Abstract
Cyclooxygenase-2 inhibitors (coxibs) are characterized by multiple molecular off-target effects and increased coronary artery disease (CAD) risk. Here, we systematically explored common variants of genes representing molecular targets of coxibs for association with CAD. Given a broad spectrum of pleiotropic effects of coxibs, our intention was to narrow potential mechanisms affecting CAD risk as we hypothesized that the affected genes may also display genomic signals of coronary disease risk. A Drug Gene Interaction Database search identified 47 gene products to be affected by coxibs. We traced association signals in 200-kb regions surrounding these genes in 84,813 CAD cases and 202,543 controls. Based on a threshold of 1 × 10−5 (Bonferroni correction for 3131 haplotype blocks), four gene loci yielded significant associations. The lead SNPs were rs7270354 (MMP9), rs4888383 (BCAR1), rs6905288 (VEGFA1), and rs556321 (CACNA1E). By additional genotyping, rs7270354 at MMP9 and rs4888383 at BCAR1 also reached the established GWAS threshold for genome-wide significance. The findings demonstrate overlap of genes affected by coxibs and those mediating CAD risk and points to further mechanisms, which are potentially responsible for coxib-associated CAD risk. The novel approach furthermore suggests that genetic studies may be useful to explore the clinical relevance of off-target drug effects.
Introduction
Selective COX-2 inhibitors (coxibs) display non-steroidal anti-inflammatory effects, which are widely used to treat chronic pain syndromes. However, long-term administration of coxibs has been found consistently to increase risk of cardiovascular events including myocardial infarction and coronary death1. Given such severe adverse effects, their use is controversially discussed in the United States and Europe2. In fact, several coxibs, such as rofecoxib, have been withdrawn from the marked for that reason3.
The recently published PRECISION trial evaluated the cardiovascular safety of celecoxib and reported no increased risk as compared to two nonselective NSAID4. However, the results have been controversially discussed for a number of reasons5, 6. For example, almost 70% stopped taking the medication during the two years of follow-up, which was entirely lost in about 25% patients4. Moreover, celecoxib was somewhat less effective than its comparators; most likely due to the relatively low dose of celecoxib used in this trial (about 200 mg/day). By contrast, studies that have shown an increase of coronary risk used higher doses (400–800 mg). Perhaps most importantly, the variants identified in our study are located in genes that are downstream of COX2 and thus affected in a similar way by the three drugs tested. Hence, our data may be relevant for NSAID in general and add to the long standing discussion of the underlying mechanisms related to both intended (analgesic) and unintended (cardiovascular) effects.
Their principle mechanism of action is to selectively inhibit the cyclo-oxygenase 2 isoform (COX-2) to reduce prostaglandin I2 and prostacyclin pro-inflammatory effects. In contrast to non-selective Cox-inhibitors, coxibs do not lower COX-1 derived thromboxane production. Since thromboxane activates platelets, selective Cox-2 inhibitors may affect unfavorably the prostacyclin (antithrombotic)/thromboxane (prothrombotic) ratio, which may explain their thrombo- and atherogenicity, and their blood pressure increasing effects3. However, coxibs display numerous other (pleiotropic or off-target) effects, which likewise could add to the untoward safety profile of the drugs3. For example, coxibs may suppress NO production, which has been related to CAD risk by genetic means7. Moreover, other Cox-inhibitors not affecting the prostacyclin/thromboxane ratio may also increase coronary event rates2. Thus, the precise mechanisms explaining cardiovascular risks of coxibs are not proven definitively2.
Genetic variants affecting disease risk can facilitate identification of drug targets8, 9. Likewise, variants may point to potential adverse effects, if risk alleles and drugs have similar functional implications10, 11. Here, we reversed this approach and systematically explored known molecular targets of coxibs for signals in genome-wide association studies (GWAS) on CAD.
The starting point of our analysis was to identify genes or gene products reported in the Drug Gene Interaction Database 12 to interact with coxibs. Considering all genes interacting with coxibs we aimed to filter out those with the potential to affect the CAD risk related to these drugs specifically by searching respective genes for genomic signals indicating CAD risk. The underlying idea is that single nucleotide polymorphisms (SNPs) may mimic drug effects in disturbing the function or regulation of a gene product, and therefore – like the drug – associate with CAD risk, even if the effect sizes might vary. The enormous statistical power of contemporary genomic analyses was thereby used to scrutinize the spectrum of pleiotropic coxib effects for those that may contribute to the increased CAD risk of long-term users of these frequently prescribed drugs. Remarkably, the strategy also led to successful identification of novel variants genome-wide significantly associated with CAD risk.
Methods
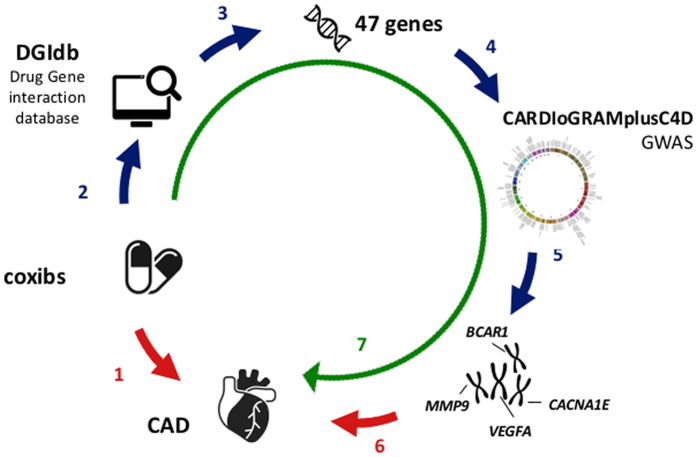
The principle approach taken in this study is illustrated in Fig. 1. The first step of the pipeline was to identify genes or gene products reported to interact with coxibs. For this we downloaded the Drug Gene Interaction Database version 2.22 (DGIdb)12. This database aims to capture genes that are known to be targeted by drugs. It is rather inclusive in that liberal criteria are used to allow entry in this database12. Thus, it may also include gene or gene products with relatively weak evidence for drug interaction. Despite this noise, we decided not to perform any manipulation on the database to avoid a bias introduced by a priori hypothesis. With this strategy, we expect that at least some of the genes reported in the database reflect true interactions which are interesting for genetic interrogation of coxib-related cardiovascular side effects.
Figure 1.
Experimental Strategy: 1. Cox 2 inhibitors (coxibs) are known to increase coronary risk. 2. All genes known to be targets of coxibs were extracted from the Drug-Gene Interaction Database (DGIdb). 3. DGIdb revealed 47 genes that interact with coxibs. 4. All common variants at the chromosomal regions representing the 47 genes were subjected to a large-scale association study. 5. Four genes displayed significance for association with CAD risk. 6. These genes are candidate risk genes for CAD. 7. It may be hypothesized that the genes affected by coxibs and here shown to associate with CAD risk participate in the adverse effects of the drugs. Some drawings were obtained and used under license from Shutterstock.com (https://www.iconfinder.com/licenses/basic). Images can be found under: https://www.shutterstock.com/image-vector/chromosomes-vector-icon-style-flat-symbol-323629910?src=library, https://www.shutterstock.com/image-vector/organ-heart-icon-310580756, https://www.shutterstock.com/image-vector/dna-icon-397249525, https://www.shutterstock.com/image-vector/pills-medication-vector-icons-set-131402543?src=library, https://www.iconfinder.com/icons/240302/find_computer_find_desktop_look_for_desktop_search_search_desktop_icon#size=128.
We included all genes reported to interact with celecoxib, etoricoxib, lumiracoxib, parecoxib, rofecoxib, and valdecoxib. The genes reported in DGIdb are shown in supplementary Table 2. This table also shows the complete output of the DGIdb query. We identified all SNPs within 200-kb surrounding the genes whose products are affected by these drugs. The 200-kb window was selected to capture most genetic variants with potential regulatory effects.
GWAS datasets
Having identified the drug gene interactions, we subsequently screened respective genes for association signals in the CARDIoGRAMplusC4D 1000 G GWAS meta-analysis on CAD13 and additional non-overlapping datasets from CARDIoGRAM14 and German MI Family Studies V (GerMIFS V). The CARDIoGRAMplusC4D 1000Genomes meta-analysis data set consists of 47 GWAS studies including data from 60,801 cases and 123,504 controls. The GWAS are imputed with the 1000Genomes phase I integrated haplotypes from December 2012 (ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20110521/) (for Methods see Nikpay et al.13).
We combined this data set13 with GWAS from CHARGE15, deCODE CAD16, CADomics14, DILGOM17, EPIC7, FRISC II GLACIER18, METISM19, MORGAM FIN20, MORGAM FRA20, MORGAM GER20, MORGAM ITA20, MORGAM UNK20, PMB7, PopGen21, SCARF SHEEP7, and STR22 that have been previously reported in references Schunkert et al.14 and/or Deloukas et al.7. Moreover, we included data from GWAS not reported before, i.e. GerMIFS V. See detailed information in supplementary Table 1. In total, this combined dataset consists of 84,813 cases and 202,543 controls.
The SNPs identified in the first stage of analysis were genotyped in additional 2,496 cases and 1,505 controls from the GerMIFS VI study (unpublished).
Statistical Methods
In the exploratory analysis of SNPs, we assumed significance for association with CAD after Bonferroni correction for the number of independent SNPs tested. We calculated the number of independent SNPs based on the linkage disequilibrium (LD) between SNPs. This method is analogue to the one proposed by Nyholt23. Nyholt et al. proposed a method to correct for multiple testing based on pairwise LD between SNPs. Because Nyholts approach, which uses spectral decomposition of pairwise LD matrices, is computationally demanding, we correct using the number of haplotype blocks. The number of independent SNPs was estimated based on the number of haplotype blocks calculated with PLINK software using the default setting of the blocks command. We calculated the haplotype blocks using the GerMIFS IV study. We do not have individual genotype data from all studies but expect this GWAS to be representative. For a total of 81,703 SNPs within the 47 gene regions tested, we identified 3,131 haplotype blocks and defined significance according to Bonferroni at a p-value threshold of 1 × 10–5.
Meta-analysis
Logistic regression, assuming an additive model, was performed on all single study data. We performed logistic regression with all genotype data available. Individuals with no genotype data or with poor quality genotype data were excluded from regression analysis. All analyses were adjusted for sex and age. Age was defined as the recruitment age for controls and the event age for cases. For meta-analysis, we calculated an ‘inverse variance weighting’- fixed-effects and a random effects model24, depending on the heterogeneity between the studies. For heterogeneity calculation Cochran’s Q was used. Threshold for heterogeneity was phet <0.01. We combined the CARDIoGRAMplusC4D 1000Genomes meta-analysis data set and non-overlapping CARDIoGRAM, GerMIFS V, and GerMIFS VI studies using an ‘inverse variance weighting’-fixed-effects model and combined effects and p-values were reported. In total we evaluated the genomic data from 87,309 CAD patients and 204,048 controls.
Functional annotation of SNPs and genes
To evaluate the functional implication of the SNPs, we identified all SNPs with an LD of r2 > 0.8 to the locus lead SNP using the HaploReg version 3 database25. We then used the Cardiogenics data set26 for a systematic search for expression quantitative trait loci (eQTLs) affecting monocyte and macrophage expression, the publicly available data from Westra et al.27, GTeX28 and over 100 studies included in the Genome-Wide Repository of Associations between SNPs and Phenotypes (GRASP) database29. In addition, we used HaploReg25 to locate SNPs in promoter and enhancer regions and performed a literature search for gene functions using Pubmed.
Identification of pleiotropic cardiovascular effects of SNPs
We used the GRASP database to screen for association signals for CAD-related phenotypes at the four loci displaying association with CAD. The GRASP database contains the association results of around 1,390 GWAS29 and is the most comprehensive GWAS database. We searched for reported associations with CAD-related traits in the GRASP database for all SNPs with LD of r2 > 0.8 with the lead SNPs. We used p < 1 × 10−3 for CAD-related traits to avoid false positive associations.
Test for enrichment
To test for enrichment, we downloaded the GSEA curated pathways database (MSigDB version c2.cp.v5.1)30 which comprises a total of 1330 pathways. We compared the number of eSNPs displaying a significant effect – after the Bonferroni correction p < 1 × 10−5 as used in our main analysis – for CAD risk in these pathways with respective eSNPs within the coxib gene subset using the Fisher exact test. SNPs were annotated the using the publicly available eQTL databases listed above.
Results
We identified in the publicly available Drug Gene Interaction Database 47 genes or gene products to interact with coxibs12 (supplementary Table 2). We studied the 200-kb region surrounding respective genes in a combined 1000Genomes imputed meta-analysis (data from CARDIoGRAM and CARDIoGRAMplusC4D) to search for SNPs showing association with CAD13. Correcting for multiple testing accounting for haplotype blocks, we considered SNPs significant with a p-value lower than 1 × 10−5. In this way, we revealed a total of four loci associated with CAD (Table 1 and Fig. 2 and supplement Table 3).
Table 1.
Association signals for CAD within 200 kb of genes reported for coxib/gene product interactions.
| Gene | Lead SNP | CAD risk allele | Frequency risk allele | OR risk allele | P-value | P-value combined analysis | Relationship of coxib to gene/gene product* | Regulatory effect (Histone marks) |
|---|---|---|---|---|---|---|---|---|
| BCAR1 | rs4888383 | T | 0.57 | 1.05 | 7.99*10−08 | 2.98*10−08 | inhibition | Promoter/Enhancer |
| MMP9 | rs7270354 | A | 0.15 | 1.06 | 6.75*10−08 | 3.34*10−08 | decreased expression | Promoter/Enhancer |
| VEGFA | rs6905288 | A | 0.60 | 1.04 | 7.44*10−07 | 8.86*10−07 | decreased expression | Enhancer |
| CACNA1E | rs556321 | C | 0.16 | 1.05 | 8.26*10−06 | 8.85*10−06 | inhibition | Promoter/Enhancer |
For each gene, the lead SNP is shown. The regulatory effects were annotated using Haploreg25.
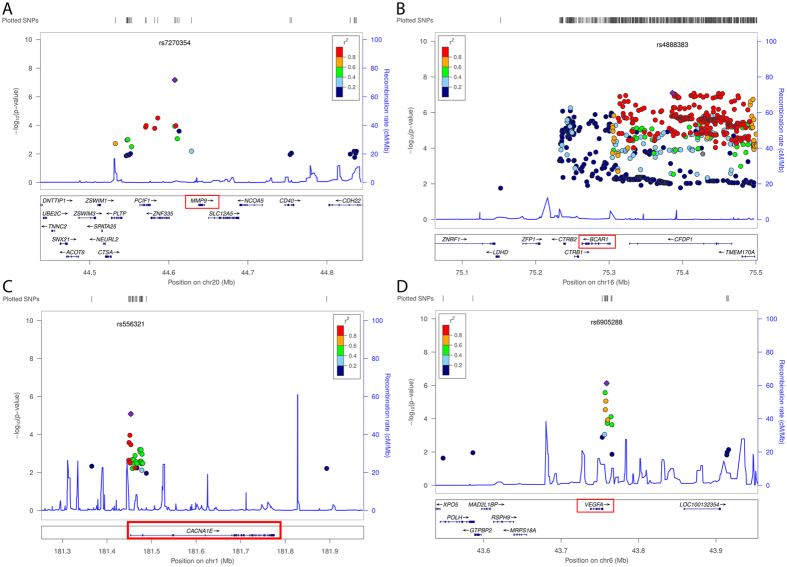
Figure 2.
Association signals for at the chromosomal regions of coxib genes (A) MMP9, (B) BCAR1, (C) CACNA1E, and (D) VEGFA using Locus Zoom53.
In the coxib pathway we identified 43 genes out of which 3 (or 7%) were found to have eSNPs displaying a significant effect – after Bonferroni correction p < 1 × 10−5 – for CAD risk. For comparison, in GSEA curated pathways out of 7076 genes only 1.5% of genes had respective eSNPs with a comparable signal for CAD risk. Hence, the set of genes on which our approach was focused was significantly enriched – by Fisher exact p-value of 0.03 – for genes with an eSNPs showing a signal for CAD.
The genes affected by coxibs and located within the loci showing SNP association for CAD are the vascular endothelial growth factor A (VEGFA), matrix metalloproteinase-9 (MMP9), breast cancer anti-estrogen resistance protein 1 (BCAR1) and the calcium channel, voltage-dependent, R type, alpha 1E subunit (CACNA1E). For the lead SNPs tagging these genes, we performed additional genotyping of 2,496 cases and 1,505 controls.
The A allele of the VEGFA lead SNP, rs6905288, is associated with CAD with a p-value of p = 8.86 × 10−7 (OR 1.04) in our data. In the GRASP database, this SNP shows genome-wide significant association with waist hip ratio and suggestive association with HDL cholesterol level, triglycerides, and systolic and diastolic blood pressure, respectively (for references and further information please see the supplement Table 4). The lead SNP is found in enhancer regions, and is associated with a decreased expression of VEGFA in adipose tissue31.
The A allele of the MMP9 lead SNP, rs7270354, is located upstream of the gene and is genome-wide significantly associated with CAD (p = 3.34 × 10−8, OR 1.06). The lead SNP and linkage disequilibrium (LD) SNPs are found in enhancer regions in several cell types. However, neither this SNP nor a SNP in LD (r2 > 0.8) is associated with another CAD related phenotype in GRASP. The CAD risk allele is associated with increased expression of MMP9 in whole blood27. The effect of the CAD risk allele is opposite of the effect of coxibs on the gene. However, it is well established that SNPs showing eQTL effects, can have opposite effect in different tissues32. Hence, the identified eQTL effect demonstrates the regulatory effect of the SNP on MMP9. Further studies are needed to identify the effect of this SNP in CAD relevant tissue as for instance smooth muscle cells.
Lead SNP, rs4888383, is located upstream of the gene BCAR1 and shows genome-wide significant association with CAD (p = 2.98 × 10−8, OR T 1.05). SNP rs4888383 is located in a potentially functional promoter region, with the CAD risk allele (T), and SNPs in high LD (r2 > 0.8), being linked to reduced expression of BCAR1 in the esophagus mucosa (GTeX). The LD SNP rs4888378, was previously associated with carotid intima media thickness (p = 6.5 × 10−7) and CAD (p = 6.53 × 10−6). In addition, the lead SNP shows suggestive association with systolic and pulse pressure, urinary albumin to creatinine ratio, and microalbuminuria (for references and further information please see supplementary Table 4).
The C allele of the CACNA1E lead SNP, rs556321, and several LD SNPs span the promoter of the gene. It is associated with CAD with a p-value of p = 8.85 × 10−6 (OR 1.05). Genetic variants in CACNA1E are reported to play a role in hypertension. LD SNPs of rs556321 are associated with blood metabolite concentrations and obesity. The CAD risk allele is associated with decreased expression of CACNA1E in blood (for references and further information see supplementary Table 4).
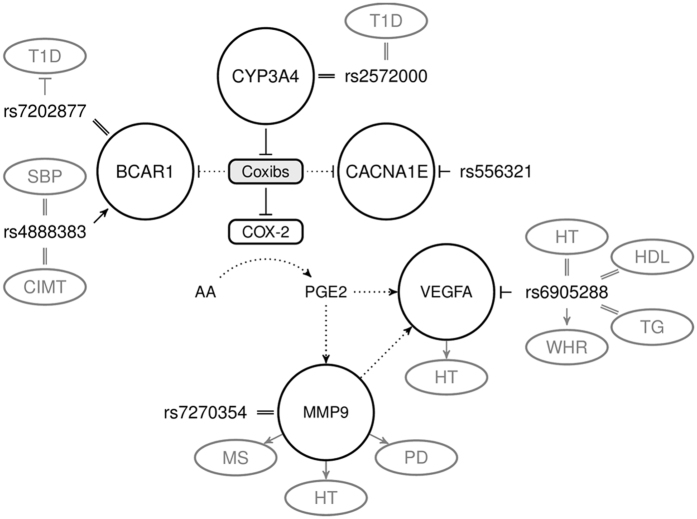
Figure 3 integrates the four genes in the functional pathways affected by coxib administration. It illustrates that VEGFA and MMP9 are downstream of the principle mechanism of COX-2 inhibition and thus may represent a class effect. BCAR1 and CACNA1E are not related to the intended pharmacological action and may thus represent off-target effects.
Figure 3.
Pharmacodynamic effects of coxib treatment related to the genetic association signals. All SNPs and their functional implication are shown based on the CAD risk allele. The rs numbers indicate the lead SNPs and their hypothesized function. rs6905288 is strongly associated with waist-to-hip-ratio and rs7202877 with type 1 diabetes (T1D). Suggestive associations were reported for rs4888383 with carotid intima media thickness (CIMT) and systolic blood pressure (SBP), for rs6905288 with hypertension (HTN), HDL-cholesterol, and triglycerides (TG), and for rs556321 with obesity (OB) and blood metabolites (BM). CAD risk allele rs556321 is associated with decreased expression of CACNA1E, rs4888383 is associated with decreased expression of BCAR1 and rs6905288 is associated with decreased expression of VEGFA. Functional links: ↑ (induction), ┬ (inhibition), ║(unknown effect). Dotted lines indicate potential intermediate functional link. AA: arachidonic acid, COX-2: Cyclooxigenase-2, PGE2: prostaglandin E2, VEGFA: vascular endothelial growth factor A, MMP9: matrix metalloproteinase-9, BCAR1: breast cancer anti-estrogen resistance protein 1, CACNA1E: calcium channel, voltage-dependent, R type, alpha 1E subunit. PD: Plaque disruption, MS = Metabolic Syndrome, T1D: type 1 diabetes, CIMT: carotid intima media thickness, SBP: systolic blood pressure, HTN: hypertension, HDL: HDL-cholesterol, TG: triglycerides, OB: obesity, BM: blood metabolites. For references see supplement.
Discussion
A data-base on drug-gene interactions lists 47 genes or gene products to be affected by administration of coxibs12, a class of drugs known for prominent coronary side effects. Analysis of the chromosomal loci harboring respective genes identified two novel loci with genome-wide significant association and two loci with robust association signals for CAD. In conjunction, it appears that coxibs affect several gene products that also play a role in modulating genetic risk of CAD. With respect to the pharmacological profile of coxibs, these data point to potential novel mechanisms for the increased myocardial infarction risk observed in long-term users of this medication.
The reason for the multitude of genes affected by inhibition of COX-2 relates in part to the variety of downstream signaling events caused by reduction of prostaglandin synthesis. VEGFA and MMP9 may be involved via this primary drug effect. BCAR1 may be affected by coxibs via other, pleiotropic mechanisms, but – like VEGFA and MMP9 – this gene has been studied functionally in the context of atherosclerosis before33–36. Thus, detection of robust genetic signals for association with CAD within these genes appears to be plausible.
Celecoxib is reported to suppress the expression of VEGFA 37. VEGFA is an important signaling molecule that mediates vasculogenesis, angiogenesis, and vascular maintenance38. VEGFA furthermore augments NO levels and, hence, may ameliorate platelet aggregation and vasoconstriction39. Inhibition of VEGFA is reported to lead to hypertension35. The lead SNP at the VEGFA locus showed multiple associations with coronary risk factors further corroborating its role in CAD.
Celecoxib is reported to inhibit the production of MMP9 in several cell types40. MMP9 is a zinc-dependent endopeptidase with tissue-specific expression known to up-regulate VEGFA34, 41. On the other hand, higher levels of MMP9 have been reported in patients with CAD33 and metabolic syndrome42. In addition, MMP9 has been linked with hypertension43, arterial stiffness43 and is reported to induce acute plaque disruption44. Further, MMP activation has been related to decreased aortic elasticity in mice via prostaglandin E2 receptor signaling45.
Celecoxib is reported to inhibit BCAR146 which belongs to the CAS family of scaffolding proteins substrate and is often linked to several forms of cancer47. In addition, the gene is reported to be associated with focal adhesions, regulation of smooth muscle cell migration, carotid intima media thickness and CAD48. It appears to be activated downstream through the angiotensin II pathway49. Interestingly, BCAR1 overexpression has been associated with celecoxib resistance in colorectal cancer46.
Celecoxib is reported to inhibit L-type calcium channels in a COX-2 independent pathway50. CACNA1E mediates the cell surface membrane potential and affects several cellular processes such as contraction and gene expression51. The lead SNP at the CACNA1E locus showed associations with other coronary risk factors. CACNA1E inhibition is also reported to lead to hypertension36 and hence, as VEGFA, is a strong candidate to explain the CAD risk of coxibs. The CAD risk SNP representing the CACAN1E locus is associated with reduced expression of the gene, which matches with the direction of effect reported for coxibs.
Taken together, our data indicate mechanistic parallelisms between chronic intake of coxibs and genetic variants affecting CAD risk, both being diverse. In line with our principle observations, we also find a large overlap of coxib related side effects and the traits related to the SNPs (see supplement Table 5). The four genes, identified in this study to associate genetically with CAD risk, are all reported to interact with celecoxib in the DGIdb. The other coxibs, however, might also alter these genes, without being studied in detail so far. Especially MMP9 and VEGFA, downstream of COX-2, are likely to be affected by the other coxibs.
On the cellular level, coxibs have been implicated to modulate angiogenesis, inflammation, cell cycle arrest, and apoptosis52, as well as focal adhesions and vascular tone50. The interaction between coxibs and the genes associated with CAD by our investigation may reinforce the implications of some of the adverse mechanisms affected by the drugs. Indeed, given the association findings reported in this study it appears plausible that the increased CAD risk is also mediated by mechanisms other than disturbing the prostacyclin/thromboxane ratio. While VEGFA and MMP9 are both downstream COX-2 inhibition, BCAR1 and CACNA1E are affected presumably through independent pathways.
In this work, we defined the risk alleles as those found more frequently in CAD cases. The definition is not based on functional testing and defining the allele more frequently found in controls as protective would be equally valid. However, exploring publically available data bases we observed genotype specific differences in gene expression which might relate to the mechanistic effects. We assigned the SNPs to VEGFA, MMP9, BCAR1, and CACNA1E because these were the genes altered by coxibs and because the SNPs are eQTLs for the respective genes. It is, however, possible that the SNPs have effects on other genes as well. Indeed, we have previously demonstrated that regulatory SNPs influence the expression of multiple genes32. Hence, we do not exclude other genes to mediate the effect on CAD for the identified loci.
Our study design has several limitations. All our findings are based on associations rather than functional testing. Here we did not test formally whether the effects of coxibs and risk alleles on respective gene products have identical directionality. This may be particularly evident, as we cannot always derive the directionality of the SNPs on expression levels of the genes that are considered as intermediary phenotypes. However, functional tests reported in the literature, as described above, are consistent with the genetic evidence provided here. Finally, we have no information in the large CARDIoGRAMplusC4D database on the intake of pain relieving medication prior to the manifestation of coronary disease. Since the association is depending on the genotype, drugs or other confounding factors are unlikely to inflate the findings. To have an effect on the association, the administration or the metabolism of the drug has to also depend on the genotype. We cannot completely exclude a pleiotropic effect, but we consider it to be very unlikely. Given the conclusive nature of the association findings, first between coxibs and CAD risk2, second between coxibs and respective gene products12, and third, between genetic variants at respective loci and CAD risk reported here, the data are highly plausible. Particularly, the strength of association with genome-wide significance for some of the gene variants may be considered to be sufficient even in a hypothesis-free analysis.
Taken together, pleiotropic or off-target effects are responsible for some adverse effects of drugs. Studying all genes known to be affected by coxibs, a class of drugs related to a prominent coronary risk, we have identified genomic mediators of CAD risk. The effects of coxibs on these genes might contribute to the unsatisfactory safety profile of this frequently used medication. Last, by exploring the mechanisms underlying the coxibs adverse effects, we revealed genome-wide significant associations for increased risk CAD at two genomic loci.
Electronic supplementary material
Acknowledgements
We thank the CARDIoGRAM and CARDIoGRAMplusC4D Consortium for contributing GWAS datasets. The study is supported by the German Federal Ministry of Education and Research (BMBF) in the context of the e:Med program (e:AtheroSysMed and sysINFLAME) and the FP7 European Union project CVgenes@target (261123). Further grants were received by the Universität zu Lübeck, and by the Fondation Leducq (CADgenomics: Understanding Coronary Artery Disease Genes, 12CVD02). This study was also supported through the Deutsche Forschungsgemeinschaft (DFG) cluster of excellence ‘Inflammation at Interfaces (I.B.) and SFB 1123. T.K. was supported by a DZHK Rotation Grant. NJS holds a chair funded by the British Heart Foundation and is a NIHR Senior Investigator. Folkert W. Asselbergs is supported by a Dekker scholarship-Junior Staff Member 2014T001 – Netherlands Heart Foundation and UCL Hospitals NIHR Biomedical Research Centre. PWF reports grants from Sanofi Aventis, grants from Lilly, grants from Novo nordisk, personal fees from Sanofi Aventis, personal fees from Lilly. LW reports institutional research grants, consultancy fees, lecutre fees, and travel support from AstraZeneca, institutional research grants, consultancy fees, lecutre fees, and travel support from Boehringer Ingelheim, institutional research grants, consultancy fees, lecutre fees, and travel support from Bristol-Myers Squibb/Pfizer, grants from Merck & Co, grants from Roche, consultancy fees from Abbott and holds two patents involving GDF-15.
Author Contributions
I.B., H.S., J.E. wrote the main manuscript. I.B. and B.R. prepared the figures. I.B., J.E., H.S. designed the study. I.B., C.W. performed the main analysis. B.R., M.K., V.T., and L.Z. helped with the analyses. S.v.A. and T.K. genotyped additional SNPs. C.J.W., M.L., P.S.W., T.Z., L.W., P.W.F., V.S., A.D., T.M., N.J.S., F.W.A., J.E., and H.S. contributed data. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10928-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schmidt M, et al. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J. 2016;37:1015–1023. doi: 10.1093/eurheartj/ehv505. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Cannon PJ. Physiology. COX-2 inhibitors and cardiovascular risk. Science. 2012;336:1386–1387. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N Engl J Med. 2016;375:2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT. Safety of Nonsteroidal Antiinflammatory Drugs. N Engl J Med. 2016;375:2595–2596. doi: 10.1056/NEJMe1614257. [DOI] [PubMed] [Google Scholar]
- 6.Nurmohamed, M. T. Therapy: Cardiovascular safety of celecoxib, naproxen and ibuprofen. Nat Rev Rheumatol, doi:10.1038/nrrheum.2017.4 (2017). [DOI] [PubMed]
- 7.CARDIoGRAMplusC4D-Consortium et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 45, 25–33, doi:10.1038/ng.2480 (2013). [DOI] [PMC free article] [PubMed]
- 8.Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 9.Tg, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schunkert H. Brotherhood of genetics and preventive medication. Eur Heart J. 2015;36:1566–1568. doi: 10.1093/eurheartj/ehv153. [DOI] [PubMed] [Google Scholar]
- 11.Ross S, et al. Association of cyclooxygenase-2 genetic variant with cardiovascular disease. Eur Heart J. 2014;35:2242–2248a. doi: 10.1093/eurheartj/ehu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith M, et al. DGIdb: mining the druggable genome. Nat Methods. 2013;10:1209–1210. doi: 10.1038/nmeth.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium CAD. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015 doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psaty BM, Sitlani C. The Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium as a model of collaborative science. Epidemiology. 2013;24:346–348. doi: 10.1097/EDE.0b013e31828b2cbb. [DOI] [PubMed] [Google Scholar]
- 16.Helgadottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 17.Inouye M, et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol Syst Biol. 2010;6 doi: 10.1038/msb.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renstrom F, et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes. 2011;60:345–354. doi: 10.2337/db10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stancakova A, et al. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes. 2009;58:1212–1221. doi: 10.2337/db08-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans A, et al. MORGAM (an international pooling of cardiovascular cohorts) Int J Epidemiol. 2005;34:21–27. doi: 10.1093/ije/dyh327. [DOI] [PubMed] [Google Scholar]
- 21.Schunkert H, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117:1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong Y, Pedersen NL, Brismar K, de Faire U. Genetic and environmental architecture of the features of the insulin-resistance syndrome. Am J Hum Genet. 1997;60:143–152. [PMC free article] [PubMed] [Google Scholar]
- 23.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotival M, et al. Integrating genome-wide genetic variations and monocyte expression data reveals trans-regulated gene modules in humans. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westra HJ, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium GT. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie R, O’Donnell CJ, Johnson AD. GRASP: analysis of genotype-phenotype results from 1390 genome-wide association studies and corresponding open access database. Bioinformatics. 2014;30:i185–194. doi: 10.1093/bioinformatics/btu273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberzon A, et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Civelek M, et al. Genetic Regulation of Adipose Gene Expression and Cardio-Metabolic Traits. Am J Hum Genet. 2017;100:428–443. doi: 10.1016/j.ajhg.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braenne I, et al. Prediction of Causal Candidate Genes in Coronary Artery Disease Loci. Arterioscler Thromb Vasc Biol. 2015;35:2207–2217. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferroni P, et al. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J Investig Med. 2003;51:295–300. doi: 10.1136/jim-51-05-17. [DOI] [PubMed] [Google Scholar]
- 34.Hoeben A, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 35.Granger JP. Vascular endothelial growth factor inhibitors and hypertension: a central role for the kidney and endothelial factors? Hypertension. 2009;54:465–467. doi: 10.1161/HYPERTENSIONAHA.109.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi K, et al. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res. 2007;100:342–353. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- 37.Wei D, et al. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.CAN-03-1945. [DOI] [PubMed] [Google Scholar]
- 38.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 39.Thijs AM, et al. Role of endogenous vascular endothelial growth factor in endothelium-dependent vasodilation in humans. Hypertension. 2013;61:1060–1065. doi: 10.1161/HYPERTENSIONAHA.111.00841. [DOI] [PubMed] [Google Scholar]
- 40.Hu M, et al. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 42.Yadav SS, et al. High serum level of matrix metalloproteinase 9 and promoter polymorphism − 1562 C:T as a new risk factor for metabolic syndrome. DNA Cell Biol. 2014;33:816–822. doi: 10.1089/dna.2014.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasmin, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25 doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 44.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichikawa, Y., Yokoyama, U. & Ishikawa, Y. Prostaglandin E2 receptor EP4 signaling in vascular smooth muscle cells decreases aortic elasticity (1123.2). The FASEB Journal28 (2014).
- 46.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkman A, van der Flier S, Kok EM, Dorssers LC. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J Natl Cancer Inst. 2000;92:112–120. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- 48.Kyaw M, et al. Src and Cas are essentially but differentially involved in angiotensin II-stimulated migration of vascular smooth muscle cells via extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase activation. Mol Pharmacol. 2004;65:832–841. doi: 10.1124/mol.65.4.832. [DOI] [PubMed] [Google Scholar]
- 49.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 50.Brueggemann LI, Mani BK, Mackie AR, Cribbs LL, Byron KL. Novel Actions of Nonsteroidal Anti-Inflammatory Drugs on Vascular Ion Channels: Accounting for Cardiovascular Side Effects and Identifying New Therapeutic Applications. Mol Cell Pharmacol. 2010;2:15–19. [PMC free article] [PubMed] [Google Scholar]
- 51.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 52.Gong L, et al. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:310–318. doi: 10.1097/FPC.0b013e32834f94cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.