 1 µM) induces a decrease of almost 60% of L-type calcium currents (Imax, Control 12.1 ± 1.8 pA/pF, n = 8; Imax, AngII 4.8 ± 0.4 pA/pF, n = 10; p < 0.05) and a ~10 mv right-shift in the channel voltage dependence of activation as seen in representative I-V plots (Fig. 6B). L-type current kinetics were studied by fitting a single exponential to the rising phase (activation) or by setting up the residual current after 50 ms of pulse (R50) at different voltages (inactivation). We did not observe differences in the activation kinetics (Fig. 6C); however, cardiomyocytes treated with AngII displayed a faster inactivation kinetics as indicated by a decreased R50 at all voltage tested (Fig. 6D). In agreement with our BRET experiments (Fig. 1E), a concentration dependent response, with an apparent IC50 of ~300 nM was observed for L-type current reduction (Fig. 6E, n = 4–10).
Figure 6.
Endogenous L-type currents from cardiomyocytes treated with AngII. (A) Representative whole-cell endogenous L-type Ca2+ current traces from cardiomyocytes control (upper traces) or treated with AngII (1 µM) for 1 hr (lower traces). Currents elicited by a voltage step protocol from –60 mV to + 50 mV in 10 mV increments, Vh = −80 mV. (B) Summary peak current I/V plots (mean ± sem) obtained from currents family as shown in (A), black line represents the best fit to a Bolztman equation (see methods). (C) Voltage-dependence of the activation time constant (τact, mean ± sem) of L-type Ca2+ currents (D) Graph showing the residual current after 50 ms of the depolarization pulse (R50, mean ± sem) versus command voltage of L-type Ca2+ currents. Faster inactivation rates result in lower R50 values. (E) Concentration-response curve of AngII-induced L-type calcium current reduction in rat cardiomyocytes, the line correspond to the best fit to Hill equation, mean values ± sem are shown. In every panel, control rat cardiomyocytes are represented with empty circles and rat cardiomyocytes treated with AngII with filled circles. (n = 4–10) *p < 0.01 with respect to control.
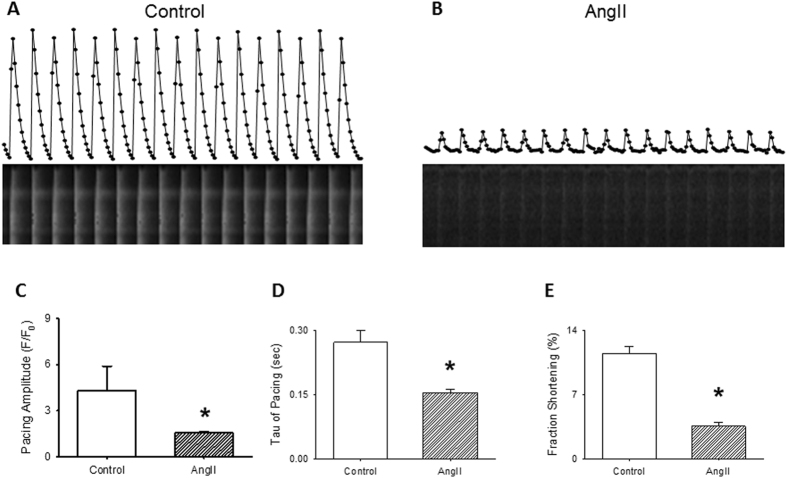
A reduction in L-type currents is predicted to have an impact on the magnitude and/or frequency of calcium transients and consequently, on cardiomyocyte contraction. To directly investigate this, we evaluated the effect of AngII on calcium mobilization and fractional shortening. Intracellular calcium was monitored in rat cardiomyocytes loaded with Fluo-4 and paced extracellularly with a 5 ms pulse at a frequency of 1 Hz. Figure 7 illustrates representative intracellular calcium transients recorded in adult rat cardiomyocytes, in control, and treated condition (AngII 1 µM, 1 hr). As shown, calcium transients from rat cardiomyocytes exposed to AngII displayed a faster transient decay as compared to control (extracted from single exponential fits to the Ca2+ transient decay; Fig. 7D) suggesting that AngII also modifies SERCA activity28. As expected from the reduced L-type calcium currents recorded, decreased calcium transient amplitudes are evident in rat cardiomyocytes treated with AngII (63.7 ± 1.3% reduction, Fig. 7C, p < 0.05) together with slower time-to-peak. Moreover, fractional shortening assessment reveals that in rat cardiomyocytes stimulated at a frequency of 1 Hz and treated with AngII, the fractional shortening was reduced to 30.7 ± 3.7% (Fig. 7E), consistent with the observed reduction of the amplitude of calcium transients (Fig. 7C).
Figure 7.
Calcium transients in cardiomyocytes treated with AngII. Representative normalized fluorescence (Fluo-4) recordings from cardiomyocytes control (A) or treated with AngII (1 µM) for 1 hr (B) and stimulated with a 5 ms external stimulus (1 Hz), individual points represent the signal for individual frames, acquire at 12 Hz. Below each fluorescence recording the respective line-scan images are shown. Bar graph (mean ± sem) of average maximal amplitude of electrically evoked Ca2+ transients (C) or time constant of Ca2+ decay (D) in cardiomyocytes control or treated with AngII (1 µM) for 1 hr. Data obtained after fitting individual calcium transients to a single exponential (n = 30–40, from 4 different cardiomyocytes preparations). *p < 0.01 with respect to control. (E) Bar graph (mean ± sem) of fractional shortening. For each bar graph, empty bars represents cardiomyocytes control and hatched bar cardiomyocytes treated with AngII (1 µM) for 1 hr. *p < 0.01 with respect to control.
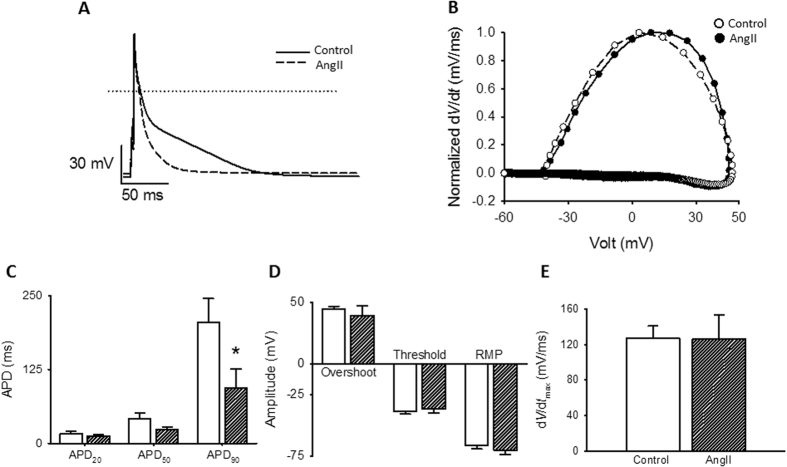
Finally, action potentials are the result of the concerted activation of many ion channels, each of which defines distinct aspects of its shape. Careful inspection of APs confers an integrative manner to investigate putative changes in other voltage-dependent ion channels as a result of AngII prolonged stimulation. Action potential duration from ventricular cardiomyocytes was determined as the time where 20%, 50% or 90% of repolarization was attained (APD20, APD50 and APD90, respectively). Representative traces of superimposed APs from rat cardiomyocytes in control and after AngII treatment are displayed in Fig. 8A. Quantification of the repolarization phase shows significant differences at APD90 for AngII treated cardiomyocytes (Fig. 8C) suggesting that AngII treatment increases potassium currents in these cells. To examine other properties of the elicited action potentials, phase-plane plots were generated by graphing the voltage derivative in time (dV/dt) versus voltage as shown in Fig. 8B. AngII treatment does not produce significant alterations in the threshold potential, the depolarization slope or the maximal depolarization voltage. Furthermore, the resting membrane potential (RMP) remains unaltered after AngII treatment (Fig. 8D and E) which evidences lack of modification of voltage dependent sodium currents by the current experimental manipulations.
Figure 8.
Action potentials from cardiomyocytes treated with AngII. (A) Representative action potentials waveforms from cardiomyocytes control (solid line) or treated with AngII (1 µM) for 1 hr (dashed line). Action potentials were elicited with a short (2 ms) depolarizing current injections (200–400 pA) at a frequency of 1 Hz. (B) Phase plot of the first derivative of membrane potential (dV/dt) against membrane potential (Vm) for the action potentials shown in (A). Control, empty symbols; AngII-treated, filled symbols. (C) Bar graphs (mean ± sem) of average action potentials duration (APD) estimated at 20%, 50% and 90% repolarization, APD20, APD50 and APD90, respectively. (D) Bar graph (mean ± sem) of maximal action potential amplitudes (Overshoot), threshold potentials and mean resting membrane potentials (RMP). (E) Bar graph (mean ± sem) of maximum rate of potentials change (dV/dt). For each bar graph, empty bars represents cardiomyocytes control and hatched bar cardiomyocytes treated with AngII (1 µM) for 1 hr. (n = 7–10) *p < 0.01 with respect to control.
Discussion
GPCR-ion channel macrocomplexes are thought to optimize transductional coupling by preventing the diffusion of signaling molecules and by modulating ion channel trafficking, either through regulation of channel surface expression or by mediating receptor-dependent internalization. The internalization process is slower than the activation of heteromeric G proteins activation (that usually occurs within seconds) and involves β-arrestin binding to phosphorylated receptors37.
The acute effects of AngII on L-type currents in cardiomyocytes have been extensively studied and appear to be species specific20, 24, to depend on the experimental approach used23 and/or on the different signaling molecules expressed27, 38, 39. In contrast, the prolonged effects of AngII on LTCC have received less attention. Prolonged AngII stimulation has been shown to increase the amount L-type current in newborn but not in adult mice cardiomyocytes40. In HL-1 myocytes, although there is a consensus that prolonged AngII-stimulation also induces an increase of L-type current, the reason for this is controversial, with a report showing a main role for β-arrestin2 40 and other showing a CREB-dependent de novo expression of CaVα1C 41. Moreover, a recent study in canine cardiomyocytes suggested a dependence on the transmural muscular layer42, suggesting the same species-specificity as for acute AngII stimulation.
Here, we show that in cardiomyocytes from adult rat AngII increases β-arrestin1 recruitment to L-type Ca2+ channel and reduces L-type Ca2+ current in a concentration-dependent manner, both processes with an EC50 and an IC50 in the order of ~100 nM, respectively (Figs 1E and 6E). This AngII concentration is orders of magnitude greater than the concentration of AngII found in plasma (in the order of pM). Nevertheless, it is in good agreement with recent literature showing that prolonged high-doses of AngII produce changes at the single cardiomyocyte level42, 43. This difference suggests that local RAS may play a fundamental role in this process since plasma levels of AngII are too low to efficiently modify LTCC levels located at the T-tubules. In fact, recent estimates of AngII levels in canine and rat T-tubules are in the order of 100 nM42, 44. Thus, the local RAS at the heart45 could be important for electrically remodeling cardiomyocytes from different muscle layers, generating regional heterogeneities in action potential morphology and contractility46. Similarly, our experiments show that prolonged stimulation with AngII leads the internalization of the L-type channel population located at the T-tubules membrane (Fig. 4).
It is generally agreed that the effects of AngII in the vasculature occur at low levels of this hormone which can be used to distinguish them from the effect of AngII in the cardiac system. This can be explained by differences in receptor affinity either by post translational modification or by the formation of tissue-specific macrocomplexes involving yet unidentified protein partners. However, more work is required to establish a clearer mechanistic understanding of this process.
It should be noted that despite differences in the magnitude of the BRET signals obtained with different constructs (Fig. 1 and Supplementary Figure 2), the overall trend was consistent with a probable LTCC-β-arrestin1 interaction. These results are supported by immunostaining data obtained from adult rat cardiomyocytes (Fig. 2). We cannot ascertain the underlying reason for the difference in BRET signal amplitude, however some weight could be assigned to potentially different orientation of the energy transfer partners as the BRET signal depends on the orientation between donor and acceptor.
More interestingly, the BRET experiments reveal a preference for β-arrestin1 recruitment after AT1R activation as compared to β-arrestin2. When activated, the AT1R recruits both β-arrestin isoforms (Fig. 1), but ERK signaling pathway activation only occurs when β-arrestin2 but not β-arrestin1 is recruited47. Thus, AT1R interaction with CaV1.2 may represent a novel form of GPCR-dependent signaling regulation in which the presence of the macromolecular complex controls the specific recruitment of β-arrestin isoforms. Understandably, this hypothesis awaits experimental corroboration.
Functionally, chronic activation of AT1R leads to the internalization of CaV1.2 in a heterologous expression system (Fig. 3) and in ventricular rat cardiomyocytes (Fig. 4). Interestingly, internalization of CaV1.2 in ventricular cardiomyocytes appears to be restricted to the population of channels present at the T-tubules known to mediate the calcium-induced calcium release process and the plateau phase of action potentials. In turn, the plasmalemma channel counterpart known to be involved in gene transcription regulation, seems unchanged by chronic stimulation with AngII. This subcellular loci-specific difference could be explained by the selective localization of AT1R at the T-tubules48 supporting the idea of the existence of an AT1R/CaV1.2 macrocomplex in these cells.
As expected from the loss of CaV1.2 signal at the T-tubule, L-type calcium currents are greatly reduced in cardiomyocytes treated with AngII (Fig. 6A). Interestingly, the remaining current display a 10 mV right-shift in the I/V curve and a faster inactivation kinetics (Fig. 6B and D), which could be a consequence either of post-translational modification of the L-type channels (as oxidation or phosphorylation due to AT1R activation) or, as the observed current depends mainly on the LTCC present at the plasmalemma, an indicative that the different population of LTCC between the T-tubules and the plasmalemma bears different auxiliary subunits that grant them with differential biophysical characteristics. Nevertheless, in accordance with the diminished calcium current, the calcium transient amplitudes (Fig. 7C) and cardiomyocyte shortening (Fig. 7E) are similarly decreased. Nonetheless, AngII treatment is not specific for L-type channels, as Ca2+-reuptake is faster in AngII-treated cardiomyocytes (Fig. 7D) likely implicating higher activity of SERCA, suggesting that AngII treatment could induce more profound changes in calcium handling than those related to mere modification of the L-type current.
Furthermore, action potential measurements (Fig. 8) allowed the monitoring of the coordinated behavior of various voltage-dependent ion channels. Despite a relatively low dependence of rat ventricular action potentials on L-type calcium currents, when compared with other species like rabbits or guinea pigs, the fact that the voltage threshold (Fig. 8D) and maximal dV/dt (Fig. 8E) were unaltered by AngII treatment clearly indicates that voltage-dependent Na+ channels were unaffected. Likewise, unchanged resting membrane potential in AngII-treated cardiomyocytes suggests that “leak” K+ channels are also unmodified. Both observations demonstrate that AngII-induced CaV1.2 channel internalization does not imply an unselective effect on ion channels. In contrast, changes in APD90 suggest that in these tissues, a voltage-dependent K+-channel is activated by chronic AT1R stimulation which is yet to be identified.
Concerning the fate of the CaV1.2 channels after prolonged AngII stimulation, those channels could be targeted either for degradation or for recycling after its internalization. In fact, a closer look of the plasmalemma-arising signal of rat cardiomyocytes treated with AngII (Figs 4 and 5 and Supplementary Figure 4) indicates a more continuous signal at the membrane edge when stained with a CaV1.2 antibody, suggesting that at least part of the channels originally in the T-tubule network could be re-targeted to the plasmalemma. At the same time, as depicted from the signal loss of CaV1.2 total intensity when AngII-treated cardiomyocytes are compared with control cardiomyocytes, part of the channels appear to be directed to degradation.
AT1R activation is well known to activate a series of intracellular signaling pathways49, those channels remaining at the membrane are likely to undergo posttranslational modification, such as PKC or CaMKII-dependent phosphorylation. Interestingly, these signals are usually associated with activation of the L-type current50, 51, suggesting that after prolonged AngII stimulation the channels in the plasmalemma, usually associated with gene transcription, are more active emphasizing the role of the process described here in cardiac remodeling.
In summary, we demonstrate a novel signaling pathway involving AT1R activation and LTCC. This AngII-dependent intracellular signaling pathway selectively recruits β-arrestin1 after receptor activation, promoting internalization of CaV1.2 channels. In adult rat cardiomyocytes, the AngII driven internalization of CaV1.2 occurs almost exclusively at the T-tubules leading to a significant reduction of L-type calcium current and calcium transient amplitudes. Functionally, the results presented here provide insights into the mechanisms by which prolonged AngII exposure causes cardiac remodeling and could have major implications for understanding the molecular mechanisms controlling the electrical transmural gradient observed in the heart.
Electronic supplementary material
Acknowledgements
We are thankful to Rocio K. Finol-Urdaneta, Jeffrey R. McArthur, Luis Michea and Andres Stutzin for constructive discussion of the manuscript. We thank to Iniciativa Cientifica Milenio. This work was supported by research grants from Fondo Nacional de Desarrollo Científico y Tecnológico (Fondecyt) 1120240 and 1160900 to DV; Fondecyt 1121078 and Millennium Institute on Immunology and Immunotherapy P09-016-F to FS. CA holds a Tier2 Canada Research Chair, and is funded by both the Canadian Institutes of Health Research (CIHR) and Natural Sciences and Engineering Research Council (NSERC).
Author Contributions
D.V., T.H., C.A., designed the project. T.H., M.E., D.M., C.M., H.M.A.V., C.C., F.L.-M. and D.V. performed the experiments. D.V., T.H. and F.S. analyzed the data. D.V. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Tamara Hermosilla and Matías Encina contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10474-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruiz-Ortega M, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 2.Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. The Journal of biological chemistry. 1998;273:17299–17302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 3.Miura S, Saku K, Karnik SS. Molecular analysis of the structure and function of the angiotensin II type 1 receptor. Hypertension research: official journal of the Japanese Society of Hypertension. 2003;26:937–943. doi: 10.1291/hypres.26.937. [DOI] [PubMed] [Google Scholar]
- 4.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 5.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Frontiers in bioscience. 2011;16:3047–3060. doi: 10.2741/3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee FJ, et al. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/S0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 7.Shukla AK, et al. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. The Journal of biological chemistry. 2010;285:30115–30125. doi: 10.1074/jbc.M110.141549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. The Journal of biological chemistry. 2004;279:48231–48237. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 9.Beedle AM, et al. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nature neuroscience. 2004;7:118–125. doi: 10.1038/nn1180. [DOI] [PubMed] [Google Scholar]
- 10.Park HW, Jung H, Choi KH, Baik JH, Rhim H. Direct interaction and functional coupling between voltage-gated CaV1.3 Ca2+ channel and GABAB receptor subunit 2. FEBS letters. 2010;584:3317–3322. doi: 10.1016/j.febslet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Kisilevsky AE, et al. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 2008;58:557–570. doi: 10.1016/j.neuron.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Kisilevsky AE, Zamponi GW. D2 dopamine receptors interact directly with N-type calcium channels and regulate channel surface expression levels. Channels. 2008;2:269–277. doi: 10.4161/chan.2.4.6402. [DOI] [PubMed] [Google Scholar]
- 13.Altier C, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nature neuroscience. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 14.Simms BA, Zamponi GW. Trafficking and stability of voltage-gated calcium channels. Cellular and molecular life sciences: CMLS. 2012;69:843–856. doi: 10.1007/s00018-011-0843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best JM, Kamp TJ. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. Journal of molecular and cellular cardiology. 2012;52:376–387. doi: 10.1016/j.yjmcc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beech DJ. Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacol Ther. 1997;73:91–119. doi: 10.1016/S0163-7258(97)87271-3. [DOI] [PubMed] [Google Scholar]
- 17.Quignard JF, et al. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. The Journal of biological chemistry. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- 18.Seki T, Yokoshiki H, Sunagawa M, Nakamura M, Sperelakis N. Angiotensin II stimulation of Ca2+ -channel current in vascular smooth muscle cells is inhibited by lavendustin-A and LY-294002. Pflugers Archiv: European journal of physiology. 1999;437:317–323. doi: 10.1007/s004240050785. [DOI] [PubMed] [Google Scholar]
- 19.Callewaert G, Hanbauer I, Morad M. Modulation of calcium channels in cardiac and neuronal cells by an endogenous peptide. Science. 1989;243:663–666. doi: 10.1126/science.2536955. [DOI] [PubMed] [Google Scholar]
- 20.Ikegami H, Endoh T, Suzuki T. Angiotensin II-induced inhibition of calcium currents in hamster submandibular ganglion neurons. Neuroscience research. 2001;41:227–232. doi: 10.1016/S0168-0102(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 21.Maturana AD, et al. Angiotensin II negatively modulates L-type calcium channels through a pertussis toxin-sensitive G protein in adrenal glomerulosa cells. The Journal of biological chemistry. 1999;274:19943–19948. doi: 10.1074/jbc.274.28.19943. [DOI] [PubMed] [Google Scholar]
- 22.De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension. 2004;44:360–364. doi: 10.1161/01.HYP.0000139914.52686.74. [DOI] [PubMed] [Google Scholar]
- 23.Ichiyanagi O, Ishii K, Endoh M. Angiotensin II increases L-type Ca2 + current in gramicidin D-perforated adult rabbit ventricular myocytes: comparison with conventional patch-clamp method. Pflugers Archiv: European journal of physiology. 2002;444:107–116. doi: 10.1007/s00424-002-0808-y. [DOI] [PubMed] [Google Scholar]
- 24.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. American journal of physiology. Heart and circulatory physiology. 2001;280:H1528–1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 25.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:976–982. doi: 10.1161/01.HYP.32.6.976. [DOI] [PubMed] [Google Scholar]
- 26.Bkaily G, et al. Angiotensin II-induced increase of T-type Ca2+ current and decrease of L-type Ca2+ current in heart cells. Peptides. 2005;26:1410–1417. doi: 10.1016/j.peptides.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Hermosilla T, et al. L-type calcium channel beta subunit modulates angiotensin II responses in cardiomyocytes. Channels. 2011;5:280–286. doi: 10.4161/chan.5.3.15833. [DOI] [PubMed] [Google Scholar]
- 28.Moreno C, et al. Cavbeta2 transcription start site variants modulate calcium handling in newborn rat cardiomyocytes. Pflugers Archiv: European journal of physiology. 2015;467:2473–2484. doi: 10.1007/s00424-015-1723-3. [DOI] [PubMed] [Google Scholar]
- 29.Altier C, et al. The Cavbeta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nature neuroscience. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, et al. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varela D, Niemeyer MI, Cid LP, Sepulveda FV. Effect of an N-terminus deletion on voltage-dependent gating of the ClC-2 chloride channel. The Journal of physiology. 2002;544:363–372. doi: 10.1113/jphysiol.2002.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauliere A, et al. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nature chemical biology. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann F, Belkacemi A, Flockerzi V. Emerging Alternative Functions for the Auxiliary Subunits of the Voltage-Gated Calcium Channels. Curr Mol Pharmacol. 2015;8:162–168. doi: 10.2174/1874467208666150507110202. [DOI] [PubMed] [Google Scholar]
- 34.Sanni SJ, et al. beta-Arrestin 1 and 2 stabilize the angiotensin II type I receptor in distinct high-affinity conformations. British journal of pharmacology. 2010;161:150–161. doi: 10.1111/j.1476-5381.2010.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw RM, Colecraft HM. L-type calcium channel targeting and local signalling in cardiac myocytes. Cardiovascular research. 2013;98:177–186. doi: 10.1093/cvr/cvt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nature reviews. Molecular cell biology. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava A, Gupta B, Gupta C, Shukla AK. Emerging Functional Divergence of beta-Arrestin Isoforms in GPCR Function. Trends in endocrinology and metabolism: TEM. 2015;26:628–642. doi: 10.1016/j.tem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Weiss S, Doan T, Bernstein KE, Dascal N. Modulation of cardiac Ca2+ channel by Gq-activating neurotransmitters reconstituted in Xenopus oocytes. The Journal of biological chemistry. 2004;279:12503–12510. doi: 10.1074/jbc.M310196200. [DOI] [PubMed] [Google Scholar]
- 39.Conforti L, Sumii K, Sperelakis N. Dioctanoyl-glycerol inhibits L-type calcium current in embryonic chick cardiomyocytes independent of protein kinase C activation. Journal of molecular and cellular cardiology. 1995;27:1219–1224. doi: 10.1016/0022-2828(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 40.Kashihara T, Nakada T, Kojima K, Takeshita T, Yamada M. Angiotensin II activates CaV 1.2 Ca2+ channels through beta-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. The Journal of physiology. 2017;595:4207–4225. doi: 10.1113/JP273883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai CT, et al. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 42.Gao J, et al. Autocrine A2 in the T-system of ventricular myocytes creates transmural gradients in ion transport: a mechanism to match contraction with load? Biophysical journal. 2014;106:2364–2374. doi: 10.1016/j.bpj.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Gao J, Cohen IS, Mathias RT. Angiotensin II Type 1 Receptor-Mediated Electrical Remodeling in Mouse Cardiac Myocytes. PloS one. 2015;10 doi: 10.1371/journal.pone.0138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saris JJ, et al. Prorenin-induced myocyte proliferation: no role for intracellular angiotensin II. Hypertension. 2002;39:573–577. doi: 10.1161/hy0202.103002. [DOI] [PubMed] [Google Scholar]
- 45.van Kats JP, et al. Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusions. Circulation. 1998;98:73–81. doi: 10.1161/01.CIR.98.1.73. [DOI] [PubMed] [Google Scholar]
- 46.Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovascular research. 1993;27:1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- 47.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. The Journal of biological chemistry. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama M, et al. Chronic ventricular myocyte-specific overexpression of angiotensin II type 2 receptor results in intrinsic myocyte contractile dysfunction. American journal of physiology. Heart and circulatory physiology. 2005;288:H317–327. doi: 10.1152/ajpheart.00957.2003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang P, Mende U. Regulators of G-protein signaling in the heart and their potential as therapeutic targets. Circ Res. 2011;109:320–333. doi: 10.1161/CIRCRESAHA.110.231423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swaminathan PD, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, et al. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c) Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.