Abstract
Necroptosis is a programmed form of inflammatory cell death involved in various pathologies, such as viral infections. In two new papers published in The EMBO Journal and EMBO Reports, Z‐DNA binding protein 1 (ZBP1) is now shown to sense RNAs during viral infection or after caspase inhibition and activate necroptosis. This may suggest that Z‐RNAs are molecular patterns for activation of necroptosis.
Subject Categories: Autophagy & Cell Death, Immunology
Pattern recognition receptors recognize foreign and endogenous nucleic acids to activate mechanisms involved in host defense against pathogens or induction of inflammation. Z‐DNA binding protein 1 (ZBP1) was first identified as an interferon‐induced cytosolic DNA sensor (Takaoka et al, 2007). In particular, ZBP1 binds nucleic acids in an unusual Z‐conformation through its Z‐DNA‐binding domains (ZBDs), termed Zα1 and Zα2. ZBDs have been shown to bind both Z‐DNA and Z‐RNA (Placido et al, 2007; Thapa et al, 2016). However, biological functions of nucleic acids in this Z‐conformation, and especially Z‐RNAs, are only partially understood. Interestingly, ZBP1 has been shown to play a critical role in necroptosis induced by mouse cytomegalovirus (MCMV) and influenza A virus (IAV; Kuriakose et al, 2016; Thapa et al, 2016). Necroptosis is a type of programmed cell death activated by specific immune receptors and infections (Cai & Liu, 2014; Sridharan & Upton, 2014). The serine/threonine protein kinase RIPK3 integrates upstream signals to become activated and phosphorylates its downstream target, the necroptosis effector mixed lineage kinase‐like (MLKL). Subsequent oligomerization of MLKL induces its insertion in membranes leading to formation of necroptotic pores and ultimately to cell lysis (Cai & Liu, 2014; Sridharan & Upton, 2014). This mechanism plays an important role in various pathologies such as cancer, neurodegeneration, and clearance of virally infected cells (Cai & Liu, 2014; Sridharan & Upton, 2014). However, viruses have evolved strategies to counteract this mechanism. For example, MCMV M45 disrupts ZBP1–RIPK3 interaction that is crucial for ZBP1‐mediated necroptosis activation by MCMV infection. Conversely, MCMV M36 (also called vICA) is believed to predispose cells to necroptosis by blocking caspase‐8, an important mediator of apoptosis known to inhibit necroptosis (Kaiser et al, 2013).
In two recent articles published in EMBO Reports and The EMBO Journal (Maelfait et al, 2017; Sridharan et al, 2017), the authors investigated the mechanism of ZBP1‐mediated activation of necroptosis by MCMV infection (Fig 1). Since the WT virus efficiently blocks this mechanism by the expression of M45, they studied a previously characterized MCMV expressing a mutated form of M45 (MCMV‐M45mutRHIM) that fails to inhibit necroptosis. By mutating or deleting ZBP1 ZBDs, the authors uncovered a crucial role of nucleic acid binding function of ZBP1 in MCMV‐M45mutRHIM‐induced necroptosis (Maelfait et al, 2017; Sridharan et al, 2017). Sridharan et al (2017) reported that although deletion of Zα1 and Zα2 ZBP1 ZBDs decreased cell death induced by MCMV‐M45mutRHIM, point mutations in these domains of residues critical for ZBP1 interaction with nucleic acids delayed cell death but did not significantly inhibit it. Conversely, mutations of these same residues by Maelfait et al (2017) prevented virus‐induced necroptosis. These discrepancies might be due to differences in the mutations performed in these two studies, as well as in distinct cell lines used. Moreover, Sridharan et al (2017) speculated that alternative ZBP1 ZBDs functions, other than nucleic acid binding, could also participate to virus‐induced necroptosis.
Figure 1. Model for ZBP1‐mediated activation of necroptosis by recognition of RNAs.
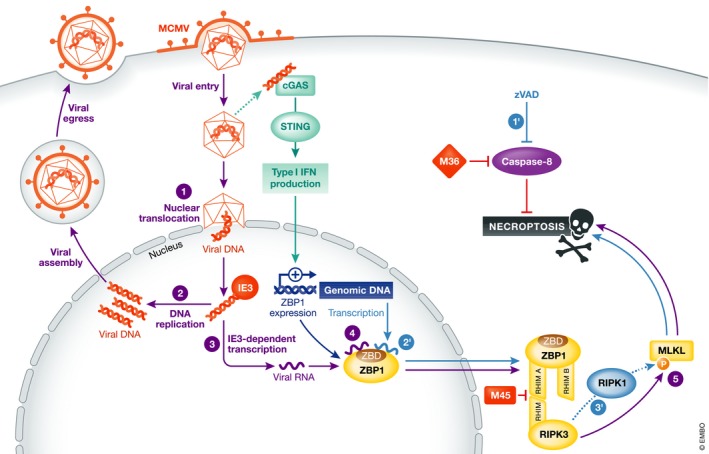
Purple circles (1–5) show ZBP1‐mediated activation of necroptosis by MCMV infection. (1, 2) Viral entry, nuclear replication, and production of progeny virus. (3) An integral part of the replication cycle is IE3‐dependent viral RNA transcription. (4) ZBP1 senses viral RNA, thus stimulating interaction with RIPK3 through its RHIM A domain. (5) RIPK3 activation induces phosphorylation of MLKL, leading to activation of necroptosis. The viral protein M36 inhibits caspase‐8‐mediated blocking of necroptosis, while M45 inhibits ZBP1–RIPK3 interaction. ZBP1 expression can be stimulated by type I IFN that can be produced, for example, after sensing of viral DNA by the cGAS‐STING pathway. Blue circles (1′–3′) show ZBP1‐mediated activation of necroptosis by endogenous RNAs upon caspase blockade. (1′) In the presence of zVAD, a caspase inhibitor, ZBP1 activates necroptosis. (2′) This is likely mediated through sensing of endogenous RNAs (Z‐RNAs?). (3′) Caspase‐inhibition‐induced necroptosis involved both RIPK3 and RIPK1.
Surprisingly, Maelfait et al (2017) showed in infected cells that ZBP1 directly bound via its ZBDs to newly synthesized RNAs but not viral DNA. Moreover, both papers showed that MCMV‐M45mutRHIM‐induced necroptosis required RNA synthesis but not viral DNA replication (Maelfait et al, 2017; Sridharan et al, 2017). Interestingly, MCMV immediate‐early protein 3 (IE3) protein, essential for the transcription of viral genes, played a crucial role in this mechanism (Maelfait et al, 2017; Sridharan et al, 2017). These results suggest that MCMV‐M45mutRHIM‐induced necroptosis is mediated by the sensing of newly transcribed RNA, possibly viral RNA, by ZBP1. Although described as a cytosolic sensor, ZBP1 was shown to shuttle between the cytoplasm and the nucleus (Deigendesch et al, 2006). Sridharan et al (2017) showed that ZBP1 and RIPK3 accumulated in the nucleus of MCMV‐infected cells concurrently with the induction of necroptosis. Moreover, MCMV‐M45mutRHIM‐induced necroptosis required trafficking of the capsid and viral genome to the nucleus of infected cells. This suggests that activation of MCMV‐M45mutRHIM‐mediated necroptosis involves nuclear recognition of newly transcribed RNA.
Necroptosis in known to participate to antiviral immunity notably by eliminating virus‐infected cells (Sridharan & Upton, 2014). In the context of MCMV‐M45mutRHIM infection, Maelfait et al showed that ZBP ZBDs play an important role in the control of viral genome replication and viral protein expression (Maelfait et al, 2017; Sridharan et al, 2017). They also investigated the function of ZBP1 ZBDs in vivo, using a model of knock‐in mice expressing ZBP1 with mutations in its ZBDs (Zbp1Zα1α2/Zα1α2). Interestingly, these mice failed to efficiently clear MCMV‐M45mutRHIM infection, unlike WT mice. These results support the idea that ZBP1‐mediated necroptosis induced by nucleic acid binding in context of viral infections plays an antiviral role in vivo. Interestingly, Maelfait et al (2017) showed that ZBP1 ZBDs were also required for necroptosis triggered by ectopic expression of ZBP1 upon caspase blockade. Strikingly, this mechanism differs at least partially from ZBP1‐dependent MCMV‐M45mutRHIM‐induced necroptosis, as it involves both RIPK1 and RIPK3, while virus‐induced necroptosis is independent on RIPK1 (Maelfait et al, 2017). However, the exact function of RIPK1 in ZBP1‐mediated activation of necroptosis upon caspase inhibition remains largely elusive. Maelfait et al (2017) also demonstrated that ZBP1 bound to endogenous RNA through its ZBDs, in a RNase‐ but not DNase‐sensitive manner, further strengthening the hypothesis of ZBP1 being a RNA sensor. The authors propose that ZBP1 senses self‐RNA, but is only able to activate necroptosis when high enough levels of downstream signaling components, like RIPK3, are available as seen when caspase‐8 is blocked.
These studies introduce the interesting concept of ZBP1 being able to recognize RNAs probably in a Z‐form (Maelfait et al, 2017; Sridharan et al, 2017; Fig 1). Yet, the mechanisms of Z‐nucleic acids formation remain elusive. Moreover, more studies are required to uncover their biological functions under normal and pathological conditions. These findings bring up the idea that Z‐nucleic acids could represent molecular patterns that activate ZBP1‐mediated cell death, as well as possibly other molecules that contain ZBDs. Understanding the mechanisms that govern formation of Z‐RNAs thus represents an emerging challenge in this field. Moreover, there is a requirement for more knowledge on the role of ZBP1, and necroptosis in general, in the host responses to infections and sterile tissue damage. Interestingly, two studies uncovered that ZBP1 senses IAV infection and is important for IAV‐induced cell death, thus suggesting an important role for this receptor in antiviral defense (Kuriakose et al, 2016; Thapa et al, 2016). Further studies on the implications of necroptosis in infection biology or in inflammation could provide information on the balance between cell death and survival, viral replication and restriction, protective immunity and deleterious inflammation.
See also: J Maelfait et al (September 2017) and H Sridharan et al (August 2017)
References
- Cai Z, Liu ZG (2014) Execution of RIPK3‐regulated necrosis. Mol Cell Oncol 1: e960759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigendesch N, Koch‐Nolte F, Rothenburg S (2006) ZBP1 subcellular localization and association with stress granules is controlled by its Z‐DNA binding domains. Nucleic Acids Res 34: 5007–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Mocarski ES (2013) Viral modulation of programmed necrosis. Curr Opin Virol 3: 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD (2016) ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1: aag2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, Rehwinkel J (2017) Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J 36: 2529–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placido D, Brown BA, Lowenhaupt K, Rich A, Athanasiadis A (2007) A left‐handed RNA double helix bound by the Z alpha domain of the RNA‐editing enzyme ADAR1. Structure 15: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan H, Upton JW (2014) Programmed necrosis in microbial pathogenesis. Trends Microbiol 22: 199–207 [DOI] [PubMed] [Google Scholar]
- Sridharan H, Ragan KB, Guo H, Gilley RP, Landsteiner VJ, Kaiser WJ, Upton JW (2017) Murine cytomegalovirus IE3‐dependent transcription is required for DAI/ZBP1‐mediated necroptosis. EMBO Rep 18: 1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T (2007) DAI (DLM‐1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448: 501–505 [DOI] [PubMed] [Google Scholar]
- Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, tenOever BR, Thomas PG, Upton JW, Balachandran S (2016) DAI senses influenza A virus genomic RNA and activates RIPK3‐dependent cell death. Cell Host Microbe 20: 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]


