Abstract
Outbreaks of the predator crown-of-thorns seastar (COTS) Acanthaster planci cause widespread coral mortality across the Indo-Pacific. Like many marine invertebrates, COTS is a nocturnal species whose cryptic behaviour during the day can affect its detectability, particularly in structurally complex reef habitats that provide many refuges for benthic creatures. We performed extensive day and night surveys of COTS populations in coral reef habitats showing differing levels of structural complexity and COTS abundance. We tested whether estimations of COTS density varied between day and night observations, and if the differences were related to changes in COTS abundance, reef structural complexity and the spatial scale of observation. Estimations of COTS density were on average 27% higher at night than during the day. Differences in COTS detection varied with changing seastar abundance but not reef structural complexity or scale of observation. Underestimation of COTS abundance in daytime was significant for a broad seastar density range, thus potentially affecting most outbreak events. Our study suggests that portions of COTS populations can be undetected during conventional surveys and control campaigns, which are exclusively conducted by day, and significantly affect the trajectory of coral reefs. Accounting for bias in COTS detection can strengthen coral reef management broadly.
Keywords: pest outbreak, Acanthaster planci, estimation bias, habitat complexity, density-dependence, contrast curve
1. Introduction
Outbreaks of the voracious coral predator crown-of-thorns seastar Acanthaster planci (COTS) cause widespread coral decline and constitute major threats to reef health across the Indo-Pacific [1,2]. Increasing reef degradation and climate change are predicted to accentuate these events, further challenging reef conservation in the twenty-first century [3–6]. COTS outbreaks can result in drastic alteration of the physical and biological structure of coral reefs [7], with consequences for valuable resources they provide to human populations [8]. For example, 42% of coral decline and a multitude of socio-economic impacts are attributed to COTS outbreaks on the Australian Great Barrier Reef [9,10]. As a result, COTS is considered as a harmful species, a pest, in many regions [1,11,12]. Consequently, COTS densities are often surveyed as part of reef monitoring programmes, and population control campaigns are frequently conducted in an attempt to reduce coral decline [13–16], despite relatively high cost and limited efficiency [1,17]. However, like many marine invertebrates, COTS are predominantly nocturnal organisms that tend to hide in crevices and remain inactive during the day, while moving and feeding on corals by night, though this behaviour can be affected by many factors [18–22]. Despite the potential effect of such cryptic behaviour on COTS detectability during surveys and control campaigns, the efficiency of COTS detection by day versus night had not been thoroughly tested across spatio-temporal scales of observation and reef environments. Because monitoring and control operations are exclusively conducted during daytime, the nocturnal behaviour of COTS could be a major source of underestimation of COTS densities, thus affecting efficiency of ecological surveys, management decisions and control efforts on Indo-Pacific reefs.
We conducted paired day and night surveys of COTS densities around the island of Moorea, French Polynesia, during one of the most intense outbreaks ever documented [7]. Here, we test for differences in COTS abundance between day and night counts at different spatio-temporal scales of observation, and across reef habitats showing a wide range in structural complexity and COTS abundance.
2. Material and methods
We conducted our study during a particularly devastating COTS outbreak that affected the island of Moorea, French Polynesia [7]. Dense adult COTS aggregations, composed predominantly of 30–45 cm diameter individuals, emerged on the north shore of the island in 2003 and gradually expanded to the entire insular reef system. By 2006, all reef habitats (fringing, barrier, outer-slope) and the three coasts of the island (north, east, west) were affected, constituting a widespread coral-mortality event. The outbreak ended in 2010, leaving reefs largely denuded of coral cover and with altered benthic and fish communities [7]. Outbreaks similar in dynamics and impacts were simultaneously observed on other proximal islands of French Polynesia. At a broader scale, these outbreaks seem to be part of a larger cycle of regional outbreaks that expanded across the Indo-Pacific, from Polynesia to the Red Sea [23].
COTS densities around Moorea were surveyed throughout the outbreak at multiple scales, including semestrial counts that were performed in triplicate 200 m2 (50 m × 4 m) permanent-transects at nine outer-reef locations consisting of three water depths (6 m, 12 m, 18 m) at each of three sites (Haapiti, Tiahura, Vaipahu) [24]. These reef habitats showed contrasting structural complexity (figure 1) and COTS abundances [7], thus providing a good test of COTS detectability across a broad range in physical habitat structure and intensity of outbreaks. Indeed, average COTS densities over the process of this study varied between nil (0 seastar 200 m−2) and one of the highest levels reported in the literature (30.3 seastar 200 m−2, equivalent to 151 650 seastar km−2). This range expands far beyond the estimated maximum sustainable density for coral communities of 1000–1500 seastar km−2 [20,25]. Between October 2007 and October 2009, we paired each of our diurnal surveys of COTS densities at the nine reef locations with a nocturnal survey performed within 12 h by the same group of divers equipped with flashlights in the same permanent-transects [24]. Diurnal surveys were performed between 09.00 and 15.00, and nocturnal surveys between 21.00 and 00.00, allowing for at least 3 h before and after sunrise and sunset. All surveys were performed on SCUBA.
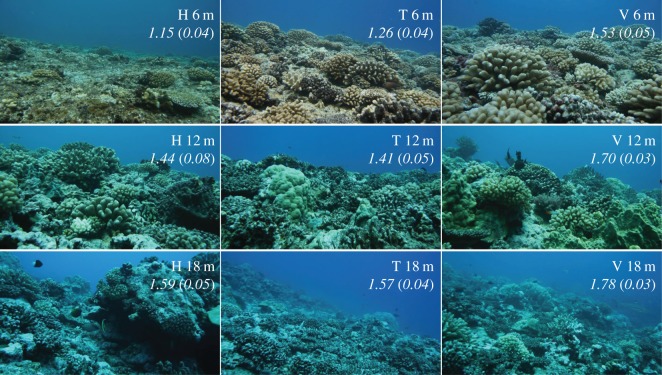
Figure 1.
Variability in reefscape across sites and depths as observed around Moorea, French Polynesia. The nine reef locations consisted of three water depths (vertically; 6 m, 12 m, 18 m) at each of three sites (horizontally; H, Haapiti; T, Tiahura; V, Vaipahu). Values in italic indicate mean (s.e.) substrate rugosity as estimated in 2008 by the chain-and-tape method. Note that reef structural complexity varies in time and space with changing coral community abundance and structure. Pictures were taken in November 2007.
We tested for differences in COTS densities between day and night counts using generalized linear mixed-effect models [26] that accounted for repeated, hierarchically designed observations performed at different dates in individually distinct transects established at specific sites and depths. Variability in response variable density was tested against the explicative factor timing-of-observation with random effects of the variables date, transect, depth and site. We tested if differences in density between day and night counts varied with the spatio-temporal scale considered by running separate models with explicative interaction terms timing-of-observation × each specific scale of observation, from individual transects (covering 200 m2 of reef area) to reef locations (approx. 1000 m2), sites (approx. 5000 m2 along continuous reef slope) and depths (approx. 5000 m2 along the reef isodepth across 20 km of coastline), and within single dates of observation versus over the entire process of the study (2 years).
We also tested whether differences in COTS density between day and night counts were correlated with changes in reef structural complexity, COTS abundance and their interaction. Reef structural complexity was quantified in 2008 using the chain-and-tape method [27] over a 10 m linear portion of each of the permanent-transects used to perform the COTS surveys [24]. A chain following the contour of the substrate was laid over the reef surface, and rugosity was calculated as the ratio between the contoured chain-length and the 10 m linear distance. As differences in COTS density correlated with COTS abundance, we used the semi-parametric contrast curve approach as a post hoc test to identify the range in seastar abundance for which estimations of COTS density differed significantly between day and night counts [28,29]. Contrast curves combine generalized linear mixed-effect model and penalized-spline statistics for an optimized representation of variability in data, including within-subject differences (in this case, observations that were replicated in time and space) and nonlinear responses [30]. Methodology and programing code for the contrast curve approach are provided in previous studies [28,29]. COTS abundance was log(x + 1) transformed before data analysis to homogenize residual variance. All statistics and graphing were coded in R (R Development Core Team) complemented by the NLME package [26].
3. Results
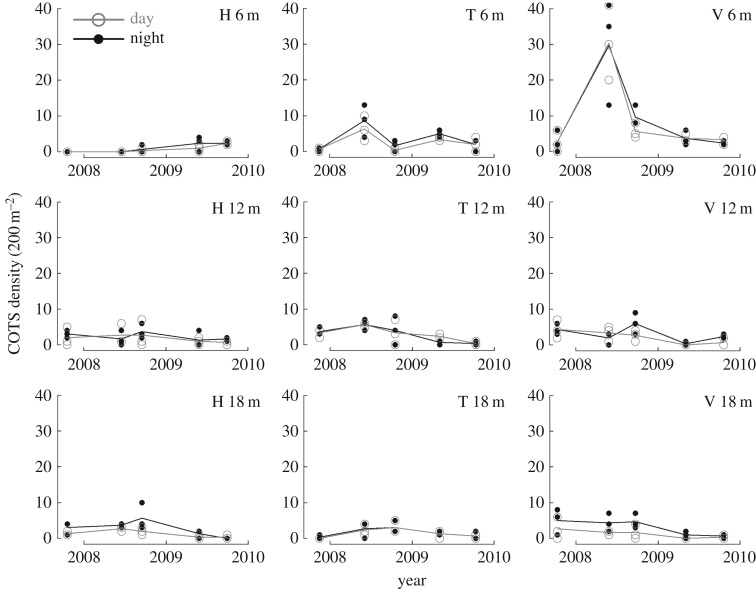
COTS densities followed similar trajectories between day and night observations (figure 2). However, significantly higher density-values were recorded at night over the process of the study and across all reef locations (p < 0.001). Overall, estimated COTS densities were on average 27% higher at night (mean = 3.3 (standard error = 0.4 s.e.) seastar 200 m−2, equivalent to 16 500 seastar km−2) than during the day (2.6 (0.4 s.e.) seastar 200 m−2, equivalent to 13 000 seastar km−2).
Figure 2.
Density trajectories of the coral-killing seastar COTS as counted by day and night time. Surveys were performed in permanent-transects established at nine reef locations that consisted of three water depths (vertically; 6 m, 12 m, 18 m) at each of three sites (horizontally; H, Haapiti; T, Tiahura; V, Vaipahu). Dots represent actual observations and lines illustrate mean trajectories.
Differences in COTS density between day and night observations were not significantly variable between different dates of observation (p = 0.078) and across spatial scales, from individual transects (p = 0.598), to reef locations (p = 0.067), sites (p = 0.154) and depths (p = 0.114). Differences between day and night counts were not correlated to changes in reef habitat complexity as measured by the rugosity index, neither in isolation (p = 0.239) nor in interaction with COTS abundance (p = 0.733). By contrast, differences between day and night counts varied with COTS density (p = 0.009). Based on the semi-parametric contrast curve approach (figure 3), COTS densities were significantly lower during day counts compared with night time over the density range 0.4–19.3 seastar 200 m−2 (equivalent to 2000–96 500 seastar km−2).
Figure 3.
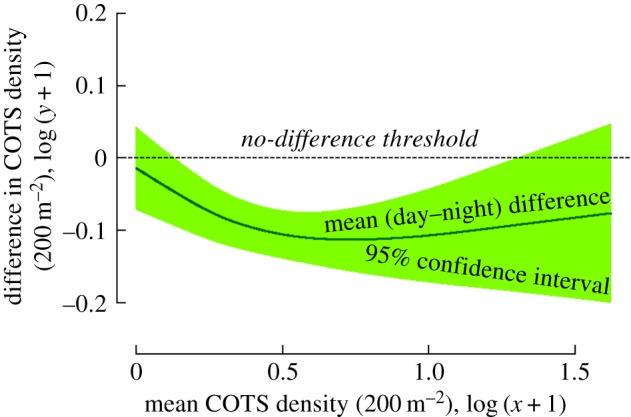
Contrast curve identifying the domain of significant difference in estimations of seastar density between day and night surveys. Densities of the coral-killing seastar COTS were estimated every six months over a period of 2 years by day and night counts performed in permanent-transects established at nine reef locations (figure 2). The semi-parametric contrast curve [28,29] represents variation in the difference between day and night estimations (difference = day density − night density, y-axis) along the seastar density range (x-axis). The domain of significant difference is identified as the portion of the covariate (x-axis) for which the 95% confidence interval of the contrast curve (shaded area) does not cross the no-difference threshold (horizontal dashed line): COTS abundance was significantly lower in day counts compared to night in the log-density range 0.13–1.31, corresponding to the COTS density interval 0.4–19.3 seastar 200 m−2.
4. Discussion
Our evaluation of COTS surveys in Moorea indicated a 27% underestimation of population densities during daytime compared to nocturnal observations. These differences in estimations of COTS density were consistent in time, space and across multiple scales of observation, but varied with changing COTS abundance. Across the wide range in reef habitat structure and COTS abundance that the reefs provided, we estimated that day counts significantly underestimated COTS density compared with night counts within the seastar abundance range of 2000–96 500 km−2. Underestimation of COTS density might be commonplace because surveys and control campaigns are exclusively conducted by day, and most reports of COTS abundance during outbreaks fall within this range [1].
Our results are in concordance with a previous investigation that found higher abundances of COTS by night due to a more cryptic behaviour of smaller (less than 20 cm diameter) seastars in daylight [21]. By contrast, a lower detection of tagged COTS individuals at night compared to daytime was reported by a recent study [11]. Discrepancy in COTS detectability at day versus night can result from different mechanisms that act in antagonistic ways, given the cryptic behaviour of COTS during daytime on one side, and the limited human sight at night on the other. In particular, these mechanisms are further influenced by numerous additional factors such as the dedicated search effort and experience of observer divers, weather conditions, various characteristics of reef habitats such as water depths and levels of structural complexity, and the abundance, size and movement of the seastars in and out of the search zone ([1,11,13,18,21,22,31], this study). The sizes of individual seastars were not measured during our surveys although, as in the recent study [11], COTS populations were composed predominantly of large (greater than 30 cm diameter) individuals. Other methodological differences included smaller sample sizes and tagging of the seastars in the recent study, whereas we performed non-invasive observations that were spatio-temporally replicated within permanent-transects without discriminating individual seastars. Besides, COTS densities were lower during the recent study [11], and surveys were performed in fragmented habitats compared with our investigation of a dynamic outbreak evolving through continuous reefs that showed a wide range in structural complexity, COTS abundance and availability in prey corals [7].
COTS outbreaks have been documented since the 1950s, but a new cycle of regional outbreaks throughout the Indo-Pacific has sparked heightened interest [1,23]. The amplitude of coral decline associated with these recent events is astounding, as might be the consequential loss in coral reef resources and ecological services [8,9]. Nevertheless, the recent outbreaks provided unique opportunities to further understanding of COTS biology and ecology, including developing strategies for detecting and controlling future outbreaks [12,14,17,32,33] and identifying ecological processes that can promote coral reef resilience in the face of these disturbances [23,34–36]. However, potential bias in COTS survey methodology might preclude efficient management of coral reef resources. Our study suggests that underestimation of COTS abundance might be commonplace, potentially resulting in under-recognition of COTS outbreaks as well as unsuccessful control efforts. Indeed, an adult COTS is estimated to consume 160–480 cm2 of coral tissue every day, and a particularly high fecundity promotes the resurgence of widespread COTS populations even from few seastars [1,20,37]. A 27% difference in COTS abundance, as estimated in our study, can thus drastically change coral reef trajectory.
COTS are expected to be increasingly more prominent protagonists of coral reefs in the near future. We advocate accounting for detection bias in conventional surveys and control campaigns, or complementing them with observations performed by night, in order to strengthen detection and control of outbreaks. Alternatively, COTS surveys can be complemented by indirect measurement protocols such as DNA-sequencing reef water samples [12,33] or monitoring the characteristic feeding scars that the seastar leaves on corals that are preyed upon [7,20,38].
Acknowledgements
This study could not have occurred without field assistance by volunteer divers from CRIOBE (Centre de Recherches Insulaires et Observatoire de l'Environnement) and Planète Urgence. We are thankful to Jane Ballard and two anonymous reviewers for improvements on the manuscript.
Ethics
This study involved non-invasive surveys of COTS populations. It was approved as part of research effort in coral reef ecology carried out at CRIOBE (Centre de Recherches Insulaires et Observatoire de l'Environnement) and IRD (Institut de Recherche pour le Développement).
Data accessibility
Crown-of-thorns seastar (COTS) density and reef substrate rugosity data used in this study is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.dk5dm [24].
Authors' contributions
M.K. participated in the conception and design of the study and data collection, analysed the data and led the writing of the manuscript; P.B. participated in data collection; M.A. participated in the conception and design of the study. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
M.K.'s Ph.D. studies were supported by grants from Polynésienne des Eaux (http://polynesienne-des-eaux.pf) and Planète Urgence (www.planete-urgence.org).
References
- 1.Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman HPA. 2014. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp). Oceanogr. Mar. Biol. 52, 133–200. (doi:10.1201/b17143-4) [Google Scholar]
- 2.Anthony KR, et al. 2015. Operationalizing resilience for adaptive coral reef management under global environmental change. Glob. Change Biol. 21, 48–61. (doi:10.1111/gcb.12700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabricius K, Okaji K, De'ath G. 2010. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29, 593–605. (doi:10.1007/s00338-010-0628-z) [Google Scholar]
- 4.Uthicke S, Logan M, Liddy M, Francis D, Hardy N, Lamare M. 2015. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 5, 8402 (doi:10.1038/srep08402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamya PZ, Byrne M, Graba-Landry A, Dworjanyn SA. 2016. Near-future ocean acidification enhances the feeding rate and development of the herbivorous juveniles of the crown-of-thorns starfish, Acanthaster planci. Coral Reefs 35, 1241 (doi:10.1007/s00338-016-1480-6) [Google Scholar]
- 6.Wolfe K, Graba-Landry A, Dworjanyn SA, Byrne M. 2017. Superstars: assessing nutrient thresholds for enhanced larval success of Acanthaster planci, a review of the evidence. Mar. Pollut. Bull. 116, 307–314. (doi:10.1016/j.marpolbul.2016.12.079) [DOI] [PubMed] [Google Scholar]
- 7.Kayal M. et al 2012. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7, e47363 (doi:10.1371/journal.pone.0047363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot R, et al. 2012. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 1, 50–61. (doi:10.1016/j.ecoser.2012.07.005) [Google Scholar]
- 9.Hundloe TA, Vanclay FM, Carter M. 1987. Economic and socio-economic impacts of the crown of thorns starfish on the Great Barrier Reef. Institute of Applied Environmental Research. Queensland, Australia: Griffith University. [Google Scholar]
- 10.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. (doi:10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNeil MA. et al 2016. Joint estimation of crown of thorns (Acanthaster planci) densities on the Great Barrier Reef. PeerJ 4, e2310 (doi:10.7717/peerj.2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MR. et al 2017. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 2004, 101038 (doi:10.1038/nature2203) [DOI] [PubMed] [Google Scholar]
- 13.Bos A, Gumanao G, Mueller B, Saceda-Cardoza M. 2013. Management of crown-of-thorns sea star (Acanthaster planci L) outbreaks: removal success depends on reef topography and timing within the reproduction cycle. Ocean Coast. Manag. 71, 116–122. (doi:10.1016/j.ocecoaman.2012.09.011) [Google Scholar]
- 14.Dayoub F, Dunbabin M, Corke P.. 2015. Robotic detection and tracking of crown-of-thorns starfish In IEEE/RSJ Int. Conf. on Intelligent Robots and Systems, 1921–1928. See http://eprints.qut.edu.au/85974/. [Google Scholar]
- 15.Dumas P, Moutardier P, Ham J, Kaku R, Gereva S, Lefèvre J, Adjeroud M. 2016. Timing within the reproduction cycle modulates the efficiency of village-based crown-of-thorns starfish removal. Biol. Conserv. 204, 237–246. (doi:10.1016/j.biocon.2016.10.027) [Google Scholar]
- 16.Nakamura M, Higa Y, Kumagai NH, Okaji K. 2016. Using long-term removal data to manage a crown-of-thorns starfish population. Diversity 8, 24 (doi:10.3390/d8040024) [Google Scholar]
- 17.Rivera-Posada J, Prattchet MS. 2012. A review of existing control efforts for Acanthaster planci; limitations to successes. Report to the Department of Sustainability, Environment, Water, Population and Communities NERP, Tropical Environmental Hub, Townsville.
- 18.Moran PJ. 1986. The Acanthaster phenomenon. Oceanogr. Mar. Biol. 24, 379–480. [Google Scholar]
- 19.Doherty PP, Davidson JJ.. 1988. Monitoring the distribution and abundance of juvenile Acanthaster planci in the central Great Barrier Reef In Proc. of the 6th Int. Coral Reef Symp., pp. 131–136.
- 20.Keesing JK, Lucas JS. 1992. Field measurement of feeding and movement rates of the crown-of-thorns starfish Acanthaster planci (L). J. Exp. Mar. Biol. Ecol. 156, 89–104. (doi:10.1016/0022-0981(92)90018-6) [Google Scholar]
- 21.Keesing JK. 1995. Temporal patterns in the feeding and emergence behaviour of the crown-of-thorns starfish Acanthaster planci. Mar. Freshw. Behav. Physiol. 25, 209–232. (doi:10.1080/10236249509378919) [Google Scholar]
- 22.De'ath G, Moran PJ. 1998. Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L) on the Great Barrier Reef: 1 patterns of activity. J. Exp. Mar. Biol. Ecol. 220, 83–106. (doi:10.1016/S0022-0981(97)00085-3) [Google Scholar]
- 23.Kayal M, Kayal E. 2017. Colonies of the fire coral Millepora platyphylla constitute scleractinian survival oases during Acanthaster outbreaks in French Polynesia. Mar. Biodivers. 47, 255–258. (doi:10.1007/s12526-016-0465-6) [Google Scholar]
- 24.Kayal M, Bosserelle P, Adjeroud M. 2017. Data from: Bias associated with the detectability of the coral-eating pest crown-of-thorns seastar (COTS) and implications for reef management. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.dk5dm) [DOI] [PMC free article] [PubMed]
- 25.Moran PJ, De'ath G. 1992. Estimates of the abundance of the crown-of-thorns starfish Acanthaster planci in outbreaking and non-outbreaking populations on reefs within the Great Barrier Reef. Mar. Biol. 113, 509–515. (doi:10.1007/BF00349178) [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1-131. See https://CRAN.R-project.org/package=nlme.
- 27.McCormick MI. 1994. Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar. Ecol. Prog. Ser. 112, 87–96. (doi:10.3354/meps112087) [Google Scholar]
- 28.Durbán M, Harezlak J, Wand MP, Carroll RJ. 2005. Simple fitting of subject-specific curves for longitudinal data. Stat. Med. 24, 1153–1167. (doi:10.1002/sim.1991) [DOI] [PubMed] [Google Scholar]
- 29.Kayal M, Vercelloni J, Wand MP, Adjeroud M. 2015. Searching for the best bet in life-strategy: a quantitative approach to individual performance and population dynamics in reef-building corals. Ecol. Complex. 23, 73–84. (doi:10.1016/j.ecocom.2015.07.003) [Google Scholar]
- 30.Ruppert D, Wand MP, Carroll RJ. 2003. Semiparametric regression. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Clements CS, Hay ME.. 2017. Size matters: predator outbreaks threaten foundation species in small marine protected areas. PLoS ONE 12, e0171569 (doi:10.1371/journal.pone.0171569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moutardier G, Gereva S, Mills SC, Adjeroud M, Beldade R, Ham J, Kaku R, Dumas P. 2015. Lime juice and vinegar injections as a cheap and natural alternative to control COTS outbreaks. PLoS ONE 10, e0137605 (doi:10.1371/journal.pone.0137605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uthicke S, Doyle J, Duggan S, Yasuda N, McKinnon AD. 2016. Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 5, 16885 (doi:10.1038/srep16885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayal M, Lenihan HS, Pau C, Penin L, Adjeroud M. 2011. Associational refuges among corals mediate impacts of a crown-of-thorns starfish Acanthaster planci outbreak. Coral Reefs 30, 827–837. (doi:10.1007/s00338-011-0763-1) [Google Scholar]
- 35.Clements CS, Hay ME. 2015. Competitors as accomplices: seaweed competitors hide corals from predatory sea stars. Proc. R. Soc. B 282, 20150714 (doi:10.1098/rspb.2015.0714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowan Z-L, Pratchett M, Messmer V, Ling S. 2017. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9, 7 (doi:10.3390/d9010007) [Google Scholar]
- 37.Harrison H, Pratchett M, Messmer V, Saenz-Agudelo P, Berumen M. 2017. Microsatellites reveal genetic homogeneity among outbreak populations of crown-of-thorns starfish (Acanthaster cf solaris) on Australia's Great Barrier Reef. Diversity 9, 16 (doi:10.3390/d9010016) [Google Scholar]
- 38.Faure G. 1989. Degradation of coral reefs at Moorea Island (French Polynesia) by Acanthaster planci. J. Coast. Res. 5, 295–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kayal M, Bosserelle P, Adjeroud M. 2017. Data from: Bias associated with the detectability of the coral-eating pest crown-of-thorns seastar (COTS) and implications for reef management. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.dk5dm) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Crown-of-thorns seastar (COTS) density and reef substrate rugosity data used in this study is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.dk5dm [24].