Abstract
Spatio-temporal patterns of population changes within and across countries have various implications. Different geographical, demographic and econo-societal factors seem to contribute to migratory decisions made by individual inhabitants. Focusing on internal (i.e. domestic) migration, we ask whether individuals may take into account the information on the population density in distant locations to make migratory decisions. We analyse population census data in Japan recorded with a high spatial resolution (i.e. cells of size 500×500 m) for the entirety of the country, and simulate demographic dynamics induced by the gravity model and its variants. We show that, in the census data, the population growth rate in a cell is positively correlated with the population density in nearby cells up to a distance of 20 km as well as that of the focal cell. The ordinary gravity model does not capture this empirical observation. We then show that the empirical observation is better accounted for by extensions of the gravity model such that individuals are assumed to perceive the attractiveness, approximated by the population density, of the source or destination cell of migration as the spatial average over a circle of radius ≈1 km.
Keywords: demography, dynamics, gravity model, migration, population census
1. Introduction
Demography, particularly spatial patterns of population changes, has been a target of intensive research because of its economical and societal implications, such as difficulties in upkeep of infrastructure [1–3], policymaking related to city planning [1,2] and integration of municipalities [3]. A key factor shaping spatial patterns of demographic dynamics is migration. Migration decisions by inhabitants are affected by various factors including job opportunities, cost of living and climatic conditions [4–6]. These and other factors are often non-randomly distributed in space, creating spatial patterns of migration and population changes over time. A number of models have been proposed to describe and predict spatio-temporal patterns of human migration [7–13].
Among these models, a widely used model is the gravity model (GM) and its variants [8,10,14,15]. The GM assumes that the migration flow from one location to another is proportional to a power (or a different monotonic function) of the population at the source and destination locations and the distance between them. The model has attained reasonably accurate description of human migration in some cases [8,16,17], as well as other phenomena such as international trades [18,19] and the volume of phone calls between cities [20,21].
Studies of migration, such as those using the GM [8,17] and other migration models [11,22], are often based on subdivisions of the space that define the unit of analysis such as administrative units (e.g. country and city). However, the choice of the unit of analysis is often arbitrary. Humans whose migratory behaviour is to be modelled microscopically, statistically or otherwise, may pay less attention to such a unit than a model assumes when they make a decision to move home. This may be particularly so for internal (i.e. domestic) migrations rather than for international migrations because boundaries of administrative units may impact inhabitants less in the case of internal migrations than international migrations. This issue is related to the modifiable areal unit problem in geography, which stipulates that different units of analysis may provide different results [23]. For example, particular partitions of geographical areas can affect parameter estimates of gravity models [24]. To overcome such a problem, criteria for selecting appropriate units of analysis have been sought [24–28]. Another strategy to address the issue of the unit of analysis is to employ models with a maximally high spatial resolution. For example, a recently proposed continuous-space GM assumes that the unit of analysis is an infinitesimally small spatial segment [12]. This approach implicitly assumes that the unit of analysis, which a modelled individual perceives, is an infinitesimally small spatial segment. In fact, humans may regard a certain spatial region, which may be different from an administrative unit and have a certain finite but unknown size, as a spatial unit based on which they make a migration decision. If this is the case, individuals may make decisions by taking into account the environment in a neighbourhood of the current residence and/or the destination of the migration up to a certain distance. Here, we examine this possibility by combining data analysis and modelling, complementing past research on the choice of geographical units for understanding human migration [24–28].
In this paper, we analyse demographic data obtained from the population census of Japan carried out in 2005 and 2010, which are provided with a high spatial resolution [29]. We hypothesize that the growth rate of the population is influenced by the population density near the current location as well as that at the focal location, where each location is defined by a 500×500 m cell in the grid according to which the data are organized. We provide evidence in favour of this hypothesis through correlation-based data analysis. Then, we argue that the GM is insufficient to produce the empirically observed spatial patterns of the population growth. We provide extensions of the GM that better fit the empirical data, in which individuals are assumed to aggregate the population of nearby cells to calculate the attractiveness of the source or destination cell of migration.
2. Methods
2.1. Dataset
We analysed demographic dynamics using data from the population census in Japan [29], which consisted of measurements from K=1 944 711 cells of size 500×500 m. The census is conducted every 5 years. We used data from the censuses conducted in 2005 and 2010 because data with such a high spatial resolution over the entirety of Japan were only available for these years. We also ran the following analysis using the data from the census conducted in 2000 (appendix A), which were somewhat less accurate in counting the number of inhabitants in each cell than the data in 2005 and 2010 [30]. In the main text, we refer to the two time points 2005 and 2010 as t1 and t2, respectively. The number of inhabitants in cell i (1≤i≤K) at time t is denoted by ni(t). We used the latitude and longitude of the centroid of each cell to define its position. Basic statistics of the data at the three time points are presented in table 1.
Table 1.
Statistics of the dataset.
| year | 2000 | 2005 | 2010 |
|---|---|---|---|
| total population | 126 925 843 | 127 767 994 | 128 057 352 |
| average number of inhabitants in a cell | 249.48 | 251.13 | 251.70 |
| median number of inhabitants in a cell | 33 | 41 | 38 |
| number of populated cells | 308 418 | 482 181 | 477 172 |
2.2. Spatial correlation
We defined the distance between cells i and j, denoted by dij, as that between the centroids of the two cells in kilometres. We measured the spatial correlation in the number of inhabitants between a pair of cells at distance d by [31]
| 2.1 |
which is essentially the Pearson correlation coefficient calculated from all pairs of cells at a distance ≈d apart. In equation (2.1), is the average number of inhabitants in an inhabited cell; is the variance of the number of inhabitants in an inhabited cell; I(d<di′j′≤d+1)=1 if d<di′j′≤d+1 (d=0,1,2,…) and I(d<di′j′≤d+1)=0 otherwise; K′ (=482 181 at time t1 and 477 172 at time t2) is the number of inhabited cells. In equation (2.1), the summations on the right-hand side are restricted to the inhabited cells i′ and j′. We suppressed the time in equation (2.1). It should be noted that C(d) can be larger than 1.
2.3. Correlation between the growth rate and the population density in nearby cells
In the analysis of the growth rate of cells described in this section, we only used focal cells i whose population size was between 10 and 100 at t1. We did so because the growth rate of less populated cells tended to fluctuate considerably and the growth rate of a more populated cell tended to be ≈0. We carried out the same set of analysis for cells whose population size was greater than 100 to confirm that the main results shown in the following sections remain qualitatively the same (appendix C). It should be noted that cell i may be partially water-surfaced.
To calculate the correlation between the rate of population growth in a cell and the population density in cells nearby, we first divided the entire map of Japan into square regions of approximately 50×50 km. The regions were tiled in a 64×45 grid to cover the whole of Japan. The minimum and maximum longitudes in the dataset were 122.94 and 153.98, respectively. Therefore, we divided the range of the longitude into 64 windows, i.e. [122.4, 123), [123, 123.5),…, [153.5, 154]. Similarly, the minimum and maximum latitudes were 45.5229 and 24.0604, respectively. We thus divided the range of the latitude into 45 windows, i.e. [24, 24,5), [24.5, 25),…, [45.5,46]. We classified each cell into one of the 64×45 regions on the basis of the coordinate of the centroid of the cell. Note that there were sea regions without any inhabitant. A region included 9600 cells at most.
The growth rate of cell i in the 5 years is given by
| 2.2 |
We denoted by Di(d) the population density at time t1 averaged over the cells j whose distance from cell i, dij, is approximately equal to d, i.e. d<dij≤d+1. We calculated the Pearson correlation coefficient between the population growth rate (i.e. Ri) and Di(d), restricted to the cells in region k, i.e.
| 2.3 |
where and are the average of Ri and Di(d) over the cells in region k, respectively. A positive value of ρk(d) is consistent with our hypothesis that the population growth rate is influenced by the population density in different cells. We remind that the summation in equation (2.3) is taken over the cells whose population is between 10 and 100. The correlation coefficient ρk(d) ranges between −1 and 1. We did not exclude water-surface cells or partially water-surface cells j from the calculation of Di(d). Finally, we defined as the average of ρk(d) over all regions excluding those with less than 20 populated cells. We decided to calculate ρk(d) for individual regions, k, and averaged it over the regions rather than to calculate the single correlation coefficient between Ri and Di(d) for the entirety of Japan. In this way, we aimed to suppress fluctuations in individual ρk(d). We show ρk(d) for each region in appendix B. We also show ρk(d) for region k such that all cells within region k and those within 30 km from any cell in region k are not in the sea in appendix B.
To examine the statistical significance of , we carried out bootstrap tests by shuffling the number of inhabitants in the populated cells at t2 without shuffling that at t1 and calculating . We generated 100 randomized samples and calculated the distribution of for each sample. We deemed the value of for the original data to be significant if it was not included in the 95% confidential interval (CI) calculated on the basis of the 100 randomized samples.
2.4. Gravity model
In the standard gravity model (GM), the migration flow from source cell i to destination cell j (≠i), Tij, is given by
| 2.4 |
where G, α, β and γ are parameters. Because α, β and γ are usually assumed to be positive, equation (2.4) implies that the migration flow is large when the source or the destination cell has many inhabitants or when the two cells are close to each other.
In addition to the GM, we investigated two extensions of the GM in which the migration flow depends on the numbers of inhabitants in a neighbourhood of cell i or j. The first extension, which we refer to as the GM with the spatially aggregated population density at the destination (d-aggregate GM), is given by
| 2.5 |
where Nj(dag) is the number of inhabitants contained in the cells within distance dag km from cell j. We remind that the distance between two cells is defined as that between the centroids of the two cells. The rationale behind this extension and the next one is that humans may perceive the population density at the source or destination as a spatial average. A similar assumption was used in a model of city growth, where cells close to inhabitant cells were more likely to be inhabited [32].
The second extension of the GM aggregates the population density around the source cell. To derive this variant of the GM, we rewrite equation (2.4) as and interpret that each individual in cell i is subject to the rate of moving to cell j, i.e. . The second extension, which we refer to as the GM with the aggregated population density at the source (s-aggregate GM), is defined by
| 2.6 |
Unless we state otherwise, we set dag=0.65 in the d-aggregate and s-aggregate GMs, which is equivalent to the aggregation of a cell with the neighbouring four cells in the north, south, east and west. We will also examine larger dag values.
Using one of the three GMs, we projected the number of inhabitants in each cell at time t2 given the empirical data at time t1. The predicted number of inhabitants in cell i at time t2, denoted by , is given by
| 2.7 |
We refer to , and as the inflow, outflow and net flow of the population at cell i, respectively.
The projection of the growth rate, denoted by , is defined by , based on which we calculated for the model. We set G=1 because the value of does not depend on G.
We measured the discrepancy between the empirical and projected data in terms of by
| 2.8 |
where and are the values of obtained for the empirical data and a model, respectively. If the relationship between ρ(d) and d is similar between the empirical data and the model, the discrepancy given by equation (2.8) takes a small value.
3. Results
3.1. Spatial distribution of inhabitants
The spatial distribution of the number of inhabitants at time t2 is shown in figure 1. The figure suggests centralization of the number of inhabitants in urban areas. We calculated the Gini index, defined by , to quantify heterogeneity in the population density across cells; it is often used for measuring wealth inequality. The Gini index at t1 and t2 was equal to 0.797 and 0.804, respectively, suggesting a high degree of heterogeneity. The survival function of the number of inhabitants in a cell at t1 and t2 is shown in figure 2. The figure suggests that a majority of cells contains a relatively small number of inhabitants, whereas a small fraction of cells has many inhabitants.
Figure 1.
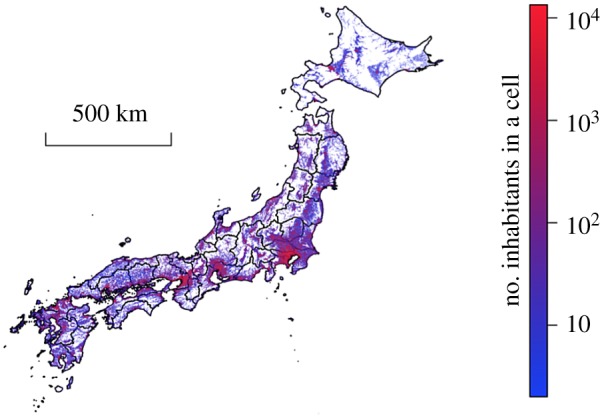
The distribution of inhabitants at time t2 (i.e. year 2010). The colour code represents the numbers of inhabitants in a cell. Vacant cells are shown in white.
Figure 2.
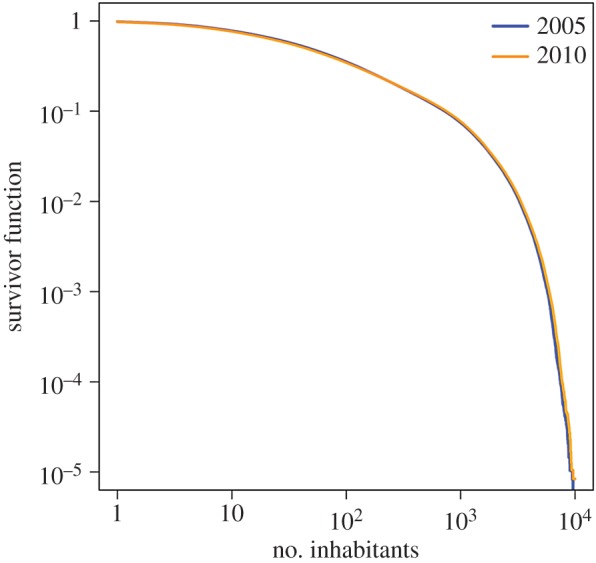
The survivor function of the number of inhabitants in a cell. The two lines almost overlap with each other.
Figure 1 suggests the presence of spatial correlation in the population density, as observed in other countries [31]. Therefore, we measured the spatial correlation coefficient in the population size between a pair of cells, C(d), where d was the distance between a pair of cells. Figure 3 indicates that C(d) is substantially positive up to d≈70 km, confirming the presence of spatial correlation. This correlation length was shorter than that observed in previous studies of data recorded in the USA [31] (≈1000 km) and spatial correlation in the population growth rate in Spain [33] (≈500 km) and the USA [34] (over 5000 km).
Figure 3.
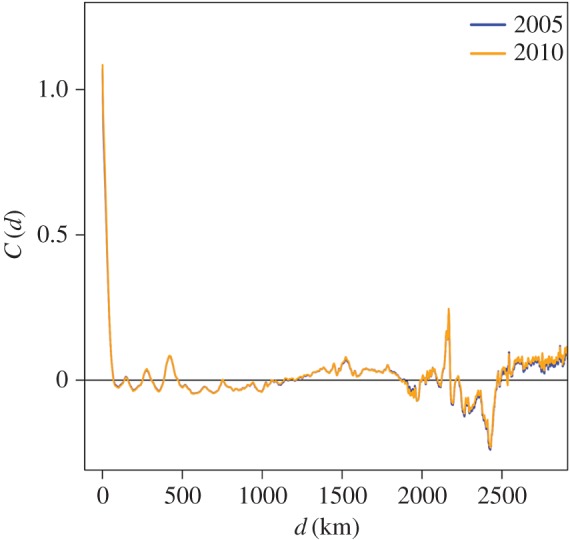
The spatial correlation in the number of inhabitants in the cell. The correlation measure C(d) is defined by equation (2.1), and d is the distance between the two cells. The two lines almost overlap with each other.
3.2. Effects of the population density in nearby cells on migration
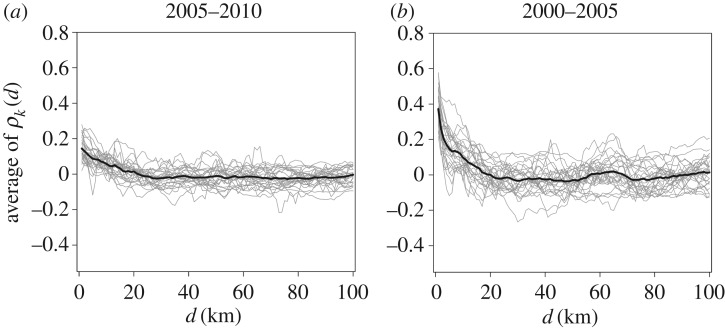
We measured , which quantifies the effect of the population in cells at distance d on the population growth in a focal cell. Figure 4 shows as a function of d. The values of were the largest at d=0. In other words, the effects of the population density within 1 km is the most positively correlated with the growth rate of a cell. This result reflects the observation that highly populated cells tend to grow and vice versa [35–37] (but see [38]). As d increased, decreased and reached ≈0 for d≥20 km. This result suggests that cells surrounded by cells with a large (small) population density within ≈20 km are more likely to gain (lose) inhabitants.
Figure 4.
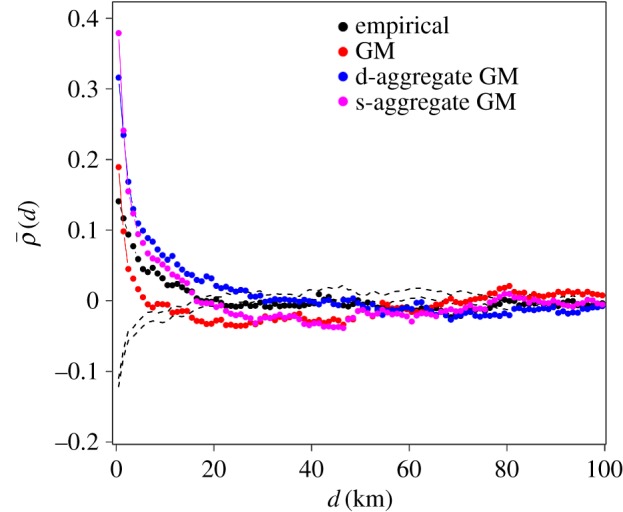
Dependence of the population growth rate in a cell on the population density at distance d, . We set α=0.4, β=0.8 and γ=1 for the GM; α=0.8, β=0.4, γ=1 and dag=0.65 km for the d-aggregate GM; α=0.4, β=0.4, γ=1 and dag=0.65 km for the s-aggregate GM. The ranges indicated by the dashed lines represent 95% confidence intervals (CIs) generated by spatially random distributions of the number of inhabitants on the inhabited cells.
The observed correlation between the population growth rate of a cell and the population of nearby cells may be explained by the combination of spatial correlation in the population density (figure 3) and positive correlation between the population growth rate and the population density in the same cell. To exclude this possibility, we measured as the partial correlation coefficient, modifying equation (2.3), controlling for the population size of a focal cell. The results were qualitatively the same as those based on the Pearson correlation coefficient (appendix D).
3.3. Gravity models
Various mechanisms may generate the dependence of the population growth rate in a cell on different cells (up to ≈20 km apart), including heterogeneous birth and death rates that are spatially correlated. Here, we focused on the effects of migration as a possible mechanism to generate such a dependency. We simulated migration dynamics using the gravity model [8,10,15] and its variants and compared the projection obtained from the models with the empirical data. We did not consider the radiation models [11,12] including intervening opportunity models [7] because our aim here was to qualitatively understand some key factors that may explain the effects of distant cells observed in figure 4 rather than to reveal physical laws governing migration.
In figure 4, we compare between the empirical data and those generated by the GM, d-aggregate GM and s-aggregate GM. Because precise optimization is computationally too costly, we set γ=1 and set α, β∈{0.4,0.8,1.2,1.6} to search for the optimal pair of α and β. For this parameter set, all models yielded positive values of , consistent with the empirical data. For the GM, decreased towards zero as d increased for d<6 km, i.e. the value of decayed faster than the empirical values. At d>6 km, generated by the GM was around zero but tended to be smaller than the empirical values. The two extended GMs yielded a decay of , which hit zero at d≈20 km, qualitatively the same as the behaviour of the empirical data. The two extended GMs generated larger values than the empirical values for d≤20 km.
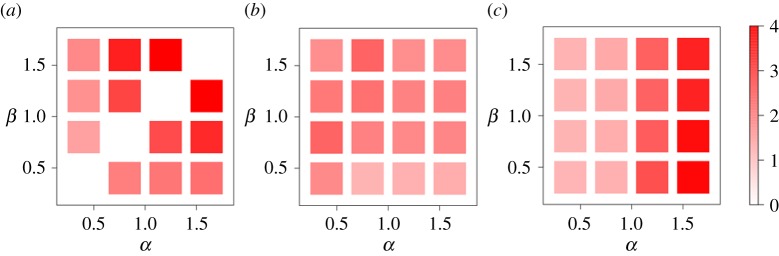
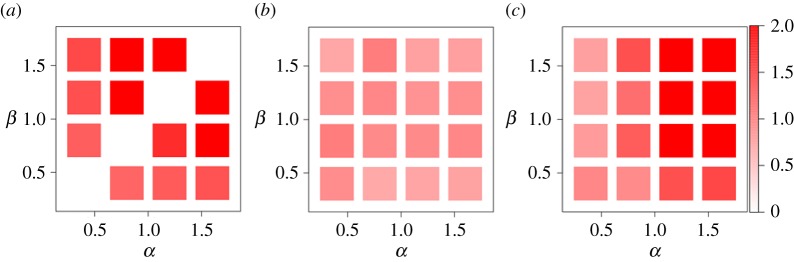
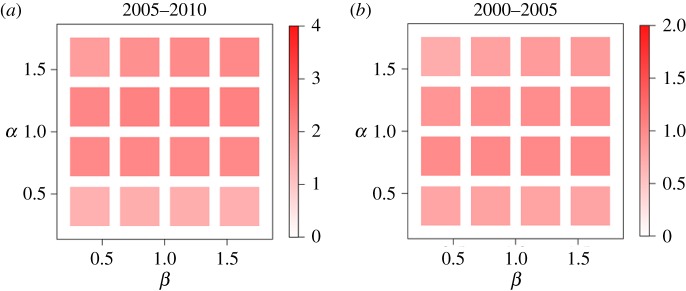
To investigate the robustness of the results against variation in the parameters of the models, we varied the parameter values as α∈{0.4,0.8,1.2,1.6} and β∈{0.4,0.8,1.2,1.6} and measured the discrepancy between the model and empirical data in terms of the discrepancy measure defined by equation (2.8). The results for the three models are shown in figure 5. The data obtained from the GM were inaccurate except when α or β was small. In addition, the minimum discrepancy for the GM (=1.469) was larger than that for the d-aggregate GM and s-aggregate GM (=1.163 and 1.161, respectively). The d-aggregate GM showed a relatively good agreement with the empirical data in a wide parameter region. The performance of the s-aggregate GM was comparable with that of the d-aggregate GM only when α=0.4 or 0.8. Our analysis suggests that aggregating nearby cells around either the source or destination of migration seems to improve the explanatory power of the GM. The performance of the d-aggregate GM was better than that of the s-aggregate GM in terms of the robustness against variation in the parameter values.
Figure 5.
The discrepancy of the GM, d-aggregate GM and s-aggregate GM from the empirical data in terms of the discrepancy measure given by equation (2.8). A dark hue represents a large discrepancy value. (a) GM. (b) d-aggregate GM. (c) s-aggregate GM. The diagonals in (a) are blank because the inflow and outflow are the same when α=β in the GM, resulting in a zero population growth rate in all cells. We set γ=1 and dag=0.65 km.
3.4. Effects of the granularity of spatial aggregation
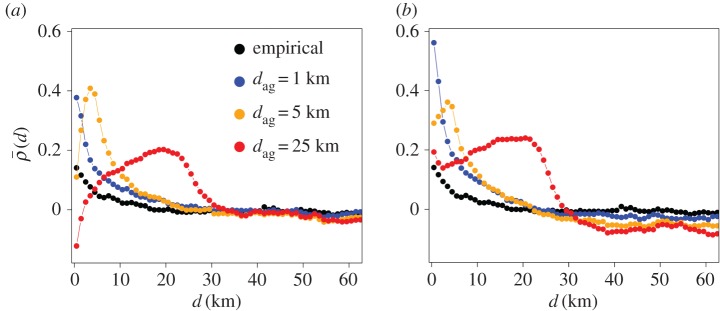
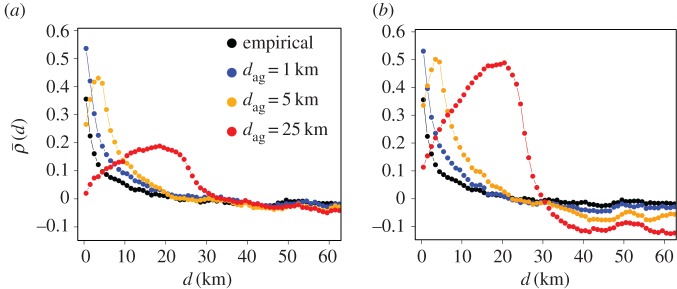
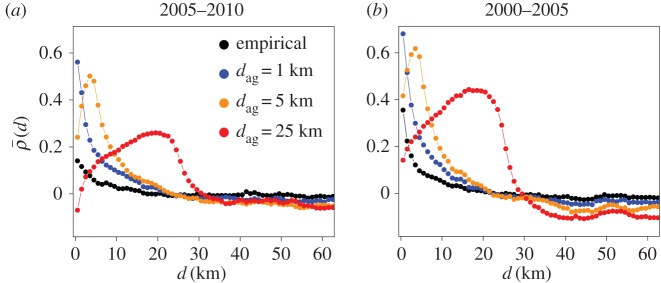
We set dag, the width for spatial smoothing of the population density at the source or destination cell in the extended GM models, to 0.65 km in the previous sections. To investigate the robustness of the results with respect to the dag value, we used dag=1, 5 and 25 km combined with the d-aggregate and s-aggregate GMs. The discrepancy between each model and the empirical data is shown in figure 6.
Figure 6.
Dependence of the population growth rate in a cell on the population density at distance d, , calculated from the empirical and numerical datafor different values of dag. (a) d-aggregate GM. We set α=0.8, β=0.4 and γ=1.0. (b) s-aggregate GM. We set α=0.4, β=0.4 and γ=1.0.
When dag=1 km, for both models, the results were similar to those for dag=0.65 km (figure 4). When dag=5 and 25 km, the behaviour of was qualitatively different, with first increasing and then decreasing as d increased, or even more complicated behaviour (i.e. s-aggregate GM with dag=25 km shown in figure 6b).
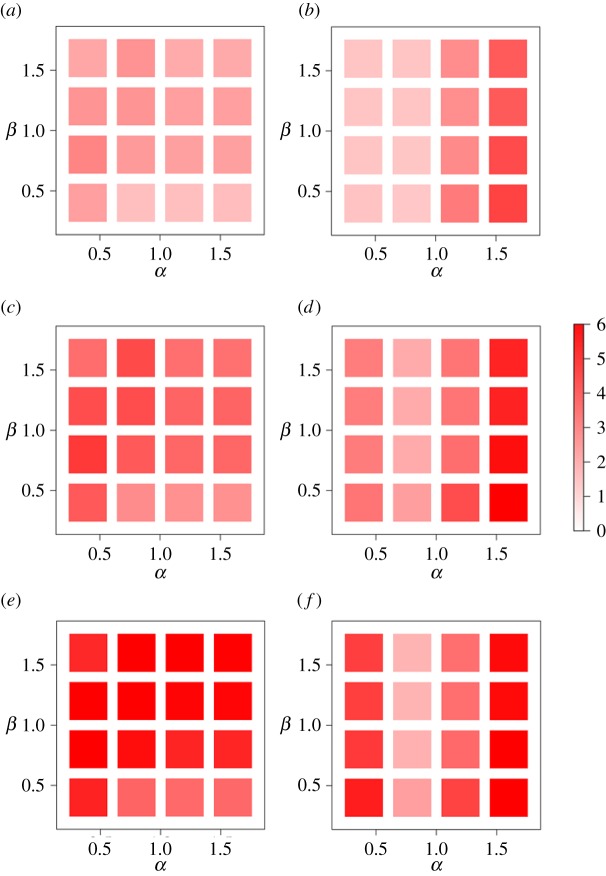
Figure 7 confirms that the results shown in figure 6 remain qualitatively the same in a wide range of α and β. In other words, the results for dag=1 (figure 7a,b) are similar to those for dag=0.65 (figure 5b,c), whereas those for dag=5 (figure 7c,d) and dag=25 (figure 7e,f) are not. We conclude that aggregating the population density at the source or destination of migration with dag=5 km or larger does not even qualitatively explain the empirical data.
Figure 7.
The discrepancy of d-aggregate GM and s-aggregate GM from the empirical data. (a) d-aggregate GM, dag=1 km. (b) s-aggregate GM, dag=1 km. (c) d-aggregate GM, dag=5 km. (d) s-aggregate GM, dag=5 km. (e) d-aggregate GM, dag=25 km. (f) s-aggregate GM, dag=25 km.
3.5. One-dimensional toy model
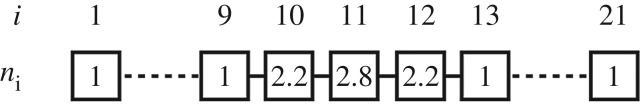
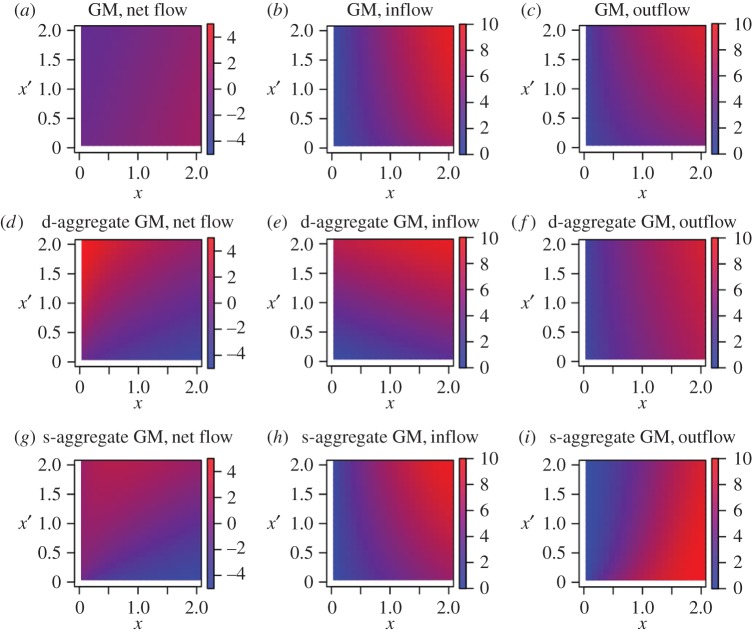
To gain further insights into the spatial inter-dependency of the population growth rate in terms of in- and out-migratory flows of populations, we analysed a toy model on the one-dimensional lattice (i.e. chain) with 21 cells (figure 8). Differently from the simulations presented in the previous sections, the current toy model assumes a flat initial population density except in the three central cells. Combined with the simplifying assumption of the one-dimensional landscape, we aimed at revealing a minimal set of conditions under which the empirically observed patterns were produced. We focused on the central cell and its two neighbouring cells, one on each side on the chain. We set the initial number of inhabitants in the central cell to x, those of the two neighbouring cells to x′ and those of the other cells to one as normalization. The distance between two adjacent cells was set to unity without a loss of generality. Then, we investigated the net flow (i.e. population growth rate), inflow and outflow of populations as a function of x and x′ using the three GMs. We set dag=1, with which we aggregated three cells to calculate the population density at the source or destination of the immigration in the two extensions of the GM.
Figure 8.
The schematic of the GM models on a chain. A square represents a cell, and ni is the initial number of inhabitants in cell i. We set x=2.8 and x′=2.2 for illustration.
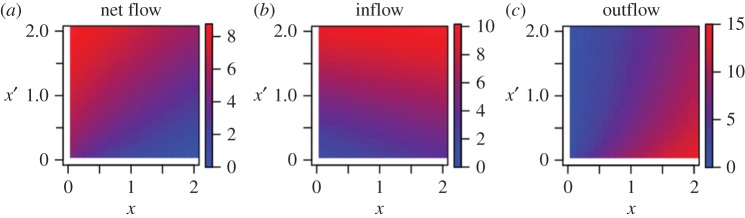
The net flow, inflow and outflow in the three models are shown in figure 9. In the GM, the net flow at the central cell heavily depended on x but negatively and only slightly depended on the population size in the neighbouring cells x′ (figure 9a). This result was inconsistent with the empirically observed pattern (figure 4). This inconsistency was due to an increase in the outflow at the central cell as x′ increased (figure 9c), whereas the inflow at the central cell was not sensitive to x′ (figure 9b).
Figure 9.
The net flow, inflow and outflow for the GM, d-aggregate GM and s-aggregate GM in the one-dimensional model with 21 cells. The initial condition is a symmetric distribution of the density of inhabitants that is uniform except in the central three cells. The initial population density is equal to x in the central cell, x′ in the neighbouring two cells and 1 in the other cells. We set G for the GM, d-aggregate GM and s-aggregate GM to 1, and , respectively, and α=0.4, β=0.6 and γ=1.0. (a) Net flow for the GM. (b) Inflow for the GM. (c) Outflow for the GM. (d) Net flow for the d-aggregate GM. (e) Inflow for the d-aggregate GM. (f) Outflow for the d-aggregate GM. (g) Net flow for the s-aggregate GM. (h) Inflow for the s-aggregate GM. (i) Outflow for the s-aggregate GM.
The patterns of migration flows for the d-aggregate and s-aggregate GMs were qualitatively different from those for the GM (figure 9d–i). In both models, the population growth rate increased as x′ increased (figure 9d,g), which is consistent with the empirically observed patterns. In the d-aggregate GM, this change was mainly owing to changes in the inflow, which increased as x′ increased (figure 9e). The outflow for the d-aggregate GM was similar to that for the GM (figure 9f). In other words, a cell surrounded by those with higher population density attracted a larger migration flow in the d-aggregate GM. By contrast, in the s-aggregate GM, changes in the population flow were mainly attributed to changes in the outflow. The inflow for the d-aggregate GM was similar to that for the GM (figure 9h) and the outflow decreased as x′ increased for the d-aggregate GM (figure 9i). In other words, a cell surrounded by those with higher population density was less likely to lose inhabitants in the s-aggregate GM.
3.6. Gravity model with the aggregation around both the source and destination cells
Lastly, we investigated an extension of the GM with the aggregation of cells around both the source and destination cells, called the sd-aggregate GM (appendix E). The behaviour of was qualitatively the same as that obtained from the d-aggregate GM, s-aggregate GM and empirical data (figure 18). In addition, the sd-aggregate GM was accurate in a wide parameter region (figure 19). We also confirmed that the discrepancy measure for the sd-aggregate GM increased as dag increased (figures 20 and 21), similar to the results for the d-aggregate and s-aggregate GMs (figures 6 and 7). The behaviour of this model on the one-dimensional toy model was also consistent with the empirical data (figure 22) because the inflow and outflow of the model were similar to those for the d-aggregate GM and s-aggregate GM, respectively.
4. Discussion
We investigated spatial patterns of demographic dynamics through the analysis of the population census data in Japan in 2005 and 2010. We found that the population growth rate in a cell was positively correlated with the population density in cells nearby, in addition to that in the focal cell. We used the gravity model and its variants to investigate possible effects of migration on the empirically observed spatial patterns of the population growth rate. Under the framework of the GM, we found that aggregating some neighbouring cells around either the source or destination of migration events considerably improved the fit of the GM model to the empirical data. The results were better when the cells around the destination cell were aggregated, in particular regarding the robustness of the results against variation in the parameter values, than when the cells around the source cell were aggregated. All the results were qualitatively the same when we set t1=2000 and t2=2005, although the census data in 2000 were less accurate than those in 2005 and 2010 (appendix A).
Aggregation of cells near the destination cell models behaviour of individuals that perceive the population of the destination cell as a sum (or average) of the population over the cells neighbouring the destination cell. Because the size of the cell is imposed by the empirical data, aggregation of cells around the destination cell is equivalent to decreasing the spatial resolution of the GM by coarse graining. Traditionally, administrative boundaries have been used as operational units of the GM [39]. A cluster identified by the city clustering algorithm may also be used as the unit [38,40]. In the continuous-space GM, the unit is assumed to be an infinitely small spatial segment [12]. However, there is no a priori reason to assume that any one of these units is an appropriate choice. Our results suggest that spatial averaging with a circle of radius dag≈1 km may be a reasonable choice as compared to a larger dag or the original cell size (i.e. 500×500 m2). Real inhabitants may perceive the population density at the destination as a spatial average on this scale. Although we reached this conclusion using the GMs, this guideline may be also useful when other migration models are used.
The present study has limitations. First, due to a high computational cost, we only examined a limited number of combinations of parameter values in the GMs. A more exhaustive search of the parameter space or the use of different migration models, as well as analysing different datasets, warrants future work.
Second, due to the lack of empirical data, we could not analyse more microscopic processes contributing to population changes. For example, because of the absence of spatially explicit data on the number of births and deaths, we did not include births and deaths into our models. However, the observed inflow and outflow were at least twice as large as the numbers of births and deaths in all the 47 prefectures in Japan (table 2). Therefore, migration rather than births and deaths seems to be a main driver of spatially untangled population changes in Japan during the observation period. The lack of data also prohibited us from looking into the effect of the age of inhabitants. In fact, individuals at a certain life stage are more likely to migrate in general [4,5]. Data on migration flows between cells, births, deaths and the age distribution, which are not included in the present dataset, are expected to enable further investigations of the spatial patterns of population changes examined in the present study.
Table 2.
The number of births, deaths, incoming inhabitants and outgoing inhabitants in the 47 prefectures in Japan between 2005 and 2009. The relative contribution of migration to demographic dynamics, denoted by RC in the table, is defined by (inflow + outflow)/(the number of births + the number of deaths + inflow + outflow). The average of RC over the 47 prefectures is 0.801. Data were obtained from refs. [41–45].
| prefecture | births | deaths | inflow | outflow | RC |
|---|---|---|---|---|---|
| Hokkaido | 206 018 | 258 620 | 1 400 785 | 1 486 704 | 0.861 |
| Aomori | 50 846 | 75 402 | 210 797 | 253 252 | 0.786 |
| Iwate | 51 415 | 74 612 | 208 183 | 237 908 | 0.780 |
| Miyagi | 98 143 | 101 836 | 569 239 | 590 600 | 0.853 |
| Akita | 37 200 | 68 304 | 132 922 | 161 071 | 0.736 |
| Yamagata | 45 724 | 67 185 | 162 073 | 182 237 | 0.753 |
| Fukushima | 84 844 | 106 394 | 299 166 | 337 621 | 0.769 |
| Ibaraki | 123 337 | 134 098 | 539 995 | 543 333 | 0.808 |
| Tochigi | 85 950 | 91 337 | 338 813 | 343 879 | 0.794 |
| Gunma | 84 469 | 93 531 | 315 194 | 323 801 | 0.782 |
| Saitama | 302 796 | 251 786 | 1 673 676 | 1 612 124 | 0.856 |
| Chiba | 259 317 | 230 495 | 1 573 082 | 1 479 729 | 0.862 |
| Tokyo | 518 801 | 481 388 | 4 228 697 | 3 855 587 | 0.890 |
| Kanagawa | 393 305 | 307 305 | 2 434 444 | 2 297 378 | 0.871 |
| Nigata | 92 614 | 123 745 | 274 628 | 301 798 | 0.727 |
| Toyama | 43 760 | 56 352 | 134 116 | 142 212 | 0.734 |
| Ishikawa | 50 547 | 52 753 | 181 465 | 190 238 | 0.783 |
| Fukui | 35 888 | 39 657 | 100 717 | 111 694 | 0.738 |
| Yamanashi | 34 806 | 42 633 | 155 103 | 166 314 | 0.806 |
| Nagano | 91 097 | 109 115 | 369 322 | 389 224 | 0.791 |
| Gifu | 88 156 | 95 085 | 323 422 | 344 227 | 0.785 |
| Shizuoka | 163 151 | 165 452 | 702 346 | 710 330 | 0.811 |
| Aichi | 347 947 | 269 444 | 1 679 203 | 1 602 590 | 0.842 |
| Mie | 78 283 | 87 013 | 305 184 | 310 744 | 0.788 |
| Shiga | 66 776 | 54 067 | 271 852 | 260 071 | 0.815 |
| Kyoto | 108 329 | 113 508 | 605 810 | 623 003 | 0.847 |
| Osaka | 383 123 | 354 039 | 2 038 886 | 2 050 292 | 0.847 |
| Hyogo | 241 991 | 239 518 | 1 092 350 | 1 099 138 | 0.820 |
| Nara | 55 617 | 59 986 | 248 748 | 271 018 | 0.818 |
| Wakayama | 38 888 | 57 061 | 134 100 | 153 523 | 0.750 |
| Tottori | 24 973 | 32 708 | 89 781 | 101 313 | 0.768 |
| Shimane | 28 992 | 43 489 | 110 090 | 122 946 | 0.763 |
| Okayama | 84 959 | 93 940 | 306 532 | 316 719 | 0.777 |
| Hiroshima | 127 862 | 131 562 | 601 952 | 613 915 | 0.824 |
| Yamaguchi | 57 910 | 83 418 | 248 565 | 266 357 | 0.785 |
| Tokushima | 30 023 | 43 472 | 122 127 | 133 197 | 0.776 |
| Kagawa | 42 961 | 52 403 | 173 267 | 182 174 | 0.788 |
| Ehime | 57 940 | 76 292 | 213 144 | 231 838 | 0.768 |
| Kochi | 28 744 | 45 893 | 122 876 | 139 076 | 0.778 |
| Fukuoka | 228 884 | 220 437 | 1 308 177 | 1 312 289 | 0.854 |
| Saga | 38 216 | 43 612 | 151 729 | 163 207 | 0.794 |
| Nagasaki | 60 771 | 76 205 | 264 659 | 306 307 | 0.807 |
| Kumamoto | 80 457 | 91 365 | 333 680 | 351 123 | 0.799 |
| Oita | 50 366 | 61 697 | 208 513 | 216 427 | 0.791 |
| Miyazaki | 50 795 | 57 628 | 226 740 | 244 558 | 0.813 |
| Kagoshima | 75 607 | 96 695 | 369 723 | 399 374 | 0.817 |
| Okinawa | 82 886 | 47 009 | 368 805 | 373 402 | 0.851 |
Third, our conclusions are based on the longitudinal data at only two time points in a single country. The strength of the current results should be understood as such.
Fourth, we did not take into account the effect of water-surface cells, which cannot be inhabited. The population density at distance d from a focal cell i, i.e. Di(d), is therefore underestimated when cell i is located near water (e.g. sea, lake, large river). Additional information about the geographical property of cells such as the water area within the cell and the land use may improve the present analysis.
Appendix A. Population changes between 2000 and 2005
In the main text, we used the data on the population census in Japan in 2005 and 2010. The data on 2000 are also publicly available although they are less accurate than those in 2005 and 2010 [30]. Here, we set t1=2000 and t2=2005 and ran the same analysis pipeline with that in the main text to examine the robustness of our results. As shown in the following, the results were qualitatively the same as those shown in the main text for (t1,t2)=(2005,2010) (figures 4–7), except for the behaviour of the GM.
In figure 10, obtained from the empirical data, the GM, d-aggregate GM and s-aggregate GM is compared. Similar to the analysis shown in the main text, for the three GMs, we set γ=1 and varied α, β∈{0.4,0.8,1.2,1.6} and used the optimized parameter values. The value for the GM was negative, contradicting the empirical data, whereas the behaviour of the d-aggregate and s-aggregate GMs was qualitatively the same as that of the empirical data.
Figure 10.
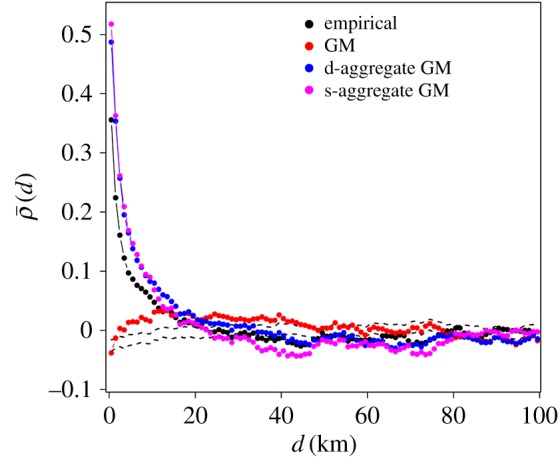
Dependence of the population growth rate in a cell on the population density at distance d, , calculated from the empirical and numerical data between 2000 and 2005. We set α=0.8, β=0.4 and γ=1 for the GM; α=0.8, β=0.4, γ=1 and dag=0.65 km for the d-aggregate GM; and α=0.4, β=1.2, γ=1 and dag=0.65 km for the s-aggregate GM. The ranges indicated by the dashed lines represent 95% CIs.
For α∈{0.4,0.8,1.2,1.6} and β∈{0.4,0.8,1.2,1.6}, the discrepancy between the model and empirical data (equation (2.8)) is shown in figure 11. The results for the GM were inaccurate for all parameter combinations that we considered (figure 11a). The d-aggregate GM yielded a good agreement with the data in a wide parameter region (figure 11b). The s-aggregate GM was accurate only for α=0.4 (figure 11c). These results are similar to those for (t1,t2)=(2005,2010) (figure 5).
Figure 11.
The discrepancy of the GM, d-aggregate GM and s-aggregate GM from the empirical data, calculated for the population change between 2000 and 2005. (a) GM. (b) d-aggregate GM. (c) s-aggregate GM. The diagonal in (a) is blank because the inflow and outflow are equal when α=β in the GM, resulting in no population change. We set γ=1 and dag=0.65 km.
We then examined the robustness of the results with respect to the dag value. The discrepancy between the models and the empirical data is shown in figure 12. For both d-aggregate and s-aggregate GMs, behaved similarly to that for the empirical data when dag=1 km but not when dag=5 km and 25 km. Figure 13 confirms this result for various values of α and β. For a wide region of the α–β parameter space, the discrepancy increased as dag increased.
Figure 12.
Dependence of the population growth rate in a cell on the population density at distance d, , calculated for the population change between2000 and 2005. We varied the values of dag. (a) d-aggregate GM. We set α=0.8, β=0.4 and γ=1.0. (b) s-aggregate GM. We set α=0.4, β=1.2 and γ=1.0.
Figure 13.
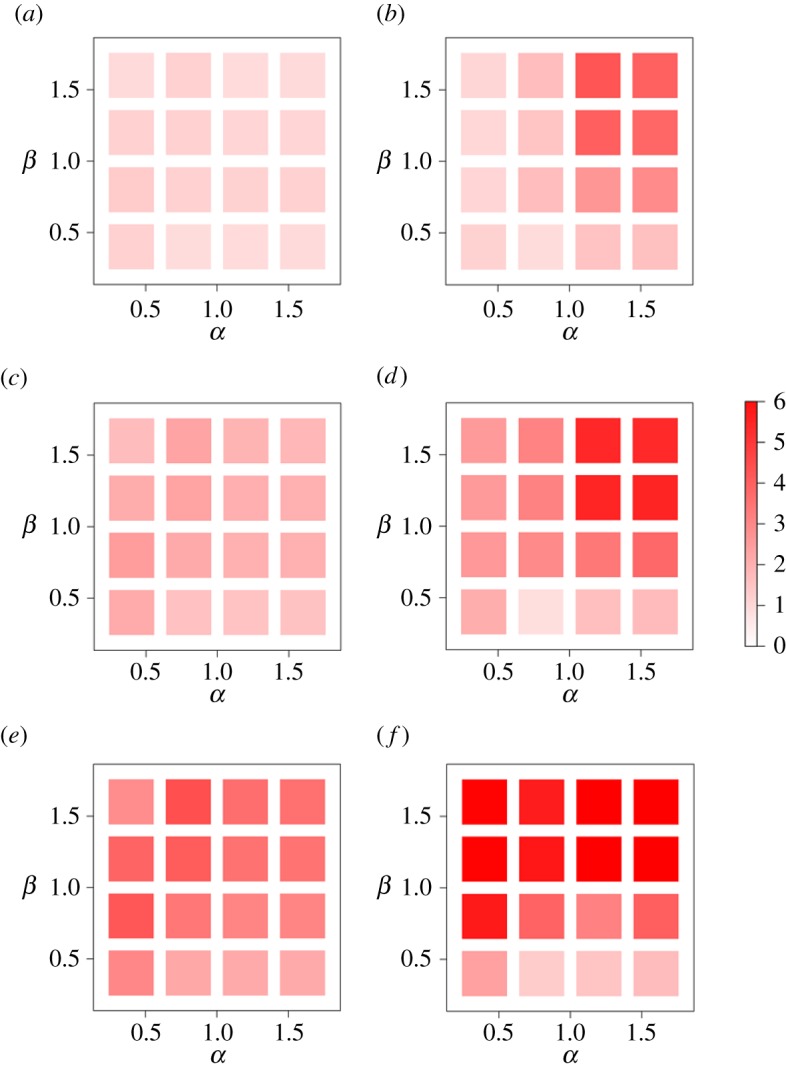
The discrepancy of d-aggregate GM and s-aggregate GM from the empirical data for the population change between 2000 and 2005. (a) d-aggregate GM, dag=1 km. (b) s-aggregate GM, dag=1 km. (c) d-aggregate GM, dag=5 km. (d) s-aggregate GM, dag=5 km. (e) d-aggregate GM, dag=25 km. (f) s-aggregate GM, dag=25 km.
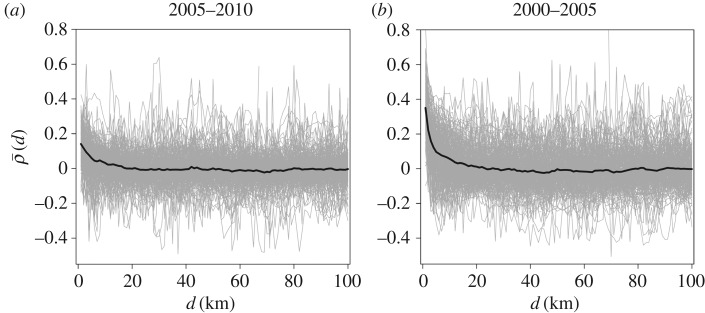
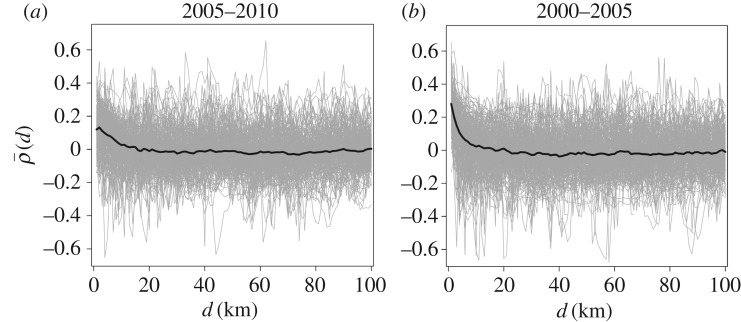
Appendix B. Plot of ρk(d) for each 50×50 km region
In the main text, we showed the values of ρk(d) averaged over all regions of size 50×50 km, denoted by (figure 4). The ρk(d) for each region k is plotted as a function of d in figure 14. The values of ρk(d) depend considerably on the region.
Figure 14.
Dependence of the population growth rate in a cell on the population density at distance d, ρk(d), calculated from the empirical data. A thin line represents ρk(d) for a region of size 50×50 km. The results for the different regions are superposed on top of each other.The thick lines represent , which is the average of ρk(d) over all the regions. The thick lines in (a) and (b) are the same as the lines with the black circles shown in figures 4 and 10, respectively. (a) (t1,t2)=(2005,2010). (b) (t1,t2)=(2000,2005).
To calculate , we used all regions. However, some regions and their nearby regions include water-surface cells, potentially biasing the estimation of . Therefore, we examined the ρk(d) values for region k such that all cells within region k and those within 30 km from any cell in region k are not in the sea. The average of ρk(d) over these regions is qualitatively the same as that shown in the main text (figure 15).
Figure 15.
Dependence of the population growth rate in a cell on the population density at distance d, ρk(d), calculated from the empirical data. We calculated ρk(d) for regions k such that all cells within 30 km from any cell in region k do not contain sea. A thin line represents ρk(d) for such a 50×50 km region. The results for the different regions are superposed on top of each other. The thicklines represent the average of ρk(d) over all the regions satisfying the aforementioned criterion. (a) (t1,t2)=(2005,2010). (b) (t1,t2)=(2000,2005).
Appendix C. Analysis of cells with more than 100 inhabitants
In the main text, we used cells whose population size was between 10 and 100. Figure 16 shows for cells whose population size was greater than 100. The behaviour of was qualitatively the same as that for the cells of the population size between 10 and 100 (figure 4).
Figure 16.
Dependence of the growth rate in a cell on the population density at distance d, ρk(d), when only the cells whose number of inhabitants is greater than 100 are considered. A thinline represents ρk(d) for a region of size 50× 50 km. The thick lines represent , which is the average of ρk(d) over all the regions. (a) (t1,t2)=(2005,2010). (b) (t1,t2)=(2000,2005).
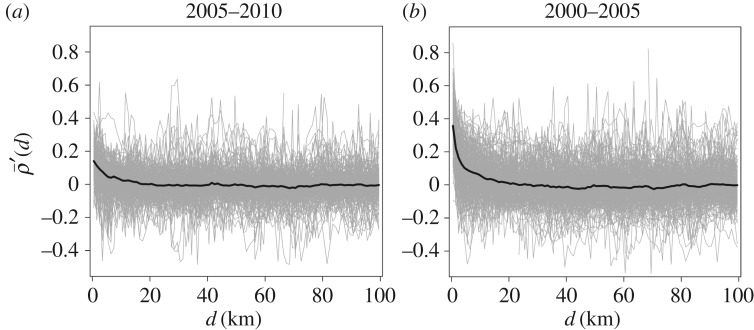
Appendix D. Analysis with the partial correlation coefficient
In the main text, we calculated ρk(d) using the Pearson correlation coefficient (equation (4)). However, the strong spatial correlation in the population size combined with the tendency that a highly populated cell grows more than sparsely populated cells do may result in spuriously large ρk(d) values. Therefore, to control for the spatial correlation in the population size, we calculated the partial correlation coefficient between the population growth rate of a cell and the population density in nearby cells, , by
| D 1 |
where cork(⋅,⋅) is the Pearson correlation coefficient between two variables in region k; Di(d) is the population density averaged over the cells at distance d from cell i in region k; Ri is the population growth rate of cell i in region k; and ni is the number of inhabitants in cell i in region k. We defined as the average of over all regions. Figure 17 shows that as a function of d behaves similarly to (figure 4).
Figure 17.
Dependence of the growth rate in a cell on the population density at distance d, , controlling for the population size of a focal cell. A thin line represents for aregion. The thick lines represent , which is the average of over all the regions. (a) (t1,t2)=(2005,2010). (b) (t1,t2)=(2000,2005).
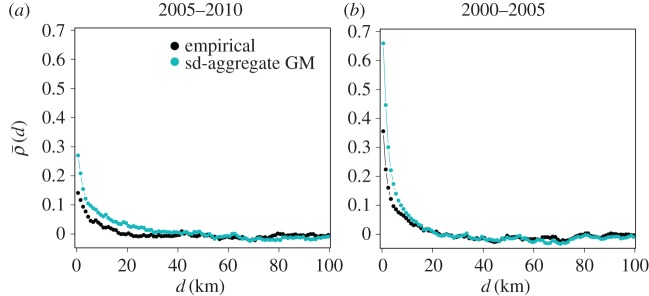
Appendix E. Gravity model with the population density aggregated around both the source and destination cells
In the main text, we aggregated the cells around either the source or destination cell but not both. Here, we carried out aggregation around both the source and destination cells. In the model, which we refer to as the GM with the aggregation around both the source and destination (sd-aggregate GM), the population flow from cell i to cell j is defined by
| E 1 |
As we present in the following, the behaviour of the sd-aggregate GM was similar to that of the d-aggregate GM (figures 4–7).
We compare between the empirical and simulated data in figure 18. The behaviour of obtained from the GM was qualitatively the same as that of the empirical data. The discrepancy between the model and empirical data (equation (2.8)) was small in a wide parameter region (figure 19). We also confirmed that the discrepancy increased as dag increased (figures 20 and 21). The net flow, inflow and outflow for the sd-aggregate GM simulated on a chain with 21 cells are shown in figure 22. The inflow and outflow for the sd-aggregate GM (figure 22b,c) were similar to those for the d-aggregate GM (figure 9e) and the s-aggregate GM (figure 9i), respectively. As a result, the net flow for the sd-aggregate GM (figure 22a) was similar to that for the d-aggregate GM (figure 9d) and the s-aggregate GM (figure 9g).
Figure 18.
Dependence of the population growth rate in a cell on the population density at distance d, , calculated from the empirical data and the numerical datagenerated from the sd-aggregate GM. (a) (t1,t2)=(2005,2010). We set α=0.4, β=1.6, γ=1 and dag=0.65 km. (b) (t1,t2)=(2000,2005). We set α=0.4, β=0.4, γ=1 and dag=0.65 km.
Figure 19.
The discrepancy of the sd-aggregate GM from the empirical data. (a) (t1,t2)=(2005,2010). (b) (t1,t2)=(2000,2005). We set γ=1 and dag= 0.65 km.
Figure 20.
Dependence of the population growth rate in a cell on the population density at distance d, , calculated for the sd-aggregate GM for different valuesof dag. (a) (t1,t2)=(2005,2010). We set α=0.4, β=1.6 and γ=1.0. (b) (t1,t2)=(2000,2005). We set α=0.4, β=0.4 and γ=1.0.
Figure 21.
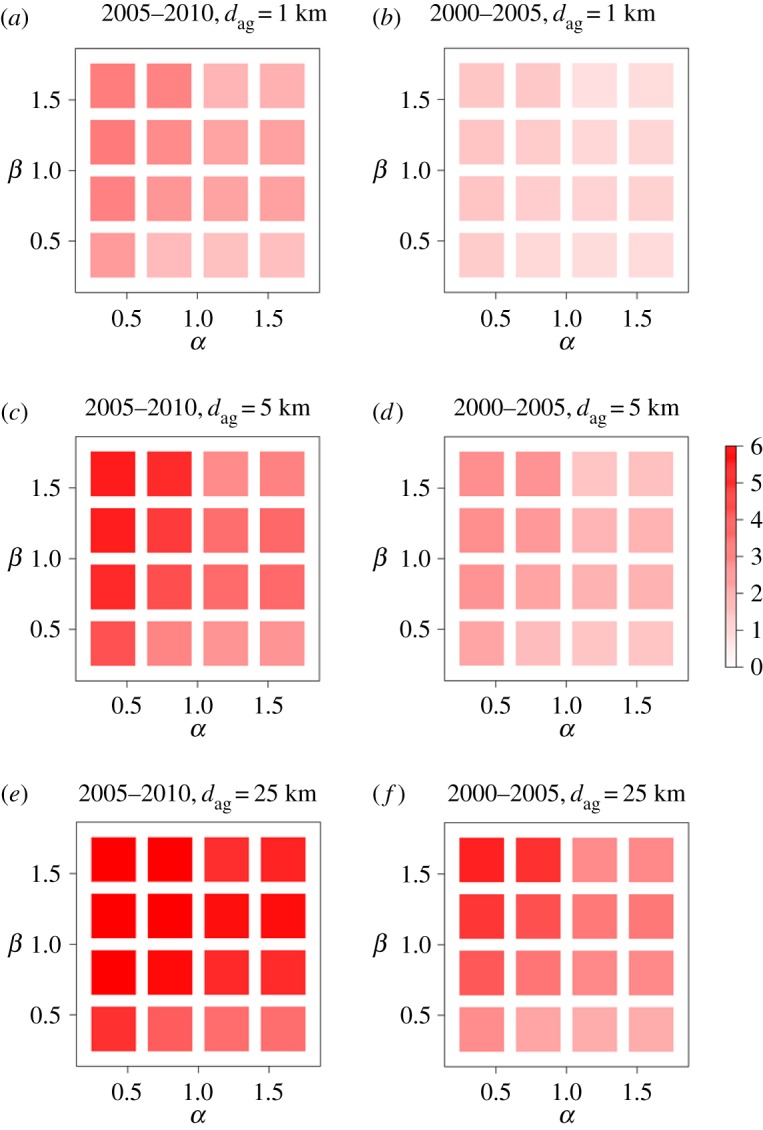
The discrepancy of the sd-aggregate GM from the empirical data. (a) (t1,t2)=(2005,2010), dag=1 km. (b) (t1,t2)=(2000,2005), dag=1 km. (c) (t1,t2)=(2005,2010), dag=5 km. (d) (t1,t2)= (2000,2005), dag=5 km. (e) (t1,t2)=(2005,2010), dag=25 km. (f) (t1,t2)=(2000,2005), dag=25 km.
Figure 22.
(a) The net flow, (b) inflow and (c) outflow for the sd-aggregate GM in the one-dimensional model. We set , α=0.4, β=0.6 and γ=1.0.
Data accessibility
Data are available from http://e-stat.go.jp/SG2/eStatGIS/page/download.html.
Authors' contributions
N.M. designed the study. K.T. collected and analysed the data. Both authors wrote the manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This work is supported by JST, CREST (grant no. JPMJCR1304). N.M. acknowledges the support provided through JST, ERATO, Kawarabayashi Large Graph Project.
References
- 1.Pallagst K. 2009. Shrinking cities in the United States of America: three cases, three planning stories. In The future of shrinking cities: problems, patterns and strategies of urban transformation in a global context (eds K Pallagst et al.), pp. 81–88. Berkeley, CA: Institute of Urban and Regional Development, University of California.
- 2.Pallagst K, Wiechmann T, Martinez-Fernandez C. 2013. Shrinking cities: international perspectives and policy implications. Abingdon, UK: Routledge. [Google Scholar]
- 3.Hara T. 2014. A shrinking society: post-demographic transition in Japan. Tokyo, Japan: Springer. [Google Scholar]
- 4.Lewis GJ. 1982. Human migration: a geographical perspective. Kent, UK: Croom Helm. [Google Scholar]
- 5.Kahley WJ. 1991. Population migration in the United States: a survey of research. Econ. Rev. 76, 12–21. [PubMed] [Google Scholar]
- 6.Beine M, Bertoli S, Moraga JF-H.2014. A practitioners’ guide to gravity models of international migration. FEDEA Working Paper 2014-03. ( doi:10.1111/twec.12265)
- 7.Stouffer SA. 1940. Intervening opportunities: a theory relating mobility and distance. Am. Sociol. Rev. 5, 845–867. (doi:10.2307/2084520) [Google Scholar]
- 8.Zipf GK. 1946. The P1P2/D hypothesis: on the intercity movement of persons. Am. Sociol. Rev. 11, 677–686. (doi:10.2307/2087063) [Google Scholar]
- 9.Cohen JE, Roig M, Reuman DC, GoGwilt C. 2008. International migration beyond gravity: a statistical model for use in population projections. Proc. Natl Acad. Sci. USA 105, 15 269–15 274. (doi:10.1073/pnas.0808185105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batty M. 2013. The new science of cities. Cambridge, MA: MIT Press. [Google Scholar]
- 11.Simini F, González MC, Maritan A, Barabási A-L. 2012. A universal model for mobility and migration patterns. Nature 484, 96–100. (doi:10.1038/nature10856) [DOI] [PubMed] [Google Scholar]
- 12.Simini F, Maritan A, Néda Z. 2013. Human mobility in a continuum approach. PLoS ONE 8, e60069 (doi:10.1371/journal.pone.0060069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barthelemy M. 2016. The structure and dynamics of cities: urban data analysis and theoretical modeling. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Anderson JE. 2011. The gravity model. Annu. Rev. Econ. 3, 133–160. (doi:10.1146/annurev-economics-111809-125114) [Google Scholar]
- 15.Rodrigue JP, Comtois C, Slack B. 2013. The geography of transport systems. Abingdon, UK: Routledge. [Google Scholar]
- 16.Karemera D, Oguledo VI, Davis B. 2000. A gravity model analysis of international migration to North America. Appl. Econ. 32, 1745–1755. (doi:10.1080/000368400421093) [Google Scholar]
- 17.Fagiolo G, Mastrorillo M. 2013. International migration network: topology and modeling. Phys. Rev. E 88, 012812 (doi:10.1103/PhysRevE.88.012812) [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya K, Mukherjee G, Saramäki J, Kaski K, Manna SS. 2008. The international trade network: weighted network analysis and modelling. J. Stat. Mech. 2008, P02002 (doi:10.1088/1742-5468/2008/02/P02002) [Google Scholar]
- 19.Kepaptsoglou K, Karlaftis MG, Tsamboulas D. 2010. The gravity model specification for modeling international trade flows and free trade agreement effects: a 10-year review of empirical studies. Open Econ. J. 3, 1–13. (doi:10.2174/1874919401003010001) [Google Scholar]
- 20.Lambiotte R, Blondel VD, De Kerchove C, Huens E, Prieur C, Smoreda Z, Van Dooren P. 2008. Geographical dispersal of mobile communication networks. Physica A 387, 5317–5325. (doi:10.1016/j.physa.2008.05.014) [Google Scholar]
- 21.Krings G, Calabrese F, Ratti C, Blondel VD. 2009. Urban gravity: a model for inter-city telecommunication flows. J. Stat. Mech. 2009, L07003 (doi:10.1088/1742-5468/2009/07/L07003) [Google Scholar]
- 22.Akwawua S, Pooler JA. 2016. An intervening opportunities model of US interstate migration flows. Geo. Res. Forum 20, 33–51. [Google Scholar]
- 23.Openshaw S. 1984. Ecological fallacies and the analysis of areal census data. Environ. Plan. A 16, 17–31. (doi:10.1068/a160017) [DOI] [PubMed] [Google Scholar]
- 24.Openshaw S. 1977. Optimal zoning systems for spatial interaction models. Environ. Plan. A 9, 169–184. (doi:10.1068/a090169) [Google Scholar]
- 25.Broadbent TA. 1970. Notes on the design of operational models. Environ. Plan. A 2, 469–476. (doi:10.1068/a020469) [Google Scholar]
- 26.Batty M. 1974. Spatial entropy. Geogr. Anal. 6, 1–31. (doi:10.1111/j.1538-4632.1974.tb01014.x) [Google Scholar]
- 27.Batty M. 1976. Entropy in spatial aggregation. Geogr. Anal. 8, 1–21. (doi:10.1111/j.1538-4632.1976.tb00525.x) [Google Scholar]
- 28.Masser I, Brown PJ. 1977. Spatial representation and spatial interaction. Pap. Reg. Sci. 38, 71–92. (doi:10.1007/BF01933513) [Google Scholar]
- 29.Portal Site of Official Statistics of Japan. See http://e-stat.go.jp/SG2/eStatGIS/page/download.html (accessed 17 March 2015).
- 30.Somu-sho Tokei-kyoku ni okeru Chiiki Mesh Tokei no sakusei. [In Japanese.] See http://www.stat.go.jp/data/mesh/pdf/gaiyo2.pdf (accessed 26 December 2016).
- 31.Gallos LK, Barttfeld P, Havlin S, Sigman M, Makse HA. 2012. Collective behavior in the spatial spreading of obesity. Sci. Rep. 2, 454 (doi:10.1038/srep00454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybski D, Ros AGC, Kropp JP. 2013. Distance-weighted city growth. Phys. Rev. E 87, 042114 (doi:10.1103/PhysRevE.87.042114) [DOI] [PubMed] [Google Scholar]
- 33.Hernando A, Hernando R, Plastino A. 2014. Space–time correlations in urban sprawl. J. R. Soc. Interface 11, 20130930 (doi:10.1098/rsif.2013.0930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernando A, Hernando R, Plastino A, Zambrano E. 2015. Memory-endowed US cities and their demographic interactions. J. R. Soc. Interface 12, 20 141 185 (doi:10.1098/rsif.2014.1185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaels G, Rauch F, Redding SJ. 2012. Urbanization and structural transformation. Q. J. Econ. 127, 535–586. (doi:10.1093/qje/qjs003) [Google Scholar]
- 36.Birchenall JA. 2016. Population and development redux. J. Popul. Econ. 29, 627–656. (doi:10.1007/s00148-015-0572-x) [Google Scholar]
- 37.Desmet K, Rappaport J. 2015. The settlement of the United States, 1800–2000: the long transition towards Gibrat’s law. J. Urban. Econ. 98, 50–68. (doi:10.1016/j.jue.2015.03.004) [Google Scholar]
- 38.Rozenfeld HD, Rybski D, Andrade JS, Batty M, Stanley HE, Makse HA. 2008. Laws of population growth. Proc. Natl Acad. Sci. USA 105, 18 702–18 707. (doi:10.1073/pnas.0807435105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barthélemy M. 2011. Spatial networks. Phys. Rep. 499, 1–101. (doi:10.1016/j.physrep.2010.11.002) [Google Scholar]
- 40.Rozenfeld HD, Rybski D, Gabaix X, Makse HA. 2011. The area and population of cities: new insights from a different perspective on cities. Am. Econ. Rev. 101, 2205–2225. (doi:10.1257/aer.101.5.2205) [Google Scholar]
- 41.Japan Geographic Data Center. 2006. Jyumin Kihon Dai-cho jinko yoran Heisei 18 nen ban. Tokyo, Japan: Japan Statistical Association; [In Japanese.] [Google Scholar]
- 42.Japan Geographic Data Center. 2007. Jyumin Kihon Dai-cho jinko yoran Heisei 19 nen ban. Tokyo, Japan: Japan Statistical Association; [In Japanese.] [Google Scholar]
- 43.Japan Geographic Data Center. 2008. Jyumin Kihon Dai-cho jinko yoran Heisei 20 nen ban. Tokyo, Japan: Japan Statistical Association; [In Japanese.] [Google Scholar]
- 44.Japan Geographic Data Center. 2009. Jyumin Kihon Dai-cho jinko yoran Heisei 20 nen ban. Tokyo, Japan: Japan Statistical Association; [In Japanese.] [Google Scholar]
- 45.Japan Geographic Data Center. 2010. Jyumin Kihon Dai-cho jinko yoran Heisei 21 nen ban. Tokyo, Japan: Japan Statistical Association; [In Japanese.] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from http://e-stat.go.jp/SG2/eStatGIS/page/download.html.