Abstract
The Yes‐associated protein (YAP) is an important transcriptional co‐activator that mediates the cellular response to mechanical and cytoskeletal cues. In two recent papers published in The EMBO Journal, Dae‐Sik Lim and colleagues show how YAP activity affects cancer formation and metastasis via a crosstalk with myocardin‐related transcription factors (MRTFs; Kim et al, 2017) and SKP2‐dependent cell cycle progression (Jang et al, 2017).
Subject Categories: Cancer, Signal Transduction, Transcription
Extracellular biophysical information such as external mechanical forces and extracellular matrix (ECM) rigidity can trigger intracellular signaling events resulting in functional outputs such as cell proliferation, differentiation, and migration through a process termed mechanotransduction (Sun et al, 2016). The ability of cells to sense and respond to mechanical stimuli during mechanotransduction is essential for several developmental processes as well as postnatal homeostasis (Jaalouk & Lammerding, 2009). On the other hand, defects in mechanotransduction influence disease courses, for example, of cardiomyopathy, muscular dystrophy, nephrotic syndrome, and cancer.
Transcription factors play a central role in integrating mechanical cues with intracellular signaling networks. Mechanical cues, for example, induce a fast translocation of specific transcriptional cofactors such as MRTFs, YAP, and TAZ from the cytoplasm to the cell nucleus where they activate specific transcriptional programs (Finch‐Edmondson & Sudol, 2016). YAP/TAZ are transcriptional co‐activators that are best known for their role in the Hippo pathway (Moroishi et al, 2015). However, their subcellular localization and hence activity can also be regulated by ECM rigidity, cell shape, and cytoskeletal tension (Moroishi et al, 2015). Upon nuclear translocation, YAP/TAZ recognize specific cis‐regulatory DNA elements via binding to transcription factors, foremost members of the TEA domain (TEAD)‐containing transcription factor family and trigger gene expression programs related to cell proliferation, survival, differentiation, and invasion (Moroishi et al, 2015). Unlike YAP/TAZ, MRTFs are transcriptional co‐activators that bind G‐actin and are thereby retained in the cytoplasm or kept inactive within the nucleus (Olson & Nordheim, 2010). Upon G‐actin polymerization, MRTFs are released from G‐actin, translocate into the nucleus, bind the transcription factor serum response factor (SRF), and control the expression of primarily cytoskeletal proteins including actin as well as numerous focal adhesion proteins (Olson & Nordheim, 2010). Two papers from the Lim laboratory provide new insight on how YAP, in response to ECM rigidity and cytoskeletal changes, promote cell cycle progression and in concert with MRTFs cancer cell invasion.
The paper published in this issue of The EMBO Journal (Jang et al, 2017) reports a novel YAP target that affects the cell cycle machinery and whose induced expression is triggered by physical cues such as ECM rigidity. Yap is known for its ability to control the expression of cell cycle regulators such as cyclin D1, E2F1 and cyclin E (Mizuno et al, 2012). Jang and co‐workers identified the S‐phase kinase‐associated protein‐2 (SKP2) as a novel YAP target. SKP2 (also called F‐box and leucine‐rich repeat protein‐1; FBXL1) is an F‐box protein of the Skp1‐Cullin1‐F‐box (SCF) ubiquitin ligase complex that targets the cell cycle inhibitors p27 and p21 for proteasomal degradation and thereby promotes cell cycle entry and G1/S transition (Uddin et al, 2016). Contrary to previous studies that attributed the high SKP2 protein levels in different human cancers to an increased protein stability, Jang and co‐workers found that Skp2 mRNA levels are regulated by mechanical cues such as cell attachment and ECM rigidity and depend on the translocation and activation of YAP, as YAP depletion results in reduced SKP2 and increased p21 and p27 levels (Fig 1; Jang et al, 2017). In support of these findings, they identified a bona fide TEAD consensus sequence in the human Skp2 gene to which YAP binds to induce transcription.
Figure 1. YAP‐dependent transcriptional regulation drives cancer formation and metastasis.
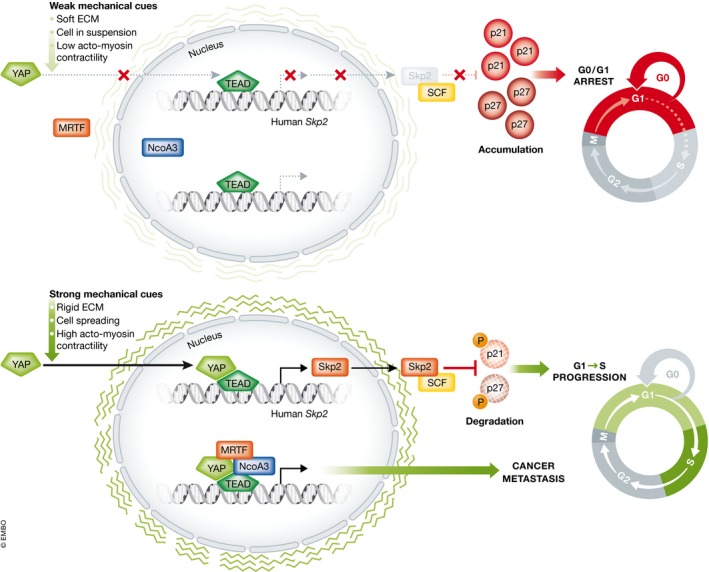
Mechanical cues such as cell density, acto‐myosin contractility, or rigid ECM increase SKP2 expression through TEAD‐YAP activity, which subsequently assembles the Skp2/Skp1‐Cullin‐F‐box (SCFS kp2) complex leading to degradation of the cell cycle inhibitors p21/p27. If YAP is inactivated, for example, when cells grow on soft substrates, SKP2 levels are low, p21/p27 proteins accumulate leading to cell cycle exit. Mechanical cues can also promote MRTF‐mediated recruitment of NCOA3 to the TEAD‐YAP complex, which can further strengthen the TEAD‐YAP‐mediated transcriptional activity.
YAP‐induced Skp2 transcription has major consequences for cell cycle progression. From a series of experiments, in which endogenous YAP was depleted together with or without the SKP2 targets p21 and p27, Jang et al (2017) concluded that SKP2 may be, at least in some cells, the key regulator of YAP depletion‐induced cell cycle exit at G0. These findings were supported by several additional observations. First, overexpression of SKP2 was sufficient to counteract cell senescence induced by p21/p27 stabilization upon YAP depletion. Second, they found a positive correlation of YAP and Skp2 expression at the mRNA level in microarray datasets from published breast cancer studies and at the protein level by immunostaining of several breast cancer tissue samples. Finally, Jang and co‐workers generated tumor spheroid‐like acini and mammospheres in 3D matrices mimicking the elastic modulus of a breast tumor and found that their formation depends on YAP as well as the downstream Skp2 transcription (Jang et al, 2017).
An interesting and unexpected observation by Jang et al (2017) was that the YAP–SKP2 signaling pathway does not operate in mice. Support for an exquisite role in human cells came from experiments showing that YAP was unable to induce Skp2 mRNA expression in different murine cell lines and that the murine Skp2 gene lacked the crucial TEAD‐binding consensus site downstream of the transcription start. Hence, the suppression of oncogene‐induced mammary tumors upon YAP gene deletion in mice (Chen et al, 2014) must be mediated by Skp2‐independent mechanism(s). Whether this Skp2‐independent mechanism(s) also operate(s) in human cancer has to be shown in future studies.
YAP/TAZ‐induced transcriptional programs also promote cancer cell invasion, which is a major reason, next to their ability to control the cell cycle, why YAP/TAZ are so potent oncogenes (Moroishi et al, 2015). A second paper from the Lim laboratory, also published in The EMBO Journal, addresses cancer metastasis and highlights how this process benefits from the cooperation of YAP/TAZ with another, well‐known transcriptional co‐activator family called MRTFs (Kim et al, 2017). Several published papers reported that MRTF‐A and YAP can directly bind each other to facilitate the expression of RhoA‐regulated genes (Yu et al, 2015) and that depletion of MRTF‐B profoundly influences TAZ expression and localization (Liu et al, 2016; Speight et al, 2016). In their paper, Kim et al (2017) show that MRTFs promote the transcriptional activity of the TEAD‐YAP complex independent of SRF and canonical Hippo signaling, as neither SRF was required for the induction of the transcriptional program nor TAZ expression or YAP phosphorylation and localization changed in MRTF‐A‐ and MRTF‐B‐manipulated cells. Interestingly, however, MRTF‐mediated activation of the TEAD‐YAP activity requires a direct interaction between MRTFs and YAP as well as the recruitment of nuclear receptor co‐activator 3 (NCOA3) to the DNA‐bound TEAD‐YAP transcription complex (Fig 1). NCOA3 is a member of steroid receptor co‐activator (SRC) family that interacts with several nuclear receptors including estrogen receptor alpha (ERα) to enhance the expression of their cognate target genes (Chang & Wu, 2012). Notably, NCOA3 is amplified or overexpressed in over 60% of breast cancer samples and other malignancies and the high NCOA3 levels were shown to correlate with cancer aggressiveness (Anzick et al, 1997). In line with these reports, Kim and colleagues found that expression of wild‐type MRTF‐B but not YAP binding‐deficient MRTF‐B spurred breast cancer cell invasion in vitro and metastasis to the lung in vivo (Kim et al, 2017). In light of the findings discussed above, the TEAD‐YAP‐MRTFs‐NCOA3 complex may also control Skp2 transcription and the cell cycle and thereby contribute at two fronts to tumor aggressiveness.
In summary, the two papers from the Lim laboratory demonstrate that ECM rigidity and cytoskeletal dynamics control not only the nuclear translocation but also the assembly of transcriptional co‐activators into large transcriptional complexes, which in turn induce the expression of gene programs that promote invasion and metastasis as well as “master genes” such as Skp2 to promote cell cycle progression. At the center stage of this regulation is YAP, whose nuclear translocation, activation, and assembly into transcriptionally active complexes are controlled by mechanical cues. Since tumor behavior is not only influenced by biophysical cues but also by metabolic stress, hormones, inflammation, it is likely that the mechanosignaling‐induced activation of transcriptional co‐activators cooperates with additional transcriptional regulators with the task to integrate all environmental cues into growth‐ and migration‐promoting signaling outputs, a realistic possibility awaiting future scrutiny.
See also: W Jang et al (September 2017) and T Kim et al (February 2017)
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277: 965–968 [DOI] [PubMed] [Google Scholar]
- Chang AK, Wu H (2012) The role of AIB1 in breast cancer. Oncol Lett 4: 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D (2014) A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev 28: 432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch‐Edmondson M, Sudol M (2016) Framework to function: mechanosensitive regulators of gene transcription. Cell Mol Biol Lett 21: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J (2009) Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim T, Koo JS, Kim SK, Lim DS (2017) Mechanical cue‐induced YAP instructs Skp2‐dependent cell cycle exit and oncogenic signaling. EMBO J 36: 2510–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Hwang D, Lee D, Kim JH, Kim SY, Lim DS (2017) MRTF potentiates TEAD‐YAP transcriptional activity causing metastasis. EMBO J 36: 520–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Chan SW, Guo F, Toloczko A, Cui L, Hong W (2016) MRTF/SRF dependent transcriptional regulation of TAZ in breast cancer cells. Oncotarget 7: 13706–13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H, Sekido Y (2012) YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle‐promoting genes. Oncogene 31: 5117–5122 [DOI] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL (2015) The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Nordheim A (2010) Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 11: 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight P, Kofler M, Szászi K, Kapus A (2016) Context‐dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton‐regulated MRTF and TAZ and TGFβ‐regulated Smad3. Nat Commun 18: 11642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Guo SS, Fässler R (2016) Integrin‐mediated mechanotransduction. J Cell Biol 215: 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM (2016) Involvement of F‐BOX proteins in progression and development of human malignancies. Semin Cancer Biol 36: 18–32 [DOI] [PubMed] [Google Scholar]
- Yu OM, Miyamoto S, Brown JH (2015) Myocardin‐related transcription factor A and yes‐associated protein exert dual control in G protein‐coupled receptor‐ and RhoA‐mediated transcriptional regulation and cell proliferation. Mol Cell Biol 36: 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]


