Abstract
Thyroid hormone responsive spot 14 (THRSP) is a small nuclear protein that responds rapidly to thyroid hormone. It has been shown that THRSP is abundant in lipogenic tissues such as liver, fat and the mammary gland in mammals. The THRSP gene acts as a key lipogenic activator and can be activated by thyroid hormone triiodothyronine (T3), glucose, carbohydrate and insulin. Here we report that chicken THRSP is also abundant in lipogenic tissues including the liver and the abdominal fat, and its expression levels increased with sex maturation and reached the highest level at the peak of egg production. Structure analysis of the THRSP gene indicates that there is a conscious estrogen response element (ERE) located in the −2390 – −2402 range of the gene promoter region. Further studies by ChIP-qPCR proved that the ERα interacts with the putative ERE site. In addition, THRSP was significantly upregulated (P < 0.05) when chickens or chicken primary hepatocytes were treated with 17β-estradiol in both the in vivo and in vitro conditions. We therefore conclude that THRSP is directly regulated by estrogen and is involved in the estrogen regulation network in chicken.
Introduction
Spot 14, also referred to as thyroid hormone responsive spot 14 (THRSP), was originally identified in rat liver1. Immunoprecipitation and tissue distribution studies demonstrated that THRSP is primarily located in the nucleus and abundant only in lipogenic tissues such as liver, fat and the mammary gland2, 3. The THRSP is a small nuclear protein that is rapidly upregulated by thyroid hormone triiodothyronine (T3) signals that induce lipogenesis4. In addition, glucose, carbohydrate, polyunsaturated fatty acids and insulin can also influence and regulate THRSP transcription5. It has been shown that THRSP, as an important transcription factor in hepatocytes, is a lipogenic activator that controls the expression of several lipogenic genes6–8. Hepatocytes transfected with a THRSP antisense oligonucleotide express decreased mRNA levels in enzymes, such as ATP-citrate lyase (ACLY), fatty acid synthase (FASN) and malic enzyme (ME), involved in the lipogenic pathway8–10. Furthermore, an increase in lipogenesis was observed in the THRSP knockout mouse and THRSP overexpression increased mRNA levels of FASN in bovine mammary epithelial cells11.
The chicken THRSP gene was first identified by microarray analysis as an expressed sequence tags (EST), and subsequently mapped to 1q41–4412. This chromosomal region in chickens also harbors quantitative trait loci (QTL) for skin and abdominal fatness13. Comparative sequence analysis among mammalian and chicken THRSP genes demonstrated a similar gene organization with 2 exons and an intron, but the chicken THRSP peptide had a low similarity (less than 30% of identities and 50% of positives) to the THRSP amino acid sequences of mammals and fishes14, 15. Furthermore, the chicken THRSP genes appear to be predominantly expressed in lipogenic tissues such as the liver and abdominal fat of feeding nestling broiler chickens, and the polymorphisms in the coding regions of the paralogous genes were associated with the abdominal fatness of chickens12, 14.
Nuclear hormone receptors are ligand-activated transcription factors that regulate gene expression by interacting with specific DNA sequences upstream of the target genes. Thyroid hormone is secreted by the thyroid gland and plays an important role in the hypothalamus-pituitary-thyroid axis. Thyroid hormones function through a group of nuclear receptors, namely the thyroid hormone receptors (TRs), which interact with the hormone receptor elements (TREs) upstream of target genes. As a thyroid hormone responsive protein, three positive TREs were identified upstream of the THRSP gene in mammalian systems16–18. Moreover, there are also carbohydrate response elements (ChoRE)4 and sterol response elements (SRE)19, 20 in the promoter of the THRSP gene. The estrogen receptors (ERs) are also nuclear hormone receptors, mediate the physiological actions of estrogen and regulate the transcription of target genes21, 22 by forming dimers with estrogen and binding to a canonical 13-bp ERE, GGTCANNNTGACC (N represents any of the nucleotides), a palindromic inverted repeat separated by any three nucleotides (NNN)23, 24. Estrogen is vitally important for sexual maturity and the development of the female reproductive system25, 26.
Our previous work on the transcriptome of the liver in chickens showed that the THRSP gene was one of the differently expressed genes, which increased by 6.24-fold in peak-laying hens (30 weeks old) compared with pre-laying hens (20 weeks old)27. It was generally suggested that the upregulated genes in the livers of laying hens were regulated by estrogen and involved in lipid metabolism given that in laying hens plasma estrogen was relative higher than in immature pullets for certain periods28, 29. Additionally, more lipoproteins, mainly very low density lipoproteins and vitellogenins, were synthesized from the hen’s liver and transported to rapidly growing oocytes30. Many differently expressed key lipid metabolism genes in our transcriptome were reported to be involved in the estrogen regulation network, such as VTG2, ApoB and very low density apolipoprotein II (APOV1). The VTG2 is a member of a small gene family encoding the yolk precursor proteins, the expression of which is strictly dependent on estrogen31–33. The mature APOV1 mRNA appears in chicken liver within a few hours after estrogen administration34. Moreover, the ERE sequence was originally identified by the conserved sequence of the estrogen-sensitive Xenopus laevis VTG2 and the chicken APOV1 genes24. THRSP has been postulated to be a lipogenic activator; however, whether the expression of THRSP is regulated by estrogen in the liver is unknown. Therefore, the objective of the present study was to investigate regulatory mechanisms of THRSP in the liver of laying hens.
Materials and Methods
Ethics Statement
The birds used in this study were Lushi green-shelled-egg chickens that were obtained from the Animal Center of Henan Agricultural University. The birds were raised in similar environmental conditions with food and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Henan Agricultural University Zhengzhou, P.R. China (Permit Number: 11–0085) and performed in accordance with the protocol outlined in the “Guide for Care and Use of Laboratory Animals” (Henan Agricultural University). All efforts had been done to decrease animals suffering. All birds were euthanized with pentobarbital.
Animals, treatment and sampling
To study the expression profiles of THRSP, 6 birds each at different development stages (10, 15, 20, 30 and 35 weeks old) were euthanized. A total of 11 tissues including the heart, liver, pectoral muscle, kidney, adrenal gland, spleen, abdominal fat, duodenum, glandular stomach, pancreas and lung were quickly removed, snap-frozen in liquid nitrogen and stored at −80 °C freezer until analysis.
To investigate the effect of 17β-estradiol on the expression of THRSP, a total of 70 birds at 10 weeks old were divided randomly into 7 groups, with 10 birds in each group. The first 3 groups of birds were injected intramuscularly (chest muscle) with 0.5 mg, 2.0 mg or 8.0 mg of 17β-estradiol (Sigma, St Louis, MO, USA) (dissolved in olive oil)/kg of body weight, respectively. The birds were euthanized 12 hours after treatment. Another 3 groups of birds were also injected with the same amounts of 17β-estradiol as it was done in the first 3 groups, but euthanized 24 hours after treatment. The last group of birds was served as control and injected with the same amount of solvent only. The liver and adipose tissues were collected and stored as mentioned above.
Primary hepatocyte culture and treatment
The method of chicken primary hepatocyte isolation was the same as described previously35, 36. In brief, hepatocytes were isolated from 18-day chicken embryonic livers. A total of 1 × 106 cells/well was seeded into 6-well plates. When the cells reached 90% confluence, cell culture medium was replaced by fresh serum-free DMEM medium containing 100 mg/mL streptomycin and 100 U/mL penicillin, and incubated for 6 hours. Cells were divided into four groups with triplicates, and were treated with 17β-estradiol dissolved in 0.1% ethanol at a final concentration of 25 nM, 50 nM or 100 nM, respectively. Cells treated with ethanol alone were used as controls. Cell culture medium was removed 24 hours after initial culture, washed twice with PBS, and followed by the addition of 1 mL of Trizol reagent (Takara, Kyoto, Japan) in each well. Cells were then collected by a cell scraper, and stored at −80 °C until use.
To further demonstrate that the expression of THRSP is regulated by estrogen, primary hepatocytes were treated with 100 nM of 17β-estradiol alone or in the presence of the ICI 182,780, an estrogen receptor antagonist at 1 μM. Cells treated with ethanol alone were used as controls. Six replicates were included in each group. Cells were collected 24 hours after treatment, and stored at −80 °C until use.
RNA extraction and reverse transcription
Total RNA was extracted from different tissues and primary hepatocyte using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Each of the RNA samples was treated with DNase I (Invitrogen #18068015) to remove trace amounts of genomic DNA according to the manufacturer’s instructions. The integrity of total RNA was confirmed by gel electrophoresis, and the RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The RNA samples with OD260/280 ratios above 1.8–2.0 were used for further study. Single-stranded cDNA was synthesized using random hexamer primers with the Thermo Scientific™ Revert Aid First Strand cDNA Synthesis kit (Thermo Scientific) following the manufacturer’s protocol. The cDNA was stored at −20 °C until use.
Chromatin immunoprecipitation (ChIP)
Liver tissues collected from birds treated with 2.0 mg /kg of estrogen were used in the Chip assay. The ChIP assay was performed using a kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. Briefly, 100 mg chicken liver tissue were cross-linked with 1% formaldehyde for 15 min at 37 °C and stopped with 2.0 M glycine. Then the samples were re-suspended in SDS lysis buffer (50 mM Tris-HCl at pH 8.0, 1% SDS, 10 mM EDTA, and protease inhibitors). Chromatin were sheared by probe sonication (5 × 10 s pulses, 30% amplitude), to an average fragment size of 200–500 bp. The lysates were cleared by centrifugation and diluted with ChIP dilution buffer (16.7 mM Tris–HCl at pH 8.1, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM NaCl, and protease inhibitors). Cell lysates were incubated with an anti-ERα (0.2 mg/mL, Invitrogen Ltd, Paisley, UK Catalog #: MA5–13065) or an anti-IgG control antibodies (Cell Signaling, Boston, MA, USA) for 12 hours at 4 °C, and then captured with the Protein G-agarose (Santa Cruz, Dallas, TX, USA). The purified DNA (IP sample) was amplified by qPCR. Meanwhile, 20% of starting chromatin without chromatin immunoprecipitation was used as input to represent the unselected DNA content. Fold Enrichment Method was used to normalize the ChIP-qPCR data. Fold Enrichment = log2 −ΔΔCt, ΔCt(THRSP) = Ct(IP)−Ct(Input) − log2 5, ΔΔCt = ΔCt(THRSP) − ΔCt(IgG).
Quantitative real-time PCR (qPCR)
The qPCR was performed using a LightCycler 96 system (Roche) by the SYBR Green method according to the method of Nolan37. Each individual sample for qPCR was run in triplicate. Chicken β-actin was used as an internal control. Each reaction contained 5 µl of SYBR Green PCR Master Mix (Takara), 3.5 µl of RNase-free water, 0.5 µl each of forward and reverse primers (10 μM) and 0.5 µl of extracted cDNA (1000 ng/μL). The amplification protocol was used as follows: denaturation at 95 °C for 5 minutes; followed by 40 amplification cycles at 95 °C for 30 seconds, annealing at 60 °C for 30 seconds and elongation at 72 °C for 20 seconds. After amplification, melting curve analysis was performed as follows: 95 °C for 10 seconds; annealing temperature of 65 °C for 20 seconds; continuous temperature gradient to 97 °C with 5 acquisitions/s. The expression levels were measured in terms of the cycle threshold (Ct) and then normalized to β-actin using the 2−ΔΔCt method. The primers for qPCR were designed by the NCBI Primers-BLAST online programs (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The forward and reverse primers for qPCR and ChIP-qPCR are shown in Table 1.
Table 1.
List of qPCR and ChIP-qPCR primers used in the study.
| Primers name | Gene | Accession number | Primer sequence (5′-3′) | Product size |
|---|---|---|---|---|
| qPCR | THRSP | NM_213577.2 | F: GTTCTGACCGACCTCACCAA | 175 |
| R: GTGGGACTTGGCACAGGAAT | ||||
| APOV1 | NM_205483.2 | F: CAATGAAACGGCTAGACTCA | 108 | |
| R: AACACCGACTTTTCTTCCAA | ||||
| THRα | NM_205313.1 | F: CATCTTCGACCTCGGCAAGT | 143 | |
| R: GGTACGTCTCCTGGCACTTC | ||||
| THRβ | NM_205447.2 | F: GTTCGCTATGACCCCGAGAG | 91 | |
| R: CTACGCCAAGACCCCCATTT | ||||
| AACS | NM_001006184.1 | F:ATGGTTCATTCAGCAGGGGG | 108 | |
| R: CCAGCCAGTCGTTGTGTAGT | ||||
| FASN | NM_205155.2 | F: TCTCTGCCATCTCCCGAACT | 184 | |
| R: GCCTTCCATTCTCTAACACA | ||||
| SCD | NM_204890.1 | F: CAAGTTCTCCGAGACGCATG | 178 | |
| R: GGGCTTGTAGTATCTCCGCT | ||||
| DIO1 | NM_001097614.1 | F:GAGCATCAGGGTGCTCCTAC | 182 | |
| R: AAGTGGGACCCCAGTTTTCG | ||||
| DIO2 | NM_204114.3 | F: GCTTCAGATCTTGCCGGTCT | 130 | |
| R: CTCCTCCAAGTTTGACCGC | ||||
| MC5R | NM_001031015.1 | F: TGTGCCTACTGTCAAGAGCA | 211 | |
| R: CTCCCAAGCATTAGACACGC | ||||
| ADHH1B | NM_001305183.1 | F: CTGAAATGACTGGCCAGGGT | 116 | |
| R: TCCCACAATCACGCTGACTC | ||||
| PIK3CA | NM_001004410.1 | F: ACACGTTCTTGTGCTGGCTA | 177 | |
| R: TAAGACAAAGGGCACACGCT | ||||
| β-actin | NM_205518.1 | F: GAGAGAAGATGACACAGATC | 116 | |
| R: GTCCATCACAATACCAGTGG | ||||
| ChIP-qPCR | APOV1 | NM_205483.2 | F: CCATTACCAAATCCGAACA | 156 |
| R: CATCTGAGACACTGGCATTT | ||||
| THRSP | NM_213577.2 | F: AGGTGCCAACAAGTTCAGC | 165 | |
| R: TCTCCATCAGCCACCTCAG |
Statistical analysis
Statistical analyses of the qPCR results were carried out using SPSS version 20.0. One-way ANOVA and repeated measures ANOVA were used for statistical analysis of relative expression levels, followed by Dunnett’s test. Graphics were drawn using Graphpad Prism 5 (Graphpad Software, San Diego, CA, USA), and differences were considered significant at P ≤ 0.05. Data were expressed as the mean ± SD.
Results
Expression patterns of THRSP in various tissues of chicken
The expression patterns of THRSP in various tissues including the heart, kidney, liver, spleen, brain, muscle and white adipose tissue of 10-week and 30-week-old chickens were detected by qPCR. The results showed that expression of THRSP mRNA was mainly confined to the liver and adipose tissue, where its abundance was the highest, followed by the pancreas and heart (Fig. 1A and B). The level of THRSP expression was greater in abdominal fat than in the liver with 10-week-old chickens, but was less in the abdominal fat at 30 weeks. Interestingly, the results showed that an expected increase of 40-fold was detected in THRSP mRNA levels in the liver at 30 weeks compared with 10 weeks (Fig. 1C). However, a dramatic decrease of 2-fold was detected in THRSP mRNA levels in adipose tissue at 30 weeks compared with 10 weeks (Fig. 1C).
Figure 1.
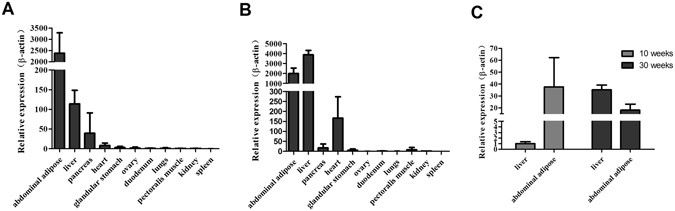
Tissue distribution of THRSP in different chicken tissues. (A) Tissue distribution of THRSP in 10 weeks old chicken tissues. (B) Tissue distribution of THRSP in 30 weeks old chicken tissues. (C) The mRNA levels of THRSP in liver and abdominal adipose of chickens at the age of 10 weeks old and 30 weeks old. The mRNA levels of the THRSP gene were normalized to the mRNA levels of β-actin gene. The data show as 1000 * 2−ΔCt and represent the mean ± SD of 6 chickens (n = 6).
Expression patterns of THRSP in the livers of chickens at different developmental stages
The expression levels of THRSP mRNAs in the livers of chickens at different developmental stages were detected by qPCR. The results showed that the expression levels of THRSP mRNA increased with sex maturation and reached the highest level at 30 weeks (Fig. 2A). According to egg production records, Lushi green-shelled-egg chickens start their first egg at an average age of 21 weeks old, and reach the peak rate of egg production at about 28 weeks. We further compared the expression pattern of the THRSP mRNA and the egg production curve of the Lushi green-shelled-egg chickens. Our data suggest a similar changing tend between the THRSP mRNA expression and the egg production (Fig. 2B).
Figure 2.
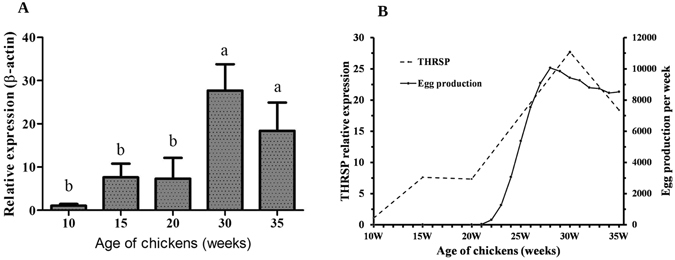
Expression patterns of THRSP in chicken livers and egg production curve analysis. (A) Expression patterns of THRSP in livers of chickens at different developmental stages. The mRNA levels of the THRSP gene were normalized to mRNA levels of β-actin in livers of chickens of different ages. Each data point represents the mean ± SD of 6 chickens (n = 6). The same letters are not significantly different, whereas different letters are significantly different. (B) Comparison between the expression curve of THRSP mRNA and the egg production curve of the Lushi green-shelled-egg chickens. Each point in the egg production curve represents the cumulative egg production of 2000 chickens in one week.
Effect of 17β-estradiol on THRSP expression in the liver and abdominal fat
The upregulation of genes involved in lipogenesis in the liver of chickens was mainly induced by estrogen because estrogen levels were increasing with sex maturation, reached peak levels before the first egg and stayed at relatively higher levels during the egg production period28, 29. Therefore, it was speculated that expression of THRSP in the liver of chickens was regulated by estrogen. To assess this, the 10-week-old juvenile hens were treated with different concentrations of 17β-estradiol for various periods. The APOV1 mRNA appears in chicken liver within a few hours after estrogen administration34, and its expression is strictly dependent on estrogen24. As a positive control, the expression levels of APOV1 mRNA increased dramatically after 17β-estradiol treatment for 12 hours and 24 hours in the liver (P < 0.01) (Fig. 3A and B). Meanwhile, the expression levels of THRSP mRNA also significantly increased in both the liver and adipose tissue after 17β-estradiol treatment for 12 hours or 24 hours (Fig. 3C–F). We also measured expression levels of three lipogenic genes including AACS, SCD and FASN in livers of birds treated with or without 17β-estradiol. All three genes were significantly upregulated in chicken livers by the estradiol treatment (Fig. 4).
Figure 3.
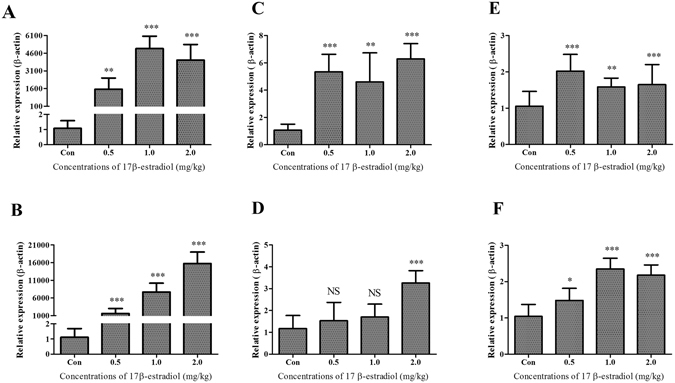
Effect of 17β-estradiol on APOV1 and THRSP mRNA expressions in the liver and abdominal fat of chicken. (A and B) APOV1 mRNA expression levels in chicken liver quantified by qPCR after different concentrations of estrogen treatment for 12 and 24 hours, respectively. (C and D) THRSP mRNA expression levels in chicken liver quantified by qPCR after different concentrations of estrogen treatment for 12 and 24 hours, respectively. (E and F) THRSP mRNA expression levels in chicken abdominal fat quantified by qPCR after different concentrations of estrogen treatment for 12 and 24 hours, respectively. Each data point represents the mean ± SD of 6 chickens (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; NS, no significant difference versus control.
Figure 4.
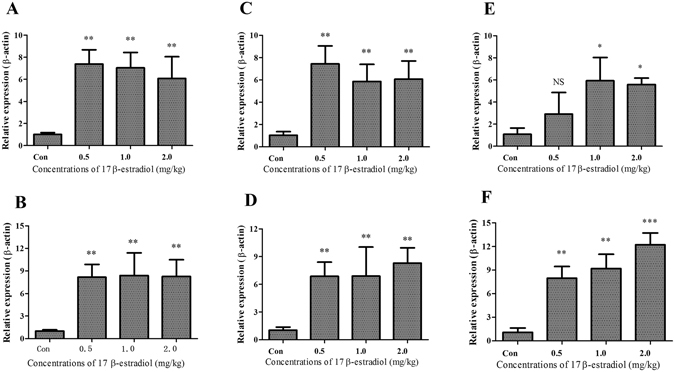
Effect of 17β-estradiol on AACS, FASN and SCD mRNA expressions in the liver of chickens. (A,C and E) AACS, FASN and SCD mRNA expression levels in chicken livers quantified by qPCR after they were treated with different doses of 17β-estradiol for 12 hours, respectively. (B,D and E) AACS, FASN and SCD mRNA expression levels in chicken livers quantified by qPCR after they were treated with different doses of 17β-estradiol for 24 hours, respectively. Each data point represents the mean ± SD of 6 chickens (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; NS, no significant difference versus control.
Effect of 17β-estradiol on THRSP expression in primary hepatocytes
To further verify whether the expression levels of THRSP were regulated by estrogen, in vitro cultured chicken embryonic primary hepatocytes were treated with different doses of 17β-estradiol. The expression levels of the APOV1, MC5R and THRSP genes were detected using qPCR. The expression levels of APOV1 mRNA, as a positive control, were significantly upregulated by 17β-estradiol treatment (Fig. 5A). As previously demonstrated35, treatment with 17β-estradiol did not change the expression of the MC5R gene in chicken embryonic primary hepatocytes (Fig. 5B). The expression levels of THRSP mRNA increased in a dose-dependent manner (Fig. 5C).
Figure 5.
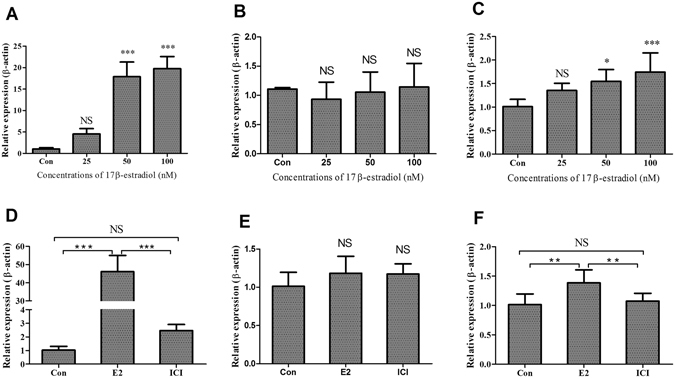
Effects of 17β-estradiol and ICI on THRSP expression in primary hepatocytes. (A,B and C) APOV1, MC5R and THRSP mRNA expression levels quantified by qPCR in primary hepatocytes treated by different doses of 17β-estradiol. (D,E and F) APOV1, MC5R and THRSP mRNA expression levels in primary hepatocytes treated by 100 nM 17β-estradiol and 10 nM ICI. Each data point represents the mean ± SD of 6 chickens (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001; NS, no significant difference versus control.
To examine the effects of estrogen on the expression of THRSP gene further, the estrogen receptor antagonist ICI 182,780 together with 17β-estradiol were used to treat the in vitro cultured chicken embryonic primary hepatocytes. The results showed that the expression of APOV1 and THRSP induced by 17β-estradiol was strongly antagonized by ICI 182,780 (Fig. 5D and F). However, the MC5R mRNA was not significantly changed by treatment with 17β-estradiol in the presence or absence of the ICI 182,780 (Fig. 5E).
Effect of 17β-estradiol on thyroid hormone signaling in the liver
The THRSP gene can be rapidly upregulated by the thyroid hormone (TH)1–4, a component of the hypothalamic-pituitary-thyroid axis that is involved in the stress responses38. Previous studies demonstrated that the activity of thyroid hormones (THs) is regulated at the transcriptional level by specific nuclear receptors that are encoded by the TH receptor-α (THRα) and TH receptor-β (THRβ) genes39, 40. To determine whether the thyroid hormone signaling participates in the regulation of THRSP in the liver, we measured the mRNA expression levels of THRα and THRβ, and other known TH target genes including Dio1, Dio2, ADH1B and PIK3CA 41, 42 in the livers after treatment with 17β-estradiol. Although expression level of THRα was significantly downregulated in livers treated with 17β-estradiol compared to control (p < 0.05), no dramatic change of any other gene measured was observed (Fig. 6). These data indicate that the increase of THRSP by 17β-estradiol do not via TH singling pathway.
Figure 6.
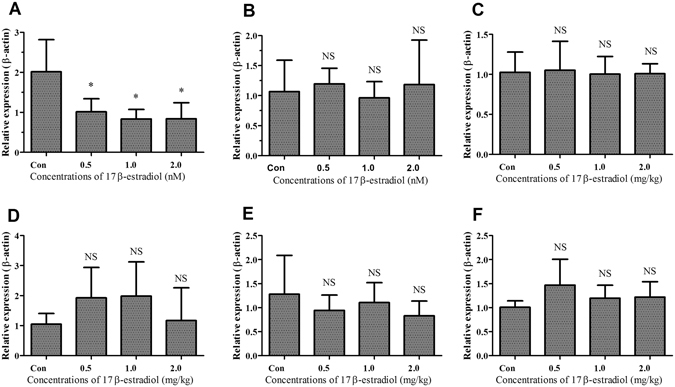
Effects of 17β-estradiol on TH relative genes expression levels in the liver. (A–F) THRα, THRβ, Dio1, Dio2, ADH1B and PIK3CA mRNA expression levels in chicken liver quantified by qPCR after they were treated with different doses of estrogen for 12 hours, respectively. Each data point represents the mean ± SD of 6 chickens (n = 6). *P < 0.05; **P < 0.01; NS, no significant difference versus control.
Identification of the putative estrogen receptor binding site in the THRSP promoter
By sequence comparison analysis, a putative estrogen receptor binding site located between −2390 and −2402 bp upstream of the THRSP promoter was found (Fig. 7A). The ChIP-qPCR analysis demonstrated that the putative ERα binding site in the THRSP promoter sequence could be detected efficiently both in Input and IP samples as it was done in the positive control of the APOV1 promoter binding site, where the gene IgG was a negative control. The fold enrichment was calculated and shown in Fig. 7B.
Figure 7.
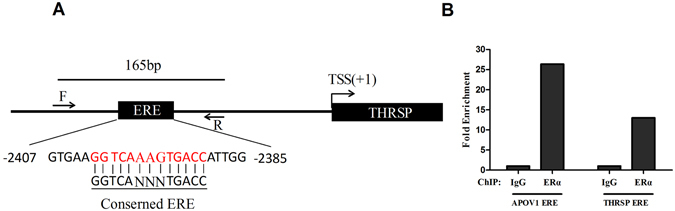
Identification of the putative estrogen receptor binding site in the THRSP promoter. (A) Location and sequence of the putative ERα binding site in the THRSP promoter. A conservative ERE was shown below the putative ERE; N represents any base. Arrows indicate the position of primers designed for qPCR amplification. (B) The binding of ERα to putative ERE regions in the THRSP promoter were analyzed by ChIP assays. After immunoprecipitation, ERE regions were amplified using qPCR. Pre-immune IgG was used as a negative control.
Discussion
Many studies have reported that THRSP is expressed predominately in lipogenic tissues such as liver, fat and the mammary gland in mammalian systems2, 3. THRSP in birds was first identified as an EST in the liver of chickens, and its expression and regulation were not well understood. Here we performed analysis of the tissue distribution of THRSP mRNA both in juvenile hens and laying hens. Results were similar to mammalian systems in that THRSP had relatively high mRNA expression levels in lipogenic tissues, liver and abdominal fat (Fig. 1). A recent study on ducks compared the mRNA expression levels of THRSP in the liver, sebum cutaneum fat and abdominal fat collected from 6-week-old ducks16, and found that THRSP had a higher mRNA expression level in fat tissue than in the liver. In our results, we also found higher mRNA expression levels in abdominal fat than in the liver in juvenile hens. However, THRSP mRNA levels were 2-fold higher in the live compared with adipose in laying hens. It is known that the liver synthesizes more than 90% of the lipids. In contrast, abdominal fat is the main storage organization. The THRSP gene has been shown to be a lipogenic gene, and mostly involved in the liver lipid synthesis biological process in birds. The increase of THRSP gene expression in the laying hens implied a more active THRSP in the livers of laying hens than that in the livers of 10-week-old pullets. In this case, we detected the expression patterns of THRSP in the liver at different developmental stages; expression patterns were similar with the chicken egg production curve. Together, these results suggest that THRSP is involved in lipid metabolism in the liver of chickens.
THRSP plays an important role in lipogenic tissues and in regulating the key genes of lipogenesis6–8. Here we reported different THRSP mRNA expression levels between natural populations of juvenile and laying hens. The difference is easily associated with different physiologically active adipose synthesis in the livers of laying hens. It is reported that egg production behavior is controlled by the hypothalamic-pituitary-gonadal axis (HPG) and sex hormones, especially estrogen. The levels of estrogen in laying hens are significantly higher than in pullets28, 29. Estrogens initiate the transcription of estrogen-dependent genes and enhance the stability of the transcripts43. Our in vivo and in vitro studies strongly suggest that estrogen upregulates the expression level of THRSP (Figs 3 and 5). Meanwhile, our data also excluded the possibility that changes of expression level of THRSP in chicken liver treated with estrogen were caused by the TH singling pathway (Fig. 6). Therefore, we believe that THRSP is involved in the estrogen regulation network. We also observed that the expression of THRα was downregulated by estradiol treatment (Fig. 6A). It has been reported that there is a striking similarity between the ERE and TRE, resulting a repression of activated ERs by TRs44, 45. A recent study suggests that increased expression of THRSP is associated with ER-positive status in mouse with breast cancer46. It seems that the downregulation of THRα might be caused by the competitive inhibition or feedback regulation of the activated ERs. Importantly, the downregulation of THRα did not lead to the activation/inhibition of the TH singling pathway.
Another study reported that progesterone significantly increased the expression level of THRSP in female subcutaneous WAT in rat47. Considering the similarity of tissue distribution patterns of THRSP, we suggest that THRSP can be upregulated by estrogen in both between the chicken and the mammals.
To date, there are many transcription factor responsive elements in the promoter of THRSP in mammals, such as TRE, ChoRE, and SRE. Thus, the THRSP gene can be quickly activated by T3 and carbohydrate4, 5. To understand further the regulation mechanism of THRSP in chickens, we analyzed the 3000 bp promoter of THRSP bases to the transcriptional start site. In our data, we found a conscious ERE in the −2434 – −2422 range (Fig. 7A). Next we found that the ERE site is a positive binding site for ERα by ChIP-qPCR. We therefore conclude that THRSP is directly regulated by estrogen and is involved in the estrogen regulation network in chicken. Nevertheless, a perturbation of the ERE by gene editing or site-directed mutagenesis may further support this conclusion.
THRSP has served as an important lipogenic gene, but the exact mechanism of THRSP involved in mammalian and avian lipid metabolism has not been well characterized. Here our study suggests that the chicken THRSP gene can be directly regulated by estrogen, which is considered to be the primary regulator of lipid metabolism (Fig. 8). This opens a new perspective in avian lipid metabolism. However, the synergistic and/or competitive actions of TH, estrogen, glucose, carbohydrate and other nutritional factors in THRSP expression need to be investigated further.
Figure 8.
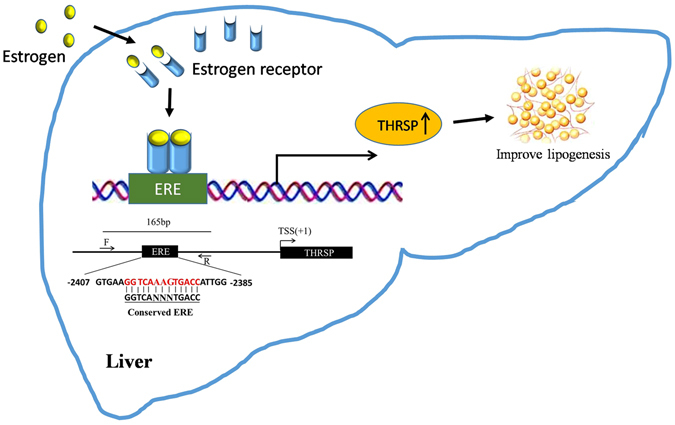
Model of estrogen-mediated regulation of THRSP.
Acknowledgements
This work was supported by the Innovation Research Team of Ministry of Education (IRT-16R23), Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-41-K04), Key Science and Technology Research Project of Henan Province (151100110800) and Henan International Cooperative Research Project (162102410030).
Author Contributions
R.J. performed the experiments and wrote the manuscript. Z.H., X.N. and T.W. participated in the management of the experimental animals and the sample collection. L.H. and L.Z. contributed to the cell culture and qPCR analyses. W.Y. and T.Y. participated in critical discussion. K.X. and L.X. conceived the study, participated in the experiment design and critical discussion. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiangtao Kang, Email: xtkang2001@263.net.
Xiaojun Liu, Email: xjliu2008@hotmail.com.
References
- 1.Seelig S, Liaw CW, Towle HC, Oppenheimer JH. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:4733–4737. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaw CW, Towle HC. Characterization of a thyroid hormone-responsive gene from rat. Journal of Biological Chemistry. 1984;259:7253–7260. [PubMed] [Google Scholar]
- 3.Jump DB, Oppenheimer JH. High basal expression and 3,5,3’-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology. 1985;117:2259–2266. doi: 10.1210/endo-117-6-2259. [DOI] [PubMed] [Google Scholar]
- 4.Tsatsos NG, Augustin LB, Anderson GW, Towle HC, Mariash CN. Hepatic expression of the SPOT 14 (S14) paralog S14-related (Mid1 interacting protein) is regulated by dietary carbohydrate. Endocrinology. 2008;149:5155–5161. doi: 10.1210/en.2008-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jump DB, Clarke SD, Macdougald O, Thelen A. Polyunsaturated fatty acids inhibit S14 gene transcription in rat liver and cultured hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8454–8458. doi: 10.1073/pnas.90.18.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towle HC, Kaytor EN, Shih HM. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annual Review of Nutrition. 2003;17:405. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 7.Aipoalani DL, O’Callaghan BL, Mashek DG, Mariash CN, Towle HC. Overlapping roles of the glucose-responsive genes, S14 and S14R, in hepatic lipogenesis. Endocrinology. 2010;151:2071–2077. doi: 10.1210/en.2009-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinlaw WB, Church JL, Harmon J, Mariash CN. Direct evidence for a role of the “spot 14” protein in the regulation of lipid synthesis. Journal of Biological Chemistry. 1995;270:16615–16618. doi: 10.1074/jbc.270.28.16615. [DOI] [PubMed] [Google Scholar]
- 9.Kinlaw WB, Tron P, Witters LA. Thyroid hormone and dietary carbohydrate induce different hepatic zonation of both “spot 14” and acetyl-coenzyme-A carboxylase: a novel mechanism of coregulation. Endocrinology. 1993;133:645–650. doi: 10.1210/endo.133.2.8102096. [DOI] [PubMed] [Google Scholar]
- 10.Brown SB, Maloney M, Kinlaw WB. “Spot 14” Protein Functions at the Pretranslational Level in the Regulation of Hepatic Metabolism by Thyroid Hormone and Glucose. Journal of Biological Chemistry. 1997;272:2163–2166. doi: 10.1074/jbc.272.4.2163. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, et al. Thyroid hormone responsive protein spot 14 enhances lipogenesis in bovine mammary epithelial cells. In Vitro Cellular & Developmental Biology - Animal. 2015;51:586–594. doi: 10.1007/s11626-014-9865-8. [DOI] [PubMed] [Google Scholar]
- 12.Cogburn LA, et al. Systems-wide chicken DNA microarrays, gene expression profiling, and discovery of functional genes. Poult Sci. 2003;82:939–951. doi: 10.1093/ps/82.6.939. [DOI] [PubMed] [Google Scholar]
- 13.Ikeobi CON, et al. Quantitative trait loci affecting fatness in the chicken. Animal Genetics. 2002;33:428–435. doi: 10.1046/j.1365-2052.2002.00911.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Carre W, Zhou H, Lamont SJ, Cogburn LA. Duplicated Spot 14 genes in the chicken: characterization and identification of polymorphisms associated with abdominal fat traits. Gene. 2004;332:79–88. doi: 10.1016/j.gene.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Grillasca JP, et al. Cloning and initial characterization of human and mouse Spot 14 genes. Febs Letters. 1997;401:38–42. doi: 10.1016/S0014-5793(96)01433-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhan K, et al. Molecular Cloning and Expression of the Duplicated Thyroid Hormone Responsive Spot 14 (THRSP) Genes in Ducks. Poultry Science. 2006;85:1746. doi: 10.1093/ps/85.10.1746. [DOI] [PubMed] [Google Scholar]
- 17.Campbell MC, Anderson GW, Mariash CN. Human spot 14 glucose and thyroid hormone response: characterization and thyroid hormone response element identification. Endocrinology. 2003;144:5242–5248. doi: 10.1210/en.2002-0008. [DOI] [PubMed] [Google Scholar]
- 18.Liu HC, Towle HC. Functional synergism between multiple thyroid hormone response elements regulates hepatic expression of the rat S14 gene. Molecular Endocrinology. 1994;8:1021–1037. doi: 10.1210/mend.8.8.7997231. [DOI] [PubMed] [Google Scholar]
- 19.Mater MK, Thelen AP, Pan DA, Jump DB. Sterol Response Element-binding Protein 1c (SREBP1c) Is Involved in the Polyunsaturated Fatty Acid Suppression of Hepatic S14 Gene Transcription. Journal of Biological Chemistry. 1999;274:32725–32732. doi: 10.1074/jbc.274.46.32725. [DOI] [PubMed] [Google Scholar]
- 20.Martel PM, et al. S14 protein in breast cancer cells: Direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Experimental Cell Research. 2006;312:278–288. doi: 10.1016/j.yexcr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Chambon P. The estrogen receptor binds tightly to ITS responsive element as a ligand-induced homodimer. Cell. 1988;55:145. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- 22.Paech K, et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 23.Walker P, Germond JE, Brownluedi M, Givel F, Wahli W. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and APOV1 genes. Nucleic Acids Research. 1984;12:8611–8626. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinhitpass L, Ryffel GU, Heitlinger E, Cato AC. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic acids research. 1988;16:647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess DRA, et al. Estrogen and Its Receptors in Efferent Ductules and Epididymis. Journal of Andrology. 2011;32:600–613. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- 26.Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. Faseb Journal. 1996;10:905–912. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- 27.Li H, et al. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. BMC Genomics. 2015;16:763. doi: 10.1186/s12864-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanabe Y, Nakamura T, Tanase H, Doi O. Comparisons of plasma LH, progesterone, testosterone and estradiol concentrations in male and female chickens (Gallus domesticus) from 28 to 1141 days of age. Endocrinologia Japonica. 1981;28:605. doi: 10.1507/endocrj1954.28.605. [DOI] [PubMed] [Google Scholar]
- 29.Williams J, Harvey S. Plasma concentrations of luteinizing hormone growth hormone, oestradiol, testosterone and androstenedione in the domestic hen from 6 weeks of age to sexual maturity. Reproduction Nutrition Development. 1986;26:515–522. doi: 10.1051/rnd:19860311. [DOI] [Google Scholar]
- 30.Schneider, W. J. Chapter 17 – Lipoprotein Receptors. Biochemistry of Lipids Lipoproteins & Membranes, 489–518 (2016).
- 31.Capony F, Williams DL. Apolipoprotein B of avian very low density lipoprotein: characteristics of its regulation in nonstimulated and estrogen-stimulated rooster. Biochemistry. 1980;19:2219–2226. doi: 10.1021/bi00551a035. [DOI] [PubMed] [Google Scholar]
- 32.Wiskocil R, et al. Coordinate regulation of two estrogen-dependent genes in avian liver. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon DA, Shelness GS, Nicosia M, Williams DL. Estrogen-induced destabilization of yolk precursor protein mRNAs in avian liver. Journal of Biological Chemistry. 1988;263:2625–2631. [PubMed] [Google Scholar]
- 34.Van HSF, Strijker R, Samallo J, Gruber M, Geert AB. Conserved sequence motifs upstream from the co-ordinately expressed vitellogenin and APOV1 genes of chicken. Nucleic Acids Research. 1986;14:8669. doi: 10.1093/nar/14.21.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren J, et al. Association of Estradiol on Expression of Melanocortin Receptors and Their Accessory Proteins in the Liver of Chicken (Gallus gallus) General & Comparative Endocrinology. 2016;240:182–190. doi: 10.1016/j.ygcen.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Fischer PWF, Marks GS. Chick embryo liver cells maintained in serum-free waymouth MD 705/1 medium. Methods in Cell Science. 1976;2:449–452. [Google Scholar]
- 37.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nature Protocols. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 38.Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–192. doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- 39.Breuker C, et al. Hepatic Expression of Thyroid Hormone-Responsive Spot 14 Protein Is Regulated by Constitutive Androstane Receptor (NR1I3) Endocrinology. 2010;151:1653–1661. doi: 10.1210/en.2009-1435. [DOI] [PubMed] [Google Scholar]
- 40.Zilz ND, Murray MB, Towle HC. Identification of multiple thyroid hormone response elements located far upstream from the rat S14 promoter. Journal of Biological Chemistry. 1990;265:8136–8143. [PubMed] [Google Scholar]
- 41.Moeller LC, Cao X, Dumitrescu AM. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nuclear Receptor Signaling. 2006;4:e020. doi: 10.1621/nrs.04020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moeller LC, Dumitrescu AM, Walker RL. Thyroid hormone responsive genes in cultured human fibroblasts. Journal of Clinical Endocrinology & Metabolism. 2005;90:936–943. doi: 10.1210/jc.2004-1768. [DOI] [PubMed] [Google Scholar]
- 43.Flouriot G, Pakdel F, Valotaire Y. Transcriptional and post-transcriptional regulation of rainbow trout estrogen receptor and vitellogenin gene expression. Molecular & Cellular Endocrinology. 1996;124:173–183. doi: 10.1016/S0303-7207(96)03960-3. [DOI] [PubMed] [Google Scholar]
- 44.Graupner G, Zhang XK, Tzukerman M. Thyroid hormone receptors repress estrogen receptor activation of a TRE. Molecular Endocrinology. 1991;5:365–372. doi: 10.1210/mend-5-3-365. [DOI] [PubMed] [Google Scholar]
- 45.Glass CK, Holloway JM, Devary OV. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988;54:313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- 46.Anderson SM. Elevated THRSP/Spot14 expression is correlated with improved clinical outcome in breast cancer which is consistent with reduced metastasis and enhanced differentiation observed in the MMTV-Neu mouse model. Cancer Epidemiology Biomarkers & Prevention. 2013;19:2525–2533. [Google Scholar]
- 47.Stelmanska E, Swierczynski J. Up-regulation of lipogenic enzyme genes expression in inguinal white adipose tissue of female rats by progesterone. Journal of Steroid Biochemistry & Molecular Biology. 2013;134:37. doi: 10.1016/j.jsbmb.2012.10.006. [DOI] [PubMed] [Google Scholar]


