Abstract
OBJECTIVES
This study sought to use a new catheter technique to split the anterior mitral valve leaflet (AML) and prevent iatrogenic left ventricular outflow tract (LVOT) obstruction immediately before transcatheter mitral valve replacement (TMVR).
BACKGROUND
LVOT obstruction is a life-threatening complication of TMVR, caused by septal displacement of the AML.
METHODS
The procedure was used in patients with severe mitral valve disease and prohibitive surgical risk. Patients either had prior surgical mitral valve ring (n = 3) or band annuloplasty (n = 1) or mitral annular calcification with stenosis (n = 1). Iatrogenic LVOT obstruction or transcatheter heart valve dysfunction was predicted in all based on echocardiography and computed tomography. Transfemoral coronary guiding catheters directed an electrified guidewire across the center and base of the AML toward a snare in the left atrium. The externalized guidewire loop was then electrified to lacerate the AML along the centerline from base to tip, sparing chordae, immediately before transseptal TMVR.
RESULTS
Five patients with prohibitive risk of LVOT obstruction or transcatheter heart valve dysfunction from TMVR successfully underwent LAMPOON, with longitudinal splitting of the A2 scallop of the AML, before valve implantation. Multiplane computed tomography modeling predicted hemodynamic collapse from TMVR assuming an intact AML. However, critical LVOT gradients were not seen following LAMPOON and TMVR. Doppler blood flow was seenacross transcatheter heart valve struts that encroached the LVOT, because the AML was split. Transcatheter heart valve function was unimpeded.
CONCLUSIONS
This novel catheter technique, which resembles surgical chord-sparing AML resection, may enable TMVR in patients with prohibitive risk of LVOT obstruction or transcatheter heart valve dysfunction.
Keywords: left ventricular outglow tract obstruction, mitral valve, structural heart disease, transcatheter mitral valve replacement, valvular heart disease
The anterior mitral valve leaflet (AML) is a mobile structure that physically separates inflow and outflow zones of the left ventricle (1). Preserving the AML during surgical mitral valve replacement can cause left ventricular outflow tract (LVOT) obstruction, either when the prosthesis struts protrude into the LVOT or when a long redundant anterior leaflet prolapses into the LVOT (2,3). In a similar manner, implantation of a transcatheter heart valve (THV) inside the native or repaired mitral valve enforces an “open position” of the AML that may encroach on the LVOT (4–7). This septal displacement of the AML is exaggerated when the aortic and mitral annular planes are acutely angulated rather than parallel, when the interventricular septum bulges toward the LVOT, when the AML is elongated, and when the implant extends or flares into the left ventricle. In this setting transcatheter mitral valve replacement (TMVR) may cause life-threatening LVOT obstruction (Figure 1A). Moreover, after TMVR an excessively long AML may prolapse anteriorly into a narrowed LVOT as in hypertrophic cardiomyopathy, or it can prolapse posteriorly and interfere with bioprosthetic heart valve opening or closing by mechanical or Bernoulli effects after surgical (3) or transcatheter mitral replacement (8). Longer AMLs are more susceptible to these effects (9). Although few data are available to guide decision-making, one-half of TMVR candidates having an intact AML (Mayra Guerrero, personal communication February 24, 2017; NCT02370511) are excluded from an ongoing clinical investigation because of the perceived risk of life-threatening LVOT obstruction.
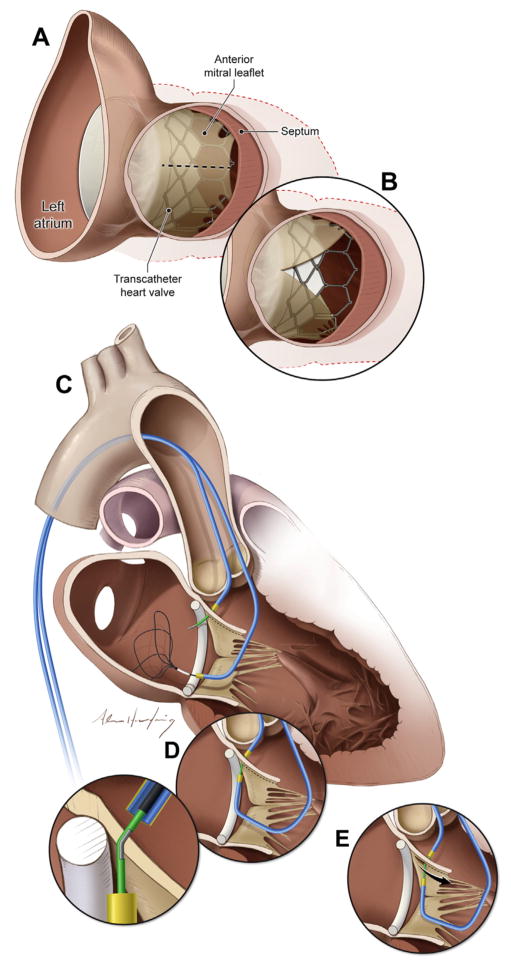
FIGURE 1. Views of the Anterior Mitral Valve Leaflet From the LVOT.
(A) In this example, transcatheter mitral valve implantation displaces the native anterior mitral valve leaflet causing LVOT obstruction. (B) After LAMPOON, LVOT obstruction is reduced and blood flows across the unobstructed struts of the implanted transcatheter heart valve. (C to E) Illustration of the LAMPOON procedure. (C) In 2 retrograde guiding catheters are positioned across the aortic valve, 1 into the LVOT and another across the mitral valve into the left atrium. The LVOT catheter directs an electrified guidewire across the base of the anterior mitral valve leaflet under echocardiographic guidance into a snare positioned through the left atrial guiding catheter. (D) Once the guidewire traverses the mitral leaflet, it is ensnared and externalized. The inset depicts a denuded and kinked section of the guidewire shaft that directs electrosurgery energy to the leaflet. (E) The guidewire is electrified and the 2 guiding catheters withdraw it to lacerate the anterior mitral leaflet lengthwise. LAMPOON = laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction; LVOT = left ventricular outflow tract.
Online Video 1 is depicted in this figure.
One approach to prevent or treat TMVR-related LVOT obstruction is pre-emptive transcoronary alcohol septal ablation (10,11), which sacrifices myocardium and risks conduction system injury and pacemaker-dependence in patients with cardiomyopathy, which is unsuitable in patients with thin interventricular septa, and which delays TMVR by 4 to 6 weeks to allow remodeling in highly symptomatic patients. Another option is surgical AML resection combined with TMVR during thoracotomy and cardiopulmonary bypass (12–14), with attendant risk and morbidity to patients already believed to be at high risk for cardiac surgery.
As an alternative, we have developed a transcatheter adjunct to TMVR using off-the-shelf equipment, and described its preclinical use (15). This technique resembles David’s (16) surgical anterior resection with chordal sparing. We create a longitudinal split of the middle scallop (A2) of the AML, immediately before TMVR. As a result, chordal attachments displace the split AML away from the LVOT after the cylindrical THV is implanted, and blood flows unobstructed across the THV stent struts (Figure 1B).
We report the initial human experience with this intentional laceration of the AML to prevent left ventricular outflow tract obstruction (LAMPOON) procedure.
METHODS
PATIENTS
TMVR with LAMPOON was performed at 2 medical centers, Emory University Hospital and Henry Ford Hospital. The institutional ethics review boards of both approved this communication. Five patients deemed inoperable and believed to have prohibitive risk of TMVR because of intact native mitral leaflets consented explicitly to this novel procedure, as clinical therapy, after consensus from the local multidisciplinary structural heart teams.
PROCEDURE PLANNING AND IMAGE GUIDANCE
Baseline electrocardiogram-gated contrast-enhanced 64-detector row cardiac computed tomography (CT) angiography was obtained to measure annular and/or annuloplasty dimensions to select a THV. Multiplanar reconstruction (Vitrea, Toshiba, Tustin, California) was performed to predict the following working projections: angle of TMVR deployment perpendicular to the prosthesis or annulus, a left anterior oblique caudal projection corresponding to a short-axis CT reconstruction to depict LAMPOON traversal position along the medial-lateral dimension, and an attainable right anterior oblique caudal projection corresponding to a 3-chamber CT reconstruction to depict LAMPOON traversal position along the leaflet base-to-tip dimension.
A predicted post-TMVR minimum LVOT area (“threatened neo-LVOT”) area was obtained on a separate workstation (Mimics, Materialise, Leuven, Belgium). TMVR was simulated in systole using cylinders corresponding to the known external length and diameter of the planned THVs, implanted at the intended depth relative to the mitral annulus (80/20 left ventricle/left atrium [LA]), and the minimum projected area recorded assuming the AML would be obstructive only where it contacts the TMVR device.
AML lengths were measured on CT and trans-esophageal echocardiography (TEE). Long leaflet length (>30 mm, compared with nominal height 18.0 to 22.5 mm of Sapien 3, Edwards Lifesciences, Irvine, California) combined with acute aortomitral angle, was considered an independent risk factor for LVOT obstruction and THV dysfunction as part of the multidisciplinary heart team evaluation, accepting the difficulty modeling this based on static CT images. TMVR with LAMPOON was performed in a biplane angiography system at 1 site, and single plane in the other. All patients underwent general anesthesia and intraprocedural TEE.
LAMPOON TECHNIQUE
The LAMPOON procedure has 3 steps (Online Video 1): leaflet traversal with a guidewire, followed by leaflet laceration, immediately followed by TMVR. These are all guided by fluoroscopy combined with TEE.
For leaflet traversal, 2 6-F coronary guiding catheters (JL3.5) are advanced across the aortic valve (Figure 1C) through 2 femoral artery sheaths. One guiding catheter is positioned retrograde in the LVOT abutting the base of A2 to direct the traversal guide-wire, and the other retrograde into the LA across the aortic and mitral valves.
In patients with mitral stenosis, we found it helpful to advance the retrograde LA catheter over a 0.014 wire rail into the transseptal catheter. The rail eases LA catheter repositioning should it prolapse into the left ventricle. The rail is formed by advancing a balloon tip catheter from a transseptal deflectable catheter (Agilis NxT medium curl, St. Jude Medical, St. Paul, Minnesota) in the LA through the main orifice of the mitral valve. The rail is a kink-resistant guidewire (Runthrough NS 0.014-inch, Terumo Interventional Systems, Somerset, New Jersey), and is externalized after snaring. Through the retrograde LA catheter, a multiloop snare (Atrieve 18/30, Argon Medical, Plano, Texas) is positioned alongside the rail and alongside the mitral coaptation surfaces.
The electrosurgical traversal technique is derived from the technique of transcaval crossing (17,18). The traversal guidewire is a 0.014-inch × 300 cm guidewire (Astato XS 20, Asahi Intecc USA, Santa Ana, California) inside an insulating polymer jacket wire convertor (Piggyback, Teleflex Vascular Solutions, Minneapolis, Minnesota), inside the retrograde LVOT guiding catheter. The external back end of the guidewire is connected via hemostatic forceps to an electrosurgery pencil and generator (ValleyLabFX, Medtronic Covidien, Minneapolis, Minnesota), set to “pure” cutting mode at 50 W. The traversal guidewire is advanced from the LVOT catheter, penetrating the base of the A2 scallop, during brief (<1 s) electrification into the prepositioned left atrial snare (Figure 1D). The wire is captured and externalized through the retrograde LA catheter.
A short segment along the middle of the guidewire shaft is noncircumferentially denuded of its polymer insulation, and then kinked, using a scalpel. This minor modification focuses electrosurgery energy required for laceration on the “inner elbow” of the guidewire (Figure 1D, inset). The radiopaque tip of the Piggyback wire converter is locked behind the denuded kinked segment for added fluoroscopic visibility and electrical insulation. The kinked segment is positioned to straddle the AML.
For laceration, both free guidewire ends are firmly pulled during electrification in a series of brief (<1 s) steps. Pulling the guidewires helps to oppose the guiding catheters, to protect the aortic valve, and to initiate laceration at the base of the AML (Figure 1E). Further tension on the guidewires completes the splitting of the AML.
ANTEGRADE TRANSSEPTAL TMVR TECHNIQUE
TMVR was performed via an antegrade transseptal route using Edwards Sapien 3 devices. The rigid TMVR guidewire was delivered into the left ventricle after first crossing the major mitral orifice using a balloon wedge endhole catheter, and atrial septostomy performed using 12-to-16-mm balloon dilatation catheters to ensure transseptal THV delivery. Tension was applied to the LAMPOON catheter system under fluoroscopy to ensure the TMVR guidewire was not entrapped. The THV was positioned in the left atrium or partway across the mitral valve before LAMPOON laceration to facilitate rapid deployment in case of early hemodynamic compromise.
TMVR was performed immediately after LAMPOON, using rapid right ventricular pacing and slow balloon inflation. After the first inflation, the THV delivery balloon was advanced slightly forward and reinflated with at least 4 ml additional inflation volume to flare the ventricular aspect of the THV stent. At the conclusion of the procedure, the iatrogenic atrial septal defect was closed based on operator discretion.
POST-PROCEDURE IMAGE AND DATA ANALYSIS
Post-procedure invasive pressure and Doppler echo-cardiography gradients were recorded across the LVOT and THV, using peak-to-peak and peak-instantaneous measurements as suggested by Geske et al. (19) for hypertrophic cardiomyopathy. Post-procedure neo-LVOT was evaluated by echocardiography. TMVR encroachment on the LVOT was measured in B-mode to determine the retrospective “threatened” LVOT diameter as if LAMPOON had not been performed, and in color Doppler mode to visualize blood flow across the THV stent struts and determine the “actual” LVOT diameter. Similarly, post-procedure CT was evaluated to measure a “threatened” LVOT area as if LAMPOON had not been performed. Data were reported as mean ± SD or as median (range) as appropriate.
RESULTS
PATIENTS
Clinical characteristics of the 5 patients are shown in Table 1. All were believed to have prohibitive surgical risk and no therapeutic alternatives. None were considered suitable for preparatory alcohol septal ablation to debulk the LVOT.
TABLE 1.
Baseline Clinical Characteristics (n = 5)
| Age, yrs | 64.8 ± 13.2 |
| Female, % | 40 |
| Severe pulmonary disease, % | 60 |
| Prior stroke, % | 40 |
| Atrial fibrillation, % | 60 |
| eGFR ml/min/1.73 m2 | 65.4 ± 11.4 |
| NT-proBNP baseline | 432.3 ± 346.9 |
| STS PROM mitral valve replacement, % | 8.2 ± 3.6 |
| NYHA CHF functional class | 3.8 ± 0.4 |
| TMVR setting | TMVR-in-ring = 3 TMVR-in-band = 1 TMVR-in-MAC = 1 |
| Primary lesion (valvular stenosis/regurgitation/mixed) | 3/1/1 |
| Ring or band nominal diameter, mm | 30.0 ± 2.8 |
| Left ventricular ejection fraction | 0.48 ± 0.14 |
| Right ventricular dysfunction, % | 80 |
| Echo septal thickness, mm | 8.8 ± 1.1 |
| Echo septal thickness amenable to alcohol septal ablation, % | 0 |
Values are mean ± SD or %.
CHF = congestive heart failure; eGFR = estimated glomerular filtration rate; MAC = mitral annular calcification; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; STS PROM = Society of Thoracic Surgeons predicted risk of mortality; TMVR = transcatheter mitral valve replacement.
The first 4 patients had undergone prior surgical mitral annuloplasty. The first had a rigid annuloplasty ring (primarily regurgitant lesion), the next 2 had semi-rigid rings (1 primarily stenotic and the other mixed regurgitant/stenotic), the fourth had a flexible posterior annuloplasty band (primarily stenotic), and the fifth had native mitral annular calcification (MAC) with stenosis.
Three (Patient #1, #2, and #4) were predicted to have prohibitive risk of LVOT obstruction or THV dysfunction from long redundant AMLs, with mean AML length 32 ± 2 mm and average peak-to-peak catheter resting LVOT gradient 7.4 ± 0.5 mm Hg. Two (Patient #3 and #5) were predicted to have life-threatening LVOT obstruction after TMVR with a predicted neo-LVOT of 67 ± 4 mm2 on the basis of multiplanar CT modeling. One required intra-aortic balloon pump at baseline because of intractable heart failure. No others required mechanical circulatory support.
LAMPOON PROCEDURE
Procedure details are shown in Table 2. A representative procedure is depicted in Online Video 2. Preparatory LAMPOON successfully split the A2 scallop of the AML in all 5 patients. Figures 2 and 3 show representative radiograph, TEE, and CT images of a patient undergoing LAMPOON and TMVR after prior surgical mitral annuloplasty. Figure 4 shows radiograph and TEE sequences of a patient undergoing LAMPOON and TMVR for MAC causing mitral stenosis. Figure 5 shows the split mitral leaflet in the interval between LAMPOON and TMVR. Figure 6 shows CT images of a patient treated for mitral stenosis caused by MAC, causing pulmonary alveolar hemorrhage. After TMVR with LAMPOON, the THV is seen spanning the entire LVOT, and the pleural and pulmonary abnormalities are dramatically improved.
TABLE 2.
Procedure Characteristics, Hemodynamics, and Imaging (n = 5)
| TMVR size, Sapien 3, mm | 26 (n = 2); 29 (n = 3) | |
|
| ||
| Crossing power, W | 50 ± 0 | |
|
| ||
| Lacerating power, W | 58 ± 11 | |
|
| ||
| LVOT catheter Judkins left length, cm | 3.9 ± 0.7 | |
|
| ||
| Left atrial retrograde catheter Judkins left length, cm | 3.7 ± 0.3 | |
|
| ||
| Procedure time, min | 214 ± 24 | |
|
| ||
| Time from catheter to valve, min | 133 ± 19 | |
|
| ||
| Time from leaflet traversal to leaflet laceration, min | 66 ± 10 | |
|
| ||
| Time from laceration to TMVR, min (range) | 3.0 (1–38) | |
|
| ||
| Fluoroscopy time, min | 116 ± 39 | |
|
| ||
| Radiation dose-area product, Gy·cm2 | 335 ± 319 | |
|
| ||
| Contrast volume, ml | 68 ± 41 | |
|
| ||
| Iatrogenic atrial septal defect closed | 4 of 5 | |
|
| ||
| Pressure, mm Hg | Before | After |
|
| ||
| Mitral valve gradient mean | 9.2 ± 4.2 | 4.6 ± 4.0 |
| LVOT gradient (range) | 7.4 ± 0.5 (7–8) | 17.6 ± 12.4 (8–39) |
| LA mean pressure | 25.8 ± 9.2 | 18.2 ± 4.8 |
| LA v-wave | 50.2 ± 15.9 | 30.0 ± 6.4 |
| PA systolic pressure | 72.8 ± 10.9 | 58.2 ± 15.4 |
|
| ||
| Computed Tomography Characteristics | ||
|
| ||
| Aortomitral plane angle, degrees, n = 5 | 123 ± 10 | |
| Neo-LVOT* predicted, mm2, n = 2 | 67 ± 4 | |
| Neo-LVOT* after TMVR, mm2, n = 2, assuming LAMPOON had not been performed | 50 ± 71 | |
|
| ||
| Echo Characteristics After TMVR | ||
|
| ||
| LVOT max native dimension B-mode after TMVR, mm | 17.6 ± 2.1 | |
| LVOT vena contracta after TMVR (includes stent-free), mm 11.8 ± 3.5 | ||
| LVOT stent-free length after TMVR, mm | 4.4 ± 4.5 | |
| LVOT obstruction threatened diameter,† % | 76 ± 24 | |
| LVOT obstructed actual diameter, % | 34 ± 15 | |
| LVOT gradient peak-instantaneous by echo, pre-discharge, mm Hg | 26 ± 11 | |
| LVOT gradient peak-instantaneous by echo, 1 month, mm Hg 17 ± 10 | ||
Values are mean ± SD (range).
Neo-LVOT is the minimum cross-sectional area of the LVOT expected after implantation of the selected valve, based on 3-dimensional computed tomography analysis. These assume there is no flow across the struts of the implanted transcatheter heart valve. Neo-LVOT is reported on the 2 patients with predicted stent encroachment rather than excessive anterior mitral valve leaflet length.
Threatened diameter refers to the diameter obstruction of the LVOT assuming there would be no flow across the struts of the transcatheter heart valve.
LA = left atrium; LAMPOON = laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction; LVOT = left ventricular outflow tract; PA = pulmonary artery; other abbreviations as in Table 1.
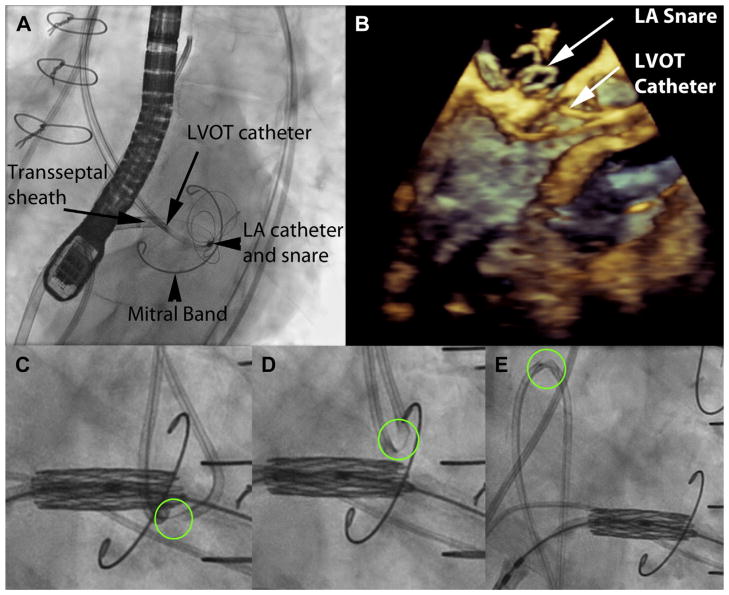
FIGURE 2. LAMPOON to Enable Transcatheter Mitral Valve Replacement Inside a Flexible Mitral Annuloplasty Band.
(A) Left anterior oblique caudal short-axis fluoroscopic projection showing 2 retrograde catheters positioned before anterior mitral valve leaflet traversal. The retrograde LVOT and LA catheters overlap in this view, as intended. The posterior mitral valve band provides a fluoroscopic marker to position the LVOT catheter along the base of the A2 scallop. The LA catheter is directing the multiloop snare and is supported by a transseptal rail through a transseptal sheath. (B) A 3-dimensional transesophageal echocardiograph of the same step, with a LVOT catheter positioned at the base of the A2 scallop, and the LA catheter pointing a multiloop snare at the other side of the A2 scallop. (C) Initiation of laceration. The kinked guidewire cutting edge is circled in green. The transcatheter heart valve is pre-positioned at the orifice of the mitral valve. The LAMPOON guidewire is pulled outward to apply tension. (D) The LAMPOON guidewire is electrified during further pulling to initiate laceration. (E) Completed laceration, with both retrograde catheters insulating the wire safely in the descending aorta. The kinked guidewire cutting edge, adjacent to the radiopaque piggyback tip marker, is seen sheathed in the catheter. LA = left atrium; other abbreviation as in Figure 1. Online Video 2 is depicted in this figure.
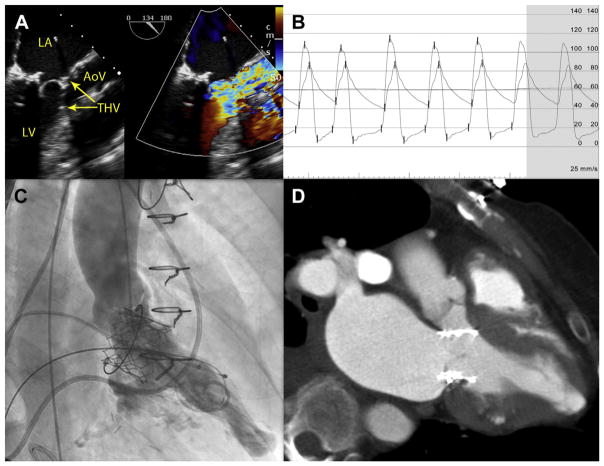
FIGURE 3. Imaging Immediately After LAMPOON for TMVR-in-Band.
These images are from the same patient as in Figure 2. (A) B-mode and color Doppler transesophageal echocardiography after LAMPOON and TMVR shows the stent struts protruding into the LVOT, and blood flow across those struts indicating successful leaflet splitting. (B) Catheter pressure measurements across the LVOT show a tolerable gradient of 16 mm Hg. (C) Left ventriculography and (D) contrast computed tomography after TMVR shows the THV encroaches completely across the LVOT and would nearly completely obstruct it if not for LAMPOON. AoV = aortic valve; LV = left ventricle; THV = transcatheter heart valve; TMVR = transcatheter mitral valve replacement; other abbreviations as in Figures 1 and 2. Online Video 2 is depicted in this figure.
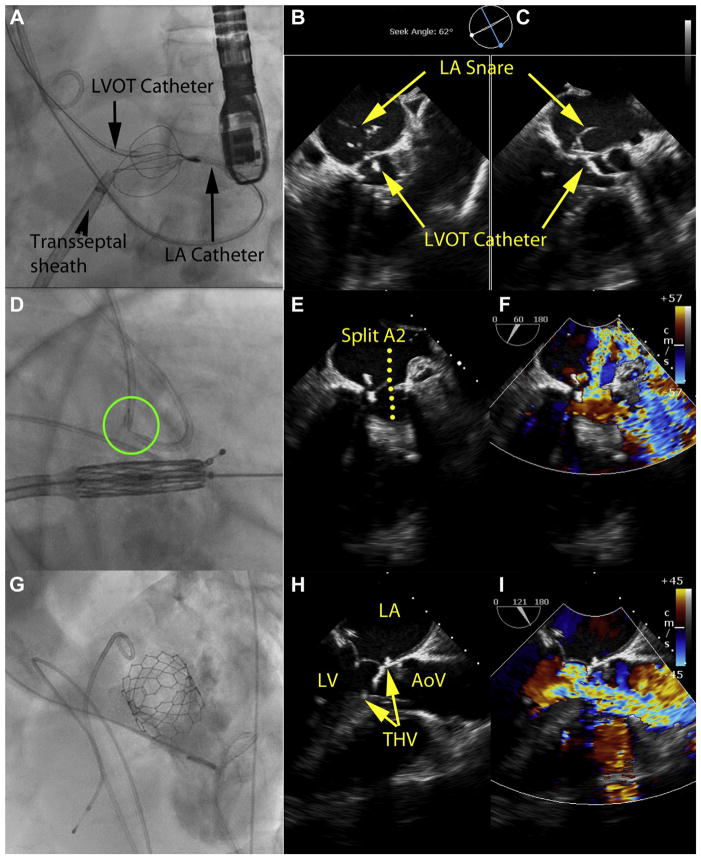
FIGURE 4. LAMPOON to Enable TMVR in Native Mitral Annular Calcification.
(A to C) Leaflet traversal, (D to F) leaflet laceration, and (G to I) imaging after LAMPOON and TMVR. (A) Guidewire traversal across the base of the anterior mitral leaflet. Four catheters are in place. An antegrade transseptal sheath, used for TMVR, is currently connected via a guidewire rail to control the retrograde LA catheter, which is used to deliver the multiloop snare on the LA side of the anterior mitral leaflet. A retrograde catheter in the LVOT is directing the traversing guidewire. There also is a pigtail catheter in the ascending aorta. (B, C) X-plane TEE immediately after leaflet traversal shows the mid-basal A2 position of the traversal system. Also evident are the 2 catheters and the LA snare. (D) The traversing guidewire tip has been externalized. The guidewire shaft is denuded, kinked, and exposed between the 2 catheters (green circle), and is electrified to slice the anterior mitral leaflet longitudinally. The THV is prepositioned for immediate deployment. (E, F) B-mode and color Doppler TEE after LAMPOON but before TMVR shows severe acute mitral valve regurgitation across the split A2 leaflet scallop. (G) Left ventriculography after TMVR shows the THV encroaches nearly completely across the LVOT and would nearly completely obstruct it if not for LAMPOON. (H, I) B-mode and color Doppler TEE after LAMPOON and TMVR shows the stent struts protruding into the LVOT, and blood flow across those struts indicating successful leaflet splitting. TEE = transesophageal echocardiography; other abbreviations as in Figures 1 to 3.
FIGURE 5. LAMPOON-Induced Anterior Mitral Leaflet Split.
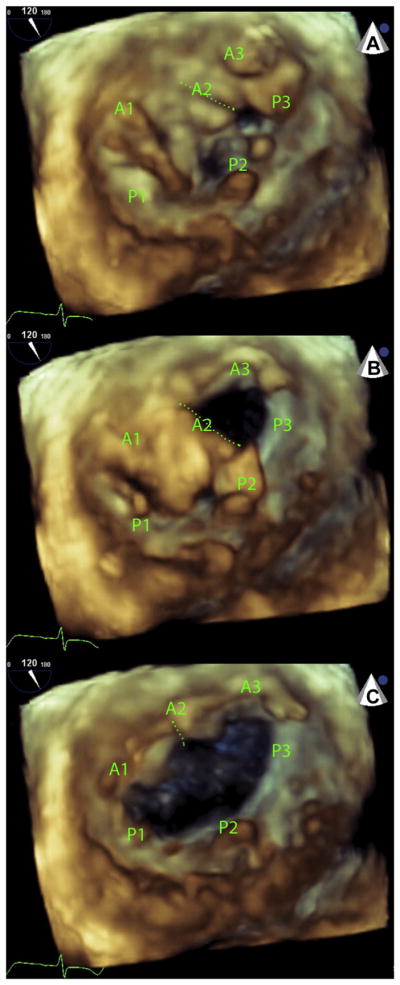
Three sequential diastolic frames of a 3-dimensional echocardiogram performed immediately after LAMPOON but before TMVR. The volume is rendered from a left-atrial “surgeons view.” (A) A laceration cleft is evident (dotted green line) at the beginning of diastole in the A2 scallop of the anterior mitral leaflet. (B) The medial half of A2 and the whole of A3 open early in diastole and then (C) the lateral half of A2 along with the whole of A1 open a fraction later in diastole. A1 to A3 and P1 to P3 represent the lateral to medial scallops of the anterior and posterior mitral leaflets, respectively. Abbreviations as in Figures 1 and 3.
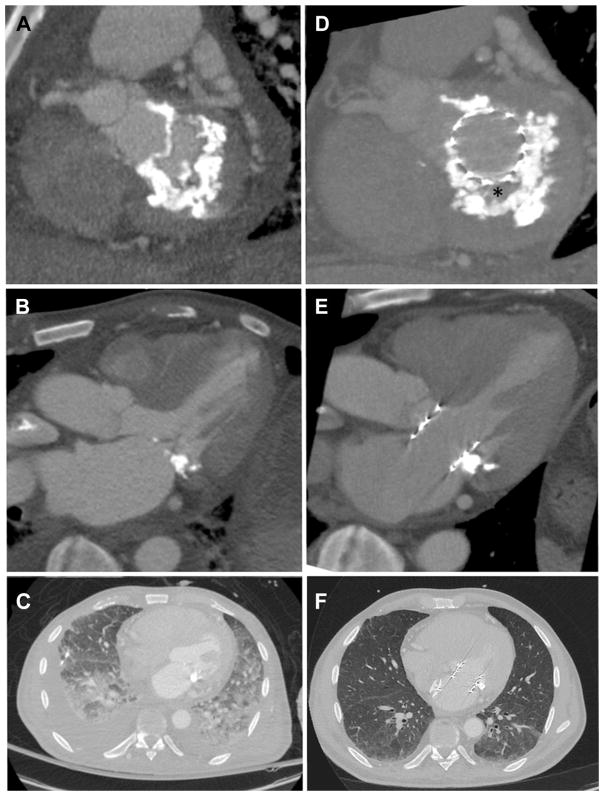
FIGURE 6. LAMPOON and TMVR in Mitral Annular Calcification.
Computed tomography scans before (A to C) and after (D to F) TMVR with LAMPOON in a patient treated for mitral annular calcification causing mitral valve stenosis. Noncalcified fibrous valve tissue (asterisk) is interposed between the THV and the mitral annular calcification (D). The transcatheter heart valve is shown to span across the LVOT (E), suggesting there would have been complete LVOT obstruction had LAMPOON not been performed. Follow-up chest images (F) show near-resolution of pulmonary alveolar hemorrhage and pleural effusion. Abbreviations as in Figures 1 and 3.
The blood pressure and heart rate did not decline in any patient during the 1-to-38-min interval between LAMPOON and TMVR. Hemodynamic details are shown in Table 2. The average post-procedure LVOT catheter gradient was 17.6 ± 12.4 mm Hg immediately after LAMPOON and TMVR in these patients otherwise expected to have intolerable LVOT obstruction or THV dysfunction.
X-plane TEE guided medial-lateral and base-to-tip positioning of the LVOT catheter before and during leaflet traversal and laceration, complemented by left anterior oblique caudal short-axis and right anterior oblique caudal pseudo-3-chamber fluoroscopic projections. A true 3-chamber extreme left anterior oblique cranial or extreme right anterior oblique caudal projection was not attainable in any patient.
POST-LAMPOON IMAGING
LAMPOON created a new jet of severe mitral regurgitation across the A2 scallop in all 5 cases (Figure 4F). After TMVR, blood flow across the THV struts was evident from the left ventricular inflow into the LVOT, which would not have been possible if the AML had not been disrupted (Figures 3A, 3C, 4G, and 4I). Echocardiography and CT details are summarized in Table 2.
SURVIVAL AND COMPLICATIONS
Four patients (80%) survived beyond 1 month (197 days [range 23 to 273 days] as of this report). Complications are described in Table 3. One patient died 23 days after TMVR with LAMPOON because of intractable right heart failure that did not improve after TMVR. There were no procedural strokes, clinically significant paravalvular leaks, or major bleeding or vascular complications of the LAMPOON and TMVR procedure.
TABLE 3.
Complications and Clinical Outcomes
| Complications (n = 5) | |
|
| |
| Valve embolization | 1 (20) |
| Paravalvular leak grade | None, n = 4 (80%) |
| Mild, n = 1 (20%) | |
| Stroke | 0 |
| Bleeding major | 0 |
| Vascular major | 0 |
| Left ventricular perforation or pseudoaneurysm | 0 |
| Hemolysis | 1 (20) |
|
| |
| Clinical outcomes (n = 5) | |
|
| |
| Length of stay after TMVR, days | 8.6 ± 5.6 |
| Intensive care unit length of stay, days | 3.2 ± 2.7 |
| Survival to hospital discharge | 5 (100) |
| Survival 30 days | 4 (80) |
| Survival ascertainment, days | 132 (23–208) |
Values are n (%), n, mean ± SD, or median (range).
Abbreviation as in Table 1.
In the flrst patient, the THV embolized immediately into the left atrium. In retrospect it was significantly undersized for the rigid annuloplasty ring. After successful TMVR using a 29-mm Sapien 3 THV, the embolized valve was secured against the LA septum using a 35-mm Amplatzer septal occluder.
Patient #5 suffered mild hemolysis, evident from low haptoglobin and elevated lactate dehydrogenase levels, and not requiring treatment. Post-procedure anemia resolved but haptoglobin remained depressed 4 months later. We suspect this is caused by mechanical red blood cell injury (20) from flow across the THV struts, because there is no paravalvular leak.
DISCUSSION
We have successfully lacerated the AML using a straightforward percutaneous technique (LAMPOON) immediately before TMVR in 5 patients. The technique resembles chordal-sparing AML resection that has become a standard in surgical mitral valve replacement (16,21). The technique succeeded in a range of different TMVR settings: mitral valve rigid ring annuloplasty, mitral valve semi-rigid ring annuloplasty, mitral valve flexible posterior band annuloplasty, and native MAC. In patients deemed ineligible for TMVR because of predicted catastrophic LVOT obstruction or THV dysfunction, LAMPOON allowed TMVR without THV dysfunction and generated LVOT obstruction that was less than otherwise predicted.
The LAMPOON technique is important because 9% to 22% of patients selected to undergo TMVR in annuloplasty rings or native MAC experience critical LVOT obstruction (14,22,23). At present at least one-third of patients seem to be excluded from TMVR out of predicted risk of LVOT obstruction caused by the displaced AML. LAMPOON may allow TMVR in most, or perhaps all such excluded patients when using commercially available (uncovered) aortic THV devices off-label. Moreover, long or redundant native mitral leaflets have occasionally interfered with THV function either by direct mechanical interposition or by creating a low-pressure Bernoulli jet that impairs THV closure. Three of 5 patients had such long and redundant AMLs. The LAMPOON technique may prevent this THV dysfunction by displacing the split mitral leaflet. Finally, the LAMPOON strategy of splitting the AML, combined with TMVR devices that allow flow across uncovered stent struts, should inform development of future dedicated TMVR devices.
Remarkably, no patient exhibited a change in heart rate or blood pressure in the short time interval between LAMPOON mitral laceration and TMVR. In each case, echocardiography revealed acute exacerbation of mitral regurgitation. Acute mitral regurgitation caused by leaflet tethering is a recognized cause of hemodynamic deterioration in antegrade trans-venous transseptal transcatheter aortic valve replacement (24), and unintentional guidewire laceration of the AML, in a tip-to-base fashion, is a recognized lethal complication of this approach (25). After LAMPOON, however, we observe a “grace period” that may reflect adaptation to left atrial volume and pressure overload from longstanding mitral stenosis or mitral regurgitation. Nonetheless, in every case we had pre-positioned the TMVR device in the left atrium to allow immediate implantation.
We did not observe severe mechanical complications of the LAMPOON procedure. One theoretical complication is injury to the aorta or aortic valve; we mitigated this risk by protecting the laceration wire surface using rigid braided guiding catheters. Another is mitral leaflet laceration through a lateral orifice of the mitral valve, which might leave a residual chorda that could prevent the split mitral leaflet from draping around the TMVR device. Another theoretical risk is of insufficiently basal traversal and laceration of the AML that causes insufficient leaflet debulking. We do not believe LAMPOON reduced stability of the THV implant; THV embolization in the first case reflected our mistaken selection of an undersized initial device. We attribute the observed low-grade hemolysis in patient #5 to nonlaminar flow across the THV struts as it spans across the LVOT. This would be classified as a mild complication according to the Mitral Valve Academic Research Criteria (26). Guidewire electrification may cause thromboembolism including cerebral thromboembolism; we mitigated this risk by anticoagulation and by selecting ablative energies used in cardiac electrophysiology procedures (27).
STUDY LIMITATIONS
Limitations of our work include the lack of a control group. We do not know with certainty that LVOT obstruction would have been clinically important without LAMPOON. However, we have compelling circumstantial evidence, such as the finding that the THV straddled the full width of the LVOT cases #3 and #5. Based on available evidence, it does not seem reasonable to offer TMVR without leaflet or septal debulking in patients with a low predicted neo-LVOT. Additional limitations are that the nature and long-term implications of THV flow disruption caused by redundant AMLs is not understood; neither is flow following LAMPOON. Given the infancy of this field, we have few data to predict post-TMVR LVOT obstruction with confidence, whether based on LVOT geometry or on specific AML configurations (length, aortomitral angle, redundancy, laxity). Despite LAMPOON there was appreciable but tolerable residual LVOT geometric obstruction and gradient in most of our patients. Relying on flow across THV struts may predispose to mechanical hemolysis, as we observed in 1 patient. Although this procedure relies on off-the-shelf catheter devices, the transcatheter valves were not designed and are not indicated for implantation in the mitral position, and are used off-label. In the mitral position, these THV devices may not perform as well as they do in the aortic position and, for example, may be subject to premature degeneration or thrombosis. Finally, the LAMPOON technique adds to the procedural complexity of antegrade transseptal TMVR, and perhaps should be undertaken in coordination with more experienced operators.
LAMPOON joins the family of transcatheter electrosurgery procedures. Originally limited to radiofrequency ablation of cardiac arrhythmias, transcatheter electrosurgery now includes atrial septal crossing by electrification of a Brockenbrough needle (28), and guidewire electrification for traversal of congenital cardiac lesions including pulmonic atresia (29) and complete aortic coarctation (30), transcaval transcatheter aortic valve replacement (17,31), and coronary CTO traversal (32), among others.
CONCLUSIONS
By splitting the AML without surgery, LAMPOON allowed successful TMVR in patients at risk of LVOT obstruction or THV dysfunction. Remarkably LAMPOON did not induce any short-term hemodynamic deterioration. Operators should use caution applying this new technique to patients. A clinical investigation of the procedure (NCT03015194) begins in 2017. The concept of disrupting the native anterior leaflet, to allow blood flow across THV struts, may inform further development of TMVR devices.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN
TMVR risks life-threatening LVOT obstruction by displacing the native anterior mitral leaflet.
WHAT IS NEW?
LAMPOON is a catheter technique to transect the anterior mitral leaflet, to prevent iatrogenic LVOT obstruction. In the 5 patients described, there was no immediate hemodynamic deterioration during the short interval between LAMPOON and TMVR. LAMPOON allowed TMVR without causing life-threatening LVOT obstruction or transcatheter heart valve dysfunction in patients believed otherwise ineligible for any treatment.
WHAT IS NEXT?
We plan a cautious multicenter investigation of LAMPOON in a larger number of patients at high risk of LVOT obstruction and prosthetic heart valve dysfunction after TMVR.
Acknowledgments
The authors thank Lauren Wheeler, Jonathan Mazal, Bill Schenke, Annette Stine, Adriana Byrnes, Sharon Howell, Ioannis Para-statidis, Frank Corrigan, Patricia Keegan, Amy Simone, Ateet Patel, Jessica Forcillo, Kelly Broxton, Neil Holtz, Deborah Dunn, Andrew Youmans, Benjamin Maples, James Lee, Nancy Sarpong, Ronnie Ramadan, Talal Alatassi, and Vicki Smith for patient care and data management; and Alan Hoofring of National Institutes of Health Division of Medical Arts.
Supported without industry funding by the clinical programs of the Structural Heart and Valve Center, Emory University Hospital; the Center for Structural Heart Disease, Division of Cardiology, Henry Ford Health System; and the Division of Intramural Research (Z01-HL006040), National Heart Lung and Blood Institute, National Institutes of Health. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular; and his employer has research contracts from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific. Dr. Greenbaum is a proctor for Edwards Lifesciences and St Jude Medical. Dr. O’Neill is a consultant for Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, and St. Jude Medical; and serves on the Board of Directors of Neovasc Inc. Dr. Thourani is a consultant for Edwards Lifesciences. Dr. Lerakis is a consultant for Edwards Lifesciences and Abbott Vascular. Dr. Kim is a consultant for Edwards Lifesciences; and a proctor for B. Braun.
ABBREVIATIONS AND ACRONYMS
- AML
anterior mitral valve leaflet
- CT
computed tomography
- LA
left atrium
- LAMPOON
laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction
- LVOT
left ventricular outflow tract
- MAC
mitral annular calcification
- TEE
transesophageal echocardiography
- THV
transcatheter heart valve
- TMVR
transcatheter mitral valve replacement
Footnotes
APPENDIX
For supplemental videos and their legends, please see the online version of this article
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose
References
- 1.Anderson RH, Spicer DE, Hlavacek AM, Cook AC, Backer CL. Wilcox’s Surgical Anatomy of the Heart. 4. New York: Cambridge University Press; 2013. [Google Scholar]
- 2.Come PC, Riley MF, Weintraub RM, et al. Dynamic left ventricular outflow tract obstruction when the anterior leaflet is retained at prosthetic mitral valve replacement. Ann Thorac Surg. 1987;43:561–3. doi: 10.1016/s0003-4975(10)60213-5. [DOI] [PubMed] [Google Scholar]
- 3.Esper E, Ferdinand FD, Aronson S, Karp RB. Prosthetic mitral valve replacement: late complications after native valve preservation. Ann Thorac Surg. 1997;63:541–3. doi: 10.1016/s0003-4975(96)01107-1. [DOI] [PubMed] [Google Scholar]
- 4.Bapat V, Pirone F, Kapetanakis S, Rajani R, Niederer S. Factors influencing left ventricular outflow tract obstruction following a mitral valve-in-valve or valve-in-ring procedure, part 1. Catheter Cardiovasc Interv. 2015;86:747–60. doi: 10.1002/ccd.25928. [DOI] [PubMed] [Google Scholar]
- 5.Hamid NB, Khalique OK, Monaghan MJ, et al. Transcatheter valve implantation in failed surgically inserted bioprosthesis: review and practical guide to echocardiographic imaging in valve-in-valve procedures. J Am Coll Cardiol Img. 2015;8:960–79. doi: 10.1016/j.jcmg.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Blanke P, Naoum C, Dvir D, et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the Neo-LVOT. J Am Coll Cardiol Img. 2017;10:482–5. doi: 10.1016/j.jcmg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Wang DD, Eng M, Greenbaum A, et al. Predicting LVOT obstruction after TMVR. J Am Coll Cardiol Img. 2016;9:1349–52. doi: 10.1016/j.jcmg.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenbaum AB, Condado JF, Eng MH, et al. Long or redundant leaflet complicating trans-catheter mitral valve replacement: case vignettes that advocate for removal or reduction of the anterior mitral leaflet. Catheter Cardiovasc Interv. 2016 doi: 10.1002/ccd.27054. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Hopmeyer J, Lefebvre XP, Schwammenthal E, Yoganathan AP, Levine RA. Importance of leaflet elongation in causing systolic anterior motion of the mitral valve. J Heart Valve Dis. 1997;6:149–59. [PubMed] [Google Scholar]
- 10.Deharo P, Urena M, Himbert D, et al. Bail-out alcohol septal ablation for left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv. 2016;9:e73–6. doi: 10.1016/j.jcin.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero M, Wang DD, O’Neill W. Percutaneous alcohol septal ablation to acutely reduce left ventricular outflow tract obstruction induced by transcatheter mitral valve replacement. Catheter Cardiovasc Interv. 2016;88:E191–7. doi: 10.1002/ccd.26649. [DOI] [PubMed] [Google Scholar]
- 12.Murashita T, Suri RM, Daly RC. Sapien XT transcatheter mitral valve replacement under direct vision in the setting of significant mitral annular calcification. Ann Thorac Surg. 2016;101:1171–4. doi: 10.1016/j.athoracsur.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Dahle G, Rein KA, Fiane AE. Edwards SAPIEN XT in native stenotic mitral valve, open technique on cardiopulmonary bypass: an option, but safe? Innovations. 2016;11:288–90. doi: 10.1097/IMI.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero M, Dvir D, Himbert D, et al. Trans-catheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first Multicenter Global Registry. J Am Coll Cardiol Intv. 2016;9:1361–71. doi: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Khan JM, Rogers T, Schenke WH, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. J Am Coll Cardiol Intv. 2016;9:1835–43. doi: 10.1016/j.jcin.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David TE. Mitral valve replacement with preservation of chordae tendinae: rationale and technical considerations. Ann Thorac Surg. 1986;41:680–2. doi: 10.1016/s0003-4975(10)63092-5. [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. 2014;63:2795–804. doi: 10.1016/j.jacc.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederman RJ, Babaliaros VC, Greenbaum AB. How to perform transcaval access and closure for transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;86:1242–54. doi: 10.1002/ccd.26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geske JB, Cullen MW, Sorajja P, Ommen SR, Nishimura RA. Assessment of left ventricular outflow gradient: hypertrophic cardiomyopathy versus aortic valvular stenosis. J Am Coll Cardiol Intv. 2012;5:675–81. doi: 10.1016/j.jcin.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Yeo TC, Freeman WK, Schaff HV, Orszulak TA. Mechanisms of hemolysis after mitral valve repair: assessment by serial echocardiography. J Am Coll Cardiol. 1998;32:717–23. doi: 10.1016/s0735-1097(98)00294-0. [DOI] [PubMed] [Google Scholar]
- 21.Reardon MJ, David TE. Mitral valve replacement with preservation of the subvalvular apparatus. Curr Opin Cardiol. 1999;14:104–10. doi: 10.1097/00001573-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Descoutures F, Himbert D, Maisano F, et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg. 2013;44:e8–15. doi: 10.1093/ejcts/ezt155. [DOI] [PubMed] [Google Scholar]
- 23.Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal trans-catheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. J Am Coll Cardiol Intv. 2016;9:1161–74. [Google Scholar]
- 24.Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–23. doi: 10.1016/j.jacc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 25.Hanzel GS, Harrity PJ, Schreiber TL, O’Neill WW. Retrograde percutaneous aortic valve implantation for critical aortic stenosis. Catheter Cardiovasc Interv. 2005;64:322–6. doi: 10.1002/ccd.20299. [DOI] [PubMed] [Google Scholar]
- 26.Stone GW, Adams DH, Abraham WT, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement. Part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66:308–21. doi: 10.1016/j.jacc.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 27.Takami M, Lehmann HI, Parker KD, Welker KM, Johnson SB, Packer DL. Effect of left atrial ablation process and strategy on microemboli formation during irrigated radio-frequency catheter ablation in an in vivo model. Circ Arrhythm Electrophysiol. 2016;9:e003226. doi: 10.1161/CIRCEP.115.003226. [DOI] [PubMed] [Google Scholar]
- 28.Bidart C, Vaseghi M, Cesario DA, et al. Radio-frequency current delivery via transseptal needle to facilitate septal puncture. Heart Rhythm. 2007;4:1573–6. doi: 10.1016/j.hrthm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal E, Qureshi SA, Chan KC, et al. Radiofrequency-assisted balloon dilatation in patients with pulmonary valve atresia and an intact ventricular septum. Br Heart J. 1993;69:347–51. doi: 10.1136/hrt.69.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almashham Y, Dahdah N, Miro J. Use of radiofrequency then stent implantation for recanalization of complete aorta coarctation. Pediatr Cardiol. 2008;29:207–9. doi: 10.1007/s00246-007-9090-2. [DOI] [PubMed] [Google Scholar]
- 31.Halabi M, Ratnayaka K, Faranesh AZ, Chen MY, Schenke WH, Lederman RJ. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: pre-clinical validation. J Am Coll Cardiol. 2013;61:1745–6. doi: 10.1016/j.jacc.2013.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson W, Harvey J, Dhawan R. E-CART (ElectroCautery-Assisted Re-enTry) of an aorto-ostial right coronary artery chronic total occlusion: first-in-man. J Am Coll Cardiol Intv. 2016;9:2356–8. doi: 10.1016/j.jcin.2016.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.