Abstract
The aim of this study was to investigate the protective role and underlying mechanisms of curcumin on glycerol-induced acute kidney injury (AKI) in rats. Glycerol (10 ml/kg BW, 50% v/v in sterile saline, i.m.) was used to induce AKI, followed by curcumin (200 mg/kg/day, p.o.) administration for 3 days. To confirm renal damage and the effects of curcumin on AKI, serum BUN, Scr, and CK as well as renal SOD, MDA, GSH-Px were measured. Additionally, morphological changes were identified by H&E staining and transmission electron microscopy. The expression of several factors including chemotactic factor MCP-1, proinflammatory cytokines including TNF-α and IL-6, as well as the kidney injury markers, as Kim-1 and Lipocalin-2 were also assessed using q-PCR. Finally, cell apoptosis in renal tissue was detected using in situ TUNEL apoptosis fluorescence staining and expression of proteins associated with apoptotic, oxidative stress and lipid oxidative related signaling pathways were detected using immunohistochemical staining and western blot. The results showed that curcumin exerts renoprotective effects by inhibiting oxidative stress in rhabdomyolysis-induced AKI through regulation of the AMPK and Nrf2/HO-1 signaling pathways, and also ameliorated RM-associated renal injury and cell apoptosis by activating the PI3K/Akt pathway.
Introduction
Acute kidney injury (AKI) is a syndrome characterized by an acute loss of renal function, and is associated with increased mortality, prolonged hospital stays and accelerated chronic kidney disease1. AKI is a relatively a newly classified disease first introduced by the emergency medical community and the international society of nephrology2, which replaced the concept of traditional acute renal failure (ARF), and was proposed in order to advance clinical diagnosis of the disease3. Despite the reversibility of the loss of renal function in most patients who survive, the mortality of AKI remains alarmingly high (over 50%)3, 4 and to date, no effective therapies to prevent or treat AKI exist.
Rhabdomyolysis (RM) is defined as a massive breakdown of skeletal muscle in which potentially large of amounts of damaging intracellular content enters into blood5. The development of RM is associated with causes such as crush syndrome, exhaustive exercise, medications, infections and toxins6–9. AKI is one of the most severe complications of RM, with approximately 15% of patients with RM developing AKI6, and 5–15% of AKI cases are attributed to RM7. Myoglobin-induced renal toxicity plays a key role in RM-associated AKI by increasing oxidative stress, inflammation, endothelial dysfunction, vasoconstriction, and apoptosis10. In recent years, research into effective therapies to prevent and recovery AKI have attracted much attention, although to date, there is no established therapy to promote recovery.
Experimental AKI induced by glycerol injection is the most commonly used experimental model of RM1, 11, 12 and is characterized by intense cortical acute tubular necrosis and inflammatory cell infiltration13. Curcumin is an active component in turmeric rhizomes (Curcuma Longa Linn), and is a polyphenolic curcuminoid that accounts for 3–5% of turmeric14 which has been shown to have antioxidant15–18, anti-inflammatory19–21, anti-apoptotic22 and anti-bacterial23 functions. The role of curcumin as a protective agent against renal injury induced by gentamicin, contrast agent, cisplatin and diabetes has been previously investigated24–32. However, the mechanisms for the anti-apoptotic and antioxidant effects of curcumin on AKI have not been elucidated, nor the effects of curcumin on experimental AKI models of RM. In this study, the protective role and underlying mechanisms of curcumin on glycerol-induced AKI in rats were investigated.
Results
Biochemical analysis of the effects of curcumin on serum and renal tissue
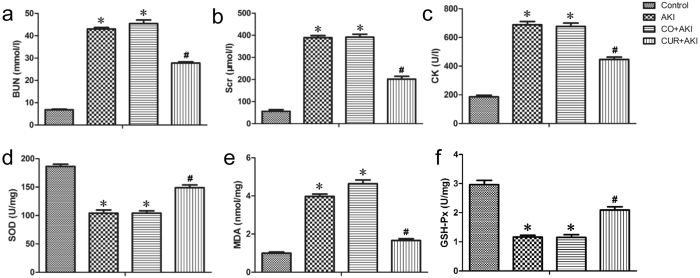
The effects of curcumin on kidney function and oxidative stress levels in rats are shown in Fig. 1. There was no significant difference in serum urea and creatinine levels between the AKI and CO + AKI (AKI treated with corn oil) groups. Glycerol injection significantly (p < 0.01) increased blood urea nitrogen (BUN), and serum creatinine (Scr) and ceatine kinase (CK) levels in both the AKI and CO + AKI groups compared with the control group (Fig. 1a,b,c). Curcumin treatment resulted in a significant reduction in BUN, Scr and CK levels in CUR + AKI (AKI treated with curcumin) group compared with the AKI and CO + AKI groups, respectively (p < 0.01).
Figure 1.
Effect of curcumin on serum BUN, Scr, CK and kidney SOD, MDA and GSH-Px in rats. Curcumin treatment on renal tissue ameliorates glycerol-induced acute kidney injury. (a,b,c) Compared with the control group, curcumin significantly reduced Scr, BUN and CK levels 72 h after glycerol injection. Statistical significance: *p < 0.01 versus the control group; #p < 0.01 versus the AKI and CO + AKI groups, respectively (n = 12). Data are represented the means ± SD. (d,e,f) Curcumin significantly reduced SOD and GSH-Px levels compared with the control group and markedly increased MDA compared with AKI and CO + AKI groups 72 h after glycerol injection. Each bar represents the mean ± SD (n = 12). Statistical significance: *p < 0.01 versus the control group; #p < 0.01 versus the AKI and CO + AKI group.
In addition, kidney tissue superoxide dismutase (SOD) enzyme activity and glutathione peroxidase (GSH-Px) enzyme activity levels decreased significantly in the AKI and CO + AKI groups compared with the control group (p < 0.01) (Fig. 1d,f). Kidney tissue MDA levels increased significantly (p < 0.01) in the AKI and CO + AKI groups compared with the control group (p < 0.01) (Fig. 1e). Curcumin treatment resulted in a significant increase in SOD and GSH-Px levels, and a reduction in MDA levels compared with the AKI and CO + AKI groups.
Effects of curcumin on renal histopathology
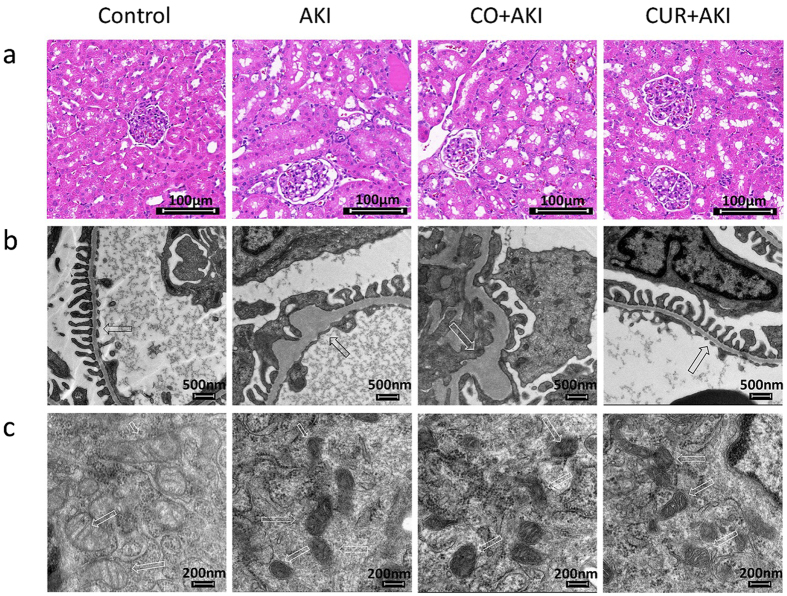
Histopathological appearances in the experimental groups and in the control group are shown in Fig. 2. Kidney sections from control rats displayed normal renal tissue structure, complete renal tubular epithelial cells and no obvious pathological changes in glomerular or renal interstitium (Fig. 2a); kidney tissue from AKI and CO + AKI rats showed kidney tubule cavity expansion and glomerular hypertrophy as well as renal tubular epithelial cell edema and interstitial inflammatory infiltration (Fig. 2a). Curcumin treatment significantly relieved severity of renal lesions and renal tubular injury in the CUR + AKI group. Specifically, tubular injury scores showed that, compared with control group, tubular injury increased in AKI and CO + AKI groups (p < 0.01) (Supplementary Fig. 1), but after curcumin treatment, this increase was signficantly negated (p < 0.01).
Figure 2.
Effect of curcumin on kidney histological morphology changes. (a) Histopathological examination of kidney tissue by H&E staining (200×). (b,c) Representative TEM photomicrographs showing GBM thickness, open slit pores and mitochondrial cristae (arrows). Control: kidney section from normal rats injected with saline; AKI and CO + AKI: Kidney section of AKI rats and AKI rats treated with corn oil showing significant tubular necrosis, cavity expansion, GBM tumefaction, mitochondrial crest loss, and reduced numbers of open slit pores. CUR + AKI: Kidney section of AKI rats treated with curcumin showing significantly improved renal morphology compared to the AKI and CO + AKI groups.
The results from TEM showed that, within the AKI and CO + AKI groups, glomerular basement membrane (GBM) thickness was increased and podocyte foot processes were effaced; the number of slit pores between the podocyte foot processes was significantly decreased (Fig. 2b); podocyte mitochondrial cristae were not apparent or disappeared; and endoplasmic reticulum expansion was evident, as were increases in secondary lysosomes (Fig. 2c). In contrast, curcumin treatment significantly ameliorated the overall lesion range. Specfically, GBM thickness was decreased, there was less fading of the podocyte foot processes and the number of open slit pores were increased compared with AKI and CO + AKI groups (Fig. 2b,c).
Effect of curcumin on renal immunohistochemistry
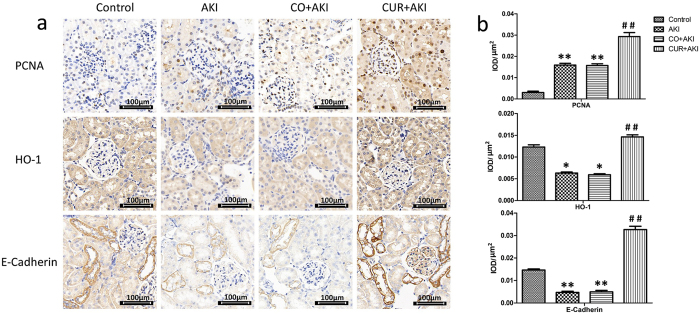
To further examine the effects of curcumin in the kidney, PCNA, HO-1 and E-cadherin expression were assessed by immunohistochemical staining (Fig. 3a and Supplementary Fig. 2a). Semi-quantitative evaluation of PCNA, HO-1 and E-cadherin expression in renal tissue were considered as the total expression of these proteins in the nucleus and cytoplasm (Fig. 3b and Supplementary Figure 2b). PCNA expression was observed in proliferating cell nuclei, and was significantly increased in AKI and CO + AKI groups compared with the control group (p < 0.01). In control group, PCNA-positive staining was weak in the kidney sections while there was a higher level of expression of PCNA in the CUR + AKI group compared with the AKI and CO + AKI groups (p < 0.01). In addition, HO-1 expression was down-regulated in AKI and CO + AKI groups compared with control group (p < 0.05); intense HO-1 staining was observed in the renal tubular epithelial cell cytoplasm in CUR + AKI group, levels were higher than that of the AKI and CO + AKI groups (p < 0.01). E-cadherin expression was also observed in tubular segments epithelial cells and significantly decreased in the AKI and CO + AKI groups compared with control group (p < 0.01). Finally, there was higher expression of E-cadherin in the CUR + AKI group compared with AKI and CO + AKI groups (p < 0.01).
Figure 3.
Expression of HO-1, PCNA and E-cadherin in kidney tissue. (a) Representative immunohistochemical images of PCNA, HO-1 and E-cadherin in sections of rat kidney from the control group, the AKI group, the CO + AKI group, and the CUR + AKI group. Curcumin treatment significantly increased expression of PCNA, HO-1 and E-cadherin in renal tissue of rats in the CUR + AKI group. (b) Semi-quantitative evaluation of PCNA, HO-1 and E-cadherin expression represented as IOD/μm2. Each bar represents means ± SD (n = 8). Statistical significance: *p < 0.01 versus the control group; #p < 0.01 versus the AKI and CO + AKI groups, respectively. IOD: integrated optical density.
Effects of curcumin on glycerol-induced renal apoptosis
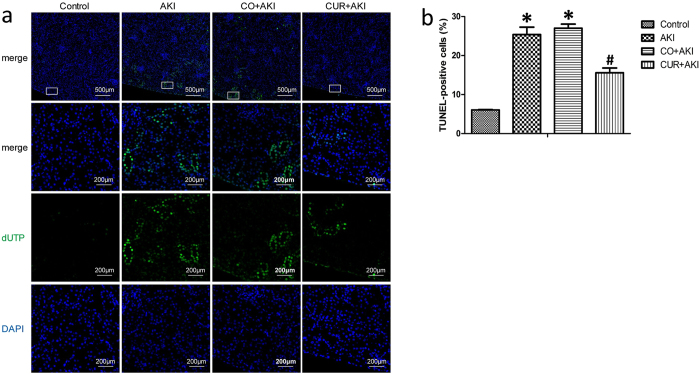
In the TUNEL assay, the nuclei of TUNEL-positive cells were stained green, indicating apoptotic cells (Fig. 4a and Supplementary Fig. 3a). The levels of apoptosis were indicated as the percentage of TUNEL-positive cells among total cells (Fig. 4b and Supplementary Fig. 3b). TUNEL-positive cells were observed mainly in the tubular area of the renal cortex, although some TUNEL-positive cells were detached from the tubular basement membrane in the lumen. Few apoptotic cells were observed in the control group, whereas the AKI and CO + AKI groups displayed more TUNEL-positive cells than the control group (p < 0.01). As expected, curcumin treatment decreased the number of TUNEL-positive cells significantly, and fewer apoptotic cells were observed in the CUR + AKI group compared with the AKI and CO + AKI groups (p < 0.01).
Figure 4.
Effect of curcumin on glycerol-induced renal apoptosis. (a) Representative in situ TUNEL fluorescence images in sections of rat kidney from the control group, the AKI group, the CO + AKI group and the CUR + AKI group. The AKI and CO + AKI groups displayed markedly increased numbers of TUNEL-positive cells than that in the control group, mainly in the tubular area of renal cortex. Curcumin treatment decreased the number of TUNEL-positive cells, and fewer apoptotic cells were observed. (b) The levels of apoptosis were indicated as the percentage of TUNEL-positive cells among total cells. Five random fields per section were examined in each experiment. Each bar represents means ± SD (n = 8). Statistical significance: *p < 0.01 versus the control group; #p < 0.01 versus the AKI and CO + AKI groups, respectively.
Effects of curcumin on the mRNA expression of inflammation and kidney injury markers in renal tissue
To examine the protective effects of curcumin from inflammation and injury in the kidney, the expression levels of monocyte chemotactic protein-1 (MCP-1), TNF-α, IL-6 and the kidney injury marker genes, Kim-1 and Lipocalin-2 (Lcn-2) were assessed by quantitative polymerase chain reaction (q-PCR) (Fig. 5a). MCP-1, TNF-α, IL-1β, Kim-1 and Lcn-2 mRNA expression were all significantly up-regulated in AKI and CO + AKI groups compared with the control group (p < 0.01) and rats in these two groups displayed a similar levels of expression for all the markers tested (Fig. 5b). Treatment with curcumin decreased the expression levels of these genes in the CUR + AKI group compared with AKI and CO + AKI groups (p < 0.01 and p < 0.05, respectively).
Figure 5.
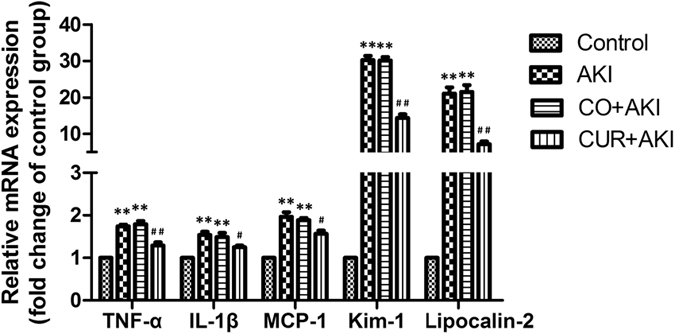
Effect of curcumin on mRNA expression of inflammatory markers and kidney injury markers in kidney tissue. Relative expression (fold) of TNF-α, IL-6, MCP-1 Kim-1 and Lcn-2 mRNA in kidney tissue of rats in the control group were detected by Q-PCR. Increased expression of TNF-α, IL-6 and MCP-1 mRNA were observed and curcumin treatment significantly decreased expression of these markers. GAPDH was used as a housekeeping gene for normalization. Data are the means ± SD (n = 10). Statistical significance: *p < 0.05, **p < 0.01 versus the control group; #p < 0.05, ##p < 0.01 versus the AKI and CO + AKI group, respectively.
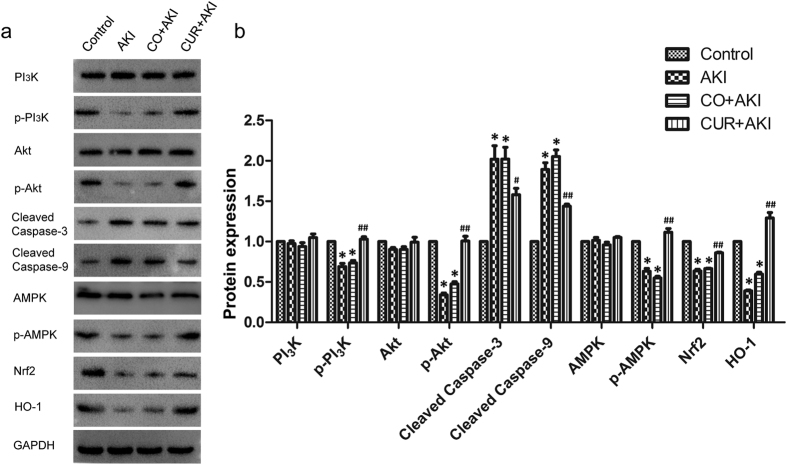
Effects of curcumin on apoptotic, oxidative stress and lipid oxidative signalling pathways in renal tissue
The potential mechanisms involved in the effects of curcumin on RM induced AKI was investigated. Protein expression of apoptotic-, oxidative stress- and lipid oxidative-related signaling pathway genes were detected by western blot (Fig. 6a). The expression of total PI3K, Akt, AMPK protein in each group was equal, while p-PI3K, p-Akt and p-AMPK were significantly lower in the AKI and CO + AKI groups compared with the control group (p < 0.01) (Fig. 6b), and was markedly up-regulated in the CUR + AKI group compared with the AKI and CO + AKI groups (p < 0.01). Down-regulation of caspase-3 and caspase-9 were observed in the CUR + AKI group compared with the AKI and CO + AKI groups (p < 0.05 and p < 0.01, respectively). Western blot analysis also showed that Nrf2 and HO-1 expression were decreased in AKI and CO + AKI groups compared with control group (p < 0.01), whereas treatment with curcumin significantly increased the expression of Nrf2 and HO-1 compared with rats in the AKI and CO + AKI groups (p < 0.01 for each).
Figure 6.
Effect of curcumin on apoptotic, oxidative stress and lipid oxidative signaling pathway associated proteins in kidney tissue. (a) Representative western blots depicting protein levels of PI3K, p-PI3K Akt, p-Akt, AMPK, p-AMPK, cleaved caspase-3, cleaved caspase-9, Nrf2 and HO-1 in kidney tissue. (b) The CUR + AKI group showed significantly increased levels of p-PI3K, p-Akt, p-AMPK, Nrf2, and HO-1, and decreased levels of cleaved caspase-3, cleaved caspase-9 compared to the AKI and CO + AKI groups. Data are the means ± SD (n = 10). Statistical significance: *p < 0.01 versus the control group; #p < 0.05, ##p < 0.01 versus the AKI and CO + AKI group.
Discussion
AKI is characterized by a rapid decline of the glomerular filtration rate and retention of nitrogenous waste products. Traumatic and non-traumatic RM are now considered to be one of the leading causes of AKI33. RM is a syndrome characterized by skeletal muscle degeneration and muscle enzyme leakage causing high mortality34. The development of RM is associated with causes such as crush syndrome, earthquakes, exhaustive exercise, medications, infections and toxins35, 36.
The most commonly used model for studying this form of ARF is obtained in the rat by intramuscular injection of glycerol, which produces a myoglobinuric state similar to clinical RM37 and is characterised by rapid increases in BUN and Scr which are associated with a marked reduction in glomerular filtration rate within 3 h after glycerol administration33. Glycerol-induced AKI in rodents is mediated by renal ischemia and myoglobin nephrotoxicity33, 38. In glycerol-induced AKI, myoglobin heme induces oxidative stress and lipid peroxidation of the proximal tubular cells, triggering the release of a series of mediators, including cytokines and chemokines, leading to leukocyte activation and subsequent tubular necrosis in the renal cortical area39–42. The symptoms that follow intramuscular injection of glycerol in the rat mimics the AKI seen in crush injury, and renal histological changes such as tubule injury are similar to those observed clinically43, 44. Therefore, this animal model was used in this study to investigate the effects of curcumin against AKI induced glycerol injection.
Curcumin is an active ingredient of polyphenolic curcuminoids extracted from the species Curcuma longa Linn. Curcumin has been well-recognized as a dietary spice for centuries, and its pharmacological activity has been studied in various animal models and clinical investigations, which have demonstrated anti-inflammatory and antioxidant effects45, 46. This study showed that curcumin improved renal function in RM-induced AKI. We evaluated kidney injury 72 h, as AKI has previously been shown to be most severe at that time point22. Rats in the AKI group exhibited significantly increased BUN, Scr and CK levels as well as significant gross and micro-morphological changes. In contrast, rats that were given curcumin exhibited markedly lower BUN, Scr and CK levels, exhibited less renal tubular epithelial cell degeneration and necrosis, and had significantly improved tubule necrosis scores and ovrerall better cellular microstructure compared to AKI rats. However, there was no significant difference between rats in the AKI and CO + AKI groups. Therefore, in the current study, corn oil played a minimal role in glycerol-induced AKI.
Furthermore, curcumin increased serum SOD and GSH-Px, a superoxide radical scavenger enzyme and a peroxide decomposition enzyme, respectively, while at the same time, decreased urinary MDA, a lipid peroxidation index marker. These data indicated that antioxidant related pathways may be activated by curcumin, thus protecting renal function against AKI47. Immunohistochemical and western blot results also showed that curcumin significantly increased expression of HO-1 and Nrf2 and suggest that curcumin treatment protects the kidney from oxidative stress by activating regulating these two proteins. Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates antioxidant genes including acting in synergy to remove reactive oxygen species (ROS) through sequential enzymatic reactions. It has been reported that curcumin can activate Nrf2 to up-regulate enzymes involved in antioxidant defense, like SOD and heme oxygenase-1 (HO-1)48, 49. In the present study, curcumin up-regulated HO-1 via activation of Nrf2, leading to lowered levels of MDA, and also increased ROS expression, which lowered SOD expression normally associated with AKI. Nrf2 binds to the antioxidant response elements (ARE) in the promoter regions of Nrf2 target genes in the nucleus, including HO-150. The induction of HO-1 has generally been considered to be an adaptive cellular response to oxidative stress51. That Nrf2-mediated HO-1 induction contributes to antioxidant capacity has been demonstrated in multiple disease models, including cardiomyopathy in type 2 diabetic mice52, a rat model of transient global cerebral ischaemia53, and endotoxin shock-induced acute lung injury in rabbits54. Recently, Nrf2/HO-1 activation has also been shown to have protective effects in aristolochic-acid-induced AKI55 and type 2 diabetic nephropathy29, as well as intestinal ischemia–reperfusion induced acute renal injury56.
The expression of proliferating cell nuclear antigen (PCNA) and E-cadherin increased significantly in AKI rats treated with curcumin. PCNA is an auxiliary protein of DNA polymerase δ which plays a fundamental role in the initiation of cell proliferation. PCNA expression is an index of renal regeneration, as its level correlates directly with the rates of cellular proliferation and DNA synthesis57. E-cadherin is a cell adhesion molecule that plays an important role in maintaining renal epithelial polarity and integrity22. Thus, results indicate that curcumin can promote cell proliferation and kidney tissue recovery.
In our study, glycerol-induced AKI increased expression of TNF-α, IL-6, MCP-1, Kim-1 and Lcn-2, all which were significantly decreased after curcumin treatment. TNF-α and IL-6 are pro-inflammatory factors58 which are expressed in injury and inflammation sites that subsequently initiate the release of other pro-inflammatory cytokines and inflammatory mediatorse59. TNF-α also activates caspase-mediated apoptosis through activation of the death receptor pathway55. It was reported that TNF-α and IL-6 expression were significantly elevated in chronic renal failure patients60 and rat AKI models using cisplatin and paraquat to induce AKI61, 62. MCP-1 is a more recently studied renal biomarker which is expressed in injury and inflammation sites and directs the recruitment of macrophages, which bind to chemokine receptors to promote macrophage adhesion and chemotaxis63. Up-regulation of MCP-1 can occur in progressive kidney disease and when interstitial inflammatory infiltration occurs63 and due to this, the role of MCP-1 in AKI has attracted increasing attention64. In AKI, pro-inflammatory cytokines and chemokines are important factors that initiate inflammation and therefore play a prominent role in the progression of AKI62. The anti-inflammatory properties of curcumin in the kidney include the suppression of the pro-inflammatory factors nuclear factor (NF)-κB and cyclooxygenase (COX)-2, as well as the up-regulation of Nrf2 and HO-131, 32. Kim-1 is a specific and sensitive biomarker of kidney injury65, 66 mRNA levels associated with this protein have been shown to increase more than any other gene after kidney injury. Furthermore, the ectodomain of Kim-1 has been shown to be cleaved from cells both in vitro and in vivo and deposited within the urine of rodents after proximal tubular injury67. Lcn-2, also named neutrophil gelatinase associated lipocalin, is a recently identified marker for acute kidney injury as well as chronic kidney disease68–70. Studies have shown that both Kim-1 and Lcn2 are induced in AKI animal models and human renal diseases that involve acute injury of the proximal tubule epithelium71–73.
Renal tubular epithelial cell apoptosis is important in the pathogenesis of RM-induced AKI. In the current study, TUNEL staining revealed that apoptotic cells in the kidneys of AKI rats treated with curcumin were markedly reduced. In addition, western blot results also showed that down-regulation of caspase-9 and caspase-3, as well as up-regulation of p-Akt were observed in the CUR + AKI group compared with the AKI and CO + AKI groups. These data suggested that curcumin treatment significantly reduce apoptosis through mediation of the PI3K/Akt signaling pathway, a cell survial pathway. We conclude that expression of phosphorylated PI3K and AKT may have inhibited the release of Bad and Bax, as well as other downstream pro-apoptotic protein caspases, resulting in the lowered levels of apoptotic cells in the CUR + AKI group74–76. Indeed, caspase-3 and caspase-9 are closely coupled to upstream, pro-apoptotic signals. Specifically, cleaved caspase-3 and cleaved caspase-9 are involved in a mitochondrial-dependent pathway that eventually leads to cell apoptosis77. In the present study, p-5′ adenosine monophosphate-activated protein kinase (p-AMPK) expression was significantly increased by curcumin treatment, which indicated that AMPK signaling pathway was activated in the CUR + AKI group. AMPK is considered a metabolic master switch, regulating several intracellular systems78, 79 and has recenty been identified as a regulator of renal hypertrophy in diabetes, a disease where it has been shown to be downregulated80, 81. Interestingly, several studies have revealed that AMPK signaling may play a specific role in renal cells82, 83. Our finding showed that activation of the AMPK signaling pathway may have produced synergistic effects with Nrf2/HO-1 signaling pathway on antioxidant responses in RM-induced AKI.
In conclusion, this study showed that curcumin exerted renoprotective effects by inhibiting oxidative stress in RM-induced AKI through activation of the AMPK and Nrf2/HO-1 signaling pathways and ameliorated RM-associated renal injury and cell apoptosis through activation the PI3K/Akt pathway. Taken together, these results indicate that curcumin might be a useful therapeutic agent for treatment of AKI, although further studie are warranted.
Methods
Chemicals
All chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise indicated.
Animal Care and Use
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Nanjing Medical University, Jiangsu, China, and methods were carried out in “accordance” with the approved guidelines. Animals were obtained from the Animal Core Facility of Nanjing Medical University, Nanjing. Sixty female Sprague Dawley (SD) rats weighting 200–250 g were housed in a 12 h dark/light cycle animal facility with controlled temperature (20–25 °C) and humidity (40–70%). Food and water were given ad libitum throughout the study.
Experimental design
Sixty female SD rats were allowed to acclimatize for 1 week after which they were deprived of water for 24 h before the experiment and randomized into four groups. Rats in the control group received half the dose of saline (10 ml/kg) in each hind limb muscle; those in the group AKI received half the dose of glycerol (10 ml/kg, 50% v/v in sterile saline) in each hind limb muscle22, 84; rats in the CUR + AKI group received curcumin 200 mg/kg/day orally as a suspension in corn oil25, 85, 86 after glycerol administration; rats in the CO + AKI group received the same daily oral volume of corn oil as the group CUR + AKI. Three days after glycerol injection, all rats were sacrificed under general anesthesia using intraperitoneal injection of pentobarbital sodium (150 mg/kg). Blood was collected through heart puncture and the right kidney was excised immediately and divided into four parts. One part was fixed in glutaraldehyde solution for TEM examination and the other was stored in −80 °C for biochemical analysis. Finally, the left kidney was fixed in 10% neutral buffered formalin for histological studies.
Sample preparation and biochemical assays
All blood samples were allowed to clot at room temperature and centrifuged at 2,000 g for 10 min to harvest serum. Serum biochemical parameters of BUN, and Scr and CK levels were measured (n = 12 per group) using the commercially available kits, BUN (995–17711), Scr (991–32593), and CK (994–64291), all from Wako Pure Chemical Industry, Japan.
The kidneys were excised, then washed in ice-cold saline and homogenized in 0.1 M Tris–HCl buffer (pH 7.4). The homogenate were first centrifuged at 10,000 g for 15 min and the supernatants were then centrifuged at 100,000 g for 1 h. The resulting supernatant (cytosolic fraction) was used for the determination of enzymatic activities and lipid peroxidation. Kidney biochemical parameters of SOD, MDA and GSH-Px were measured spectrophotometrically (Eon, BioTeK, USA) using the commercially available kits, SOD (A001-1), MDA (A003-1), and GSH-Px (A005), all from Jiancheng Bioengineering Institute, Nanjing, China.
Histopathological examination
After sacrifice and dissection, kidneys from each rat were rapidly excised and then perfused in saline solution. Kidneys of rats from the different groups were fixed in 10% neutral buffered formalin for 24 h, dehydrated in graded alcohol and embedded in paraffin. The paraffin embedded tissues were then cut into 5-μm thick sections and stained with hematoxylin and eosin (H&E). Renal tubule injury was scored semi-quantitatively according to a scoring system described previously87, 88. Briefly, renal injury was defined by necrotic lysis, tubule dilation, cast formation, sloughing of cellular debris into the tubule lumen, or naked tubule basement membrane. Tubules in the boundary area between the renal cortex and medulla, and in the outer strip of the outer medulla were included. Renal injury scores were determined by the percentage of tubules injured: 0, no injury; 1, <20%; 2, 21–50%; 3, >50%; and 4, total destruction of all epithelial cells. Five random fields for each kidney slide (Five slides per animal) were examined (n = 8 per group).
Immunohistochemical staining
For immunohistochemical analysis, the paraffin sections were first dewaxed after which heat-mediated antigen retrieval was performed by microwaving sections for 20 min in 10 mM sodium citrate, pH 6.0. Sections were allowed to cool for 15 min, followed by a brief wash in deionized water, and then rinsed twice in PBS. Sections were incubated for 30 min in 5% goat serum in DPBS containing 0.1% Tween and 0.5% BSA. The sections were incubated overnight at 4 °C with primary antibody PCNA (sc-56), HO-1 (sc-390991) and E-Cadherin (sc-71007); all from Santa Cruz Biotechnology, CA, USA, at the appropriate dilution. The secondary antibody Dako REAL EnVisio Detection System (K5007, DAKO, Denmark) was used to detect primary antibodies. The specimens were then counterstained with hematoxylin for 1 min. All sections were incubated under the same conditions with the same concentration of antibodies and at the same time, in order to have comparable immunostaining among the different experimental groups. Tissue sections in the boundary area between the renal cortex and medulla were observed and photographed with a microscope and were semi-quantified by Image-Pro Plus 6.0 software. The integrated optical density (IOD) of each photograph was collected. Five fields of each slice (Five slides per animal) were randomly selected for blinded measuring (n = 8 per group). Images were quantified by the immunoreactive area (IA) in μm2 and the IOD. Staining intensity (SI) for each image was calculated as SI = IOD/IA and mean with standard deviation was obtained for each series.
TEM examination
Each specimen was prepared at all steps with an electron microscope reagent to generate ultrathin sections, and examined under a transmission electron microscope (Tecnai G2 Spirit Bio TWIN, FEI Ltd., USA). Electron micrographs of five to ten glomeruli per kidney were randomly examined for each experiment (n = 6 per group).
In situ TUNEL fluorescence staining assay
The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay was performed according to the manufacturer’s instructions (11684817910, Roche, Switzerland). Kidney tissue was fixed in 10% formalin overnight, dehydrated, embedded in paraffin, cut into 4 µm-thick sections, and placed on a numbered polylysine-coated glass slide. TUNEL-positive cells, which were stained green; nuclei were stained with DAPI to observe the nature of the TUNEL-positive cells. TUNEL-positive cells and total cells in the boundary area between the renal cortex and medulla were counted and analyzed using Image-Pro Plus 6.0 software. Five random fields per slide (Five slides per animal) were examined in each experiment (n = 8 per group).
Q-PCR
Total mRNA was extracted from renal cortical tissue sample using TRIzol Reagent (B5704–1, Takara, Dalian, China) according to the manufacturer’s instructions, followed by treatment with DNase I (2212, Takara) according to the manufacturer’s instructions. RNA quality and quantity were determined using a Spectro-photometer (NanoDrop 2000c, Thermo Scientific, USA) and cDNA was synthesized immediately after using PrimeScriptTM RT reagent Kit (RR037A, Takara, Dalian, China), according to the manufacturer’s instructions. Q-PCR was performed using the Light Cycler PCR QC Kit (Roche, Switzerland) and the 7300 Real-Time PCR System (LC96, Roche). PCR primers are listed in Supplementary Table 1. Data analysis was performed using GraphPad Prism 5 software (n = 10 per group).
Western Blot
Western blotting was performed according to methods previously described22. Briefly, 50 ug of total lysate from kidney tissue were subjected to 15% polyacrylamide gel electrophoresis and transferred to cellulose acetate membranes. The membranes were blocked with 1× casein solution for approximately 4 h and then incubated with rabbit monoclonal anti-P-AMPK (Ser485) (2537), anti-P-Akt (Ser473) (4058), anti-Akt (4685), anti-cleaved caspase-3 (Asp175) (9664) and anti-Cleaved Caspase-9 (Asp330) (7237), all from Cell signaling technology; or rabbit polyclonal anti-Nrf2 (sc-722), anti-GAPDH (H-12) (sc-166574) and mouse monoclonal anti-HO-1 (sc-390991) anti-AMPK (sc-398861), all from Santa Cruz Biotechnology; or PI3K (ab86714) and p-PI3K (ab182651), both from Abcam Biotechnology, in blocking buffer for 2 h at room temperature. Membranes were then washed three times with Tris-buffered saline-Tween-20 (TBST), incubated with goat anti-rabbit IgG-HRP secondary antibody (sc-2004, Santa Cruz Biotechnology) and imaged by ChemiDoc XRS + Molecular Imager (Bio-Rad, USA). All images were using the free image analyzing software Image J 1.42 (n = 10 per group).
Statistical analysis
Statistical analysis was performed using SPSS v.16. Results values are means ± standard error (SE) and all statistical comparisons were made by means of one-way ANOVA test followed by Duncan’s multiple range test post hoc analysis. Student’s t-test was used to compare differences between means. p values of <0.05 were considered significant.
Electronic supplementary material
Supplementary information for Effect of curcumin on glycerol-induced acute kidney injury in rats
Acknowledgements
We would like to thank the staff members of who organised this study, as well as our colleagues and all the study staff for their enormous efforts in collecting and ensuring the accuracy and completeness of the data. This work was supported by Science and Technology Development Key Projects Fund of Nanjing Medical University (2016NJMUZD019 and 2016NJMUZD020).
Author Contributions
J.D.W. and X.X.P. performed animal experiments and drafted the manuscript. H.L.F., Y.Z. and Q.T.H. helped perform pathological experiments and experiments regarding molecular biological techniques. Y.J.D. provided essential help help in all animal experiments. Y.Y. and Q.C. performed biochemical assays on serum and renal tissue. D.B. provided constructive suggestions on the experimental design and revised the manuscript. D.R.H. designed the study, edited the manuscript and supervised the study. All authors reviewed and approved the final the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jindao Wu and Xiongxiong Pan contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10693-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dan Bao, Email: jndwbaodan@njmu.edu.cn.
Daorong Hou, Email: houdaorong@njmu.edu.cn.
References
- 1.Zager RA. Marked protection against acute renal and hepatic injury after nitrited myoglobin + tin protoporphyrin administration. Translational research: the journal of laboratory and clinical medicine. 2015;166:485–501. doi: 10.1016/j.trsl.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Critical Care. 2006;10 doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney International. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 4.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 5.Mai MH, EI-Gowelli HM. Montelukast abrogates rhabdomyolysis-induced acute renal failure via rectifying detrimental changes in antioxidant profile and systemic cytokines and apoptotic factors production. European journal of pharmacology. 2012;683:294–300. doi: 10.1016/j.ejphar.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Better OS. The crush syndrome revisited (1940–1990) Nephron. 1990;55:97–103. doi: 10.1159/000185934. [DOI] [PubMed] [Google Scholar]
- 7.Wu GL, et al. Exhaustive swimming exercise related kidney injury in rats-protective effects of acetylbritannilactone. Int J Sports Med. 2012;33:1–7. doi: 10.1055/s-0031-1284397. [DOI] [PubMed] [Google Scholar]
- 8.Vanholder R. SM, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 9.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803–811. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 10.Panizo N, et al. Molecular Mechanisms and Novel Therapeutic Approaches to Rhabdomyolysis-Induced Acute Kidney Injury. Kidney & blood pressure research. 2015;40:520–532. doi: 10.1159/000368528. [DOI] [PubMed] [Google Scholar]
- 11.Wilson DR, et al. Glycerol induced hemoglobinuric acute renal failure in the rat. 3. Micropuncture study of the effects of mannitol and isotonic saline on individual nephron function. Arce Nephron. 1967;4:337–355. doi: 10.1159/000179594. [DOI] [PubMed] [Google Scholar]
- 12.Abul-Ezz SR, Walker PD, Shah SV. Role of glutathione in an animal model of myoglobinuric acute renal failure. Proc Natl Acad Sci USA. 1991;88:9833–9837. doi: 10.1073/pnas.88.21.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clinical journal of the American Society of Nephrology: CJASN. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augustyniak A, et al. Natural and synthetic antioxidants: an updated overview. Free Radical Research. 2010;44:1216–1262. doi: 10.3109/10715762.2010.508495. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa S, et al. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Research. 2004;24:563–569. [PubMed] [Google Scholar]
- 16.Dinkovakostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Molecular Nutrition& Food Research. 2008;52:128–138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese V. Curcumin and the cellular stress response in free radical-related diseases. Molecular Nutrition & Food Research. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The International Journal of Biochemistry&Cell Biology. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueki M, et al. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Journal of Bioscience and Bioengineering. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Wang ME, et al. Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. The Journal of Nutritional Biochemistry. 2012;23:1352–1366. doi: 10.1016/j.jnutbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Geng XD, et al. Biological Membrane-Packed Mesenchymal Stem Cells Treat Acute Kidney Disease by Ameliorating Mitochondrial-Related Apoptosis. Scientific reports. 2017;7 doi: 10.1038/srep41136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mun SH, et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20:714–718. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa), in streptozotocin induced diabetic rats. Molecular and Cellular Biochemistry. 1998;166:169–175. doi: 10.1023/A:1006819605211. [DOI] [PubMed] [Google Scholar]
- 24.Farombi EO, Ekor M. Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food and Chemical Toxicology. 2006;44:1443–1448. doi: 10.1016/j.fct.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Ali BH, et al. Curcumin has a palliative action on gentamicin-induced nephrotoxicity in rats. Fundam. Clin. Pharmacol. 2005;19:473–477. doi: 10.1111/j.1472-8206.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 26.Buyuklu M, et al. Protective effect of curcumin against contrast induced nephropathy in rat kidney: what is happening to oxidative stress, inflammation, autophagy and apoptosis? Eur Rev Med Pharmacol Sci. 2014;18:461–470. [PubMed] [Google Scholar]
- 27.Negretteguzman M, et al. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1–16. doi: 10.1155/2015/917435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwan KB, et al. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med J. 2016;57:664–673. doi: 10.3349/ymj.2016.57.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim ZS. Renoprotective effect of curcumin against the combined oxidative stress of diabetes and nicotine in rats. Molecular Medicine Reports. 2016;13:3017–3026. doi: 10.3892/mmr.2016.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin K, et al. Comparative in vivo evaluations of curcumin and its analog difluorinated curcumin against cisplatin-induced nephrotoxicity. Biol.Trace Elem. Res. 2014;157:156–163. doi: 10.1007/s12011-014-9886-x. [DOI] [PubMed] [Google Scholar]
- 31.Ueki M, et al. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J. Biosci. & Bioeng. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 33.Parekh R, Care DA, Tainter CR. Rhabdomyolysis: advances in diagnosis and treatment. Emergency medicine practice. 2012;14:1–15. [PubMed] [Google Scholar]
- 34.Kim JH, et al. N-acetylcysteine attenuates glycerol-induced acute kidney injury by regulating MAPKs and Bcl-2 family proteins. Nephrol Dial Transplantat. 2010;25:1435–1443. doi: 10.1093/ndt/gfp659. [DOI] [PubMed] [Google Scholar]
- 35.Daher EF, et al. Differences in community, hospital and intensive care unit-acquired acute kidney injury: observational study in a nephrology service of a developing country. Clinical Nephrology. 2012;78:449–455. doi: 10.5414/CN107167. [DOI] [PubMed] [Google Scholar]
- 36.Thiel G, Wilson DR, Arce ML, Oken DE. Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron. 1967;4:276–297. doi: 10.1159/000179588. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CH, Kurtz TW, Waldinger TP. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res. 1977;40:178–182. doi: 10.1161/01.RES.40.2.178. [DOI] [PubMed] [Google Scholar]
- 38.Zager RA, Burkhart KM, Conrad DS, Gmur DJ. Iron, hemeoxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995;48:1624–1634. doi: 10.1038/ki.1995.457. [DOI] [PubMed] [Google Scholar]
- 39.Zager RA, Burkhart KM. Differential effects of glutathione and cysteine on Fe2+, Fe3+, H2O2 and myoglobin-induced proximal tubular cell attack. Kidney Int. 1998;53:1661–1672. doi: 10.1046/j.1523-1755.1998.00919.x. [DOI] [PubMed] [Google Scholar]
- 40.Moore KP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. The Journal of biological chemistry. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 41.Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392. doi: 10.1038/sj.ki.5000315. [DOI] [PubMed] [Google Scholar]
- 42.Oken DE, Arce ML, Wilson DR. Glycerol-induced hemoglobinuric acute renal failure in the rat. Journal of Clinical Investigation. 1966;45:724–735. doi: 10.1172/JCI105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein JH, Lifschitz MD, Barnes LD. Current concepts on the pathophysiology of acute renal failure. American Journal of Physiology. 1978;234:171–181. doi: 10.1152/ajprenal.1978.234.3.F171. [DOI] [PubMed] [Google Scholar]
- 44.Boonla O, et al. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Wu W, Geng H, Liu Z, Li H, Zhu ZZ. Effect of curcumin on rats/mice with diabetic nephropathy: a systematic review and meta-analysis of randomized controlled trials. J. Tradit. Chin. Med. 2014;34:419–429. doi: 10.1016/S0254-6272(15)30041-8. [DOI] [PubMed] [Google Scholar]
- 46.DeRubertis FR, Craven PA, Melhem MF, Salah E. M. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- 47.Balogun E, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013;39:101–121. doi: 10.1002/biof.1072. [DOI] [PubMed] [Google Scholar]
- 49.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 50.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J. Mol. Cell. Cardiol. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Lee H, et al. Sevoflurane post-conditioning increases nuclear factor erythroid 2-related factor and haemoxygenase-1 expression via protein kinase C pathway in a rat model of transient global cerebral ischaemia. Br. J. Anaesth. 2015;114:307–318. doi: 10.1093/bja/aeu268. [DOI] [PubMed] [Google Scholar]
- 53.Yu JB, et al. Role of Nrf2/ARE pathway in protective effect of electroacupuncture against endotoxic shock-induced acute lung injury in rabbits. PloS one. 2014;9 doi: 10.1371/journal.pone.0104924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang SJ, Meng JP, Qu Y, Liu YB. The progress on the signal transduction pathways of apoptosis. Chin J Comp Med. 2007;17:297–301. [Google Scholar]
- 55.Sun Q, et al. Protective effect of ginsenoside Rb1 against intestinal ischemia- reperfusion induced acute renal injury in mice. Plos one. 2013;8 doi: 10.1371/journal.pone.0080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shalaby RH, Rashed LA, Ismaail AE, Madkour NK, Elwakeel SH. Hematopoietic stem cells derived from human umbilical cord ameliorate cisplatin-induced acute renal failure in rats. Am J Stem Cells. 2014;3:83–96. [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, et al. Attenuation of inflammation by Emodin in lipopolysaccharide- induced acute kidney injury via inhibition of Toll-like receptor 2 signal pathway. Kidney Diseases. 2015;9:202–208. [PubMed] [Google Scholar]
- 58.Wan L, Bellomo R, Di GD, Ronoco C. The pathogenesis of septic acute renal failure. Curt Opin Crit Care. 2003;9:496–502. doi: 10.1097/00075198-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Lee BT, et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrology. 2015;16:1–6. doi: 10.1186/s12882-015-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI200215606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu SY, Yeh TY, Lin SY, Peng FC. Unfractionated bone marrow cells attenuate paraquat-induced glomerular injury and acute renal failure by modulating the inflammatory response. Scientific reports. 2016;6 doi: 10.1038/srep23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MJ, Tam FW. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta. 2011;412:2022–2030. doi: 10.1016/j.cca.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 63.Chen HH, et al. Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion. J Cell Physiol. 2014;229:1202–1211. doi: 10.1002/jcp.24554. [DOI] [PubMed] [Google Scholar]
- 64.Bonventre JV. Kidney injury molecule-1 (kim-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Invest Suppl. 2008;241:78–83. doi: 10.1080/00365510802145059. [DOI] [PubMed] [Google Scholar]
- 65.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 66.Bailly V, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 67.Bolignano D, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 69.Dvergsten J, Manivel JC, Correa-Rotter R, Rosenberg ME. Expression of clusterin in human renal diseases. Kidney Int. 1994;45:828–835. doi: 10.1038/ki.1994.109. [DOI] [PubMed] [Google Scholar]
- 70.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2004;286:552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 71.Viau A, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rached E, et al. Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin A in vivo and in vitro. Toxicol. Sci. 2008;103:371–381. doi: 10.1093/toxsci/kfn040. [DOI] [PubMed] [Google Scholar]
- 73.Atif F, Yousuf S, Stein DG. Anti-tumor effects of progesterone in human glioblastoma multiforme: role of PI3K/Akt/mTOR signaling. The Journal of Steroid Biochemistry & Molecular Biology. 2015;146:62–73. doi: 10.1016/j.jsbmb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annual review of physiology. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 75.Chalah A, Khosravi-Far R. The mitochondrial death pathway. Advances in Experimental Medicine and Biology. 2008;615:25–45. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- 76.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochimica Et Biophysica Acta. 2011;1813:235–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 77.Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol. 2013;366:152–162. doi: 10.1016/j.mce.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews. Molecular Cell Biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kume S, Koya D, Uzu T, Maegawa H. Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee MJ, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:617–627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 81.Lee MJ, et al. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal. 2010;22:65–70. doi: 10.1016/j.cellsig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee HJ, et al. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. The Journal of Biological Chemistry. 2012;287:4451–4461. doi: 10.1074/jbc.M111.278325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim HY, Yokozawa T, Nakagawa T, Sasaki S. Protective effect of c-aminobutyric acid against glycerol-induced acute renal failure in rats. Food and Chemical Toxicology. 2004;42:2009–2014. doi: 10.1016/j.fct.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 84.Chuang SE, Chen AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food and Chemical Toxicology. 2000;38:991–995. doi: 10.1016/S0278-6915(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 85.Buyuklu M, et al. Protective effect of curcumin against contrast induced nephropathy in rat kidney: what is happening to oxidative stress, inflammation, autophagy and apoptosis? European Review for Medical and Pharmacological Sciences. 2014;18:461–470. [PubMed] [Google Scholar]
- 86.Yang CW, et al. Preconditioning with erythropoietin protects against subsequent ischemia- reperfusion injury in rat kidney. FASEB J. 2003;17:1754–1755. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
- 87.Melnikov VY, et al. Neutrophil-independent mechanisms of caspase-1-and il-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110:1083–1091. doi: 10.1172/JCI0215623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang DW, et al. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/636053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information for Effect of curcumin on glycerol-induced acute kidney injury in rats