Abstract
We previously identified serine protease HtrA1 as a down-regulated gene in epithelial ovarian cancer (EOC), but the functional consequence of loss of HtrA1 in EOC remains largely unclear. Here, we report that loss of HtrA1 attenuates anoikis - a critical physiological barrier for tumor metastasis. In response to loss of anchorage, HtrA1 expression was up-regulated in SKOV3 cells, resulting in autocatalytic activation of HtrA1. Stable knockdown of HtrA1 in SKOV3 and TOV21G cells resulted in resistance to anoikis due to enhanced activation of EGFR/AKT pathway. In suspended SKOV3 cells, enhanced expression of HtrA1 inhibited EGFR/AKT pathway, leading to increased cell death, while protease inactive mutant HtrA1 failed to result in either the inhibition of EGFR/AKT pathway or increased cell death, suggesting the requirement of HtrA1 protease activity in regulating anoikis. Immunoprecipitation and immunofluorescence assays revealed that HtrA1 interacted with EGFR not only on the cell membrane but also in the nucleus. Most importantly, down-regulation of HtrA1 significantly enhanced the peritoneal dissemination of SKOV3ip1 cells in NOD/SCID mice, with increased p-EGFR level in corresponding tumor nodules compared to that in xenografts originated from the control cells. Taken together, these data reveal for the first time a novel function of HtrA1 in promoting anoikis by attenuating activation of EGFR/AKT pathway that may contribute to its metastasis suppression capacity, thus providing a possible explanation for the aggressive nature of human ovarian tumors with down-regulated HtrA1.
Keywords: anoikis, HtrA1, apoptosis, metastasis, ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death among gynecologic diseases in Western countries (1, 2). Over 70% of patients with EOC are diagnosed at advanced stage, characterized by intraperitoneal carcinomatosis with massive ascites. The extensive intra-abdominal disease is difficult to eradicate completely by surgery. Most patients require aggressive chemotherapy and suffer from relapsed cancer (3, 4). Understanding the molecular mechanisms that contribute to the metastatic dissemination of ovarian cancer has important implications in eradicating this life-threatening disease.
We previously identified serine protease HtrA1 as a down-regulated gene in a majority of EOC (5), but the functional consequence of loss of HtrA1 in EOC remains largely unclear. Increasing evidence suggests the involvement of HtrA1 down-regulation in tumor progression and metastasis. HtrA1 expression is frequently down-regulated in metastatic foci of various tumors compared to primary tumors, such as melanoma (6), prostate cancer (7, 8), sarcoma (9), and lung cancer (10). Additionally, higher loss of HtrA1 is strongly associated with poor prognosis in human mesothelioma (11) and glioma (12). HtrA1 expression is also down-regulated in metastatic ovarian cancer compared to normal ovary tissue, suggesting a possible modulating role of HtrA1 in ovarian cancer metastasis (13). However, to our best knowledge, the mechanisms by which loss of HtrA1 promotes the development of secondary tumors have not been reported.
As a physiological barrier to developing metastasis, epithelial cells normally undergo apoptosis, by a process termed “anoikis”, due to loss of contact with extracellular matrix (14-16). Acquisition of resistance to anoikis is therefore a prerequisite for EOC cells to survive in ascitic fluids before forming metastatic foci. Interestingly, HtrA1 is recognized as a pro-apoptotic factor in EOC (5, 17). Forced expression of full-length HtrA1 is sufficient to induce apoptosis of ovarian cancer cells. Considering down-regulation of pro-apoptotic proteins renders anoikis resistance in breast cells (18), we hypothesized that HtrA1 might influence metastasis by regulating detachment-induced apoptosis. Here we provide evidence for a previously unknown role of HtrA1 in regulating metastasis of ovarian cancer, which links down-regulation of HtrA1 with increased resistance to anoikis through deregulated EGFR survival pathway.
Materials and Methods
Cell culture, transfection and drug treatment
Human ovarian cancer cell lines SKOV3, TOV21G and human embryonal kidney cell line HEK293T were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). OV167 cell line was established as described previously (17). SKOV3.ip1 cell line was a kind gift from Dr. Ellen Vitetta. Cells were transfected with plasmids using Lipofectamine Plus (Invitrogen). Human EGF was purchased from R&D Systems (Minneapolis, MN). Erlotinib was purchased from Chemie Tek Inc (Indianapolis, IN). PD98059 and LY294002 were purchased from Calbiochem (San Diego, CA).
Plasmids
Protease active wide type (WT) HtrA1, protease inactive mutant (SA) HtrA1, and protease active Mac25-deleted (Δ Mac25) construct in pcDNA3.1 plasmids were generated as previously described (17, 19). Lentiviral plasmids SFFV-Luciferase was kindly provided by Dr. Yasuhiro Ikeda. HtrA1 targeting shRNA (sh1 and sh2) and non-targeting shRNA (NT) were purchased from Sigma-Aldrich.
Western blot analysis
Western blot analysis was performed as described previously (17). Cell lysates were analyzed with the following antibodies: anti-HtrA1, p-EGFR (Tyr992), EGFR, p-AKT, AKT, p44/42 MAPK, MAPK, C-caspase-3, and β-actin. Anti-HtrA1 antibody was rabbit polyclonal antibody raised as previously described (19). Monoclonal antibody against β-actin was purchased from Sigma-Aldrich. All other antibodies were obtained from Cell Signalling Technology (Danvers, MA). Densitometric analysis was performed for immunoblots from three independent experiments using Scion Image software (Frederick, MD). Expression values of phosphorylated signals were normalized with corresponding total expression and indicated as fold-change over the controls.
Anoikis assay
Anoikis was induced as previously described (20). Specifically, cells were transferred from standard, adhesive plates to ultra-low cluster (ULC) plates (Corning, NY) and incubated for the indicated time points at the density of 0.5-1×106 cells/ ml. Cells were allowed to reattach in adhesive plates, cultured overnight and followed by Coomassie staining or MTT assay performed as previously described (17).
Flow cytometry
1×106 of cells were incubated with 2 μg/ml PI solution at room temperature for 15 min in the dark. Apoptotic cells were quantified by flow cytometry (Becton Dickinson, San Jose, CA) using a single laser, emitting excitation light at 488 nm.
Immunoprecipitation
HEK293T cells were transiently co-transfected with EGFR plus WT, SA or ΔMac25 HtrA1 expression plasmids, and 24 h later, lyzed on ice for half an hour. Immunoprecipitates were obtained using either mouse anti-EGFR or rabbit anti-HtrA1 antibodies conjugated to protein A/G–agarose (Santa Cruz, CA).
Immunostaining and confocal microscopy
Immunofluorescence staining with EGFR, HtrA1, and Hoechst DNA dye in OV167 and SKOV3 cells was done as described previously (21). Laser-scanning confocal microscopy was performed on a Zeiss LSM510 with krypton-argon and helium-neon lasers.
In vivo metastasis assay
All mice were handled according to the Guide for the Care and Use of Laboratory Animals. The procedures were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic College of Medicine. Female NOD/SCID mice (National Cancer Institute-Frederick Cancer Research and Development Center), aged 4 to 6 weeks, were used for this study with 6 mice in each group. SKOV3ip1 cells were re-engineered to express luciferase (SKOV3ip-luc). 2.5×106 of SKOV3ip-luc cells with NT or HtrA1 shRNA were injected into the abdomen of mice. Bioluminescent reporter imaging was performed to monitor the seeding of SKOV3ip-luc cells on the 7th, 14th, and 21st day after injection. Bioluminescent signals were recorded using the Xenogen IVIS System. On the 28th day after inoculation, all mice were sacrificed due to generation of ascites and laparotomy was performed.
Immunohistochemical studies
The tumors were fixed in 10% paraformaldehyde for 24 h and paraffin-embedded. Then 4 μm-thick consecutive sections were cut for immunohistochemistry for HtrA1, p-EGFR, and C-caspase-3 as previously described (17). The positive cells were counted from five randomly selected high-power fields, and expressed as percentages of total cells counted.
Statistical analysis
Two-tailed Student's t test and ANOVA followed by Newman-Keuls test were performed using Prism 3.0 (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
Results
Expression of HtrA1 is up-regulated during anoikis
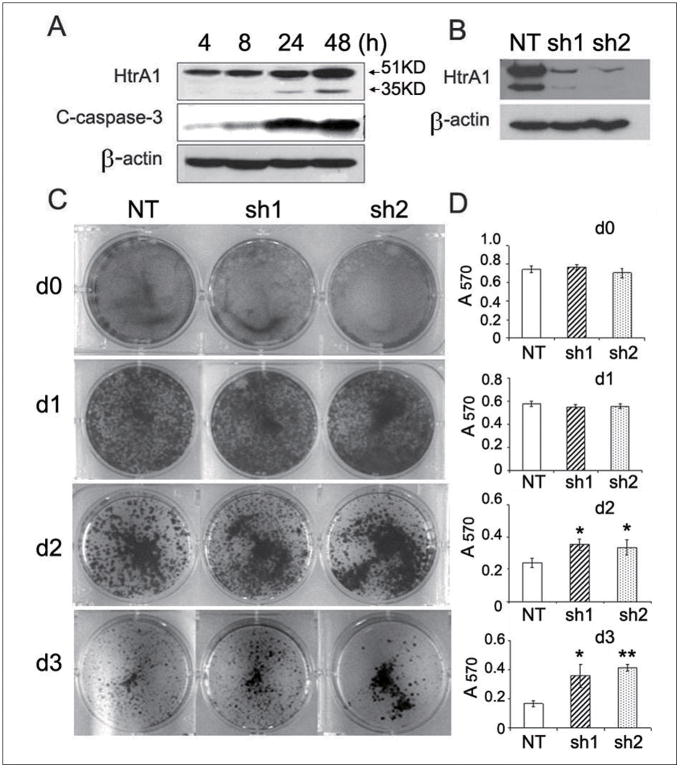
To elucidate the effects of HtrA1 on anoikis, we first examined the effects of detachment on SKOV3 cells with endogenous HtrA1 expression. Western blot analysis confirmed activation of apoptosis upon attachment withdrawal and showed C-caspase-3 increasing in a time-dependent manner (Fig. 1A). Twenty fours hours after detachment, HtrA1 expression was upregulated (Fig. 1A), resulting in auto-activation with increasing 35KD proteolytic fragment, as previously reported (17).
Figure 1.
Loss of HtrA1 attenuates anoikis. A, up-regulated expression of HtrA1 induced by loss of attachment. SKOV3 cells were cultured in suspension for indicated time points. Immunoblot was performed with antibodies against HtrA1, C-caspase-3, or β-actin. B, knockdown of HtrA1 in SKOV3 cells with HtrA1 shRNAs (sh1 and sh2). C-D, Down-regulated HtrA1 leads to increased cell survival of suspended cells. 1×106 of SKOV3 clonal cells (NT, sh1 and sh2) were grown in suspension for 0-3 d and subsequently transferred into 6-well adhesive plates followed by Coomassie staining (C) or MTT assay (D). Each column displays the mean±s.d. from 3 separate experiments. *, P<0.05, **, P<0.01
Down-regulation of HtrA1 renders cell resistance to anoikis in vitro
We next determined the effect of loss of HtrA1 on anoikis in ovarian cancer cells. Previously described SKOV3 clonal cells with non-targeting (NT) or HtrA1 shRNA (sh1 and sh2) were used (21). Western blot analysis confirmed efficient knockdown of HtrA1 by sh1 and sh2 (Fig. 1B). Cells were prevented from attachment for 0 to 3 days, and subsequently transferred to adhesive plates. Strikingly, Coomassie staining demonstrated that down-regulation of HtrA1 led to a significantly increased cell survival compared to the control after 48 h in suspension (Fig. 1C). MTT assay indicated a ∼1.5-fold protection from cell death upon 48 h by knockdown of HtrA1, reaching ∼2.5-fold by 72 h (Fig. 1D). Consistent with these results higher levels of C-caspase-3 were observed in NT cells compared to HtrA1 knockdown cells after cell detachment (Supplementary Fig. S1). Similar results were obtained in TOV21G cells (Supplementary Fig. S2). Soft agar assay revealed increased numbers of colonies in HtrA1 knockdown cells compared to the control cells, suggesting that loss of HtrA1 promotes anchorage-independent growth (Supplementary Fig. S3). These data suggest a novel role for loss of HtrA1 in mediating anoikis resistance.
HtrA1 down-regulation attenuates anoikis through EGFR signaling
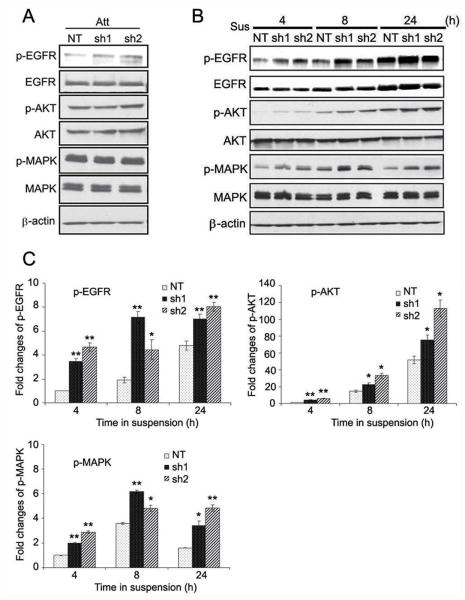
In an attempt to elucidate the mechanism by which loss of HtrA1 confers cell resistance to anoikis, we compared the survival signals between HtrA1 expressing and knockdown cells. We first examined the potential involvement of EGFR signaling, because EGFR is commonly over-expressed in many human malignant tumors of epithelial origin and often associated with an aggressive phenotype implying a poor prognosis (22). When cells were grown on adhesive plates, there was a mild increase in p-EGFR in HtrA1 knockdown cell lines, sh1 and sh2, without remarkable influences on the activities of its downstream mediators, AKT and MAPK signals (Fig. 2A). Interestingly, when cells were cultured in suspension, there was a significant increase in the level of EGFR phosphorylation in two HtrA1 knockdown cell lines, sh1 and sh2. Densitometric analysis indicated a 4-, 3-, and 1.5-fold higher levels of p-EGFR in sh1 and sh2 cells than in NT control cells at 4, 8, and 24 h post-suspension, respectively (Fig. 2B-C). Moreover, this activation translated into increased activation of its downstream mediators. The levels of both p-AKT and p-MAPK were at least 1.5-fold higher in cells lacking HtrA1 than in the controls at each time point (Fig. 2B-C). These data suggest that loss of HtrA1 results in increased activity of EGFR pathway, which may play a role in resistance to anoikis.
Figure 2.
Down-regulation of HtrA1 leads to increased activity of EGFR pathway. A, mild increase of EGFR activation in HtrA1 knockdown cells under attachment condition (Att). B-C, significantly enhanced activation of EGFR pathway in HtrA1 knockdown cells during suspension culture. 1×10 6 of SKOV3 clonal cells (NT, sh1 and sh2) were grown in suspension (Sus) for the indicated hours. Levels of indicated signals were determined by Western blot analysis (B) and densitometry analysis of p-EGFR, p-AKT, and p-MAPK levels was performed (C). Expression values of phosphorylated signals were normalized with corresponding total expression and indicated as fold-change over the controls (SKOV3 with stable transfection of non-targeting shRNA). Bar graphs represent mean±s.d. from 3 separate experiments. *, P<0.05, **, P<0.01
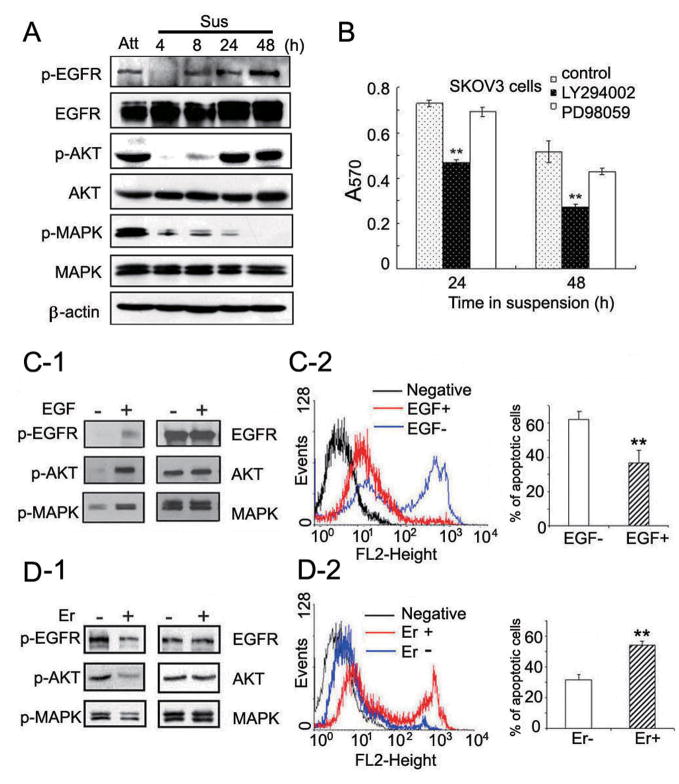
Next, we performed time course experiments to examine EGFR signaling in more detail in SKOV3 cells before and after suspension. The results showed that loss of contact led to remarkably decreased activities of EGFR, AKT, and MAPK signals in the early period of suspension (Fig. 3A, 4 and 8h time points). Phosphorylation of EGFR was almost undetectable at 4 h post-suspension, but gradually increased after 8 h, suggesting a rapid recovery of EGFR activity. The p-AKT level showed similar kinetics to that of p-EGFR. By contrast, the p-MAPK level gradually decreased. These results are consistent with what was seen with suspended NT cells alone (Fig 1B, NT at 4, 8 and 24 hr), suggesting that AKT signaling may be more tightly regulated by EGFR during anoikis and it may preferentially contribute to cell survival compared to MAPK signaling. Indeed, when suspended cells were treated with AKT and MAPK inhibitors separately, AKT inhibitor, LY294002, but not MAPK inhibitor, PD98059, led to dramatically decreased cell survival (Fig. 3B). We obtained similar results with TOV21G cells (Supplementary Fig. S4A-B). We further assessed the effects of EGF (a ligand for EGFR) and Erlotinib (a phosphorylation inhibitor of EGFR) on cell signaling and anoikis. In the presence of EGF (20ng/ml), the activity of EGFR/AKT pathway increased (Fig. 3C-1), leading to a decreased ratio of apoptotic cells with positive PI staining (Fig. 3C-2). As shown in the bar graph, the percentage of apoptotic cells decreased from 61.8% to 38.2% after exposure to EGF. In turn, treatment with Erlotinib (5 μM) resulted in dramatically decreased activity of EGFR/AKT pathway (Fig. 3D-1), leading to an increased ratio of apoptotic cells from 31.5% to 53.6% (Fig. 3D-2). Similar results were obtained with TOV21G cells by MTT assay after exposure to EGF or Erlotinib (Supplementary Fig. S4 C-D). These data demonstrate that EGFR/AKT pathway plays a critical role in cell survival after cell detachment.
Figure 3.
EGFR/AKT pathway plays a crucial role in cell survival during suspension. A, changes of EGFR signaling before and after cell detachment. 1×106 of SKOV3 cells were cultured under attachment condition (Att) or suspension condition (Sus) for the indicated time points. Levels of indicated signals were determined. B, inhibition of AKT pathway leads to decreased cell survival in suspended cells. 1×106 of suspended SKOV3 cells were treated with DMSO, LY294002 (20 μM), or PD98059 (20 μM) separately for indicated time points. Cell survival was assessed by MTT assay. C-D, Effects of EGF and Erlotinib on cell survival during suspension culture. SKOV3 cells were cultured in serum-free medium with or without 20 ng/ml EGF (C1-2), or in regular medium with or without 5 μM Erlotinib (D1-2) for 24 h, respectively. Levels of indicated signals were determined by Western blot analysis (C-1, D-1). Cell apoptosis were quantified by flow cytometry according to positive PI staining (C-2, D-2). Cells without adding PI solution served as negative control (black peaks). Red peaks indicate cells under treatment with EGF or Erlotinib. Blue peaks indicate cells without any treatment. Bar graph shows the percentages of apoptotic cells. Each column displays the mean±s.d. from 3 separate experiments. *, P<0.05, **, P<0.01.
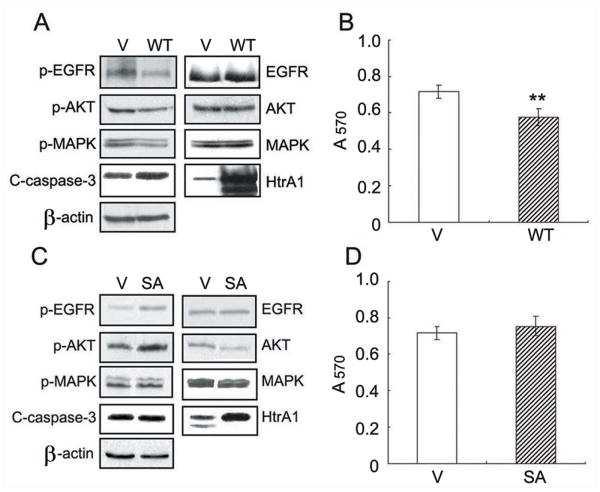
HtrA1 Protease activity is required for inhibition of EGFR signaling
Since down-regulation of HtrA1 enhanced EGFR activity in suspended cells, we surmised that over-expression of HtrA1 may lead to attenuation of EGFR activity. SKOV3 cells transiently transfected with empty vectors or WT HtrA1 were subject to anoikis assay. As expected, over-expression of HtrA1 attenuated the recovery of EGFR activity during suspension, with lower levels of p-AKT and p-MAPK but higher level of C-caspase-3 (Fig. 4A). MTT assay showed a significantly increased cell death in cells over-expressing HtrA1 (Fig. 4B).
Figure 4.
HtrA1 protease activity is required for promoting anoikis and inhibiting EGFR signaling. A-B, effects of forced expression of HtrA1 on anoikis. SKOV3 cells transiently transfected with vector or WT HtrA1 were cultured in forced suspension for 24 h. The activities of indicated signals were examined by Western blot analysis (A) and cell survival was determined by MTT assay (B). C-D, effects of SA mutant HtrA1 on anoikis. SKOV3 cells were transiently transfected with empty vector or SA mutant HtrA1 and cultured in forced suspension for 24 h, followed by immunoblot with indicated antibodies (C) or MTT assay (D). Data are expressed as mean±s.d. and represent 3 independent trials performed in triplicate. *, P < 0.05, **, P < 0.01
In order to conclusively prove that HtrA1 serine protease activity is necessary for regulating anoikis, SKOV3 cells were transiently transfected with SA mutant HtrA1 and subject to anoikis assay. Interestingly, SA mutant HtrA1 resulted in a mild increase in the activity of EGFR/AKT pathway as well as a mild increase in cell survival, although without achieving statistical significance (Fig. 4C-D).
HtrA1 interacts with EGFR
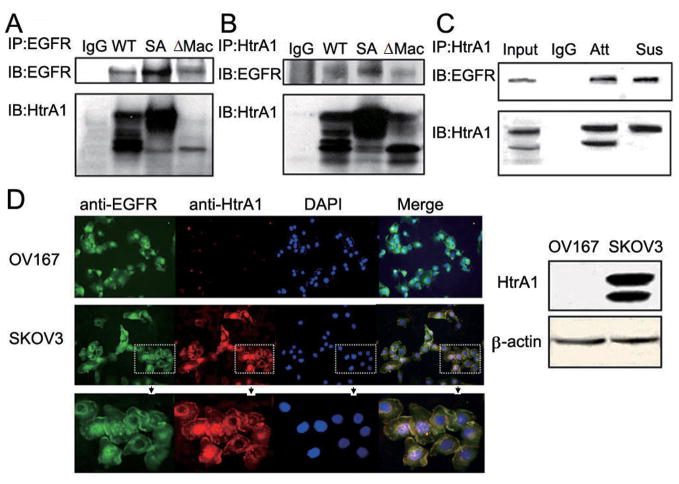
Our previous studies have shown that HtrA1 is not only a secreted protein, but also localized to the cytoplasm and associates with tubulins within the cell (21). To determine whether it can interact with EGFR, we co-expressed EGFR with WT HtrA1 in HEK293T cells. As shown in Fig. 5A (lane 2), immunoprecipitation with anti-EFGR antibody showed that HtrA1 was co-precipitated with EGFR. To further investigate whether this HtrA1-EGFR interaction depends on HtrA1 protease activity and whether the active 35KD product, ΔMac25 HtrA1 (17), can also interact with EGFR, we co-expressed EGFR with SA mutant or ΔMac25 HtrA1 in HEK293T cells. As shown in Fig. 5A (lane 3 and 4), both SA mutant and ΔMac25 HtrA1 were co-precipitated with EGFR. These results were consistent with our reverse immunoprecipitation with anti-HtrA1 antibody. EGFR was co-precipitated with WT, SA mutant and ΔMac25 HtrA1 (Fig. 5B). To rule out the possibility that these interactions were due to over-expression artifacts, we examined the association between endogenous HtrA1 and EGFR in SKOV3 cell lysates. As shown in Fig. 5C (lane 3), EGFR was successfully pulled down by endogenous HtrA1. To confirm that HtrA1 can associate with EGFR also in suspended cells, SKOV3 cells were cultured in suspension for 8 h before cell lysates were collected. As shown in Fig. 5C (lane 4), again EGFR was co-precipitated with HtrA1. All these data strongly demonstrate that both full length HtrA1 and its 35KD active product can associate with EGFR, independent of HtrA1 serine protease activity. Immunoflourescence in SKOV3 cells showed co-localization of HtrA1 and EGFR on the cell membrane (Fig. 5D). Interestingly, in a few cells the co-localization of HtrA1 and EGFR in the nucleus was also observed, which was verified by the co-immunoprecipitation of HtrA1 and EGFR in the nuclear fractions from SKOV3 cells (Supplementary Fig. S5).
Figure 5.
HtrA1 interacts with EGFR. A-B, exogenous HtrA1 and EGFR associate with each other. Plasmids encoding WT HtrA1, SA mutant HtrA1 or ΔMac25 HtrA1 were co-transfected with EGFR separately into HEK293T cells. After 24 h, immunoprecipitation was performed with mouse anti-EGFR antibody (A) or rabbit anti-HtrA1 antibody (B). Normal IgG was used as the negative control. C, endogenous HtrA1 associates with endogenous EGFR. SKOV3 cells were cultured under attachment condition (Att) or suspension condition (Sus) for 8 h, followed by immunoprecipitation with rabbit anti-HtrA1 antibody. D, immunolocalization of HtrA1 and EGFR. SKOV3 cells were fixed with 4% PFA and stained for the expression of EGFR (green) and HtrA1 (red). Nuclei were counterstained with DAPI (blue). OV167 cells which do not express HtrA1 served as negative controls. HtrA1 co-localizes with EGFR on the cell membrane in SKOV3 cells (see merged images). The bottom panel shows magnification of regions in dashed square from the middle panel. The right panel shows expression levels of HtrA1 in OV167 and SKOV3 cells.
Down-regulation of HtrA1 promotes peritoneal dissemination of ovarian cancer cells in vivo
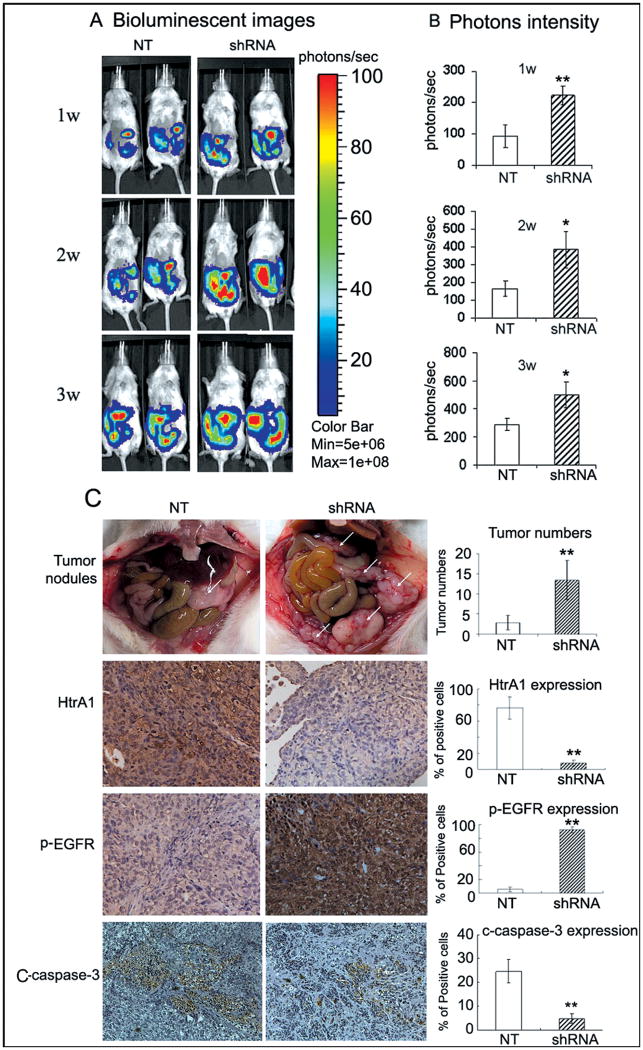
SKOV3ip1 cells were re-engineered to express luciferase (SKOV3ip-luc). HtrA1 downregulated batch clones were generated with HtrA1 shRNA lentivirus (sh2). Batch clones expressing NT shRNA served as controls. Western blot analysis confirmed efficient knockdown of HtrA1 expression by HtrA1 shRNA (Supplementary Fig. S6). To mimic the in vivo process of abdominal dissemination, cells with NT or HtrA1 shRNA were injected intraperitoneally into the NOD-SCID mice. One week post injection, the bioluminescence in the abdominal regions of mice in HtrA1 shRNA group was 2.4-fold higher than in NT group and maintained at a higher level during the whole observation period (Fig. 6A-B). Tumor nodules were identified in 5 of 6 (83.3%) mice in each group. The pattern of tumor formation differed dramatically in mice challenged with NT and HtrA1 shRNA transfectants. The latter produced a significantly higher incidence of tumor formation, characterized with small-volume nodules throughout the peritoneal cavity (Fig. 6C). NT transfectants showed a mean colony number of 3 (range 1-5) per mouse, whereas shRNA transfectants showed a mean colony number of 14 (range 7-20) per mouse. Pathologic review confirmed tumor metastasis into neighboring organs such as liver, diaphragm and occasionally pancreas in both groups. However, mice in shRNA group developed nine metastatic foci outside peritoneal cavity, 3-fold higher than that in NT group. No metastases were found in lungs in each group. Immunohistochemistry with anti-HtrA1 antibodies revealed that the expression of HtrA1 was maintained at a significantly lower level in tumors derived from HtrA1 knockdown cells. As shown in Fig. 6C, most cancer cells were positive for HtrA1 in NT control group (76.33 ± 13.71 %), while the positive ratio of HtrA1 in shRNA group was only 7.50 ± 3.87 % (P<0.0001). Importantly, anti-p-EGFR and anti-C-caspase-3 immunostaining demonstrated significantly higher levels of p-EGFR accompanied by lower levels of C-capsase-3 in HtrA1 knockdown xenografts.
Figure 6.
Down-regulation of HtrA1 promotes peritoneal dissemination of ovarian cancer cells. A, bioluminescent images in mice. 2.5×106 of SKOV3ip-luc cells with NT or HtrA1 shRNA were injected i.p. into the NOD-SCID mice. Abdominal colonization was measured using bioluminescence at 1, 2, and 3 weeks post injection. B, bar Graph showing photon intensity. C, pathological characteristics of dissemination in HtrA1 knock-down xenografts. Significantly higher numbers of tumor nodules were observed in mice from shRNA group compared to those from NT group as indicated by white arrows. Bar graph shows the average number of tumor nodules per mouse. Expression of HtrA1, p-EGFR, and C-capase-3 in tumor sections were analyzed with immunohistochemistry, photographed at ×200magnification. Schematic representation shows the quantified expression level of HtrA1 and p-EGFR. Data are expressed as mean±s.d. *, P < 0.05, **, P < 0.01
Discussion
Understanding anoikis seems to be of particular importance for cancer research as malignant cells, once begin to metastasize, they have obviously acquired properties rendering them resistant to anoikis (23, 24). In patients with advanced ovarian cancer, the peritoneal cavity accumulates large amounts of ascites containing tumor cells. These cells are shed from the primary tumor, and subsequently can attach to the omentum and invade the peritoneal mesothelial wall, forming secondary lesions throughout the peritoneal cavity. It has been reported that after the inoculation of cancer cells into the peritoneal cavity, the first adhesion of cancer cells to the peritoneum takes place on day 5 (25). This suggests that before forming metastases, EOC cells must acquire anoikis resistance to survive for a few days in ascites. Predictably, induction of anoikis was shown to suppress peritoneal dissemination (26). Understanding the precise mechanisms responsible for the deregulation of anoikis is of great importance for prevention and treatment of metastatic ovarian cancer.
Here we identified HtrA1 as a novel mediator regulating anoikis in EOC cells. We found that HtrA1 expression was up-regulated in response to stress triggered by the absence of adhesion signals, leading to autocatalytic activation (Fig. 1A). Strikingly, Coomassie staining and MTT assay demonstrated that loss of HtrA1 resulted in 1.5- and 2.5-fold protection from anoikis by 48 and 72 h respectively after cell detachment (Fig. 1C-D). With HtrA1 down-regulated cells producing more colonies, soft-agar assay reinforced our notion that loss of HtrA1 attenuates the need for anchorage (Supplementary Fig. S2). HtrA1 down-regulation renders cells more resistant to anoikis, suggesting that up-regulation of HtrA1 expression contributes to cell death. Hence, our results constitute the first report about an inhibitory role of HtrA1 for tumor cell survival under anoikis conditions. In agreement with previous studies (27, 28), our in vivo experiments demonstrated that the protection from anoikis endows EOC cells with increased metastatic potential. When injected intraperitoneally, HtrA1 knockdown SKOV3ip1 cells formed 4.7-fold more tumor colonies compared to the controls. All these data confirmed our hypothesis that down-regulation of HtrA1 confers cell resistance to anoikis and finally promotes the abdominal dissemination of ovarian cancer.
Over-expression of EGFR occurs in 17% to 62% of ovarian cancer (29, 30). Activation of EGFR has been associated with protection from anoikis in various cell types (31-35). Consistently, we found that in SKOV3 cells which over-express EGFR, EGFR activation alleviated the requirement of matrix engagement for cell survival, which can be enhanced by EGF and attenuated by Erlotinib treatment (Fig.3C-D). The observed protection effect of EGFR may account for the reported close correlation between over-expressed EGFR and ovarian cancer metastasis (22). However, the mechanism responsible for activation of EGFR after detachment is not understood yet. Autonomous LPA production by ovarian cancer cells can induce constitutive pro-HB-EGF shedding and EGFR activation (36). Both LPA and HB-EGF were found significantly elevated in ascitic fluid from ovarian cancer patients (37), suggesting the possibility of transactivation of EGFR by LPA. Other possible mechanisms may be some ligand-independent activation pathways, including interaction with integrins formed at cell-cell contacts (38), “cross-talk” with chemokine G-protein-coupled receptor (39), or Redox mediated transactivation (34).
Both AKT and MAPK are recognized as crucial effectors contributing to cell survival under anoikis condition in numerous cell types (14, 31-33). However, the specific functions of these two pathways vary greatly depending on the tissue and cell contexts. In the case of suspended SKOV3 cells, we provide evidence that AKT plays a more crucial role in cell survival during anoikis. Along with the recovery of EGFR activity, only AKT but not MAPK activity increased. Moreover, incubation of cells with an AKT inhibitor (LY 294002), but not MAPK inhibitor (PD98059), resulted in dramatic cell death in suspended cells (Fig. 3B, Supplementary Fig. S3A-B). Accumulating evidence suggests that AKT plays a central role in anoikis resistance in ovarian cancer. The anoikis resistance conferred by both RAB25 and TrkB was also found to be associated with activation of the PI3K/AKT pathway (27, 40). Activation of AKT not only leads to pro-mitotic transcriptional activation but maybe more importantly, portrays multiple inhibitory effects on the apoptotic machinery such as inactivation of caspase-9, phosphorylation of the pro-apoptotic protein Bim and Bad, up-regulation of Bcl-Xl and Mcl-1, as well as activation of nuclear factor-κB (NF-κB) (41-43).
In the present study, we have delineated a novel molecular link between EGFR and HtrA1. Down-regulation of HtrA1 enhanced the activation of EGFR in suspended cells (Fig. 2), resulting in increased cell survival (Fig. 1C-D), while forced expression of HtrA1 resulted in attenuated EGFR activation, leading to decreased cell survival during suspension culture (Fig. 4A-B). These data strongly suggest that HtrA1 acts up-stream of EGFR, inhibits EGFR activation and finally promotes anoikis. The observation of significantly higher level of p-EGFR in HtrA1 down-regulated xenografts further confirmed this inhibition role in vivo. SA mutant HtrA1 failed to cause increased cell death, indicating the requirement of HtrA1 protease activity. The reason why we did not observe a significantly increased cell survival in SA mutant HtrA1 transfectants may be due to the effect of endogenous HtrA1 and limited transfection efficiency. The secreted HtrA1 was shown to regulate many intra- and extra-cellular biological effects (44-45). In our study, immunoprecipitation and confocal assays demonstrated that HtrA1, as well as its 35KD active form, can bind to EGFR on the cell membrane (Fig. 5). Interestingly, in a few cells the co-localization of HtrA1 and EGFR in the nucleus was also observed. These findings are consistent with recent reports that both HtrA1 and EGFR have been detected in cell nucleus (46, 47). Accumulating evidence reveal that nuclear EGFR is involved in several different cellular processes that are important in cancer progression, such as gene transcription associated with cell proliferation, DNA synthesis and repair, and even cell resistance to chemotherapy (48, 49). More recently, high nuclear EGFR is reported to be associated with poor prognosis in ovarian cancer (50). Co-localization of HtrA1 and EGFR in the nucleus implies that nuclear HtrA1 may act as a regulator of nuclear EGFR, which may influence the process of ovarian cancer metastasis. However, how HtrA1 regulates EGFR and what is the contribution of this interaction in nucleus to anoikis needs further investigation.
In summary, we report here a novel property of HtrA1 in promoting detachment-induced cell death – anoikis and identify EGFR as its new downstream signal. Down-regulation of HtrA1 in ovarian cancer leads to the relief of inhibiting action on EGFR, confers cell resistance to anoikis, and eventually promotes intraperitoneal dissemination. These results strongly indicate HtrA1 as well as its targeted EGFR pathway as rational molecular targets for preventing and inhibiting metastasis in ovarian cancer.
Supplementary Material
Acknowledgments
Grant support: This work was funded by grants from the National Cancer Institute CA12340 (to V.S. and J.C.), the Mayo Clinic Bernard and Edith Waterman Center for Cancer Genetics (to V.S.) and the Ovarian Cancer Research Fund PEO/MC/01.08 (to X. H.)
We thank Dr. Zhixue Liu (Emory University, GA) and members of the Shridhar lab for stimulating discussions; Dr. Ikeda Yasuhiro (Mayo Clinic, MN) for SFFV-Luc plasmids.
Abbreviations
- C-caspase-3
cleaved caspase-3
- HtrA
high temperature requirement A
- ΔMac25
Mac25-deleted
- WT
wide type
- SA
serine-to-alanine mutant at catalytic site, position 328
- EGFR
Epithelial growth factor receptor
- MAPK
Mitogen-Activated Protein Kinases
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
The authors declare no conflict of interest.
Notes: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Aletti GD, Dowdy SC, Gostout BS, et al. Quality improvement in the surgical approach to advanced ovarian cancer: the Mayo Clinic experience. J Am Coll Surg. 2009;208:614–20. doi: 10.1016/j.jamcollsurg.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Sehouli J, Senyuva F, Fotopoulou C, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol. 2009;99:424–7. doi: 10.1002/jso.21288. [DOI] [PubMed] [Google Scholar]
- 5.Chien J, Staub J, Hu SI, et al. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. 2004;23:1636–44. doi: 10.1038/sj.onc.1207271. [DOI] [PubMed] [Google Scholar]
- 6.Baldi A, De Luca A, Morini M, et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene. 2002;21:6684–8. doi: 10.1038/sj.onc.1205911. [DOI] [PubMed] [Google Scholar]
- 7.Yu YP, Landsittel D, Jing L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–9. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 8.Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Segal NH, Pavlidis P, Noble WS, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol. 2003;21:1775–81. doi: 10.1200/JCO.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 10.Esposito V, Campioni M, De Luca A, et al. Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer Res. 2006;26:3455–9. [PubMed] [Google Scholar]
- 11.Baldi A, Mottolese M, Vincenzi B, et al. The serine protease HtrA1 is a novel prognostic factor for human mesothelioma. Pharmacogenomics. 2008;9:1069–77. doi: 10.2217/14622416.9.8.1069. [DOI] [PubMed] [Google Scholar]
- 12.Kotliarov Y, Steed ME, Christopher N, et al. High-resolution global genomic survey of 178 gliomas reveals novel regions of copy number alteration and allelic imbalances. Cancer Res. 2006;66:9428–36. doi: 10.1158/0008-5472.CAN-06-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets. 2009;9:451–68. doi: 10.2174/156800909788486704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–64. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–85. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature. 2004;430:973–4. doi: 10.1038/430973a. [DOI] [PubMed] [Google Scholar]
- 17.Chien J, Aletti G, Baldi A, et al. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. 2006;116:1994–2004. doi: 10.1172/JCI27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons MJ, Patel P, Brat DJ, Colbert L, Vertino PM. Silencing of TMS1/ASC promotes resistance to anoikis in breast epithelial cells. Cancer Res. 2009;69:1706–11. doi: 10.1158/0008-5472.CAN-08-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu SI, Carozza M, Klein M, Nantermet P, Luk D, Crowl RM. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J Biol Chem. 1998;273:34406–12. doi: 10.1074/jbc.273.51.34406. [DOI] [PubMed] [Google Scholar]
- 20.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 21.Chien J, He X, Shridhar V. Identification of tubulins as substrates of serine protease HtrA1 by mixture-based oriented peptide library screening. J Cell Biochem. 2009;107:253–63. doi: 10.1002/jcb.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–43. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 23.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77(4):477–8. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 24.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–57. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck RC. Walker 256 tumor implantation in normal and injured peritoneum studied by electron microscopy, scanning electron microscopy, and autoradiography. Cancer Res. 1973;33:3181–8. [PubMed] [Google Scholar]
- 26.Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L) Cancer Res. 2001;61:4837–41. [PubMed] [Google Scholar]
- 27.Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–6. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 28.Devoogdt N, Rasool N, Hoskins E, Simpkins F, Tchabo N, Kohn EC. Overexpression of protease inhibitor-dead secretory leukocyte protease inhibitor causes more aggressive ovarian cancer in vitro and in vivo. Cancer Sci. 2009;100:434–40. doi: 10.1111/j.1349-7006.2009.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–81. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen JS, Jakobsen E, Hølund B, Bertelsen K, Jakobsen A. Prognostic significance of p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian cancer. Int J Gynecol Cancer. 2004;14:1086–96. doi: 10.1111/j.1048-891X.2004.14606.x. [DOI] [PubMed] [Google Scholar]
- 31.Reginato MJ, Mills KR, Paulus JK, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–40. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 32.Jost M, Huggett TM, Kari C, Rodeck U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell. 2001;12:151927. doi: 10.1091/mbc.12.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demers MJ, Thibodeau S, Noël D, et al. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J Cell Biochem. 2009;107:639–54. doi: 10.1002/jcb.22131. [DOI] [PubMed] [Google Scholar]
- 34.Giannoni E, Fiaschi T, Ramponi G, Chiarugi P. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene. 2009;28:2074–86. doi: 10.1038/onc.2009.77. [DOI] [PubMed] [Google Scholar]
- 35.Zoppi N, Barlati S, Colombi M. FAK-independent alphavbeta3 integrin-EGFR complexes rescue from anoikis matrix-defective fibroblasts. Biochim Biophys Acta. 2008;1783:1177–88. doi: 10.1016/j.bbamcr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto S, Hirata M, Yamazaki A, et al. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004;64:5720–7. doi: 10.1158/0008-5472.CAN-04-0811. [DOI] [PubMed] [Google Scholar]
- 37.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–91. [PubMed] [Google Scholar]
- 38.Yu X, Miyamoto S, Mekada E. Integrin alpha 2 beta 1 dependent EGF receptor activation at cell-cell contact sites. J Cell Sci. 2000;113:2139–47. doi: 10.1242/jcs.113.12.2139. [DOI] [PubMed] [Google Scholar]
- 39.Porcile C, Bajetto A, Barbieri F, et al. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–53. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543–52. doi: 10.1111/j.1349-7006.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann J. Molecular mechanisms of “detachment-induced apoptosis--Anoikis”. Apoptosis. 2002;7:247–60. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 42.Idogawa M, Adachi M, Minami T, Yasui H, Imai K. Overexpression of BAD preferentially augments anoikis. Int J Cancer. 2003;107:215–23. doi: 10.1002/ijc.11399. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard V, Harnois C, Demers MJ, et al. B1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis. 2008;13:531–42. doi: 10.1007/s10495-008-0192-y. [DOI] [PubMed] [Google Scholar]
- 44.Launay S, Maubert E, Lebeurrier N, et al. HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ. 2008;15:1408–16. doi: 10.1038/cdd.2008.82. [DOI] [PubMed] [Google Scholar]
- 45.Canfield AE, Hadfield KD, Rock CF, Wylie EC, Wilkinson FL. HtrA1: a novel regulator of physiological and pathological matrix mineralization? Biochem Soc Trans. 2007;35:669–71. doi: 10.1042/BST0350669. [DOI] [PubMed] [Google Scholar]
- 46.Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 47.Clawson GA, Bui V, Xin P, Wang N, Pan W. Intracellular localization of the tumor suppressor HtrA1/Prss11 and its association with HPV16 E6 and E7 proteins. J Cell Biochem. 2008;105:81–8. doi: 10.1002/jcb.21804. [DOI] [PubMed] [Google Scholar]
- 48.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–9. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–8. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia W, Wei Y, Du Y, et al. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009;48:610–7. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.