Abstract
Objective
Since publication of the ARMA trial in 2000, use of tidal volumes (VT) ≤6 mL/kg predicted body weight (PBW) with corresponding plateau airway pressures (PPlat) ≤30 cmH2O has been advocated for acute lung injury (ALI). However, compliance with these recommendations is unknown. We therefore investigated VT (mL/kg PBW) and PPlat (cmH2O) practices reported in studies of ALI since ARMA using a systematic literature review (i.e. not a meta-analysis).
Data Sources
PubMed, Scopus, and EMBASE.
Study Selection
Randomized controlled trials (RCTs) and non-randomized studies (NRS) enrolling ALI patients from May 2000 to June 2013 and reporting VT.
Data Extraction
Whether the study was an RCT or NRS and performed or not at an ARDSNetwork center; in RCTs, the pre- and post-randomization VT (mL/kg PBW) and PPlat (cmH2O) and whether a VT protocol was used post-randomization; in NRSs, baseline VT and PPlat.
Data Synthesis
Twenty-two RCTs and 71 NRSs were included. Since 2000 at ARDSNetwork centers, routine VT was similar comparing RCTs and NRSs (p=0.25) and unchanged over time (p=0.75) with a mean value of 6.81(95%CI: 6.45,7.18). At non-ARDSNetwork centers routine VT was also similar when comparing RCTs and NRSs (p=0.71), but decreased (p=0.001); the most recent estimate for it was 6.77(6.22,7.32). All VT estimates were significantly >6 (p≤0.02). In RCTs employing VT protocols, routine VT was reduced in both ARDSNetwork (n=4) and non-ARDSNetwork (n=11) trials (p≤0.01 for both), but even post-randomization was >6 [6.47(6.29,6.65) and 6.80(6.42,7.17), respectively; p≤0.0001 for both)]. In 59 studies providing data, routine PPlat, averaged across ARDSNetwork or non-ARDSNetwork centers was significantly <30 (p≤0.02).
Conclusion
For clinicians treating ALI since 2000, achieving VT ≤6 mL/kg PBW may not have been as attainable or important as PPlat ≤30 cmH2O.. If so, there may be equipoise to test if VT ≤6 mL/kg PBW are necessary to improve ALI outcome.
Keywords: acute respiratory distress syndrome, acute lung injury, mechanical ventilation, tidal volume, ARDS
Introduction
Mechanical ventilation, although a lifesaving therapy for acute lung injury (ALI), including the adult respiratory distress syndrome (ARDS), can also aggravate injury.1–3 During the 1990s, randomized controlled trials (RCTs) studied different tidal volume (VT) levels to minimize such injury.4–8 The largest trial (ARMA), conducted by the ARDS Network (ARDSNet), reported that a protocol reducing VT to 4 to 6 mL/kg predicted body weight (PBW) and plateau pressures (PPlat) to ≤30 cmH2O, significantly decreased mortality from 40 to 31% when compared to a protocol targeting a “traditional” control VT of 12 mL/kg PBW (See Appendix 1 for the methods used in patients randomized to the low tidal volume arm of the ARMA trial).9 Since the ARMA trial was published in 2000, this low VT (LVT) protocol10,11 (also referred to as the lung protective ventilation protocol12) has been advocated by the ARDSNet (Appendix 2), as well as the Surviving Sepsis Campaign Guidelines (SSC) 13, the Institute for Healthcare Improvement,14 and the German Sepsis Society.15 Adherence to mechanical ventilation with VT ≤6 mL/kg PBW has also been stipulated for use in RCTs testing new therapies for ALI and has even been recommended for mechanically ventilated patients without ALI.16,17
Despite these recommendations, evidence suggests that physicians have not routinely maintained VT ≤6 mL/kg PBW in patients with ALI. Surveys in the United States (US),18 Ireland,19 Finland,20 Mexico,21 and Canada22 over the past ten years reported routine VT (±SD) in ALI patients greater than 6 mL/kg PBW (7.0±1.6, 8.4±2.0, 8.6±1.9, 8.3±3.0, and 9.0±2.5, respectively). Recently published RCTs of ALI/ARDS from the US and Europe beginning enrollment in 2006 also reported routine VT ≥6 mL/kg PBW (7.3±1.5 and 8.1±1.8, respectively averaged over study groups).23,24 However, routine PPlat in these studies were ≤30 cmH2O. We therefore hypothesized that since publication of the ARMA trial, although widely advocated, VT ≤6 mL/kg PBW have not routinely been used in patients. To test this hypothesis, we performed a systematic literature review (i.e. not a meta-analysis) and analyzed VT and accompanying PPlat used for ALI in RCTs and non-randomized studies (NRS) conducted since ARMA.
Materials and Methods
Literature Search
PubMed, Scopus, and EMBASE databases were searched employing the search terms and strategy summarized in Appendix 3. The search was limited to human subjects, the English language, and publication dates from May 2000 until June 2013. References from retrieved reports were examined for additional studies. Both RCTs and NRSs (i.e. observational studies or uncontrolled treatment studies) were analyzed if the patient population studied met diagnostic criteria for ALI 25; the date of initial enrollment followed publication of the ARMA trial (May 4, 2000); and the average routine VT for the population studied were reported. Two authors (DJ and PQE) independently reviewed all citations and abstracts followed by relevant full text articles to determine study suitability. Disagreements were resolved by consensus. This study was reviewed by local IRB and was deemed exempt from approval.
Data Extraction
Extracted data from selected studies included the following: the number of enrolled patients; patient ages and sex; VT (in mL/kg PBW); PPlat; risk factors for ALI; baseline PaO2:FiO2 ratios; and Acute Physiology and Chronic Health Evaluation II scores (APACHE-II), Lung Injury scores (LIS), and Simplified Acute Physiology scores (SAPS II); reported mortality rates; exclusion criteria; whether the trial took place at a single center or multiple centers; geographic location; location of the trial at either an ARDSNet or non-ARDSNet center; and for RCTs, whether an LVT protocol based on the ARMA trial or similar criteria was employed following patient randomization. For RCTs VT pre-randomization and the first VT reported post-randomization (if available) were recorded whereas for NRSs the baseline VT was recorded. For studies providing data for individual patients, group mean values were calculated. Pre-randomization and baseline VT and PPlat were considered routine ones. For studies in which VT were provided in mL/kg or total mL only, attempts were made to contact corresponding authors to obtain VT in mL/kg PBW. Tidal volumes based on ideal body weight (IBW) were considered comparable to ones based on PBW.9,26–34 If only VT in mL/kg total body weight was available, then a conversion factor was applied based on previous literature documenting a difference of +1.55 mL/kg between VT per PBW and VT per total body weight.20 This conversion was necessary for one RCT and nine non-RCTs.35–44 For 56 studies providing VT in mL only, investigators from 4 subsequently provided it in mL/kg PBW or mL/kg IBW. 29,35,39,45 Remaining studies were excluded.
Data Analysis
All analyses were done using R packages meta and metafor unless stated otherwise. For studies with more than one group, VT and PPlat data were first combined to generate study level summary statistics.26–28,30,31,46–51 This was justified because there was little difference between treatment groups within each study. Random-effect meta-analysis and meta-regression were performed with inverse-variance weighting and estimated using restricted maximum likelihood. While these were the most appropriate methods for our analysis, this is not a standard meta-analysis since tidal volume is not the experimental treatment or outcome, and it is similar across treatment groups. The month and year of initial enrollment for studies were used for analysis. For two RCTs and six NRSs only initial years of enrollment were available for analysis (Tables E1 and E2).38,43,51–56 The 95% confidence bands were estimated and plotted in Figures 2 and 4. The change in VT (pre- versus post-randomization) were analyzed via a special bivariate meta-analysis random effect model57 using SAS version 9.3. Two-sided p-values ≤0.05 were considered significant.
Figure 2.
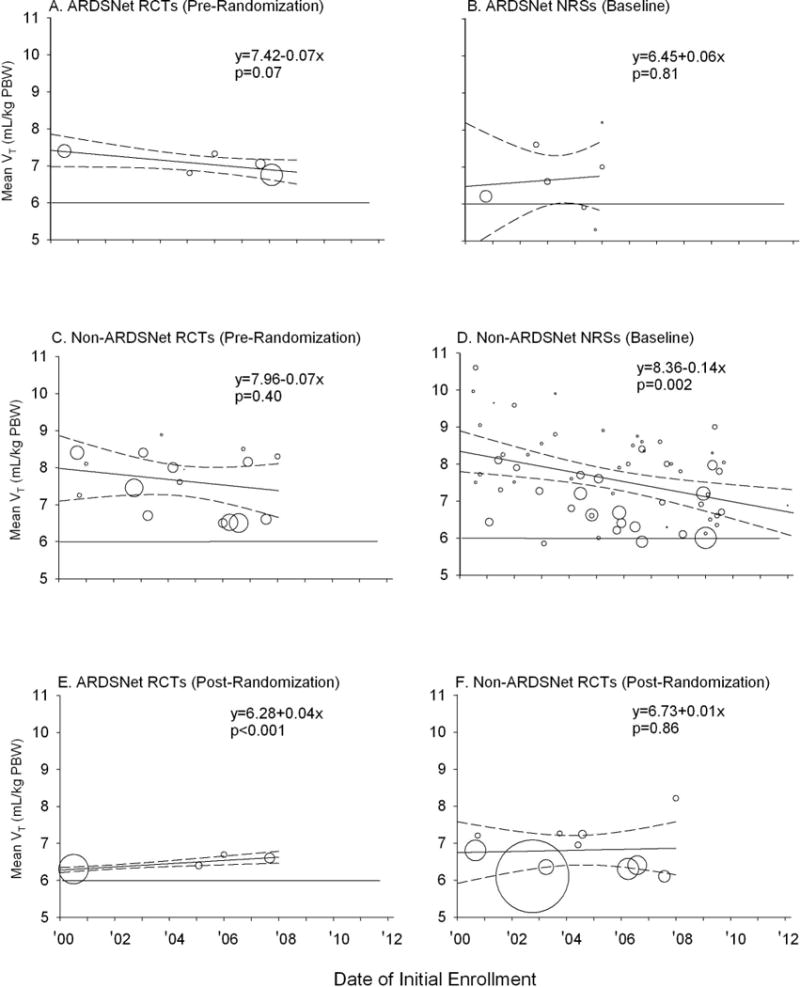
Mean tidal volume (VT) for the various types of centers and trials are shown. The area of each circle is proportional to the inverse of the variance associated with each mean VT value. The solid diagonal line in each panel represents the weighted regression line and the dashed lines represent the 95% CIs for the relationship between baseline VT and the date of initial enrollment into trials in each group. Equations for the weighted regression lines and p-values for the slope of the line are shown for each group of studies. A horizontal line denoting a 6 mL/kg PBW VT is shown in each panel for reference to the VT targeted in the ARDSNet LVT protocol. Panels E and F show data post-randomization VT in RCTs employing low VT protocols.
Figure 4.
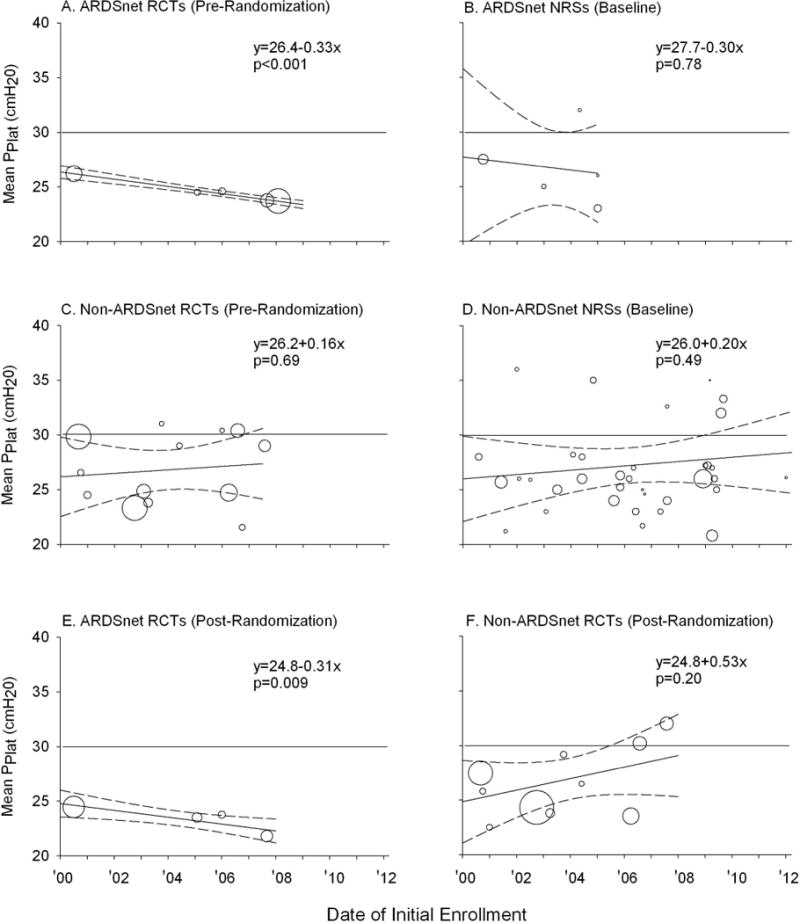
Mean plateau pressure (PPlat) for the various types of centers and trials are shown. The area of each circle is proportional to the inverse of the variance associated with each mean PPlat value. The solid diagonal line in each panel represents the weighted regression line and the dashed lines represent the 95% CIs for the relationship between baseline PPlat and the year of the start of enrollment for each group of trials. Equations for the weighted regression lines and p-values for the slope of the line are shown. These equations were available for all groups of studies except for nonrandomized studies at ARDSNet centers (Panel B) in which there were too few studies to fit a regression line. A horizontal line denoting a PPlat of 30 cmH2O is included for reference. Panels E and F show data post-randomization PPlat in RCTs employing low VT protocols.
Results
Literature Search and Study Characteristics
A literature search yielded 3,328 references. After independent review and subsequent discussion, the authors assessing individual studies (DSJ and PQE) were in agreement that 93 of these studies met inclusion criteria (Figure 1). Of these, 22 were RCTs23,24,43,52,58–75 (totaling 7,503 patients) and 71 were NRSs 19–22,26–42,44–51,53–56,76–113 (totaling 5,376 patients). Characteristics of these trials and studies are listed in Tables E1 and E2 in the electronic supplement while patient baseline data are listed in Tables E3 to E6.
Figure 1.
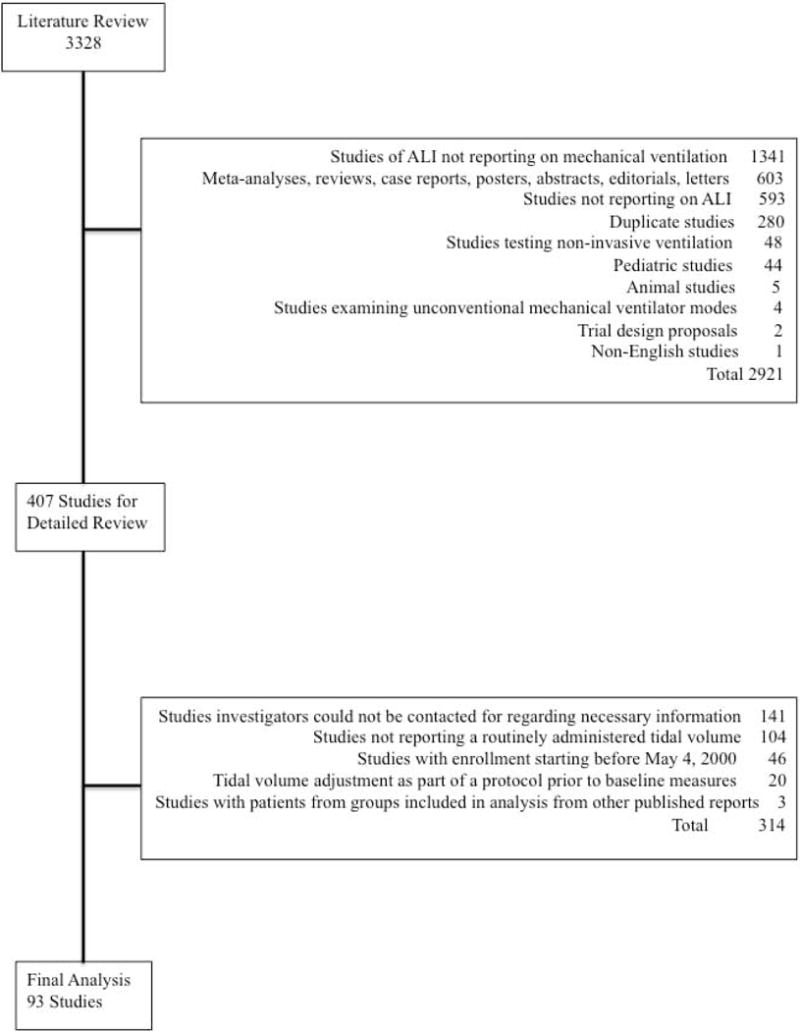
This figure is a flow chart that summarizes the results of the literature search employed and the selection of trials for analysis in this study. From 3,328 references, 93 studies (22 randomized controlled trials and 71 non-randomized studies) met criteria for inclusion.
Routine Tidal Volumes
We first examined the mean VT (ml/kg PBW) routinely prescribed for patients in four groups of studies: pre-randomization in RCTs or at baseline in NRSs at ARDSNet (Figure 2, Panels A and B, respectively) or non-ARDSNet centers (Figure 2, Panels C and D, respectively). The area of each circle in Figure 2 is proportional to the inverse of the variance associated with the mean VT value for each study. A weighted regression line with 95% confidence intervals (CI) for the relationship over the study period (May 2000 to June 2013) between VT and date of initial study enrollment is shown for each of the four groups of trials. Baseline VT was not significantly associated with baseline PaO2:FiO2 or PPlat (each p=ns) (Tables E5 and E6). Other baseline measures of disease severity were reported in fewer than 50% of studies and were not analyzed. Across the study period, routine mean VT were >6 ml/kg PBW in 87 of the 93 studies analyzed. At ARDSNet centers, while the value of routinely prescribed VT decreased over the study period in a pattern that approached significance for RCTs [rate of annual change in VT (mL/kg PBW) (95% CI)] [−0.07 (−0.14, 0.006), p=0.07], routine VT did not change significantly in NRSs [0.06 (−0.40, 0.52), p=0.81]. At non-ARDSNet centers, routine VT did not change significantly in RCTs [−0.07 (−0.25, 0.10), p=0.40] but did decrease significantly in NRSs [−0.14 (−0.22, −0.05), p=0.002]. Since changes in VT comparing RCTs versus NRSs at either ARDSNet or non-ARDSNet centers were not significantly different (p=0.60 and 0.58, respectively), we calculated common slopes to increase the detection of significant reductions over time. After combining RCTs and NRSs, the mean annual VT change over the study period was still not significant for ARDSNet centers [−0.03 (−0.21, 0.15), p=0.75] but was for non-ARDSNet centers [−0.13 (−0.20, −0.05), p=0.001].
We then compared routine VT at ARDSNet and non-ARDSNet centers to the widely recommended goal of 6 mL/kg PBW. At ARDSNet centers, where routine VT have not changed significantly over the study period, VT was significantly higher than 6 mL/kg PBW [6.81 (6.45, 7.18), p≤0.0001]. Furthermore, at non-ARDSNet centers, where there have been significant decreases in VT over the study period, even the estimated mean VT (95% CI) at the time of the most recent study did not meet this goal and was significantly greater than 6 mL/kg PBW [6.77 (6.22, 7.32); p=0.006].
Changes in Tidal Volumes Following Randomization in RCTs
We next examined how VT changed from pre- to post-randomization in RCTs. Four of 5 ARDSNet and 11 of the 14 non-ARDSNet center RCTs reported post-randomization VT and also stipulated use of a LVT protocol during the post-randomization period (Table E1). Three non-ARDSNet center RCTs that reported post-randomization VT did not stipulate such a protocol (Table E1). For RCTs stipulating an LVT protocol, all ARDSNet23,59,64,71,73 and four non-ARDSNet centers24,58,63,67 described employing the ARDSNet LVT protocol while five non-ARDSNet centers targeted a VT of 6 mL/kg PBW52,60,61,72,74 and five non-ARDSNet centers targeted a VT of 6 to 8 mL/kg PBW.62,65,68,69,75 Figure 3 shows mean (±SE) VT pre- and post-randomization for ARDSNet RCTs (Figure 3A) and for non-ARDSNet RCTs that either did (Figure 3B) or did not (Figure 3C) stipulate the use of an LVT protocol. In RCTs employing an LVT protocol, post-randomization VT increased over time at ARDSNet centers [slope: 0.04 (0.02, 0.07), p=0.0003] but not at non-ARDSNet centers [slope: 0.01 (−0.15, 0.18), p=0.86] (Figure 2, Panels E and F). Across RCTs, mean (95% CI) changes in VT from pre- to post-randomization were highly significant for ARDSNet and non-ARDSNet RCTs employing an LVT protocol [−0.67 (−1.206, −0.14), p=.01, and −0.66 (−0.96, −0.36), p<0.0001, respectively] but not for non-ARDSNet trials not reporting using a protocol [0.03 (−0.56, 0.62), p=0.91]. Despite significant reductions in VT in RCTs stipulating an LVT protocol, the mean (95% CI) post-randomization VT at ARDSNet [6.47 (6.29, 6.65)] and non-ARDSNet [6.80 (6.42, 7.17)] centers were still significantly greater than 6 mL/kg PBW over the study period (both p≤0.0001).
Figure 3.
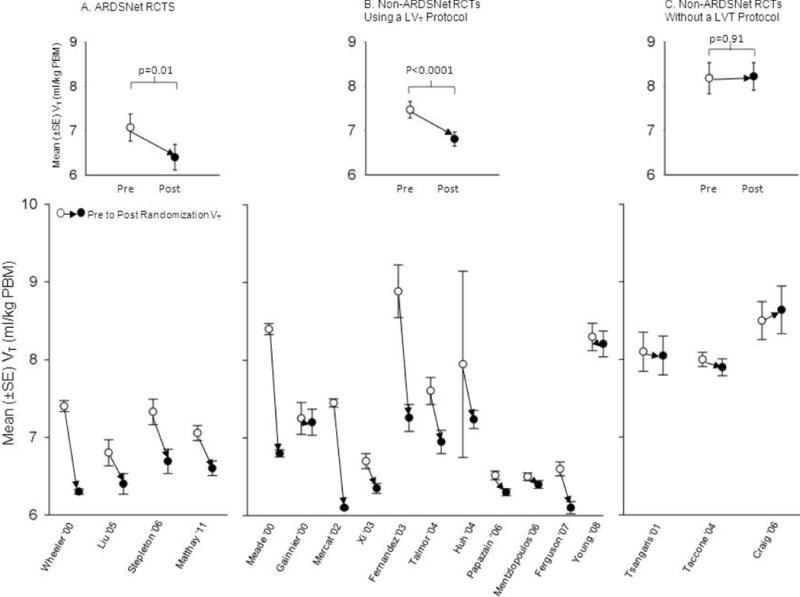
This figure compares mean tidal volumes (VT, ±SE) pre-randomization (open circles) and post-randomization (closed circles) in RCTs at ARDSNet centers (Figure 3A) and at non-ARDSNet centers that either did (Figure 3B) or did not (Figure 3C) stipulate the use of a low VT protocol for patients after randomization. The figures in the insets in each panel show the estimated mean pre- and post-randomization VT in each group of trials with p-values reflecting the significance for the change in VT.
Routine Plateau Pressures
Eighteen RCTs and 41 NRSs reported PPlat. Figure 4 shows the routine mean PPlat in patients pre-randomization in RCTs and at baseline in NRSs at ARDSNet (Panels A and B, respectively) and non-ARDSNet (Panels C and D, respectively) centers. Routine PPlat were ≤30 cmH2O in 48 out of 59 studies. In ARDSNet RCTs, although routine PPlat was already ≤30 cmH2O at the start of the decade (26.4, (25.8,26.9) p<0.0001), it decreased further across the years studied [rate of annual change in PPlat (cmH2O) (95% CI)] [−0.33 (−0.42, −0.25), p<0.0001]. In ARDSNet NRSs, and in non-ARDSNet RCTs and NRSs, routinely prescribed PPlat did not change significantly [−0.30 (−2.36, 1.76), p=0.78; 0.16 (−0.62, 0.94), p=0.69; and 0.20 (−0.37, 0.76), p=0.49, respectively] and the mean values over the study period were all significantly <30 cmH2O [26.6(23.7,29.6), p=0.02; 26.8(25.1,28.6), p=0.0003; and 27.2(25.6,28.8); p≤0.0007, respectively].
In RCTs that stipulated use of an LVT protocol, PPlat post-randomization decreased over the study period at ARDSNet [−0.31(−0.55, −0.08), p=0.009) but not at non-ARDSNet centers [0.53(−0.29,1.34), p=0.20] (Figure 4, Panels E and F). Post-randomization mean PPlat were ≤30 cmH2O in all but two of these trials.
Discussion
Since the ARMA trial, routine VT administered to ALI patients at academic centers has decreased significantly at non-ARDSNet but not at ARDSNet centers. However, when routine VT were either averaged over this time period for ARDSNet centers [mean (95%CI) mL/kg PBW] [6.81 (6.45, 7.18)], or estimated at the time of the most recent RCTs or NSRs at non-ARDSNet centers [6.77 (6.22, 7.32)], these remained 10 to 15% significantly greater than the 6 mL/kg PBW VT widely recommended based on the ARDSNetwork LVT protocol.9 Highlighting differences between actual and recommended practice, in RCTs at both ARDSNet and non-ARDSNet centers stipulating an LVT protocol post-randomization, enrolled patients on average have had their routine VT reduced significantly. However, even after these reductions, VT in these trials [6.47 (6.29, 6.65) and 6.80 (6.42, 7.17) respectively] remained significantly greater than 6 mL/kg PBW.
Failure to routinely employ the ARDSNet LVT and to achieve its stated VT goal has frequently been invoked as evidence that physicians do not effectively incorporate clinical research results and thereby potentially jeopardize patient care.114,115 In 2009, investigators at the University of Washington noted “…since the publication of the landmark randomized trial demonstrating the efficacy of lung protective ventilation (LPV), a large proportion of patients with ALI still receive mechanical ventilation with tidal volumes above the goal of 6 mL/kg predicted body weight. Barriers to the delivery of LPV include concern about adverse effects of low tidal volumes, inadequate knowledge of the LPV protocol, under-recognition of ALI, and an unwillingness of the bedside physician to relinquish control of the ventilator.”11 However, there are alternative explanations for this discrepancy.
One explanation is that rather than targeting a specific VT level, physicians adjust VT based on airway pressures such as a PPlat ≤30 cmH2O. 84,116 Titrating to such a PPlat does not always necessitate the level of VT recommended with the LVT protocol. In the absence of higher airway pressures, physicians may be more concerned about the potential adverse effects of lower VT (e.g. patient-ventilator dyssynchrony, hypercapnia, and hypoxemia).12 Analysis of physician practice including ARMA trial pre-randomization data, suggests that physicians have routinely adjusted VT based on airway pressures or measures of lung compliance.117–120 Notably, the widely advocated SSC sepsis bundles,121 (in contrast to the SSC guidelines), directed titration of VT to achieve a PPlat ≤30 cmH2O but did not recommend a specific VT level. This component of the bundles has been well adhered.122,123 Consistent with such practice, in the present study, although baseline VT were greater than those targeted in the ARDSNet LVT protocol, baseline PPlat were ≤30 cmH2O in the majority of studies.
Concerns regarding the design and interpretation of the ARMA trial may have also limited acceptance of the LVT protocol. At the trial’s publication, questions were raised as to whether it had demonstrated benefit with low VT as opposed to introducing harm with the high “traditional” control VT of 12 mL/kg PBW.124 Post-randomization airway pressures (mean±SE) in these controls (34.1±0.4 cmH2O) were greater than before randomization in these subjects (30.3±0.6 cmH2O) as well as in control patients not having their VT raised after randomization in other VT trials (≤30 cmH2O).120 Such differences have confounded meta-analyses of VT trials and prevented a definitive conclusion about the benefit of the ARDSNet LVT protocol.125 By not including a control group in ARMA representing conventional therapy, the trial was unable to demonstrate that the LVT protocol improved survival compared to routine care.126 A recent large observational study did report that adherence to the ARDSNet LVT protocol based on reductions in VT and PPlat alone or together, was associated with reduced mortality in ALI patients.127 However, this study’s observational design and use of both VT and PPlat to assess adherence to the ARDSNet protocol potentially complicate its interpretation.
In contrast to routine care, our findings suggest that application of LVT protocols are frequently stipulated in RCTs investigating new therapies for ALI and that their use was associated with patients receiving significantly lower VT after enrollment than before. On the one hand, these reductions suggest that the presence and use of LVT protocols may increase prescription of lower tidal volumes.128 However, this discrepancy in VT use comparing pre- and post-randomization periods also raises concerns. First, administration of VT during the investigation of a new therapy that on average differ from routine ones may confound interpretation of that therapy’s effects during later clinical use. Such discrepancies may have the greatest impact for new therapies related to ventilator management. For example, maneuvers to promote airway recruitment might result in greater improvements in oxygenation in the setting of lower VT, which increases the potential for atelectasis, compared to higher VT. The second concern relates to the potential for practice misalignments to develop.120 As noted above, several lines of evidence indicates that physicians routinely titrate VT based on airway pressures (e.g. PPlat) and the underlying severity of lung injury as reflected by lung compliance. Patients with less severe lung injury and better lung compliance receive higher VT than patients with more severe disease and lower compliance. Prior analysis of the ARMA trial showed that randomizing patients into fixed treatment groups (i.e. low or high VT groups) disrupted this relationship between routinely applied VT and the severity of disease and created practice misalignments.120 Stipulating use of the ARDSNet LVT protocol or a similar one in trials would introduce this same risk and further confound extrapolation of trial results clinically.
The findings that both routine VT as well as post-enrollment ones in RCTs employing LVT protocols have remained greater than 6 mL/kg PBW raises the possibility that this widely advocated goal may be unachievable in many ALI patients. Even at ARDSNet centers where routine adoption of LVT protocols might be most expected, routine and post-enrollment VT did not change after ARMA and remained on average between 6.5 and 7.0 mL/kg PBW. While VT has decreased over the past decade at non-ARDSNet centers, they started at higher levels (p=0.01) than at ARDSNet centers and, based on the most recent estimates, remain significantly greater than 6 mL/kg PBW.
This study has limitations. First, while we extracted recorded pre-randomization and baseline VT for both RCTs and NRSs, it is possible that with routine care these may have decreased following admission and reached those targeted by the LVT protocol. However, such reductions have not occurred in other studies that examined use of the LVT protocol at later time points.128 Second, inability of most ventilators to allow adjustment of VT in mL/kg PBW may have resulted in the application VT different from 6 mL/kg PBW for some patients in which this level was in fact intended. However, the fact that average VT were significantly greater than 6 mL/kg PBW for all comparisons made, suggests that these differences were not related to obstacles arising from ventilator calibration alone. Third, in the present study PPlat data were unavailable in several trials. It is possible that in these trials, PPlat were sufficiently >30 cmH2O to raise concern regarding the routine VT that was being employed. However, mean PPlat was consistently ≤30 cmH2O in the majority of trials providing data. The absence of reported PPlat from some trials may also suggest that parameters other than airway pressure were employed to manage VT. However this absence does not necessarily mean that PPlat was not measured and employed by clinicians. Fourth, it is unclear why in ARDSNetwork RCTs post-randomization VT increased while PPlat decreased overtime. Although a decrease in the severity of lung injury of patients post-randomization in these trials over time might explain this pattern, there was insufficient data to explore this. Finally, it is possible that our search strategy, that included English language publications only, may have omitted relevant studies for analysis. However, the terminology we employed was broad and yielded more than 3300 studies for review.
Conclusions
Minimizing injury related to mechanical ventilation during management of patients with ALI is important. However, more than a decade has passed since ARMA was published and adherence to the ARDSNet LVT protocol and application of VT of ≤6 mL/kg PBW are still not routine despite being advocated as the standard of care. Physicians do however routinely employ VT associated with PPlat ≤30 cmH2O. One interpretation of these findings is that clinicians caring for patients with ALI are more focused on limiting PPlat – and if PPlat is ≤30 cmH2O, they are less focused on limiting VT. Ultimately, studies employing mixed methods (e.g. surveys, focus groups, cross-sectional audits of barriers/facilitators) may be needed to fully understand why VT ≤6 mL/kg PBW is not being employed by clinicians. However, if there is equipoise among clinicians regarding the need for VT ≤6 mL/kg PBW to improve the outcome of patients with ALI, but this strategy is being advocated for all cases, then a trial testing it is necessary. Such a trial would be needed not only to guide future care but also because a VT ≤6 mL/kg PBW has not yet been compared to routine care.
Supplementary Material
Acknowledgments
This research was supported by the Division of Intramural Research of the Clinical Center at the National Institutes of Health. There are no conflicts of interests to report for the authors.
Drs. Eichacker, Jaswal, Li, and Natanson received support for article research from NIH and disclosed government work. Dr. Leung lectured for Astra-Zeneca (Speaking Honoraria). Dr. Sun disclosed government work. Dr. Cui received support for article research from NIH. Dr. Kern received support for article research from NIH.
Appendix 1
In the group treated with lower tidal volumes, the tidal volume was reduced to 6 ml per kilogram of predicted body weight within four hours after randomization and was subsequently reduced stepwise by 1 ml per kilogram of predicted body weight if necessary to maintain plateau pressure at a level of no more than 30 cm of water. The minimal tidal volume was 4 ml per kilogram of predicted body weight. If plateau pressure dropped below 25 cm of water, tidal volume was increased in steps of 1 ml per kilogram of predicted body weight until the plateau pressure was at least 25 cm of water or the tidal volume was 6 ml per kilogram of predicted body weight. For patients with severe dyspnea, the tidal volume could be increased to 7 to 8 ml per kilogram of predicted body weight if the plateau pressure remained 30 cm of water or less. Plateau pressures were measured with a half-second inspiratory pause at four-hour intervals and after changes in the tidal volume or positive end-expiratory pressure. Plateau pressures of more than 50 cm of water in the patients in the group treated with traditional tidal volumes and of more than 30 cm of water in patients in the group treated with lower tidal volumes were allowed if the tidal volume was 4 ml per kilogram of predicted body weight or if arterial pH was less than 7.15.
Appendix 2
NIH NHLBI ARDS Clinical Network
Mechanical Ventilation Protocol Summary
INCLUSION CRITERIA
Acute onset of
PaO2/FiO2 ≤300 (corrected for altitude)
Bilateral (patchy, diffuse, or homogeneous) infiltrates consistent with pulmonary edema
No clinical evidence of left atrial hypertension
PART I: VENTILATOR SETUP AND ADJUSTMENT
Calculate predicted body weight (PBW) Males = 50 + 2.3 [height (inches) – 60] Females = 45.5 + 2.3 [height (inches) – 60]
Select any ventilator mode
Set ventilator settings to achieve initial VT = 8 mL/kg PBW
Reduce VT by 1 ml/kg at intervals ≤2 hours until VT = 6 mL/kg PBW.
Set initial rate to approximate baseline minute ventilation (not >35 bpm).
Adjust VT and RR to achieve pH and plateau pressure goals below.
PLATEAU PRESSURE GOAL: ≤30 cmH2O
Check PPlat (0.5 second inspiratory pause), at least q4h and after each change in PEEP or VT.
If PPlat >30 cmH2O: decrease VT by 1mL/kg steps (minimum = 4 mL/kg).
If PPlat <25 cmH2O and VT <6 mL/kg, increase VT by 1 ml/kg until PPlat >25 cmH2O or VT = 6mL/kg.
If PPlat <30 cmH2O and breath stacking or dyssynchrony occurs: may increase VT in 1 mL/kg increments to 7 or 8 mL/kg if PPlat remains <30 cmH2O
Appendix 3: Search terms and strategies for the three data bases employed (i.e. Pubmed, EMBASE and Scopus)
PubMed
“respiratory distress syndrome, adult” [majr] OR “acute lung injury”[majr] OR “acute respiratory distress syndrome”[tiab] OR “adult respiratory distress syndrome”[tiab] OR “acute lung injury”[tiab] OR ARDS[tiab] OR ALI[tiab] Filters: Clinical Trial; Comparative Study; Meta-Analysis; Publication date from 2000/01/01; Humans; English; Adult: 19+ years
(“respiratory distress syndrome, adult”[majr] OR “acute lung injury”[majr] OR “acute respiratory distress syndrome”[tiab] OR “adult respiratory distress syndrome”[tiab] OR “acute lung injury”[tiab] OR ARDS[tiab] OR ALI[tiab]) AND (“clinical trials as topic”[mesh] OR “meta-analysis as topic”[mesh] OR “epidemiologic studies”[mesh]) Filters: Publication date from 2000/01/01; Humans; English; Adult: 19+ years
(“respiratory distress syndrome, adult”[majr] OR “acute lung injury”[majr] OR “acute respiratory distress syndrome”[tiab] OR “adult respiratory distress syndrome”[tiab] OR “acute lung injury”[tiab] OR ARDS[tiab] OR ALI[tiab]) AND (“non-randomized” OR nonrandomized OR “non-randomised”) Filters: Publication date from 2000/01/01; Humans; English; Adult: 19+ years
EMBASE
adult respiratory distress syndrome’/exp/mj OR ‘acute lung injury’/exp/mj AND (‘clinical study’/exp OR ‘comparative study’/exp OR ‘meta analysis’/exp OR ‘meta analysis (topic)’/exp) AND ([adult]/lim OR [aged]/lim) AND [humans]/lim AND [english]/lim AND [2000–2014]/py
‘adult respiratory distress syndrome’/exp/mj OR ‘acute lung injury’/exp/mj AND ([cochrane review]/lim OR [controlled clinical trial]/lim OR [meta analysis]/lim OR [randomized controlled trial]/lim OR [systematic review]/lim) AND ([adult]/lim OR [aged]/lim) AND [humans]/lim AND [english]/lim AND [2000–2014]/py
Scopus
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(“clinical trial”)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(randomized trial OR randomised trial)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(controlled trial)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(multicenter OR “multi-center”)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(meta-analysis)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(“comparative study”)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
(TITLE(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) OR ABS(“adult respiratory distress syndrome” OR “acute respiratory distress syndrome” OR “acute lung injury”) AND TITLE-ABS-KEY(“non-randomized” OR nonrandomized OR “non-randomised”)) AND PUBYEAR > 1999 AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) AND (LIMIT-TO(EXACTKEYWORD, “Adult”))
Footnotes
Copyright form disclosures: Dr. Welsh disclosed that she does not have any potential conflicts of interest.
References
- 1.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–34. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 3.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–24. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 4.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568–78. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 5.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 6.Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998;338:355–61. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 7.Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–8. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Kallet RH, Corral W, Silverman HJ, Luce JM. Implementation of a low tidal volume ventilation protocol for patients with acute lung injury or acute respiratory distress syndrome. Respir Care. 2001;46:1024–37. [PubMed] [Google Scholar]
- 11.Cooke CR, Kahn JM, Watkins TR, Hudson LD, Rubenfeld GD. Cost-effectiveness of implementing low-tidal volume ventilation in patients with acute lung injury. Chest. 2009;136:79–88. doi: 10.1378/chest.08-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32:1289–93. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Healthcare Improvement. Prevent excessive inspiratory plateau pressures. 2011 [Google Scholar]
- 15.Reinhart K, Brunkhorst FM, Bone HG, et al. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinare Vereinigung fur Intensiv- und Notfallmedizin (DIVI)) Ger Med Sci. 2010;8 doi: 10.3205/000103. Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–9. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 17.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care. 2013;17:R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevransky JE, Martin GS, Mendez-Tellez P, et al. Pulmonary vs nonpulmonary sepsis and mortality in acute lung injury. Chest. 2008;134:534–8. doi: 10.1378/chest.08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAuley D. Acute lung injury and the acute respiratory distress syndrome in Ireland: A prospective audit of epidemiology and management. Crit Care. 2008;12 doi: 10.1186/cc6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linko R, Okkonen M, Pettila V, et al. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med. 2009;35:1352–61. doi: 10.1007/s00134-009-1519-z. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 23.Stapleton RD, Martin TR, Weiss NS, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–62. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 26.Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care. 2010;55:282–7. [PubMed] [Google Scholar]
- 27.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–9. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 28.Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–8. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 29.Constantin JM, Jaber S, Futier E, et al. Respiratory effects of different recruitment maneuvers in acute respiratory distress syndrome. Crit Care. 2008;12:R50. doi: 10.1186/cc6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–55. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 31.Akoumianaki E, Lyazidi A, Rey N, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013;143:927–38. doi: 10.1378/chest.12-1817. [DOI] [PubMed] [Google Scholar]
- 32.Katsiari M, Koulouris NG, Orfanos SE, Maguina N, Sotiropoulou C, Koutsoukou A. Intercomparison of three recruitment maneuvers in acute respiratory distress syndrome: the role of Body Mass Index. Minerva Anestesiol. 2012;78:675–83. [PubMed] [Google Scholar]
- 33.Cepkova M, Brady S, Sapru A, Matthay MA, Church G. Biological markers of lung injury before and after the institution of positive pressure ventilation in patients with acute lung injury. Crit Care. 2006;10:R126. doi: 10.1186/cc5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutsoukou A, Perraki H, Orfanos SE, et al. History of mechanical ventilation may affect respiratory mechanics evolution in acute respiratory distress syndrome. Journal of Critical Care. 2009;24:626.e1–.e6. doi: 10.1016/j.jcrc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Tongyoo S, Vilaichone W, Ratanarat R, Permpikul C. The effect of lateral position on oxygenation in ARDS patients: a pilot study. J Med Assoc Thai. 2006;89(Suppl 5):S55–61. [PubMed] [Google Scholar]
- 36.Pulletz S, Kott M, Elke G, et al. Dynamics of regional lung aeration determined by electrical impedance tomography in patients with acute respiratory distress syndrome. Multidisciplinary Respiratory Medicine. 2012;7 doi: 10.1186/2049-6958-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl CA, Moller K, Schumann S, et al. Dynamic versus static respiratory mechanics in acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2006;34:2090–8. doi: 10.1097/01.CCM.0000227220.67613.0D. [DOI] [PubMed] [Google Scholar]
- 38.Simonato M, Baritussio A, Ori C, et al. Disaturated-phosphatidylcholine and surfactant protein-B turnover in human acute lung injury and in control patients. Respir Res. 2011;12:36. doi: 10.1186/1465-9921-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: The ECMOnet score. Intensive Care Med. 2013;39:275–81. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estenssoro E, Dubin A, Laffaire E, et al. Impact of positive end-expiratory pressure on the definition of acute respiratory distress syndrome. Intensive Care Med. 2003;29:1936–42. doi: 10.1007/s00134-003-1943-4. [DOI] [PubMed] [Google Scholar]
- 41.Bellani G, Messa C, Guerra L, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med. 2009;37:2216–22. doi: 10.1097/CCM.0b013e3181aab31f. [DOI] [PubMed] [Google Scholar]
- 42.Lesur O, Langevin S, Berthiaume Y, et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med. 2006;32:1167–74. doi: 10.1007/s00134-006-0235-1. [DOI] [PubMed] [Google Scholar]
- 43.Tsangaris I, Galiatsou E, Kostanti E, Nakos G. The effect of exogenous surfactant in patients with lung contusions and acute lung injury. Intensive Care Med. 2007;33:851–5. doi: 10.1007/s00134-007-0597-z. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Song Z, Zhou X, et al. A 12-month clinical survey of incidence and outcome of acute respiratory distress syndrome in Shanghai intensive care units. Intensive Care Med. 2004;30:2197–203. doi: 10.1007/s00134-004-2479-y. [DOI] [PubMed] [Google Scholar]
- 45.Robak O, Schellongowski P, Bojic A, Laczika K, Locker GJ, Staudinger T. Short-term effects of combining upright and prone positions in patients with ARDS: a prospective randomized study. Crit Care. 2011;15:R230. doi: 10.1186/cc10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang SY, Dabbagh O, Gajic O, et al. Contemporary ventilator management in patients with and at risk of ALI/ARDS. Respiratory Care. 2013;58:578–88. doi: 10.4187/respcare.01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;41:472–80. doi: 10.1097/CCM.0b013e31826ab377. [DOI] [PubMed] [Google Scholar]
- 48.Kao KC, Yang CT, Hu HC, et al. Resolution of organ functional scores to predict the outcome in adult acute respiratory distress syndrome patients receiving high-frequency oscillatory ventilation. J Trauma. 2011;71:1536–42. doi: 10.1097/TA.0b013e3182332102. [DOI] [PubMed] [Google Scholar]
- 49.Lu Q, Zhang M, Girardi C, Bouhemad B, Kesecioglu J, Rouby JJ. Computed tomography assessment of exogenous surfactant-induced lung reaeration in patients with acute lung injury. Crit Care. 2010;14:R135. doi: 10.1186/cc9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevransky JE, Martin GS, Shanholtz C, et al. Mortality in sepsis versus non-sepsis induced acute lung injury. Crit Care. 2009;13:R150. doi: 10.1186/cc8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolthuis EK, Kesecioglu J, Hassink LH, Determann RM, Korevaar JC, Schultz MJ. Adoption of lower tidal volume ventilation improves with feedback and education. Respir Care. 2007;52:1761–6. [PubMed] [Google Scholar]
- 52.Mentzelopoulos SD, Theodoridou M, Malachias S, et al. Scanographic comparison of high frequency oscillation with versus without tracheal gas insufflation in acute respiratory distress syndrome. Intensive Care Med. 2011;37:990–9. doi: 10.1007/s00134-011-2162-z. [DOI] [PubMed] [Google Scholar]
- 53.Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med. 2010;38:1845–51. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu CW, Lee DL, Lin SL, Sun SF, Chang HW. The initial response to inhaled nitric oxide treatment for intensive care unit patients with acute respiratory distress syndrome. Respiration. 2008;75:288–95. doi: 10.1159/000101478. [DOI] [PubMed] [Google Scholar]
- 55.Jog S, Patel D, Dravid T, et al. Early application of high frequency oscillatory ventilation in ‘H1N1 influenza’ related ARDS is associated with better outcome: A retrospective study. Intensive Care Med. 2013;39:1146–7. doi: 10.1007/s00134-013-2878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 58.Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–9. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 59.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 60.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 61.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez R, Trenchs X, Klamburg J, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008;34:1487–91. doi: 10.1007/s00134-008-1119-3. [DOI] [PubMed] [Google Scholar]
- 63.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–23. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kesecioglu J, Beale R, Stewart TE, et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:989–94. doi: 10.1164/rccm.200812-1955OC. [DOI] [PubMed] [Google Scholar]
- 66.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302:1977–84. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 67.Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13:R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xi XM, Jiang L, Zhu B. Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl) 2010;123:3100–5. [PubMed] [Google Scholar]
- 69.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 70.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–6. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 71.Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mentzelopoulos SD, Malachias S, Zintzaras E, et al. Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur Respir J. 2012;39:635–47. doi: 10.1183/09031936.00158810. [DOI] [PubMed] [Google Scholar]
- 73.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 75.Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–13. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 76.Kallet RH, Jasmer RM, Pittet JF, et al. Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med. 2005;33:925–9. doi: 10.1097/01.ccm.0000162382.59289.9c. [DOI] [PubMed] [Google Scholar]
- 77.Decailliot F, Demoule A, Maggiore SM, Jonson B, Duvaldestin P, Brochard L. Pressure-volume curves with and without muscle paralysis in acute respiratory distress syndrome. Intensive Care Med. 2006;32:1322–8. doi: 10.1007/s00134-006-0265-8. [DOI] [PubMed] [Google Scholar]
- 78.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 79.Yilmaz M, Iscimen R, Keegan MT, et al. Six-month survival of patients with acute lung injury: prospective cohort study. Crit Care Med. 2007;35:2303–7. doi: 10.1097/01.ccm.0000284505.96481.24. [DOI] [PubMed] [Google Scholar]
- 80.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–8. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann M, Bein T, Arlt M, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rios FG, Estenssoro E, Villarejo F, et al. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Crit Care. 2011;15:R201. doi: 10.1186/cc10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levesque E, Hoti E, Jiabin J, et al. Respiratory impact of paracentesis in cirrhotic patients with acute lung injury. J Crit Care. 2011;26:257–61. doi: 10.1016/j.jcrc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 84.Young MP, Manning HL, Wilson DL, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med. 2004;32:1260–5. doi: 10.1097/01.ccm.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- 85.Mentzelopoulos SD, Roussos C, Zakynthinos SG. Static pressure volume curves and body posture in acute respiratory failure. Intensive Care Med. 2005;31:1683–92. doi: 10.1007/s00134-005-2838-3. [DOI] [PubMed] [Google Scholar]
- 86.Sakr Y, Vincent JL, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 87.Wolthuis EK, Korevaar JC, Spronk P, et al. Feedback and education improve physician compliance in use of lung-protective mechanical ventilation. Intensive Care Med. 2005;31:540–6. doi: 10.1007/s00134-005-2581-9. [DOI] [PubMed] [Google Scholar]
- 88.Reutershan J, Schmitt A, Dietz K, Unertl K, Fretschner R. Alveolar recruitment during prone position: Time matters. Clinical Science. 2006;110:655–63. doi: 10.1042/CS20050337. [DOI] [PubMed] [Google Scholar]
- 89.Hinkson CR, Benson MS, Stephens LM, Deem S. The effects of apparatus dead space on P(aCO2) in patients receiving lung-protective ventilation. Respir Care. 2006;51:1140–4. [PubMed] [Google Scholar]
- 90.Cepkova M, Kapur V, Ren X, et al. Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest. 2007;132:836–42. doi: 10.1378/chest.07-0409. [DOI] [PubMed] [Google Scholar]
- 91.Mikkelsen ME, Dedhiya PM, Kalhan R, Gallop RJ, Lanken PN, Fuchs BD. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008;53:455–61. [PubMed] [Google Scholar]
- 92.Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med. 2008;34:75–81. doi: 10.1007/s00134-007-0847-0. [DOI] [PubMed] [Google Scholar]
- 93.Phillips CR, Chesnutt MS, Smith SM. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med. 2008;36:69–73. doi: 10.1097/01.CCM.0000295314.01232.BE. [DOI] [PubMed] [Google Scholar]
- 94.Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Critical Care Med. 2008;36:1043–8. doi: 10.1097/01.CCM.0b013e318168fcab. [DOI] [PubMed] [Google Scholar]
- 95.Quenot JP, Mentec H, Feihl F, et al. Bedside adherence to clinical practice guidelines in the intensive care unit: the TECLA study. Intensive Care Med. 2008;34:1393–400. doi: 10.1007/s00134-008-1059-y. [DOI] [PubMed] [Google Scholar]
- 96.Mauri T, Coppadoro A, Bellani G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med. 2008;36:2302–8. doi: 10.1097/CCM.0b013e3181809aaf. [DOI] [PubMed] [Google Scholar]
- 97.Demory D, Arnal JM, Wysocki M, et al. Recruitability of the lung estimated by the pressure volume curve hysteresis in ARDS patients. Intensive Care Med. 2008;34:2019–25. doi: 10.1007/s00134-008-1167-8. [DOI] [PubMed] [Google Scholar]
- 98.Pohlman MC, McCallister KE, Schweickert WD, et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit Care Med. 2008;36:3019–23. doi: 10.1097/CCM.0b013e31818b308b. [DOI] [PubMed] [Google Scholar]
- 99.Bloos F, Muller S, Harz A, et al. Effects of staff training on the care of mechanically ventilated patients: a prospective cohort study. Br J Anaesth. 2009;103:232–7. doi: 10.1093/bja/aep114. [DOI] [PubMed] [Google Scholar]
- 100.Allardet-Servent J, Forel JM, Roch A, et al. Pulmonary capillary blood flow and cardiac output measurement by partial carbon dioxide rebreathing in patients with acute respiratory distress syndrome receiving lung protective ventilation. Anesthesiology. 2009;111:1085–92. doi: 10.1097/ALN.0b013e3181b8f676. [DOI] [PubMed] [Google Scholar]
- 101.Allardet-Servent J, Forel JM, Roch A, et al. FIO2 and acute respiratory distress syndrome definition during lung protective ventilation. Crit Care Med. 2009;37:202–7. e4–6. doi: 10.1097/CCM.0b013e31819261db. [DOI] [PubMed] [Google Scholar]
- 102.Marik PE, Machare Delgado E, Baram M, Gradwell G, Romeo S, Dutill B. Effect of airway pressure release ventilation (APRV) with pressure support (PS) on indices of oxygenation and ventilation in patients with severe ARDS: A cohort study. Critical Care and Shock. 2009;12:43–8. [Google Scholar]
- 103.Craig TR, Duffy MJ, Shyamsundar M, et al. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med. 2010;38:114–20. doi: 10.1097/CCM.0b013e3181b43050. [DOI] [PubMed] [Google Scholar]
- 104.Mentzelopoulos SD, Malachias S, Kokkoris S, Roussos C, Zakynthinos SG. Comparison of high-frequency oscillation and tracheal gas insufflation versus standard high-frequency oscillation at two levels of tracheal pressure. Intensive Care Med. 2010;36:810–6. doi: 10.1007/s00134-010-1822-8. [DOI] [PubMed] [Google Scholar]
- 105.Arnal JM, Paquet J, Wysocki M, et al. Optimal duration of a sustained inflation recruitment maneuver in ARDS patients. Intensive Care Med. 2011;37:1588–94. doi: 10.1007/s00134-011-2323-0. [DOI] [PubMed] [Google Scholar]
- 106.Herasevich V, Tsapenko M, Kojicic M, et al. Limiting ventilator-induced lung injury through individual electronic medical record surveillance. Crit Care Med. 2011;39:34–9. doi: 10.1097/CCM.0b013e3181fa4184. [DOI] [PubMed] [Google Scholar]
- 107.Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 108.Dongelmans DA, Paulus F, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Adaptive support ventilation may deliver unwanted respiratory rate-tidal volume combinations in patients with acute lung injury ventilated according to an open lung concept. Anesthesiology. 2011;114:1138–43. doi: 10.1097/ALN.0b013e31820d8676. [DOI] [PubMed] [Google Scholar]
- 109.Monnet X, Bleibtreu A, Ferre A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–7. doi: 10.1097/CCM.0b013e31822f08d7. [DOI] [PubMed] [Google Scholar]
- 110.Guervilly C, Forel JM, Hraiech S, et al. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012;40:1539–45. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 111.Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: The PaO2/FiO2 ratio under a standard ventilatory setting – A prospective, multicenter validation study. Intensive Care Med. 2013;39:583–92. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 112.Rodriguez PO, Bonelli I, Setten M, et al. Transpulmonary pressure and gas exchange during decremental PEEP titration in pulmonary ARDS patients. Respiratory Care. 2013;58:754–63. doi: 10.4187/respcare.01977. [DOI] [PubMed] [Google Scholar]
- 113.Pham T, Combes A, Roze H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 114.Dennison CR, Mendez-Tellez PA, Wang W, Pronovost PJ, Needham DM. Barriers to low tidal volume ventilation in acute respiratory distress syndrome: survey development, validation, and results. Crit Care Med. 2007;35:2747–54. doi: 10.1097/01.CCM.0000287591.09487.70. [DOI] [PubMed] [Google Scholar]
- 115.Chia JY, Clay AS. Effects of respiratory-therapist driven protocols on house-staff knowledge and education of mechanical ventilation. Clin Chest Med. 2008;29:313–21. vii. doi: 10.1016/j.ccm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Kalhan R, Mikkelsen M, Dedhiya P, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34:300–6. doi: 10.1097/01.ccm.0000198328.83571.4a. [DOI] [PubMed] [Google Scholar]
- 117.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:21–30. doi: 10.1097/01.ccm.0000150652.91411.66. [DOI] [PubMed] [Google Scholar]
- 118.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003;167:1304–9. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 119.Carmichael LC, Dorinsky PM, Higgins SB, et al. Diagnosis and therapy of acute respiratory distress syndrome in adults: an international survey. J Crit Care. 1996;11:9–18. doi: 10.1016/s0883-9441(96)90015-5. [DOI] [PubMed] [Google Scholar]
- 120.Deans KJ, Minneci PC, Suffredini AF, et al. Randomization in clinical trials of titrated therapies: Unintended consequences of using fixed treatment protocols. Crit Care Med. 2007;35:1509–16. doi: 10.1097/01.CCM.0000266584.40715.A6. [DOI] [PubMed] [Google Scholar]
- 121.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–74. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 122.Shiramizo SC, Marra AR, Durao MS, Paes AT, Edmond MB, Pavao dos Santos OF Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One. 2011;6:e26790. doi: 10.1371/journal.pone.0026790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rinaldi L, Ferrari E, Marietta M, et al. Effectiveness of sepsis bundle application in cirrhotic patients with septic shock: a single-center experience. J Crit Care. 2013;28:152–7. doi: 10.1016/j.jcrc.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 124.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–4. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 125.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Annals of Internal Medicine. 2009;151:566–76. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 126.de Prost N, Ricard JD, Saumon G, Dreyfuss D. Ventilator-induced lung injury: historical perspectives and clinical implications. Ann Intensive Care. 2011;1:28. doi: 10.1186/2110-5820-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Umoh NJ, Fan E, Mendez-Tellez PA, et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36:1463–8. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


