Abstract
A host of studies support that younger, better performing adults express greater moment-to-moment blood oxygen level-dependent (BOLD) signal variability (SDBOLD) in various cortical regions, supporting an emerging view that the aging brain may undergo a generalized reduction in dynamic range. However, the exact physiological nature of age differences in SDBOLD remains understudied. In a sample of 29 younger and 45 older adults, we examined the contribution of vascular factors to age group differences in fixation-based SDBOLD using (1) a dual-echo BOLD/pseudo-continuous arterial spin labeling (pCASL) sequence, and (2) hypercapnia via a computer-controlled gas delivery system. We tested the hypothesis that, although SDBOLD may relate to individual differences in absolute cerebral blood flow (CBF), BOLD cerebrovascular reactivity (CVR), or maximum BOLD signal change (M), robust age differences in SDBOLD would remain after multiple statistical controls for these vascular factors. As expected, our results demonstrated that brain regions in which younger adults expressed higher SDBOLD persisted after comprehensive control of vascular effects. Our findings thus further establish BOLD signal variability as an important marker of the aging brain.
Introduction
The study of lifespan development, cognition, and brain signal variability continues to gain momentum in cognitive neuroscience1–7 via EEG, MEG, and fMRI. In particular, a host of studies support that younger, better performing adults express greater moment-to-moment BOLD signal variability in various cortical regions3, 8–12. Overall, an emerging view states that brain signal variability may index a more effective, flexible system, and that the aging brain may undergo a generalized reduction in dynamic range1, 13.
However, the exact physiological nature of age differences in BOLD signal variability remains understudied. In particular, age differences in vascular properties could provide one potential reason why BOLD signal variability appears generally reduced in older adults1, 13, 14. Aging is known to be associated with hardening of blood vessel walls throughout the body15, 16; accordingly, increased rigidity in vessels of the brain could lead to a change in neurovascular coupling, with a decreased vascular response to a given level of metabolic demand17. Past attempts to address physiological confounds in BOLD variability studies involved the use of various proxy measures and techniques, such as manual and semi-automated independent component analysis (ICA)18 denoising pipelines, PHYCAA+19, and mixed-model control of level and change in observed blood pressure and heart rate3, 8–10, 12. However, more direct measures of vascular factors may be needed to support principled interpretations of age differences in BOLD signal variability. Specifically, it remains to be seen whether controlling for vascular rigidity and reactivity20, 21 would eliminate observed age group differences in BOLD signal variability.
Respiratory manipulations offer an excellent opportunity to examine this issue because such manipulations induce changes in BOLD signal via controlled vascular challenge. In particular, hypercapnia (i.e., breathing increased concentrations of CO2) leads to robust changes in cerebral blood flow (CBF) throughout gray matter via the vasodilatory properties of CO2 22, 23. Hypercapnia yields substantial increases in BOLD signal throughout the brain that, in combination with quantification of the concomitant evoked change in CBF and end-tidal O2 concentrations, can be used to characterize the vascular component of the BOLD signal through a calibrated fMRI model22, 24–28. BOLD responses to hypercapnia can be used to estimate (1) BOLD cerebrovascular reactivity (CVR), defined as the increase in signal per unit of vasodilatory signal or mmHg CO2, and (2) the maximum possible BOLD signal change (M). CVR is thought to be an indicator of vascular health in the brain since it is a measure of vasodilatory capacity of brain blood vessels, and has been found to be reduced in stroke, carotid artery occlusion, and Alzheimer’s disease17, 29–31. M corresponds to the BOLD signal that would be obtained from complete elimination of deoxygenated hemoglobin from cerebral veins; it represents the dynamic range of the BOLD signal, and appears reduced in older adults17, 32. CBF, BOLD CVR, and M represent a comprehensive index of potential vascular contributions to BOLD, thus allowing us to address how accounting for such vascular factors may impact age group differences in BOLD signal variability. Although a host of studies have examined how various types of vascular scaling impact standard analyses of age differences in mean BOLD signals17, 33, 34, no study to date has examined whether typically found age differences in BOLD signal variability1, 13 remain robust after comprehensive control of vascular factors.
Accordingly, in a sample of younger and older adults scanned during fixation, we tested the hypothesis that although higher BOLD signal variability (SDBOLD_fix) in younger adults may relate to CBF, BOLD CVR, or M (acquired via dual-echo BOLD/pseudo-continuous arterial spin labeling (pCASL) and hypercapnia), robust age group differences in BOLD variability would remain after multiple statistical controls for these vascular parameters. Given that BOLD CVR and M are typically (and in the present study) measured via hypercapnia during rest, we focus here only on fixation-based BOLD variability to better ensure that cognitive states under which all brain measures of interest are acquired (i.e., BOLD variability, CBF, BOLD CVR, and M) are comparable. Further, we also examine SDBOLD_fix within data that have been carefully denoised prior to estimation of age differences or vascular effects; as such, only relatively artifact-free SDBOLD_fix data are analyzed in our models of interest.
Methods
Participants
Acquisitions were originally conducted in 31 young (10 females, 19–32 yrs, mean age = 23.74 ± 2.90 yrs) and 51 older (34 female, 55–72 yrs, mean age = 63.18 ± 4.82 yrs) healthy participants on a Siemens TIM Trio 3 T magnetic resonance imaging (MRI) system (Siemens Medical Solutions, Erlangen, Germany) using the vendor-supplied 32-channel receive-only head coil for all acquisitions (see ref. 17). All participants gave informed consent, the local ethics committee (Comité mixte d’éthique de la recherche du Regroupement Neuroimagerie/Québec) approved the study, and all methods were performed in accordance with the relevant approved guidelines and regulations. See further details below regarding how the final sample (n = 74, 29 young adults (9 females), 45 older adults (32 females)) was determined in the context of the current study.
Exclusion criteria for this study included claustrophobia, cardiac disease, hypertension or taking medication to lower blood pressure, neurological or psychiatric illness, smoking, excessive drinking (more than 2 drinks per day), thyroid disease, diabetes, asthma, and using a regular treatment known to be vasoactive or psychoactive. Participants were all nonsmokers, or had been nonsmokers for at least 5 years. Older participants met with a geriatric MD to ensure that they did not meet any of the exclusion criteria for the study. All participants completed a short neuropsychological screening battery to assess normal cognition. The cognitive characterization of this cohort has been published before and results can be found in Table 1 of Gauthier et al.17. Older adults participants also completed the Mini-Mental State Examination35 to screen for global cognitive decline; no participant scored less than 26.
Table 1.
Multivariate PLS model peak activations, bootstrap ratios, and cluster sizes for regions showing increased BOLD signal variability with age.
| Region | Hem | MNI coordinates | BSR | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Precentral gyrus | R | 48 | 8 | 44 | 4.87 | 49 |
| Precuneus | L | −4 | −64 | 32 | 4.86 | 167 |
| Anterior cingulate | L | 0 | 32 | 24 | 3.90 | 45 |
| Superior medial gyrus | L | 0 | 52 | 8 | 3.64 | 20 |
| Middle temporal gyrus | R | 56 | −48 | 12 | 3.61 | 18 |
| Middle occipital gyrus | L | −44 | −76 | 36 | 3.39 | 16 |
| Angular gyrus | L | −56 | −68 | 24 | 2.83 | 16 |
Note: SD = standard deviation; BOLD = blood oxygen level-dependent; Hem = hemisphere; MNI = Montreal Neurological Institute; BSR = bootstrap ratio (model salience/bootstrapped standard error).
Whenever possible, participants needing eyesight correction were asked to wear contact lenses on the day of the MRI experiment. For those without contact lenses, eyesight was corrected to the nearest possible 0.50 D using MRI-compatible glasses. For participants with significant hearing losses, written instructions were projected onto a screen at the end of the bore that could be seen by participants through a mirror.
MRI data
The current paper examines rest block data from a previously published block design task paradigm17. In total, there were five 60 sec rest blocks available for analysis in the current study (total = 300 sec). Rest blocks from this paradigm were of focus given that hypercapnia measures were also collected during resting-state (see below), thus matching the cognitive states of BOLD and vascular data as closely as possible.
Magnetic resonance image acquisition
Sessions included two anatomical, 1 × 1 × 1 mm magnetization prepared rapid gradient echo (MPRAGE) acquisitions with repetition time (TR)/echo time (TE)/flip angle = 2300 ms/3 ms/9 deg, 256 × 240 matrix, and a generalized autocalibrating partially parallel acquisition (GRAPPA) acceleration factor of two36. Older participants had an additional fluid attenuated inversion recovery (FLAIR) acquisition to estimate the presence and severity of white matter lesions. FLAIR acquisition parameters included TR/TE/flip angle = 9000 ms/107 ms/120 with echo train length of 15, an inversion time of 2500 ms, 512 × 512 matrix for an in-plane resolution of 0.43 × 0.43 mm and 25 slices of 4.8 mm. White matter hyperintensities were quantified using the scale from Wahlund et al.37. Only participants with scores of 0 or 1, corresponding to no or few small lesions, were included in this study. The score average and standard deviation was 0.67 ± 0.48 in the older group.
Functional image series were acquired using a dual-echo pseudo-continuous arterial spin labeling acquisition38 to measure changes in CBF and BOLD signal simultaneously. The parameters used include: TR = 3000 ms, TE #1 = 10 ms, TE #2 = 30 ms, flip angle = 90 deg, with 4 × 4 mm in-plane resolution and 11 slices of 7 mm (1 mm slice gap) on a 64 × 64 matrix (at 7/8 partial Fourier). GRAPPA acceleration factor = 2, postlabeling delay = 900 ms, label offset = 100 mm, Hanning window-shaped right frontal pulse with duration/space = 500 ms/360 ms, flip angle of labeling pulse = 25 deg, slice-selective gradient = 6 mT/m, tagging duration = 1.5 seconds38.
We conducted two imaging sub-sessions within the same overall session. In the first sub-session, MPRAGE, FLAIR, and fMRI data were acquired. In the second sub-session, MPRAGE and the hypercapnia functional acquisition were performed. Participants were taken out of the scanner in between these two acquisition segments to either put on, or take off the hypercapnia mask, depending on the order of acquisitions. Participants were allowed to move or stretch during this pause to ensure greater comfort, especially in the older participants.
Hypercapnic manipulation
Hypercapnic manipulations were achieved using a computer-controlled gas delivery system in combination with a sequential gas delivery circuit (RespirAct system; Thornhill Research Inc, Toronto, Ontario, Canada). The RespirAct system allows independent control of end-tidal partial pressure of CO2 (PCO2) and end-tidal partial pressure of O2 (PO2) using a feed-forward physiological model, using as input the measured or predicted baseline O2 consumption and CO2 production of a subject39. PCO2 was targeted to be 40 mm Hg at baseline and 45 mm Hg during the hypercapnia blocks. These values were maintained throughout each block. PO2 was targeted to be 100 mm Hg throughout the experiment. Gas was sampled continuously (via RespirAct) at the mouth and analyzed for PCO2 and PO2. During the hypercapnic stimulation, volunteers breathed through the circuit via a soft plastic mask sealed to the face using adhesive dressing (Tegaderm 3 M Healthcare, St. Paul, MN, USA), as necessary to prevent gas leakage. Participants were asked to breathe deeply enough to empty the fresh gas compartment of the breathing circuit at every breath during the functional acquisitions (to ensure delivery of the entire gas dose delivered by the machine and stable end-tidal values throughout the block). Generally, subjects did not have difficulty complying with this requirement.
Participants underwent the manipulation twice during the study, once outside the scanner before the imaging session for acclimation, and once during the MRI session. Subjects were interviewed after the acclimation session to assess their level of respiratory discomfort using the 7-point scale published by Banzett et al.40. Subjects reporting a subjective rating of 5 or greater (moderate discomfort or greater) were not invited to continue in the study (2 cases). The average discomfort rating over all subjects was 2.22 ± 1.03, corresponding to only slight discomfort that could be maintained for long periods.
Data preprocessing
fMRI and ASL data were preprocessed with FSL 541, 42 and Neurolens (www.neurolens.org). Pre-processing included: motion-correction with spatial smoothing (8 mm full-width at half maximum Gaussian kernel) and high-pass filtering (0.01 Hz). The CBF signal was isolated from the series of first echoes using linear surround subtraction43, and the BOLD signal was extracted using linear surround addition of the second echo series43–45 in Neurolens. Registration of TE = 30 ms (i.e., BOLD) functional images to high-resolution participant-specific T1 images, and from T1 to 2 mm standard space (MNI 152_T1) was carried out using FLIRT. These same spatial transformation matrices were then used to register the TE = 10 ms (i.e., ASL) data, to minimize normalization errors due to bright scalp signal typical of ASL images.
Beyond standard preprocessing steps, we subsequently examined all functional volumes for artifacts via independent component analysis (ICA) within-run, within-person, as implemented in FSL/MELODIC18. Noise components were targeted according to several key criteria: (a) Spiking (components dominated by abrupt time series spikes ≥6 SDs); (b) Motion (prominent edge or “ringing” effects, sometimes [but not always] accompanied by large time series spikes); (c) Susceptibility and flow artifacts (prominent air-tissue boundary or sinus activation; typically represents cardio/respiratory effects); (d) White matter (WM) and ventricle activation46; (e) Low-frequency signal drift47; (f) High power in high-frequency ranges unlikely to represent neural activity (≥75% of total spectral power present above 0.13 Hz;); and (g) Spatial distribution (“spotty” or “speckled” spatial pattern that appears scattered randomly across ≥25% of the brain, with few if any clusters with ≥10 contiguous voxels [at 4 × 4 × 4 mm voxel size]). Examples of these various components we typically deem to be noise can be found in supplementary materials in Garrett et al.48. By default, we utilize a conservative set of rejection criteria; if manual classification decisions are difficult due to the co-occurrence of apparent “signal” and “noise” in a single component, we typically elect to keep such components, which helps guard against potential concerns that ICA denoising may remove “signal” of interest49. Two independent raters of noise components were utilized, and >90% inter-rater reliability was required on separate data before denoising decisions were made on the current data. Components identified as artifacts were then regressed from corresponding fMRI runs using the FSL regfilt command. The use of ICA denoising had dramatic effects in our past research, effectively doubling the predictive power of BOLD signal variability8. Thus, calculating BOLD signal variance from relatively artifact-free BOLD time series permits the examination of what is more likely meaningful brain signal dynamics. Furthermore, as the current study seeks to identify possible vascular biases in existing methods, we elected to preprocess the data using the same techniques as in previously published studies3, 8, 12, 50.
The pre-processed flow-weighted time series from the 10 ms TE (short echo) was also denoised using the same process to ensure comparable removal of motion and other artifact components (e.g., drifts, spikes). All the same exclusion criteria were used for these datasets, and two additional rejection criteria were added to take into account ASL-specific artifacts, likely due to tag and fat saturation instabilities. The components presumably due to tag effects took the form of large areas of highly positively or negatively correlated signals. These areas did not follow anatomical boundaries and were typically largest in the bottom slices of the volume. The fat saturation components were a crescent-shaped area of the same shape as the back of the head shifted into occipital areas.
Computation of SDBOLD during fixation blocks (SDBOLD_fix)
One of several BOLD signal variance measures utilized in fMRI research (e.g., amplitude of low frequency fluctuations, or ALFF51), we focused on a modified voxel-wise time series standard deviation from fixation block data (SDBOLD_fix). SDBOLD does not require continuous data, making it an effective measure of signal variation in concatenated (discontinuous) block data such as those utilized in the current study. We do not employ Fourier-based measures such as ALFF in the present study explicitly due to our use of concatenated (discontinuous) block data. The validity of frequency-specific power estimates taken via ALFF relies on continuous time. Due to the concatenated (discontinuous) block nature of our data, only local within-block data would be amenable to frequency-specific power estimation in our study (30 secs per block; using a rule of thumb minimum 3 cycles of any estimable frequency = only frequencies ≥0.10 Hz are estimable), thus precluding estimation of power within a typical bandpass range in BOLD data (0.01 to 0.10 Hz).
To compute SDBOLD_fix from the current fixation block data, we first performed a block-normalization procedure to account for residual low-frequency artifacts. We normalized all fixation blocks such that the overall 4D mean (x*y*z*time) across brain and block was 100. For each voxel, we then subtracted the block mean and concatenated across all blocks. Finally, we calculated voxel standard deviations across this concatenated time series8–10, 48. Our computation of SDBOLD differs from resting-state-fluctuation analysis (RSFA52, another time series SD estimation method) in that the original RSFA does not normalize for 4D means, nor blocks, in the same manner. So-called “normalized” RSFA33 does, however, remove the entire time-series mean prior to SD calculation, but this is not relevant for a block design such as ours as we do not consider meaningful the differences between discontinuously acquired blocks, given that extreme mean differences between blocks can dramatically inflate SD estimates8, 9. Critically, regardless of the extent that SDBOLD_fix and RSFA may relate mathematically, our examination of SDBOLD occurs only after extensive multi-stage data denoising routines. Accordingly, for the remainder of the current paper, our use of the terms “SDBOLD_fix” or “BOLD signal variability” thus refer to signal variability resulting from already cleaned, denoised, relatively artifact-free, and multiply normalized time series data.
As noted above, the fixation blocks we analyzed in the current study are extracted from an alternating block design (fixation-task-fixation-task). We chose to examine only fixation blocks in the current study to maintain maximum overlap in “brain or cognitive state” between BOLD, and baseline CBF and hypercapnia data. Hypercapnia data are typically, as in the current study, collected only off-task. This ensures that any comparison of age prediction by both SDBOLD_fix and vascular measures are not confounded by presumed differences in cognitive state.
However, one potential concern regarding SDBOLD_fix in the present study may be that because fixation blocks alternate between task blocks, it is possible that the temporal variability in any given fixation block may be impacted by the signal dynamics of the immediately preceding task block. If so, a preceding block should have the greatest impact on variance within the first portion of the succeeding block (i.e., due to BOLD signal (or brain state) spillover from the preceding block), rather than within later portions of the succeeding block. However, we showed extensively in past work10 that the split-half reliability of SDBOLD_fix values from concatenated first and concatenated second block halves for fixation block-based SDBOLD_fix estimation was near unity in younger and older adult groups (each r = ~0.97). This suggests that estimation of SDBOLD from fixation blocks is robust despite embedding within a broader alternating block-design study. We thus apply the same logic in the current study when linking SDBOLD_fix to vascular measures obtained during resting periods.
Vascular parameter estimation
Baseline CBF, CVR and M values were obtained using Neurolens and in-house Matlab code (for M). These vascular and metabolic parameters were selected for the current study since they have been previously shown to robustly capture several important aspects of age-related differences in hemodynamics21, 44, 53–55. Fractional changes in BOLD and CBF signals were then determined for hypercapnia by fitting a general linear model (GLM) to the respective signals and dividing the estimated effect size by the estimated constant term. Model fits used a single-gamma hemodynamic response function with parameters described by Glover56 and included linear, quadratic, and cubic polynomials to represent baseline signal and drifts.
Absolute resting CBF was determined from the pseudocontinuous arterial spin labeling data using the approach described by Wang et al.,57 assuming blood-brain partition coefficient = 0.9, labeling efficiency = 0.80, blood T1 = 1.49 seconds, and gray matter T1 = 1.4 seconds. For this computation, the baseline ASL difference signal estimated in the GLM fit for each gas manipulation was divided by the corresponding unsubtracted baseline EPI signal from the ASL series, computed in a similar GLM fit carried out on the unsubtracted EPI series. The unsubtracted baseline EPI signal from the ASL series is used here as a surrogate for the fully relaxed magnetization that can alternately be acquired in the form of what is termed an M0 scan. To account for incomplete recovery of longitudinal magnetization during the sequence TR of 3 seconds, baseline EPI estimates from gray matter ROIs were corrected using the gray matter T1 value cited above. The resultant ratio was converted to absolute CBF units based on the parameters above. CVR was obtained by dividing the percent BOLD signal changes during the hypercapnia manipulation by the increase in end-tidal PCO2 values during this manipulation44, 58, 59. BOLD CVR was used rather than CBF CVR since BOLD CVR was shown earlier to be a more sensitive measure of vascular change in aging17.
| 1 |
The generalized calibration model (GCM) method28, 44 was utilized to obtain M estimates for each subject, using ROI-averaged values for relative CBF and BOLD signal changes during hypercapnia.
| 2 |
where α is the flow-volume coupling during hypercapnia and is assumed to have a value of 0.1860 and β is the influence of deoxygenated hemoglobin on transverse relaxation61. A β value of 1.5 was used here. SVO2 is the venous oxygen saturation and is calculated from end-tidal O2 concentrations (PO2) and CBF using a series of equations described previously28, 44.
Multivariate statistical analyses and determination of regions for brain parameter estimation
To examine multivariate relations between SDBOLD_fix and age group during rest blocks, we employed a “Task PLS” analysis62, 63. Task PLS begins by calculating a between-subject covariance matrix (COV) between experimental conditions/groups and each voxel’s SDBOLD_fix. COV is then decomposed using singular value decomposition (SVD).
| 3 |
This decomposition produces a left singular vector of experimental condition/group weights (U), a right singular vector of brain voxel weights (V), and a diagonal matrix of singular values (S). This analysis produces orthogonal latent variables (LVs) that optimally represent relations between experimental conditions/groups and voxel-wise SDBOLD values. Each LV contains a spatial activity pattern depicting the brain regions that show the strongest relation to condition/group contrasts identified by the LV. Each voxel weight (in V) is proportional to the covariance between voxel SDBOLD and the condition/group contrast. In the current study, only one LV was estimable, given that we examined a single condition (fixation) across two age groups (i.e., a single PLS-derived contrast captures the associated age group effect on SDBOLD).
Significance of detected relations between multivariate spatial patterns and conditions/groups was assessed using 1000 permutation tests of the singular value corresponding to each LV. A subsequent bootstrapping procedure revealed the robustness of voxel saliences across 1000 bootstrapped resamples of the data64. By dividing each voxel’s mean salience by its bootstrapped standard error, we obtained “bootstrap ratios” (BSRs) as normalized estimates of robustness. We thresholded BSRs at conservative values of ±2.70, which exceeds a 99% confidence interval.
Typically, to obtain a PLS-based summary measure of each participant’s robust expression of a particular LV’s spatial pattern, one would calculate within-person “brain scores” by multiplying each voxel (i)’s weight (V) from each LV (j) (produced from the SVD in equation (1)) by voxel (i)’s SDBOLD value, for each condition/group (k) within person (l), and summing over all (n) brain voxels:
| 4 |
This is equivalent to the vector multiplication of V by a subject’s vector of SDBOLD values for all voxels. However, in the current study, to test the impact of vascular effects on age differences in SDBOLD_fix, we wanted to constrain all subsequent analyses only to brain scores calculated from regions thresholded by bootstrapping procedures that expressed higher SDBOLD_fix in younger adults. We then utilized these same thresholded regions for extracting single, averaged vascular parameters (absolute CBF, BOLD CVR, and M). This allowed us to address in a spatially specific manner whether higher SDBOLD_fix typically seen in younger vs. older adults can be accounted for by vascular factors.
To restrict all multivariate analyses to grey matter (GM) from the denoised images (which were analyzed whole brain), we masked our functional data with the GM tissue prior provided in FSL (resampled to 4 mm). We localized thresholded peaks from PLS model output by submitting resulting MNI coordinates to the Anatomy Toolbox (version 1.8) in SPM8, which applies probabilistic algorithms to determine the cytoarchitectonic labeling of MNI coordinates65, 66.
Final regression models
Given our primary goal to test whether PLS-derived age differences in SDBOLD_fix could be accounted for by vascular factors, we fit a series of subsequent regression models in which age group and either one or all of the vascular predictors (absolute baseline CBF, BOLD CVR, and M) predicted SDBOLD_fix. Finally, we evaluated results using 1000 bootstrap resamples (with replacement) of the data.
Treatment of distributions, and subsequent univariate and multivariate outlier detection
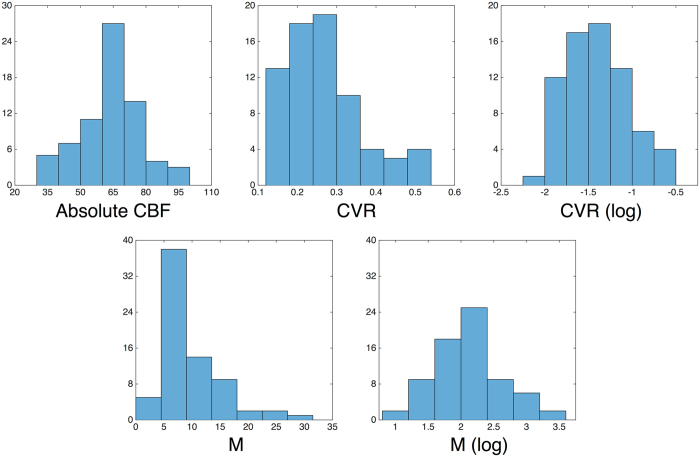
Inspection of distributions occurred for all variables prior to all regression model runs. Univariate outlier detection was performed on all measures prior to regression modeling noted above; cases that were greater than ± 2.5 SDs from sample means on any variable were removed. The removal of univariate outlier cases yielded an effective sample size of n = 74 (29 young, 45 older) prior to multivariate outlier detection (see below). Following this step, BOLD CVR and M distributions still deviated from Gaussian normality (1-sample Kolmogorov-Smirnov Test, both ps < 0.05) and were both right skewed. We thus log transformed the BOLD CVR and M variables, which produced Gaussian distributions for both (1-sample Kolmogorov-Smirnov Test, both ps > 0.10). CBF, and pre- and post-transformed CVR and M distributions, are noted in Fig. 1).
Figure 1.
Histograms of absolute CBF, and pre- and post-transformed CVR and M values utilized in Models 1–4. Note: CBF = cerebral blood flow; CVR = cerebrovascular reactivity; M = maximal BOLD signal change.
Multivariate normality was then assessed for the full regression model run (Table 2, Model 4) by estimating the Mahalanobis distance (D 2) for each subject. As Mahalanobis distances are approximately Χ2 distributed, we compared our D 2 values (df = 4, where df represents the number of predictors in the model (age group, absolute CBF, BOLD CVR, and M)) to a reference Χ2 distribution within a Q-Q plot. Extreme values in the tail of the D 2 distribution that departed from the reference distribution were removed, while maintaining that no more than ~5% of the model cases be held out, no matter how extreme the absolute D 2 value; in our model, this amounted to a reduction of three cases out of 74 (final n = 71; 28 young, 43 older adults). To maintain comparability of samples, all sub-models (Table 2, Models 1–3) were run using the same n = 71 that remained after multivariate outlier targeting for the full model. All regression models and outlier detection steps were run using SPSS 23 (IBM, Inc.).
Table 2.
Univariate models.
| Model | Dependent variable | Predictor | b | Bootstrap 95% CI | se | t | p | Zero- order | Partial | Semi- Partial | VIF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SDBOLD brain score | Age Group | −0.89 | (−1.16, −0.59) | 0.14 | −6.21 | 3.67 * 10 −8 | −0.61 | −0.60 | −0.60 | 1.09 |
| Absolute CBF | 0.00 | (−0.01, 0.01) | 0.01 | −0.44 | 0.66 | 0.13 | −0.05 | −0.04 | 1.09 | ||
| 2 | SDBOLD brain score | Age Group | −0.69 | (−1.09, −0.34) | 0.17 | −3.97 | 1.77 * 10 −4 | −0.61 | −0.43 | −0.37 | 1.66 |
| BOLD CVR | 0.40 | (−0.08, 0.80) | 0.24 | 1.66 | 0.10 | 0.51 | 0.20 | 0.16 | 1.66 | ||
| 3 | SDBOLD brain score | Age Group | −0.75 | (−1.06, −0.43) | 0.16 | −4.73 | 1.16 * 10 −5 | −0.61 | −0.50 | −0.45 | 1.37 |
| M | 0.24 | (−0.12, 0.55) | 0.16 | 1.49 | 0.14 | 0.44 | 0.18 | 0.14 | 1.37 | ||
| 4 | SDBOLD brain score | Age Group | −0.67 | (−1.03, −0.031) | 0.19 | −3.57 | 6.79 * 10 −4 | −0.61 | −0.40 | −0.34 | 1.90 |
| Absolute CBF | 0.00 | (−0.01, 0.01) | 0.01 | −0.39 | 0.70 | 0.13 | −0.05 | −0.04 | 1.15 | ||
| BOLD CVR | 0.29 | (−0.21. 0.80) | 0.27 | 1.08 | 0.28 | 0.51 | 0.13 | 0.10 | 2.01 | ||
| M | 0.17 | (−0.24, 0.53) | 0.17 | 0.99 | 0.33 | 0.44 | 0.12 | 0.09 | 1.64 | ||
| 5 | CorrSD−CBF | Age Group | −0.05 | (−0.12, 0.02) | 0.04 | −1.30 | 0.20 | −0.16 | n/a | n/a | n/a |
| 6 | CorrSD−CVR | Age Group | −0.10 | (−0.17, −0.02) | 0.04 | −2.50 | 0.02 | −0.29 | n/a | n/a | n/a |
Note: SD = standard deviation; CI = confidence interval; BOLD = blood oxygen level-dependent; YA = young adults; OA = older adults; CBF = cerebral blood flow; CVR = cerebrovascular reactivity; M = maximal BOLD signal change; VIF = variance inflation factor. Significant p-values are in bold font. “Zero-order”, “partial,” and “semi-partial” columns reflect effect sizes in Pearson’s correlation metric. Levene’s test for equality of variances was insignificant in all models (ps ranged from 0.12 to 0.95). Finally, the frequency of males and females was significantly different in the young (20 male, 8 female) and older (11 male, 32 female) groups (Chi square = 14.49, p = 1.41 * 10−4); however, sex had no predictive effect in any model reported above (all ps ranged from 0.21 to 0.52), and also had no appreciable effect on the unique effect of any predictor of interest. We thus report all final models above without further control for participant sex. Finally, there were no robust interactions between age group and any vascular parameter in Models 1–4 (all ps > 0.25).
Results
PLS results
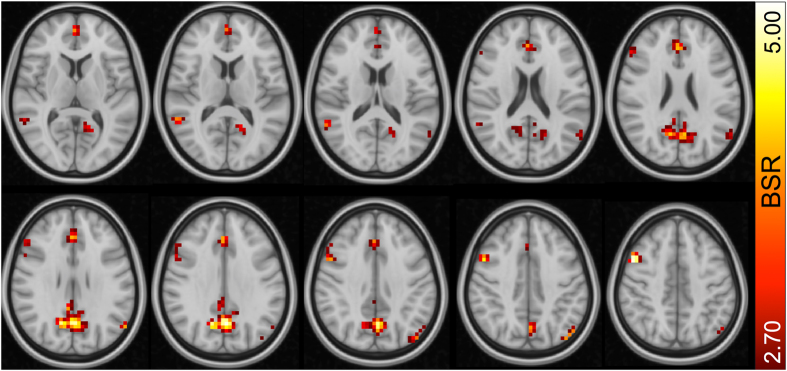
We first ran a multivariate partial least squares (PLS) model (see Methods for details) to test for regions in which younger adults expressed greater SDBOLD_fix, and we found a single robust latent variable (permuted p = 0.012) representing this relationship. The thresholded brain pattern (Fig. 2) highlights regions in which younger adults (YA) expressed higher SDBOLD_fix. This model revealed several regional effects that are in general accordance with previous studies. In particular, precuneus, anterior cingulate, and DLPFC often exhibit higher SDBOLD_fix in younger adults7–10, 67. These convergent findings position the current dataset well for subsequent examination of whether the effect of significantly higher SDBOLD_fix in younger adults can be eliminated by accounting for vascular factors. A complete list of bootstrapped cluster peaks can be found in Table 1.
Figure 2.
Regions expressing greater SDBOLD in younger vs. older adults. Note: BSR = bootstrap ratio. From top left, slices are shown from Z = 8 to Z = 44 in 4 mm increments.
Regression models linking age and vascular measures to SDBOLD_fix
Next, we extracted baseline CBF, BOLD CVR, and M from the regions showing higher SDBOLD_fix levels in younger adults in the PLS model noted above. We then fit several models using 1000 resamples (with replacement) of the data, regressing SDBOLD-based brain scores (from the age-based PLS model above) on age group and the various vascular measures. This allowed us to test whether initial age differences in SDBOLD could be attributed to vascular factors. We began by running separate models with age group and each vascular predictor (to give each vascular predictor a maximal chance to account for variance in SDBOLD_fix), and then a full model with age group and all vascular predictors together. As hypothesized, our results indicated that age differences in SDBOLD_fix remained regardless of whether age was pitted against single or against multiple vascular predictors (Table 2, Models 1–4). BOLD CVR (r = 0.51) and M (r = 0.44) indeed expressed moderate zero-order relations to SDBOLD (but only weakly for CBF, r = 0.13), although this initial predictive utility for vascular parameters was largely eliminated when controlling for age group (unique (semi-partial) rs = −0.04 (CBF), 0.16 (BOLD CVR), and 0.14 (M)). However, this in turn indicates that a certain amount of predictive variance attributed to vascular parameters was shared with age group, reflected in differential reductions in the zero-order predictive utility of age (r = −0.61) across models (semi-partial rs; Model 1 = −0.60; Model 2 = −0.37; Model 3 = −0.45; Model 4 = −0.34) in Table 2.
Distributional analyses of links between SDBOLD_fix and CVR/CBF
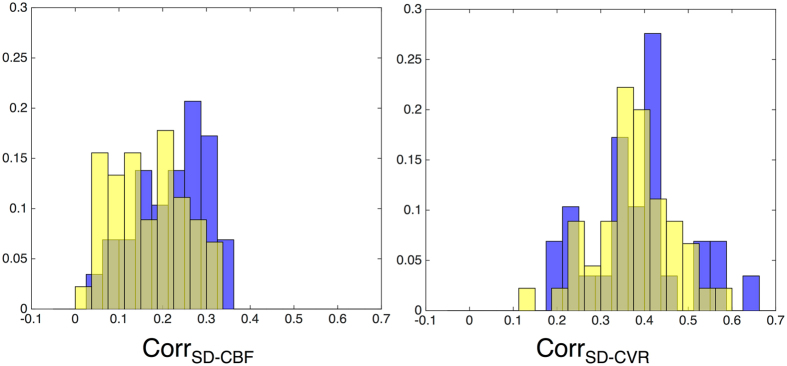
Next, we examined the within-person correlations between SDBOLD_fix and CBF, and SDBOLD_fix and CVR, across all voxels in the brain, to identify the range of relations between SDBOLD_fix and vascular measures across persons and age groups. While very recent improvements in ASL acquisitions and analysis also make voxel-wise M estimation more feasible, the current data only allow reliable M estimation through averaging across multiple voxels in an ROI17. Resulting distributions can be found in Fig. 3. We find that there is not only a remarkably wide range in correlation between SDBOLD_fix and vascular measures across subjects, but also minimal (Table 2, Model 5) or modest (Table 2, Model 6) effect sizes representing differences between age groups in these values. This suggests that on average, age groups are not dramatically different in how SDBOLD_fix and vascular parameters relate across the brain, and that the strength of these relations varies widely across persons. These findings have direct relevance to future approaches seeking voxel-wise, within-subject calibration of fMRI signal variance. The logic of within-person calibration relies on the presumed reliability of relationship between BOLD and vascular measures. Recent work may however make it possible to obtain more robust calibrated fMRI estimates in the future68, 69.
Figure 3.
Histograms of within-subject voxel-wise correlations of SDBOLD-CBF and SDBOLD-CVR relations for younger (blue) and older (yellow) adults. Note: Y-axis values represent normalized histogram proportions due to different sample sizes in each age group (young adults, n = 29; older adults n = 42).
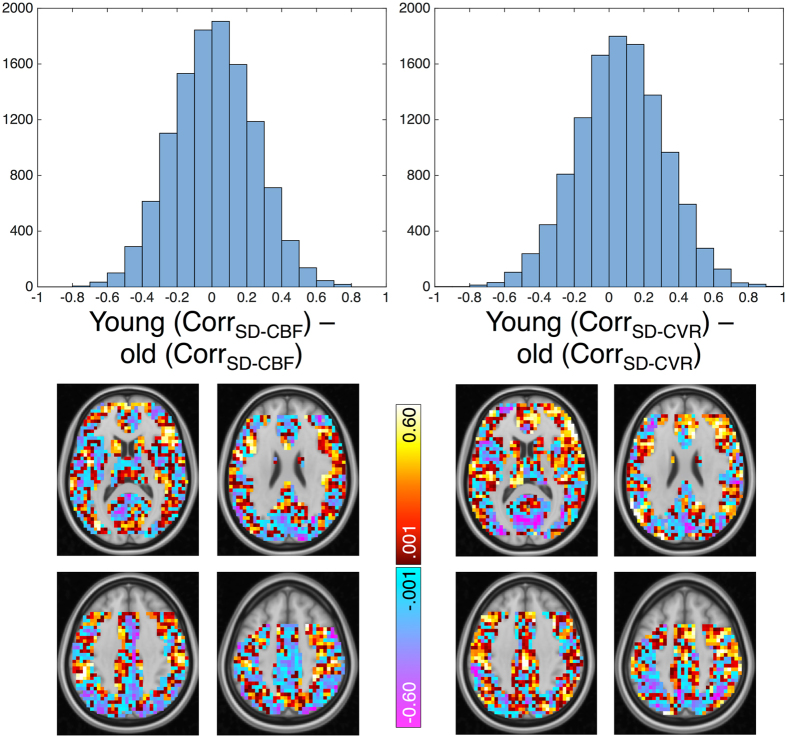
An alternative approach to visualizing differential coupling between SDBOLD_fix, CBF, and CVR can occur at the voxel (rather than subject) level, in which voxel correlations are computed across age group members. One can then visualize whether SDBOLD-vascular coupling differs by age group in each voxel. Raw age group differences (young minus old) in coupling are plotted in Fig. 4. Here, one can see that there are profound regional differences in whether younger or older adults show the tightest coupling between SDBOLD_fix and vascular factors. Despite a modest group difference at the whole brain level (Table 2, Model 6), voxel effects are much more varied in direction. Thus, whether across regions within-person and groups, or within-voxel across persons and groups, the direction and strength of coupling of SDBOLD_fix and vascular parameters varies greatly. At present, these findings highlight the challenges of simple voxel-wise vascular control of SDBOLD_fix in the study of age differences.
Figure 4.
Differential voxel-wise coupling strength between SDBOLD, and CBF and CVR in younger and older adults. Note: Correlations between SDBOLD-CBF, and SDBOLD-CVR are computed for each voxel, across age group members. Voxel histograms represent a young minus old group difference of these voxel-wise correlation values (upper row), which are then plotted in the brain maps below each histogram (lower row). Slices for each distribution represent Z = 12 (upper left), 24 (upper right), 36 (lower left), and 48 (lower right). Orientation of images: right is right. Missing data in frontal regions are a result of partial coverage and angulation restrictions for adequate tagging in pCASL data. For complete description of both histrograms and spatial representation, these maps are intentionally unthresholded.
Discussion
Overall, our past work demonstrates that brain signal variability may index a more effective, flexible system, and that the aging brain may undergo a generalized reduction in dynamic range1. However, the veracity of these claims requires that age-related reductions in SDBOLD_fix exist beyond vascular factors that may distinguish younger from older adults12, 17, 20, 21, 32, 54, 70–72. In the present study, using dual-echo BOLD/ASL, hypercapnia, and multivariate statistical methods, we examined whether lower BOLD cortical signal variability in older relative to younger adults could be accounted for by vascular parameters (CBF, CVR, and M) that are known to affect BOLD signal amplitude in older adults17. Our results demonstrated that SDBOLD_fix remained significantly higher in younger adults after accounting for vascular effects, no matter which model we ran (Table 2, Models 1–4). These findings converge with past work attempting to control indirectly for physiological confounds in BOLD variability via techniques such as manual and semi-automated independent component analysis (ICA)18 denoising pipelines, PHYCAA+19, and mixed-model control of level and change in observed blood pressure and heart rate8–10, 12. In all cases, reported age effects were highly robust over and above such vascular controls, providing initial support for a neural basis for age differences in BOLD signal variability.
The study of age-related BOLD fluctuations have recently come under some scrutiny, including calls to utilize resting BOLD signal variability directly as a “vascular scaling factor” due to moderate correlations between exogenous physiological parameters (heart rate, blood pressure, respiration) and resting BOLD fluctuations (but in data that had not been physiologically denoised14). In our opinion, and based on our current results, one should not consider BOLD fluctuations directly as a logical proxy for vascular scaling, even though BOLD fluctuations correlate with vascular parameters to some extent. Doing so effectively attributes all signal fluctuations to a single source (vasculature), which has no basis for support in the literature. It is also trivial that simple exogenously measured physiological parameters should correlate with BOLD; this topic has been the target of a variety of denoising pipelines and techniques over the past 15 years19, 73, 74. The primary question in the present study is thus not whether BOLD fluctuations may relate to any physiological or vascular measure, but instead whether there is any meaningful information in BOLD signal variability over and above the influence of well-measured, brain region-specific vascular parameters. We have argued in past work that vascular issues are unlikely to account fully for BOLD variability effects, including various studies showing that BOLD fluctuations predict cognition separately within older adults12 and within younger adults1, 48, in which the role of vascular differences may be relatively small. However, what is necessary is the adequate treatment of the vascular/physiological components of BOLD fluctuations prior to the interpretation of such fluctuations. In the current study, we attempted to parameterize vascular effects in regions in which SDBOLD_fix was higher in younger adults, and investigate whether we could eliminate these age differences in BOLD signal variability via vascular control. Our results demonstrate that higher BOLD signal variability in younger adults cannot be fully accounted for by individual differences in CBF, BOLD CVR, or BOLD dynamic range (M), thus further supporting the principled examination of age differences in BOLD fluctuations1, 12, 13.
Seeking the most effective method(s) of vascular “control”
The most flexible form of vascular control when examining BOLD signal variability data would be to perform it at the single voxel level, which could then occur prior to and irrespective of any model of choice (like any preprocessing step). However, there are several issues with this approach at present, and we are not yet at the point where a specific correction strategy can be recommended. First, voxel-wise vascular control of BOLD parameters presumes a predictable relation between vascular measures and BOLD, within and across regions and subjects. In the present study, the correlations between SDBOLD_fix and CBF, and between SDBOLD_fix and CVR, varied greatly across subjects, with some participants showing very weak (near zero) correlation values, and others showing very strong relations (see Fig. 3). This result converges with recent work on young adults also suggesting inconsistent within-subject spatial relations between BOLD RSFA/ALFF and CVR75, 76. Although the reasons for such subject-wise variation are not yet clear, the lack of robust SDBOLD_fix-vascular relations in all subjects guarantees that voxel-wise scaling will have differing and unpredictable effects across subjects and brain regions. Similarly, when examining age group differences in across-subject correlations of SDBOLD_fix-CBF and SDBOLD_fix-CVR for each voxel (Fig. 4), these correlations also varied greatly in strength and direction. This result highlights that while there are many brain regions in which older adults express greater SDBOLD_fix-vascular coupling than in younger adults, this pattern is not consistent and provides a source of relatively unpredictable variation when enacting vascular “control” in aging studies of BOLD dynamics. Combined, these findings argue against any simple utilization of voxel-wise vascular control of SDBOLD_fix. Furthermore, as the BOLD signal arises from a combination of CBF, blood volume and oxidative metabolism, correction by a single component of the BOLD signal may not offer adequate correction for complex age-related vascular/metabolic differences. However, future work could pursue the nature of these very interesting regional differences in SDBOLD_fix-vascular relations. Improved measurement of the voxel-wise M parameter would allow this type of correction, but given the non-linear combination of low SNR measurements required, this was not possible with the current data.
One straightforward and often utilized way to improve the reliability of potentially noisy vascular estimates is to extract average CBF, CVR, and M values from multi-voxel ROIs17, 21, 77, 78, and either perform voxelwise or ROI-based correction of BOLD with these average values, or covary the effects of vascular parameters from effects of interest. This approached has been used in the past with task-based fMRI to show a reduced age-related difference in brain activity21. In the present study, we took this voxel-averaged model covariation approach, extracting average CBF, CVR, and M values from those thresholded voxels that expressed higher SDBOLD_fix in younger adults. Covariation also seems a more appropriate statistical control because the effect of potentially unpredictable or unreliable vascular covariates would be handled accordingly at the model level, rather than scaled at the voxel level. We further performed log transformation (to achieve a Gaussian distributional form) and uni- and multivariate outlier detection for each vascular variable. Combined, voxel averaging and data cleaning were intended to give each vascular parameter its best opportunity to account for age-related variance in SDBOLD_fix (see Table 2); importantly however, robust age differences in SDBOLD_fix remained in all models we ran. Overall, future studies could use the current approach to take into account age-related vascular effects in studies of BOLD variability. While it might be unrealistic to obtain all three parameters in most contexts, BOLD CVR may be the most accessible and useful covariate given the present results. However, high quality calibrated fMRI studies would benefit most from using the M parameter, as this parameter determines the BOLD signal amplitude and is therefore theoretically the optimal parameter to best account for vascular effects.
Potential caveats and future directions
There are important caveats to the current study that can be minimized in future work. First, the older group used here is healthier than groups typically recruited in aging studies due to the comprehensive list of exclusion criteria. Participants with controlled high blood pressure and high cholesterol were excluded from this study to prevent medication-related impacts on vascular measures such as CBF and CVR. Because of this however, the results presented likely represent a lower bound on the importance of vascular parameters on SDBOLD_fix. Less healthy cohorts could show a larger reduction of SDBOLD_fix after accounting for vascular parameters. Second, our finding that vascular factors cannot fully account for age differences in SDBOLD_fix demonstrated the impact of vascular effects in already comprehensively denoised data. It is thus possible that the impact of vascular parameters on attenuating age differences in SDBOLD_fix may have increased if our data were not already denoised. However, we wanted to test our primary research questions using a preprocessing pipeline typical of our past work in BOLD variability, which argued for careful denoising to optimize age and cognition-based effects1, 8, 9, 12. It is also far simpler to acquire standard fMRI data and denoise them than it is to also acquire gas inhalation-based hypercapnia dataset for any study of interest. In the current study, we did both.
Third, the post-label delay utilized in the present study (900 ms) is not optimal for older populations with slower blood flow, potentially leading to an underestimation of baseline CBF and possible errors in M estimation. These factors may be minimized in the present sample given their above-average health status. This may however contribute to differential relationships between CBF, M and SDBOLD_fix (it would not, however, have an impact on the relationship between CVR and SDBOLD_fix). Future studies could use multiple delay pCASL data to yield more accurate CBF and M estimates. While BOLD CVR is a sensitive marker of vascular aging, the M parameter is in theory the most relevant parameter for investigating the impact of declining vascular health on the amplitude of BOLD signal fluctuations (since it represents explicitly the BOLD dynamic range). Future studies could also use more recent BOLD calibration models45, 68, 79 to investigate these effects further by not only allowing measurement of vascular effects in BOLD, but also allowing one to account for the impact of resting metabolism. Such models help to better determine dynamic range given that BOLD signal amplitude depends partly on how much deoxyhemoglobin is present at rest.
Fourth, many papers examining vascular effects in BOLD fluctuation amplitudes have utilized resting-state data14, 33, 34, whereas we have examined fixation block data as a proxy for resting state. Although it has been argued that fixation block data (from a broader block design study) may not be a valid proxy for resting state given that external cognitive engagement may modify resting activity80–82, there has been no evidence to date that such an effect exists for BOLD variability measures; in fact, ongoing work in our group suggests that pre- and post-task resting-state BOLD variability, and fixation block-based BOLD variability, are all very highly correlated and show very similar spatial patterns in relation to aging (Grady and Garrett, under review). Finally, future studies could examine the extent to which age- and cognition-related differences in artifact-free BOLD signal variability (in particular, independent of hypercapnia-based vascular effects) correlates with artifact-free EEG/MEG signal variability (e.g., power, entropy, dimensionality). This would permit a more comprehensive assessment of the neural basis of age- and cognition-related differences in brain signal dynamics14. Interestingly however, increasing work on the vascular basis of EEG/MEG suggests that hypercapnia may impact some aspects of electrical brain signals and their associated brain states in healthy young adults83, 84. Thus, future multi-modal assessment of brain dynamics may benefit from covarying hypercapnia-based vascular effects from both BOLD and electrical signals prior to examining age differences in brain dynamics to avoid biasing model results.
Conclusion
Overall, our results demonstrated that brain regions in which younger adults expressed higher SDBOLD_fix remained robust after accounting for vascular effects1, 12, 13. Our findings thus further establish and promote the principled study of aging-related BOLD dynamics.
Acknowledgements
This work was supported by the Max Planck UCL Centre for Computational Psychiatry and Ageing Research (DDG, UL), an Emmy Nöther group grant from the German Research Foundation (DDG), and CIHR (RDH, CJG).
Author Contributions
D.D.G., R.D.H., and C.J.G. designed research and formulated research hypotheses. D.D.G., R.D.H., and C.J.G. contributed new analytic tools. D.D.G. and C.J.G. analyzed data; and D.D.G., U.L., R.D.H., and C.J.G. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garrett DD, et al. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neuroscience & Biobehavioral Reviews. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balsters, J. H., Robertson, I. H. & Calhoun, V. D. BOLD Frequency Power Indexes Working Memory Performance. Front Hum Neurosci7 (2013). [DOI] [PMC free article] [PubMed]
- 3.Burzynska AZ, et al. White Matter Integrity Supports BOLD Signal Variability and Cognitive Performance in the Aging Human Brain. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0120315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He BJ. Spontaneous and Task-Evoked Brain Activity Negatively Interact. J. Neurosci. 2013;33:4672–4682. doi: 10.1523/JNEUROSCI.2922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mišić B, et al. Coordinated Information Generation and Mental Flexibility: Large-Scale Network Disruption in Children with Autism. Cereb. Cortex. 2015;25:2815–2827. doi: 10.1093/cercor/bhu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswal BB, et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett D, Kovacevic N, McIntosh AR, Grady CL. Blood Oxygen Level-Dependent Signal Variability Is More than Just Noise. J. Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett D, Kovacevic N, McIntosh AR, Grady CL. The Importance of Being Variable. J. Neurosci. 2011;31:4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett D, Kovacevic N, McIntosh AR, Grady CL. The Modulation of BOLD Variability between Cognitive States Varies by Age and Processing Speed. Cereb. Cortex. 2013;23:684–693. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guitart-Masip M, et al. BOLD Variability is Related to Dopaminergic Neurotransmission and Cognitive Aging. Cereb. Cortex. 2016;26:2074–2083. doi: 10.1093/cercor/bhv029. [DOI] [PubMed] [Google Scholar]
- 12.Garrett DD, et al. Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc. Natl. Acad. Sci. USA. 2015;112:7593–7598. doi: 10.1073/pnas.1504090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady CL, Garrett DD. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging and Behavior. 2013;8:274–283. doi: 10.1007/s11682-013-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsvetanov KA, et al. The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Hum. Brain Mapp. 2015;36:2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown WR, Thore CR. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and Applied Neurobiology. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke MF, Hashimoto J. Mechanical Factors in Arterial Aging. Journal of the American College of Cardiology. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier CJ, et al. Age dependence of hemodynamic response characteristics in human functional magnetic resonance imaging. Neurobiology of Aging. 2013;34:1469–1485. doi: 10.1016/j.neurobiolaging.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann CF, Smith SM. Probabilistic Independent Component Analysis for Functional Magnetic Resonance Imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 19.Churchill NW, Strother SC. PHYCAA+: An optimized, adaptive procedure for measuring and controlling physiological noise in BOLD fMRI. NeuroImage. 2013;82:306–325. doi: 10.1016/j.neuroimage.2013.05.102. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, et al. Alterations in Cerebral Metabolic Rate and Blood Supply across the Adult Lifespan. Cereb. Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, et al. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. NeuroImage. 2013;78:415–425. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoge RD, et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magn Reson Med. 1999;42:849–863. doi: 10.1002/(SICI)1522-2594(199911)42:5<849::AID-MRM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Hoge RD, et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl. Acad. Sci. USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito H, Kanno I, Ibaraki M, Suhara T, Miura S. Relationship between baseline cerebral blood flow and vascular responses to changes in PaCO 2measured by positron emission tomography in humans: implication of inter-individual variations of cerebral vascular tone. Acta Physiologica. 2008;193:325–330. doi: 10.1111/j.1748-1716.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 26.Mark CI, et al. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn. Reson. Med. 2010;64:749–756. doi: 10.1002/mrm.22405. [DOI] [PubMed] [Google Scholar]
- 27.Tancredi FB, et al. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2-induced cerebrovascular reactivity. J. Magn. Reson. Imaging. 2012;36:312–321. doi: 10.1002/jmri.23658. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier CJ, Hoge RD. A generalized procedure for calibrated MRI incorporating hyperoxia and hypercapnia. Hum. Brain Mapp. 2012;34:1053–1069. doi: 10.1002/hbm.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vis JB, et al. Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. Journal of Cerebral Blood Flow & Metabolism. 2015;35:1015–1023. doi: 10.1038/jcbfm.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marstrand JR, et al. Cerebral Perfusion and Cerebrovascular Reactivity Are Reduced in White Matter Hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.STR.0000012808.81667.4B. [DOI] [PubMed] [Google Scholar]
- 31.Girouard H. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. Journal of Applied Physiology. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 32.De Vis JB, et al. Age-related changes in brain hemodynamics; A calibrated MRI study. Hum. Brain Mapp. 2015;36:3973–3987. doi: 10.1002/hbm.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu P, et al. A comparison of physiologic modulators of fMRI signals. Hum. Brain Mapp. 2013;34:2078–2088. doi: 10.1002/hbm.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di X, Kannurpatti SS, Rypma B, Biswal BB. Calibrating BOLD fMRI Activations with Neurovascular and Anatomical Constraints. Cereb. Cortex. 2013;23:255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Griswold MA, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 37.Wahlund LO, et al. A New Rating Scale for Age-Related White Matter Changes Applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.STR.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 38.Wu W-C, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn. Reson. Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 39.Slessarev M, et al. Prospective targeting and control of end-tidal CO 2and O 2concentrations. J. Physiol. (Lond.) 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respiration Physiology. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-X. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. NeuroImage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier CJ, Desjardins-Crépeau L, Madjar C, Bherer L, Hoge RD. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. NeuroImage. 2012;63:1353–1363. doi: 10.1016/j.neuroimage.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO2 using a generalized calibration model for hypercapnia and hyperoxia. NeuroImage. 2012;60:1212–1225. doi: 10.1016/j.neuroimage.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 46.Birn RM. The role of physiological noise in resting-state functional connectivity. NeuroImage. 2012;62:864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Smith AM, et al. Investigation of Low Frequency Drift in fMRI Signal. NeuroImage. 1999;9:526–533. doi: 10.1006/nimg.1999.0435. [DOI] [PubMed] [Google Scholar]
- 48.Garrett D, McIntosh AR, Grady CL. Brain Signal Variability is Parametrically Modifiable. Cereb. Cortex. 2014;24:2931–2940. doi: 10.1093/cercor/bht150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bright MG, Murphy K. Is fMRI ‘noise’ really noise? Resting state nuisance regressors remove variance with network structure. NeuroImage. 2015;114:158–169. doi: 10.1016/j.neuroimage.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrett DD, McIntosh AR, Grady CL. Moment-to-moment signal variability in the human brain can inform models of stochastic facilitation now. Nat Rev Neurosci. 2011;12:612–612. doi: 10.1038/nrn3061-c1. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 52.Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. NeuroImage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. Journal of Cerebral Blood Flow & Metabolism. 2010;30:1094–1099. doi: 10.1038/jcbfm.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ances BM, et al. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum. Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohtasib RS, et al. Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. NeuroImage. 2012;59:1143–1151. doi: 10.1016/j.neuroimage.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 56.Glover GH. Deconvolution of Impulse Response in Event-Related BOLD fMRI1. NeuroImage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, et al. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 58.Forbes ML, et al. Assessment of Cerebral Blood Flow and CO2 Reactivity After Controlled Cortical Impact By Perfusion Magnetic Resonance Imaging Using Arterial Spin-Labeling in Rats. Journal of Cerebral Blood Flow & Metabolism. 1997;17:865–874. doi: 10.1097/00004647-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Graham GD, et al. BOLD MRI monitoring of changes in cerebral perfusion induced by acetazolamide and hypercarbia in the rat. Magn. Reson. Med. 1994;31:557–560. doi: 10.1002/mrm.1910310514. [DOI] [PubMed] [Google Scholar]
- 60.Chen JJ, Pike GB. MRI measurement of the BOLD-specific flow–volume relationship during hypercapnia and hypocapnia in humans. NeuroImage. 2010;53:383–391. doi: 10.1016/j.neuroimage.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Boxerman JL, et al. The intravascular contribution to fmri signal change: monte carlo modeling and diffusion-weighted studiesin vivo. Magn. Reson. Med. 1995;34:4–10. doi: 10.1002/mrm.1910340103. [DOI] [PubMed] [Google Scholar]
- 62.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial Pattern Analysis of Functional Brain Images Using Partial Least Squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 64.Efron, B. & Tibshirani, R. An introduction to the bootstrap. (Chapman & Hall/CRC, 1993).
- 65.Eickhoff SB, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 66.Eickhoff SB, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 67.Burzynska AZ, et al. A Scaffold for Efficiency in the Human Brain. J. Neurosci. 2013;33:17150–17159. doi: 10.1523/JNEUROSCI.1426-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wise RG, Harris AD, Stone AJ, Murphy K. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. NeuroImage. 2013;83:135–147. doi: 10.1016/j.neuroimage.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tancredi FB, Lajoie I, Hoge RD. Test-retest reliability of cerebral blood flow and blood oxygenation level-dependent responses to hypercapnia and hyperoxia using dual-echo pseudo-continuous arterial spin labeling and step changes in the fractional composition of inspired gases. J. Magn. Reson. Imaging. 2015;42:1144–1157. doi: 10.1002/jmri.24878. [DOI] [PubMed] [Google Scholar]
- 70.Handwerker DA, Gazzaley A, Inglis BA, D’Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum. Brain Mapp. 2007;28:846–859. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gauthier CJ, et al. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiology of Aging. 2015;36:304–314. doi: 10.1016/j.neurobiolaging.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glover GH, Li T-Q, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 74.Marx M, Pauly KB, Chang C. A novel approach for global noise reduction in resting-state fMRI: APPLECOR. NeuroImage. 2013;64:19–31. doi: 10.1016/j.neuroimage.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Golestani AM, Wei LL, Chen JJ. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: Validation in healthy adults. NeuroImage. 2016;138:147–163. doi: 10.1016/j.neuroimage.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lipp I, Murphy K, Caseras X, Wise RG. Agreement and repeatability of vascular reactivity estimates based on a breath-hold task and a resting state scan. NeuroImage. 2015;113:387–396. doi: 10.1016/j.neuroimage.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donahue MJ, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J. Magn. Reson. Imaging. 2013;38:1129–1139. doi: 10.1002/jmri.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brundel M, et al. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. Journal of Diabetes and its Complications. 2012;26:205–209. doi: 10.1016/j.jdiacomp.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Bulte DP, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. NeuroImage. 2012;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sami S, Miall RC. Graph network analysis of immediate motor-learning induced changes in resting state BOLD. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sami S, Robertson EM, Miall RC. The time course of task-specific memory consolidation effects in resting state networks. J. Neurosci. 2014;34:3982–3992. doi: 10.1523/JNEUROSCI.4341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohmann G, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall EL, et al. The effect of hypercapnia on resting and stimulus induced MEG signals. NeuroImage. 2011;58:1034–1043. doi: 10.1016/j.neuroimage.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 84.Wang D, et al. Comparing the effect of hypercapnia and hypoxia on the electroencephalogram during wakefulness. Clinical Neurophysiology. 2015;126:103–109. doi: 10.1016/j.clinph.2014.04.012. [DOI] [PubMed] [Google Scholar]