Abstract
Quantitative imaging modalities for the analysis of hypoxia in brain tumors are lacking. The objective of this study was to generate absolute maps of tissue ptO2 from [18F]-FMISO images in glioblastoma and less aggressive glioma patients in order to quantitatively assess tumor hypoxia. An ancillary objective was to compare estimated ptO2 values to other biomarkers: perfusion weighted imaging (PWI) and tumor metabolism obtained from 1H-MR mono-voxel spectroscopy (MRS). Ten patients with glioblastoma (GBM) and three patients with less aggressive glioma (nGBM) were enrolled. All patients had [18F]-FMISO and multiparametric MRI (anatomic, PWI, MRS) scans. A non-linear regression was performed to generate ptO2 maps based on normal appearing gray (NAGM) and white matter (NAWM) for each patient. As expected, a marked [18F]-FMISO uptake was observed in GBM patients. The ptO2 based on patient specific calculations was notably low in this group (4.8 ± 1.9 mmHg, p < 0.001) compared to all other groups (nGBM, NAGM and NAWM). The rCBV was increased in GBM (1.4 ± 0.2 when compared to nGBM tumors 0.8 ± 0.4). Lactate (and lipid) concentration increased in GBM (27.8 ± 13.8%) relative to nGBM (p < 0.01). Linear, nonlinear and ROC curve analyses between ptO2 maps, PWI-derived rCBV maps and MRS-derived lipid and lactate concentration strengthens the robustness of our approaches.
Introduction
Hypoxia is a critical component of the glioblastoma (GBM) microenvironment and has been associated with both poor prognosis and resistance to various therapies1. There is a marked correlation between hypoxia, vascular dysfunction and tumor aggressiveness2. It is also known, since the 1950’s, that the low oxygenation observed in the tumor plays a major role in the resistance to X-ray radiation therapy3. The conventional treatment of GBM is surgical resection when possible, followed by radiotherapy along with concomitant chemotherapy. Despite the rigorous treatment protocol, the median survival fails to exceed 15 months4. Multi-parametric MRI or CT imaging are routinely used for tumor diagnosis, for the planning of radiation treatment but also for tumor follow-up after treatment. However, molecular imaging with positron emission tomography (PET) tracers sensitive to various biological parameters have now enlarged the range of functional information available for more targeted treatments and treatment efficacy determination5. For instance, biological target volume (BTV) used in radiotherapy could provide additional relevant data for dose painting in biologically active sub-regions or to boost radiotherapy6. Several approaches have been published over the last decade to estimate tumor hypoxia, or tumor oxygenation, including Eppendorf probes, electron paramagnetic resonance (EPR), 19F-MRI, MRI quantitative BOLD and another recently developed MRI technique based on lipid relaxation enhancement termed MOBILE [for review see Corroyer-Dulmont et al., 2015]7. Until now, PET imaging has been demonstrated to be the most rigorous (and “gold standard”) approach to map tumor hypoxia. Yet, PET is a quantitative imaging technique and remains the most sensitive and specific approach. One of the most common hypoxia-specific tracers in PET imaging is 3-[18 F]-fluoro-1-(2-nitro-1-imidazolyl)-2-propanol ([18F]-FMISO)8. However, it is well known that the relationship between oxygen tension and [18F]-FMISO uptake is non-linear9 and estimation of ptO2 (oxygen pressure in the tissue) remains mandatory for advanced use of FMISO maps since ptO2 maps are of greater biological pertinence. For instance, the accurate calculation of dose modulation for radiotherapy based on the Oxygen Enhancement Ratio effect requires ptO2 mapping10. Two main models have been proposed in the literature to convert [18F]-FMISO images into ptO2 maps11, 12. The former is a complex approach that uses many parameters to relate FMISO uptake to oxygen partial pressure but that was validated at the preclinical level. The latter is a simpler approach but needs to be validated in various situations.
To the best of our knowledge, adapted and validated mathematical models have never been employed in patients suffering from glioma. Here, we have initially employed in GBM patients the mathematical model previously used for head and neck cancers12. Nonetheless, this model turned out misfit. Therefore, the aim of the present study was to propose a new method of mathematical modeling to build-up ptO2 maps from [18F]-FMISO images for individual patient. Ten patients with GBM (GBM group) and three others patients with less aggressive glioma (nGBM group) were included and in which the proposed model was validated through the use of indirect estimates of tumor oxygenation from multimodal imaging; namely MRS and rCBV maps which were used to reflect anaerobic tumor metabolism and vascular status, respectively.
Results
Description of patients and images
GBM patients were characterized by the extravascular leakage of Gd-DOTA, hyperintensities on FLAIR images, a well-defined [18F]-FMISO uptake, an elevated rCBV and a decrease in the NAA signal concomitant to an increase in the lactate and lipid signal (Fig. 1A, two uppers panels and Fig. 1B). In contrast, nGBM were characterized by no extravasation of Gd-DOTA, hyperintensities on FLAIR, basal [18F]-FMISO uptake corresponding to the washout of the radiopharmaceutical in oxic conditions, nearly normal rCBV and a MRS profile compatible with that of less aggressive tumors (Fig. 1A, lower panel and Fig. 1B). The mean tumor volume based on contrast enhancement is 36.4 ± 34.7 cm3 in GBM group. Additionally, the edema region quantified in T2w-Flair contrast is 113.1 ± 70.0 cm3 and 25.8 ± 18.4 cm3 for both GBM and nGBM patients. Quantitatively, the ratio of [18F]-FMISO was 1.4 ± 0.2 for GBM and 0.9 ± 0.1 for nGBM (p < 0.001). The rCBV was 1.4 ± 0.2 for GBM and 0.8 ± 0.4 for nGBM (p < 0.001) (Fig. 1C,D).
Figure 1.
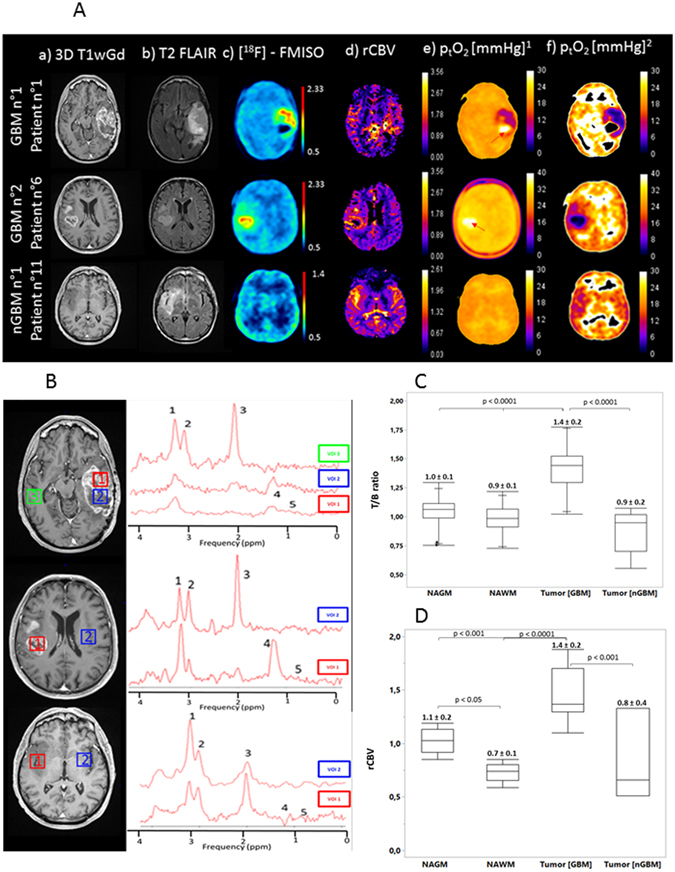
(A) Representative axial 3D T1wGd (a), fluid attenuation inversion recovery (FLAIR) (b), [18F]-FMISO (c), rCBV maps (d), representative absolute ptO2 maps (e) on the basis of the values of (a,b and c), proposed by Toma-Dasu and coll. and adjusted ptO2 (f) maps with empirical approach (non-linear regression) of two glioblastoma (GBM) and one less aggressive glioma (nGBM) patients. (B) 1H-MR mono-voxel spectroscopy was performed (Te = 144 ms) in tumor and/or necrotic tissue as well as non-tumor tissue for the same patients. Spectral peak locations and VOIS: (1) choline, (2) creatine, (3) NAA (N-acetyl aspartate, (4) lactate and (5) lipids. (C) Box plot of T/B ratio and rCBV (D) quantification in NAGM, NAWM and GBM and nGBM tumors areas.
Estimation of ptO2
We firstly used the unknown parameters initially described12 to calculate the ptO2 value from the [18F]-FMISO uptake. With this empirical approach, the mean ptO2 in NAGM and NAWM were 19.7 ± 0.5 and 20.9 ± 0.4 mmHg.
In the contrast-enhanced tumor of GBM group, the mean ptO2 was 15.2 ± 1.1 mmHg; in the nGBM patient, the volume of edema was delineated and the mean ptO2 was found to be 19.4 ± 0.4 mmHg. Necrotic foci of the tumors in GBM patients also appeared abnormally elevated (arrows Fig. 1A(e)). Given that the retention of [18F]-FMISO in a hypoxic cell occurs only when the partial pressure of oxygen is less than 10 mmHg13 and that no [18F]-FMISO metabolism should have occurred in the necrotic region of the tumor as well as in the health tissue, we hypothesized that the initial parameters reported for the operational equation would require further adaptation for normal and pathological brain tissues.
Adaptation of the model for intracerebral tissues
We consequently determined the equation by fixing the ptO2 in two regions (NAGM and NAWM) which allowed a hyperbolic function to convert the [18F]-FMISO uptake to absolute values of ptO2. For each patient, a, b and c were estimated. The mean values were 1.5, 0.7 and 9.9 respectively.
After fitting the data and generating ptO2 maps (Fig. 1A(f)), the mean ptO2 was 29.0 ± 5.7 and 41.5 ± 6.5 mmHg in NAGM and NAWM, respectively (p < 0.001). The mean estimated ptO2 in the tumors was 4.8 ± 1.9 mmHg in the GBM group and 33.3 ± 11.3 mmHg for the nGBM group (p < 0.001) (Fig. 2A). No significant differences were found between the oxygen status in NAGM and nGBM tissues.
Figure 2.
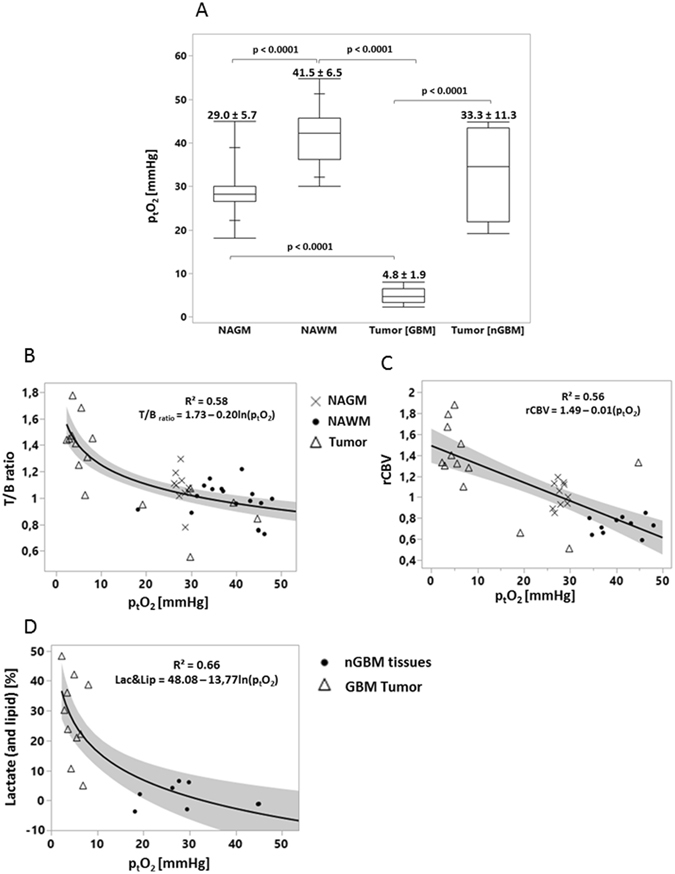
Box plot of adjusted ptO2 values (medians and the four quartiles as well as individual results) for each region of interest (NAGM, NAWM, GBM tumor and nGBM tumor) and the corresponding absolute values as mean ± sd (Fig. 2A). Correlation regressions between [18F]-FMISO uptake (T/B ratio), ptO2, rCBV and the lipids and lactate concentration. (B) Non-linear fit between [18F]-FMISO uptake and adjusted ptO2 values. Each point represent a volume of interest (x NAGM | • NAWM | Δ Tumor). (C) Linear fit between rCBV (calculated from PWI-MRI imaging) and ptO2 (estimated form [18F]-FMISO uptake). (D) Non-linear fit between Lactate and lipid [%] (calculated from MRS) and ptO2.
Comparison of ptO2 maps with rCBV maps and 1H-MRS metabolites
Linear and logarithmic regressions were applied between [18F]-FMISO, ptO2, rCBV, lactate (and lipid) concentration (Fig. 2B–D). A significant non-linear relationship was found between the [18F]-FMISO uptake (T/B ratio) and affined ptO2 across tumoral, NAGM and NAWM areas (R² = 0.58) (Fig. 2B). More interestingly, in the same areas, the correlation was also demonstrated between rCBV and ptO2 (R² = 0.56) (Fig. 2C). On the other hand, the correlation between ptO2 and lactate/lipid concentrations was also significantly evidenced (R² = 0.66) (Fig. 2D).
ROC analyses for tumor and tissue type detection
The ROC curves were performed to assess the ability of the various estimates to discriminate between GBM and nGBM patients (Fig. 3). The AUC value was 0.94 for CBV (threshold = 1.24); 0.96 for [18F]-FMISO (threshold = 1.16). Interestingly; the AUC was 1.00 for ptO2 (threshold = 8.07 mmHg).
Figure 3.
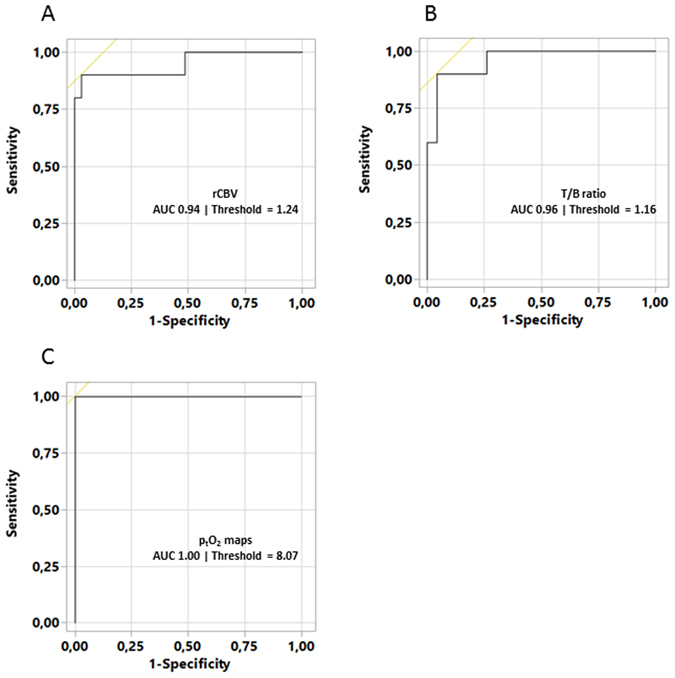
ROC curve analyses for discrimination of GBM and nGBM) using rCBV (A), T/B ratio maps (B) and affined ptO2 maps (C). The AUC and ROC threshold (for best sensitivity and specificity) values are reported in each ROC histogram.
Discussion
In GBM, hypoxia has been identified as an independent factor of poor prognosis1. We present here an approach to estimate ptO2 maps calibrated for individual patients and based on [18F]-FMISO PET that markedly discriminates severely hypoxic GBM from nGBM; ptO2 maps correlate well with other indices of oxygen status and may suggest avenues to explore personalized radiotherapy.
In the present study, we demonstrate that the model initially proposed by Toma-Dasu and his group12 for head and neck cancer to calculate intracerebral ptO2 requires further adaptation in the specific instance of brain tumors. After an ad hoc calculation of the parameters for individual patients based on the values of [18F]-FMISO retention in their corresponding NAGM and NAWM, we calculated ptO2 in NAGM as 29.0 ± 5.7 mmHg and that of NAWM as 41.5 ± 6.5 mmHg. Measurements in the tumor of GBM subjects revealed a mean ptO2 of 4.8 ± 1.9 mmHg that is in good agreement with the measurements obtained with oxygen sensitive probes14. However, one should mention that the assessment of tissue oxygen with probes remains problematic due to the high spatial heterogeneity observed in GBM patients. Additionally, in the nGBM group, the calculated ptO2 was 33.3 ± 11.3 mmHg in the tumoral tissue. This value is closed to that of NAGM and NAWM values and also concords with previous probe-based results15. Our findings clearly demonstrate the existence of severe hypoxia in GBM tumors, which may, in turn, explain the limited response to radiation therapy.
In terms of rCBV status, a marked and significant difference was observed between nGBM and GBM, which is in agreement with the literature16. The reproducibility of rCBV maps is reliable in non-pathological brain regions (NAGM and NAWM) in the two groups of patients (rCBVGM = 1.1 ± 0.2 and rCBVWM = 0.7 ± 0.1 respectively). Additionally, we failed to detect outright perfusion abnormalities in the nGBM group. In contrast, rCBV was increased in the GBM group (p < 0.05). The correlation between rCBV and ptO2 revealed an inverse linear relationship. These results confirm those of Cher and colleagues17 and those of Gerstner and colleagues18 based on histological and MRI markers of the vasculature and hypoxia assessed by [18F]-FMISO PET and reinforce the idea that the vessels observed in GBM are poorly functional; the result is a drastic fall in ptO2 values. We also applied the ROC curve analysis on GBM and nGBM patients discrimination, and observed that the sensitivity and specificity were improved when ptO2 was used instead of [18F]-FMISO and rCBV maps.
A correlation was also found between ptO2 values and the lactate and lipid concentrations, which may reflect an intense anaerobic metabolism resulting with lactate production in highly hypoxic tissues.
In the present study, we have developed a mathematical algorithm to enable the calculation of absolute ptO2 in tumoral tissues at the individual level. The values so obtained are in accordance with other, albeit indirect, estimations of oxygenation status, namely blood volume and indices of anaerobic metabolism. As radiosensitivity parameters that take into account the OER, and the non-linearity between ptO2 and [18F]-FMISO, we believe that the present ptO2 maps should be used rather than [18F]-FMISO to evaluate the impact of dose escalation on tumor control probability (TCP) models.
The principal features of our investigation are undernoted. i) The determination of the three factors necessary to calculate ptO2 from [18F]-FMISO maps and, these, in individual ROI and in individual subjects. ii) Our innovative approaches allow in terms of tissue oxygenation, a marked discrimination between the values in GBM (severely hypoxic) and less aggressive glioma as well as apparently normal brain tissue, be it white or gray matter. The overall findings would encourage prospective study.
However, our proof of concept has some limitations. First, the calculation of ptO2 is based on a mathematical model. Although other models have been proposed in the literature9, 11, the relationship between [18F]-FMISO uptake and ptO2 remains hyperbolic. The advantage of our approach is that it requires two regions of interest for calibration of the model.
Another limitation of this study is that [18F]-FMISO is trapped into hypoxic cells when ptO2 is less than 10 mmHg19 rendering the calculation of ptO2 less adapted for low grade gliomas.
Finally, in our small cohort, [18F]-FMISO uptake was only observed in GBM but not in less malignant gliomas (grade II and III). These findings concord with numerous published studies in which, GBM display invariably an increased uptake of [18F]-FMISO20, 21. Nonetheless, it is less evident that less aggressive glioma develop hypoxia. Cher and colleagues22 reported that all the GBM showed a high uptake of [18F]-FMISO, however they reported that only one patient of grade III shows a positive uptake of [18F]-FMISO. In another study23, the authors reported a positive uptake in grade III but the uptake observed in grade IV was significantly higher. Our results concord with these observations since a very severe hypoxia was obtained for GBM and not for less aggressive grades, however, our proposed model needs to be tested in a larger cohort of GBM and less aggressive gliomas. Additionally, this hypoxic marker and its conversion could be a powerful tool for stratification in radiation treatment.
In the present study, the main goal was to analyze the feasibility of adapting a model, formerly developed for head and neck to brain tumors, as a proof of concept. Future investigations are needed to test this hypothesis on the tumor control improvement with radiotherapy ought to benefit from the wealth of information based on functional imaging. Molecular imaging with positron emission tomography (PET) tracers is sensitive to various biological parameters have now enlarged the range of functional information available for more targeted treatments planning by defining a so called “ biological target volume “ (BTV) treatment efficacy determination. The BTV could provide additional relevant data for dose painting in biologically active sub-regions or to boost radiotherapy.
Methods
Patients
This study was part of a prospective monocentric clinical trial called HYPONCO, funded by INCa (Institut National du Cancer). The study was approved by both the ethics committee (CPP Nord-Ouest III) and AFSSAPS (Agence française de sécurité sanitaire des produits de santé) and registered under EUDRACT number 2009-015543-16. More information’s are available bellow: (NTC01200134 - https://www.clinicaltrials.gov/ct2/show/NCT01200134). All procedures performed in the study were in accordance with ethical standards of both research committees. All patients are included between September 10, 2010 and November 16, 2012 after having been fully informed of the study and provided signed, informed consent.
Ten patients with confirmed GBM (based on the classification of the World Health Organization (WHO)) and three patients with less aggressive glioma were investigated. The characteristics of the patients are summarized (Table 1). After the discovery of a glioma (based on anatomical MRI), PET imaging was employed to detect regions of hypoxia. The day following PET imaging, an MRI study (anatomic, vascular and metabolic) was performed. PET images and anatomical 3D T1w-Gd images were used to guide the placement of the volumes of interest (VOI) for MRS.
Table 1.
Patients and tumor characteristics.
| No | Sex | Age | KP | Tumor Location | CE | T/B | WHO Grade | Histology |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 28 | 100 | Parietal, Temporal, Insular, L | Yes | Yes | IV | Glioblastoma |
| 2 | M | 58 | 90 | Temporal, R | Yes | Yes | IV | Glioblastoma |
| 3 | M | 64 | 80 | Frontal, L | Yes | Yes | IV | Glioblastoma |
| 4 | M | 52 | 80 | Frontal, R | Yes | Yes | IV | Glioblastoma |
| 5 | M | 62 | 80 | Occipital, CC, L | Yes | Yes | IV | Glioblastoma |
| 6 | M | 67 | 90 | Temporal, Insular, R | Yes | Yes | IV | Glioblastoma |
| 7 | M | 56 | 70 | Occipital, CC, Temporal, L | Yes | Yes | IV | Glioblastoma |
| 8 | M | 63 | 90 | Frontal, L | Yes | Yes | IV | Glioblastoma |
| 9 | F | 71 | 70 | Parietal, Occipital, R | Yes | Yes | IV | Glioblastoma |
| 10 | M | 46 | 80 | Frontal, CC, R, L | Yes | Yes | IV | Glioblastoma |
| 11 | M | 51 | 100 | Temporal, Insular, R | No | No | III | Oligoastrocytoma |
| 12 | M | 58 | 90 | Hemisphere, R, Brain-stem | Yes | No | III | Gliomatosis |
| 13 | M | 54 | 100 | Frontal, R | No | No | II | Oligodendroglioma |
M: Male, F: Female, KP: Karnofsky’s performance scale, R: Right, L: Left, CC: Corpus callosum. CE: Contrast enhancement after Gadolinium, T/B: Tissue to blood ratio ≥1.2, WHO: World Health Organization.
Positron emission tomography/CT Scan
[18F]-FMISO was synthesized by the LDM-TEP group (UMR6301-ISTCT, Cyceron) based on methods previously described24. Acquisitions performed on a General Electric Discovery VCT 64 PET/CT camera, lasted 20 min and were carried out 120 minutes after the intravenous injection of ≈5 MBq/kg of [18F]-FMISO. The attenuation-corrected images were reconstructed with an OSEM 2D algorithm (9 subsets and 2 iterations) and filtered in 3D with a Butterworth filter. Two blood samples were taken and the plasma averaged at the time of imaging for the calculation of T/B maps according to the following formula:
| 1 |
Magnetic Resonance Imaging
Anatomical imaging
Investigations were performed with a Signa HDxt 1.5 T (GE Healthcare) in the radiology department of university hospital of Caen. After a scout view, an axial T2w FLAIR (fluid attenuated inversion recovery) sequence was performed to assess vasogenic edema.
PWI
Dynamic T2*-weighted EPI images were acquired 30 s prior to and during the first pass of an intravenous bolus injection of 0.1 mmol/kg of Gd-DOTA (Dotarem®, Guerbet, France) and thereafter 60 s (24 slices, 40 repetitions, slice spacing: 5.5 mm, pixel resolution 2.19 × 2.19 mm, TR/TE = 2375/60 ms). Variations of the T2* signal in the tissue, which are proportional to the concentrations of the contrast agent, were calculated with in-house macros based on ImageJ software as: ∆R2 * (t) = −ln(S(t)/S0), where S0 = the signal intensity before contrast agent injection, and S(t) = the signal intensity over time. Then, CBV maps were generated by integrating the area under the γ-variate fitted curves to avoid any effect of recirculation of the contrast agent. Images were then normalized by dividing CBV maps by the mean value of the normal-appearing contralateral side (so as to compute relative CBV maps). Thereafter, a 3D T1-weighted sequence was performed for tumor contrast enhancement (124 slices, slice spacing 1.5 mm, pixel resolution 1.01 × 1.01 mm, TR/TE = 9.3/3.6 ms).
Mono-voxel 1H-MRS
A standard PRESS (point resolved spectroscopy) sequence was used to measure regional metabolic differences in tumor and non-tumor tissues at several echo times [35 ms (all VOI), 144 ms (all VOI), 288 ms (partial VOI), 432 ms (partial VOI) and 576 ms (partial VOI)] with mono-voxel MRS. Only 144 ms has been retained to avoid the impact of macromolecular resonances. The VOI were first placed on the hyperperfused area identified on Gd-enhanced T1w images (tumor active areas), followed by the contralateral, mirror site (non-tumor tissue). For each VOI, 2048 points were sampled with a frequency of 2500 Hz to obtain the NMR spectra. After a frequency correction of all spectra, residual water components and potential lipids (5.4 ppm) were suppressed with a HLSVD (Hankel-Lenczos singular value decomposition) filter (frequency band of 4.2 ppm to 7.5 ppm). Frequency bands of interest for each metabolite around the principal resonance peaks were defined: for choline [Cho (3.35–3.10ppm)]; creatine [Cr (3.10–2.88 ppm)] and N-acetyl aspartate [NAA (2.24–1.87 ppm)]. We defined a frequency band [lactate and lipid (1.87–0.68 ppm)] that includes resonance of lactate (doublet at 1.3 ppm), major lipid resonances at 1.3ppm [CH2 group] and at 0.9 ppm [CH3 group]. The selection of these bandwidths is in accord with previously described in vitro and in vivo NMR studies of brain metabolites25. Areas of each frequency band of interest were then expressed as a percentage of the total area of the real value of spectra from 0 to 5.9 ppm. These proportions of area were taken as an index for the concentration of the metabolites. Lactate and lipid are pooled as it is difficult to distinguish the lactate doublets from lipids at 1.3 ppm. Accordingly we chose not to dissociate these compounds and to use a scale expressed as a percentage of the total area in order to obtain more precise indices of concentration, albeit with lower specificities. Pre-processing of the spectra was carried out on JMRUI 5.0 software.
MRI, MRS and PET imaging co-registration
The PMOD 3.1® image fusion software was used to co-register the MRS volume of interest, [18F]-FMISO, ptO2 maps and rCBV to the reference sequence 3D T1w-Gd. The co-registration process was performed automatically, by a trilinear interpolation with rigid matching and normalized mutual information.
Definition of tumor region of interest
For the GBM group, the severely hypoxic areas were determined from segmented [18F]-FMISO with values of T/B ratio >1.226. Active tumor areas were segmented manually on 3D T1w-Gd images with no necrotic foci (which is formerly defined as an anoxic region) and used to assess vascular abnormalities. For the nGBM subjects, defined by the absence of both an increased [18F]-FMISO uptake and, vascular abnormalities, the region of interest (ROI) corresponding to the FLAIR hyperintensities was taken as the tumor. The edema is defined as a region with hyperintensities on T2w images, which encompasses the tumor core but also invaded tissue that cannot be considered anymore as a healthy tissue.
Conversion of FMISO uptake into absolute values of the partial pressure of oxygen (ptO2) in brain tissue
The mathematical model12 initially proposed to convert [18F]-FMISO images into absolute ptO2 maps is:
| 2 |
where u is the uptake as assessed by the T/B ratio and a, b and c are tissue-dependent, reaction-specific parameters. Based on the model described by Toma-Dasu and colleagues in neck and head tumors, which are also hypoxic12, a, b and c were 10.9, 10.7 and 2.5 respectively.
The novel approach to convert [18F]-FMISO to ptO2 maps
After co-registration of all images, a processing step was performed to segment normal appearing gray matter (NAGM) and normal appearing white matter (NAWM) so as to generate masks (SPM8 software). The edematous zones were avoided. Three patients are exemplified in Fig. S1. The median T/B value of [18F]-FMISO uptake was measured in NAGM and NAWM region and ptO2 was fixed at 30 mmHg27 and 60 mmHg28 respectively. A least-square error minimization (Levenberg-Marquardt non-linear regression) was then performed under Matlab 2012b® to adjust a, b and c to the fixed ptO2 in NAGM and NAWM respectively to calibrate the equation. Thereafter, we calculated ptO2 in the whole brain by the model based on the estimates in normal appearing regions.
Statistical analyses
Box plots, linear and logarithmic regressions, Tuckey’s HSD test and ROC curves analysis were performed using JMP Pro10 software (SAS). Further statistical analyses are detailed in the legends to figures. Data are presented either as mean ± sd or median and extremes of range.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
Centre National de la Recherche Scientifique (CNRS), the Université de Caen Normandie (UCN), the Conseil Régional de Basse-Normandie (CRBN), the Fondation Elen, the Institut National du Cancer (INCa), the Agence Nationale de la Recherche-labex IRON (ANR-11-LABX-0018–01).
Author Contributions
Study design: A.C., J.S.G. and S.V. Data analyses: A.C., S.C., F.K., N.D., S.V. Collection of data: J.M.C. Manuscript writing: A.C., O.T. and S.V. Critical revision of the manuscript: E.P., L.B., E.T.M. Approval of the final version: All authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08646-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spence AM, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F] fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin. Cancer Res. 2008;14:2623–2630. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans M, et al. The relationship among hypoxia, proliferation, and outcome in patients with de novo glioblastoma. Transl. Oncol. 2010;3:160–169. doi: 10.1593/tlo.09265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray LH, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 4.Chinot OL, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 5.Ling CC, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:551–560. doi: 10.1016/S0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 6.Piroth MD, et al. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. 2012;188:334–339. doi: 10.1007/s00066-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 7.Corroyer-Dulmont A, et al. Imaging modalities to assess oxygen status in glioblastoma. Front. Med. 2015;2 doi: 10.3389/fmed.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreau A, et al. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J, et al. A robotic system for 18F-FMISO PET-guided intratumoral pO2 measurements. Med. Phys. 2009;36:5301–5309. doi: 10.1118/1.3239491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.South CP, et al. Dose prescription complexity versus tumor control probability in biologically conformal radiotherapy. Med. Phys. 2009;36:4379–4388. doi: 10.1118/1.3213519. [DOI] [PubMed] [Google Scholar]
- 11.Bowen SR, et al. Characterization of PET hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl. Med. Biol. 2011;38:771–780. doi: 10.1016/j.nucmedbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toma-Dasu I, et al. Dose prescription and treatment planning based on FMISO-PET hypoxia. Acta Oncol. 2012;51:222–230. doi: 10.3109/0284186X.2011.599815. [DOI] [PubMed] [Google Scholar]
- 13.Fleming IN, et al. Imaging tumour hypoxia with positron emission tomography. Br. J. Cancer. 2015;112:238–250. doi: 10.1038/bjc.2014.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collingridge DR, et al. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1999;53:127–131. doi: 10.1016/S0167-8140(99)00121-8. [DOI] [PubMed] [Google Scholar]
- 15.Lally BE, et al. The interactions of polarographic measurements of oxygen tension and histological grade in human glioma. Cancer J. 2006;12:461–466. doi: 10.1097/00130404-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Boxerman JL, et al. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 17.Cher LM, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J. Nucl. Med. 2006;47:410–418. [PubMed] [Google Scholar]
- 18.Gerstner, E. et al. ACRIN 6684: Assessment of tumor hypoxia in newly diagnosed GBM using 18F-FMISO PET and MRI. Clin Cancer Res, May 16 (2016). [DOI] [PMC free article] [PubMed]
- 19.Gross MW, et al. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int. J. Cancer. 1995;61:567–73. doi: 10.1002/ijc.2910610422. [DOI] [PubMed] [Google Scholar]
- 20.Hirata K, et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging. 2012;39:760–770. doi: 10.1007/s00259-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 21.Bekaert L, et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging. 2017;44:1383–1392. doi: 10.1007/s00259-017-3677-5. [DOI] [PubMed] [Google Scholar]
- 22.Cher LM, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-Fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–418. [PubMed] [Google Scholar]
- 23.Yamamoto, Y. et al. Hypoxia assessed by 18F-Fluoromisonidazole positron emission tomography in newly diagnosed gliomas. 33, 621–625 (2012). [DOI] [PubMed]
- 24.Valable S, et al. Complementary information from magnetic resonance imaging and 18F-fluoromisonidazole positron emission tomography in the assessment of the response to an antiangiogenic treatment in a rat brain tumor model. Nucl. Med. Biol. 2011;38:781–793. doi: 10.1016/j.nucmedbio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Govindaraju V, et al. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::AID-NBM619>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Szeto MD, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009;69:4502–4509. doi: 10.1158/0008-5472.CAN-08-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou H, et al. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: A multisite EPR oximetry with implantable resonators. J. Magn. Reson. 2012;214:22–28. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokes BT. Traumatically induced alterations in the oxygen fields in the canine spinal cord. Exp. Neurol. 1982;75:665–677. doi: 10.1016/0014-4886(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.


