Abstract
German cockroaches are major household allergens that can trigger allergic airway inflammatory diseases with sensitive T-cell responses. Although the use of immune modulatory biologics, such as antibodies, to mediate allergic responses has recently been examined, only systemic administration is available because of the size limitations on intranasal administration. Here we utilized a cell-permeable peptide, dNP2, to deliver the cytoplasmic domain of cytotoxic T-lymphocyte antigen-4 (ctCTLA-4) through the airway epithelium to modulate Th2 responses in a German cockroach extract (GCE)-induced allergic airway inflammation model. The intranasal delivery efficiency of the dNP2-dTomato protein to the lungs was higher in GCE-induced asthmatic lung parenchymal cells compared to the sham cells. Intranasal administration of the dNP2-ctCTLA-4 protein inhibited airway hyper-responsiveness and reduced airway inflammation and remodeling, including goblet cell metaplasia and collagen deposition around the bronchi. The number of infiltrated cells, including eosinophils, and the levels of IL-4, IL-5, IL-13 and IFN-γ in the lungs were significantly reduced, presumably owing to inhibition of Th2 differentiation. However, intranasal administration of CTLA4-Ig did not inhibit airway inflammation. These results collectively suggest that dNP2-ctCTLA-4 is an efficient intranasally applicable candidate biologic for treating allergic asthma.
Introduction
Allergic asthma is a severe chronic pulmonary disease that causes major health problems, including critically obstructed inhalation and exhalation, excess mucus, coughing, wheezing, chest tightness and pain.1 Several causative allergens have been described, including house dust mites,2 pollen3 and cockroaches.4
German cockroaches are one of the most common allergens in urban environments, and its extracts (GCE) are widely used for allergic airway inflammation models.5 GCE contains serine protease and Bla g peptide, which can activate T cells directly or through antigen-presenting cells.6 GCE can prime Th2 through CD103+ dendritic cells7 by promoting expression of T-cell immunoglobulin mucin domain 4 (TIM4).8 GCE-mediated Th2 responses induce asthmatic pathology through secretion of cytokines, such as IL-4, IL-5 and IL-13, which induce airway inflammation, hyper-responsiveness and remodeling.9, 10 These characteristics mimic human asthmatic features as a model system.
Despite the importance of Th2 cells and B-cell-mediated immunoglobulin E (IgE) production in asthma, T-cell-specific immune modulatory drugs are not still available for topical asthma therapy. Systemic injection of monoclonal antibodies, such as anti-IgE (omalizumab), anti-IL-5 (mepolizumab), anti-IL-13 (anrukinzumab) and anti-TGF-β (daclizumab), have been studied for asthma treatment.11 However, systemic injection of these monoclonal antibodies has drawbacks, including high cost and adverse effects, such as injection site reactions and potentially increased vulnerability to infections.11, 12 In addition, antibodies can only be applied systemically because of tight junctions in the respiratory endothelium and the biomolecule size limitations.13
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is an important immune checkpoint protein that inhibits T-cell activation.14, 15 CTLA-4 binds to co-stimulatory molecules on the surface of antigen-presenting cells, such as CD80 and CD86, with much higher efficiency than the co-stimulatory receptor, CD28, on T cells.16 This interaction induces negative signaling and inhibits T cells.17 Abatacept is a recombinant protein consisting of the CTLA-4 extracellular domain conjugated with the immunoglobulin G constant region (CTLA-4-Ig). It can inhibit allergic airway inflammation in an ovalbumin (OVA)-induced asthma model with reduced Th2 responses, such as eosinophilic infiltration, Th2 cytokine expression and decreased levels of serum IgE, through intravenous or intraperitoneal injections.18, 19, 20
Recently, we reported a novel human-derived cell-penetrating peptide, dNP2, that could efficiently escape blood vessels to access resident cells in various tissues, including the brain.21 We also generated dNP2-ctCTLA-4, a recombinant protein consisting of dNP2 and the cytoplasmic domain of CTLA-4. This molecule ameliorated autoimmune encephalomyelitis in mice and reduced Th1 and Th17 cells in the spinal cord.21
In this study, we hypothesized that dNP2 could efficiently deliver a protein to asthmatic lung resident cells via intranasal administration and that dNP2-ctCTLA-4 could regulate Th2 inflammation. We analyzed the efficiency of dNP2 and Hph-1 with respect to intranasal protein delivery. In addition, we demonstrated the advantage of dNP2-ctCTLA-4 over CTLA4-Ig via intranasal administration for treating allergic asthma. We investigated the immune modulatory effects of dNP2-ctCTLA-4 in a GCE-induced chronic asthma model and showed that it can control airway inflammation, hyper-responsiveness and the remodeling process via inhibition of Th2 responses.
Materials and methods
Animals
Eight-week-old female BALB/c mice were purchased from Orient Bio (Daejon, South Korea). All mice were housed in a specific pathogen-free animal facility. The mice were maintained on a 12-h light–dark cycle with regular chow and autoclaved water. GCE-induced asthma experiments were approved by the Animal Research Ethics Board of Yonsei University, and intranasal protein delivery efficiency experiments were approved by the Animal Care and Use Committees and Ethics Board for Animal Research of Hanyang University.
dNP2 recombinant protein purifications
All recombinant proteins were purified as previously described.21 In brief, Escherichia coli BL21 (DE3) Star pLysS cells were transformed with pRSET-b plasmids encoding dNP2 recombinant protein. The colony was cultured in ampicillin-containing Luria-Bertani broth media at 37 °C, and protein expression was induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). The proteins were purified by Ni-NTA affinity chromatography (Qiagen, Hilden, Germany) and desalted using a PD-10 column (GE Healthcare, Chicago, IL, USA). All proteins were stored at −80 °C.
German cockroach extract preparations
German cockroach extract was prepared as previously described.22 The pulverized Blattela germanica were defatted in 200 ml ether/ethyl acetate and slowly stirred at 4 °C overnight in phosphate-buffered saline (PBS) with 6 mM β-mercaptoethanol and 1 mg ml−1 1-phenyl-3(2-thiazolyl)-2-thiourea. The supernatant was filtered through a 0.22-μm filter after centrifugation and then lyophilized.
Generation of the GCE-induced asthma model
Eight-week-old BALB/c mice were sensitized by intranasal administration of 120 μg GCE or PBS (Sham) twice a week for 3 weeks. Mice were treated with 10 μg of dNP2-ctCTLA-4, dNP2-EGFP, CTLA4-Ig or PBS intranasally every time GCE was administered. To confirm intranasal delivery efficiency in the lungs, 100 μg of dTomato, Hph-1-dTomato, dNP2-dTomato or PBS was intranasally administered at day 21 for 15 min. The mice were killed, and the delivery efficiency of the proteins and the pathological changes in the lungs were analyzed.
Airway hyper-responsiveness analysis
Airway hyper-responsiveness was measured 4 days after the last GCE administration using a flexiVent 5.1 small animal ventilator (SCIREQ, Montreal, PQ, Canada). The mice were challenged with a saline control aerosol followed by various concentrations of methacholine (MeCh) (3.1, 6.25, 12.5, 25 and 50 mg ml−1). The aerosol was delivered for 10 s, and regular ventilation was maintained during that time. Measurements were made twice at 1 min intervals following each concentration of MeCh aerosol.
Bronchoalveolar lavage fluid analysis
The mouse lungs were washed with 1 ml of Hank’s balanced salt solution (HBSS) with an intubated tube to collect bronchoalveolar lavage (BAL) fluid. Total cells in the BAL fluids were counted with a hemocytometer. Then, BAL fluids were centrifuged, and the cells were prepared on slides using cytocentrifugation at 1000 r.p.m. for 3 min. All slides were stained with a Hemacolor staining kit (Merck Millipore, Darmstadt, Germany). The cells were differentially counted until the total counted number reached at least 200, using standard hemocytologic procedures to count macrophages and eosinophils.
Lung lysate analysis
The lungs were harvested after BAL fluid collection and homogenized using T-PER tissue protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA). After homogenization, whole suspensions were incubated at 4 °C for 30 min and centrifuged at 2500 r.p.m. for 10 min. Supernatants were filtered with a 0.45-μm filter to analyze cytokine levels.
Histological analysis
Lung tissues were prepared as paraffin blocks and sectioned to 3-μm thickness. Slide glasses with attached sections were stained with periodic acid-Schiff or Masson’s trichrome methods. Stained tissues were analyzed with a microscope (Olympus BX40, Olympus, Center Valley, PA, USA).
Th2 differentiation assay
Mouse naive CD4 T cells were isolated from 8-week-old BALB/c mice using magnetic activated cell sorting (MACS, Naive CD4 T cell isolation kit, mouse, Miltenyi, Bergisch Gladbach, Germany). Then, 2.5 × 105 of the cells were seeded on anti-CD3/anti-CD28 antibody (0.1 μg per well)-coated 96-well plates. The cells were incubated in the presence of anti-IFN-γ neutralizing antibody (5 μg ml−1), IL-2 (50 U ml−1), IL-4 (30 ng ml−1) and PBS, 1 μM of dNP2-EGFP or 1 μM of dNP2-ctCTLA-4 for 6 days. On day 6, the cells were analyzed by flow cytometry after re-stimulation, protein transport inhibitor treatment and intracellular staining with anti-mouse IFN-γ-FITC and anti-mouse IL-4-PE FACS antibodies.
Statistical analysis
All data were statistically analyzed by one-way ANOVA using Prism 6 software (GraphPad). P-values <0.05 were considered statistically significant.
Results
Protein delivery efficiency of dNP2 into the lung resident cells via intranasal administration
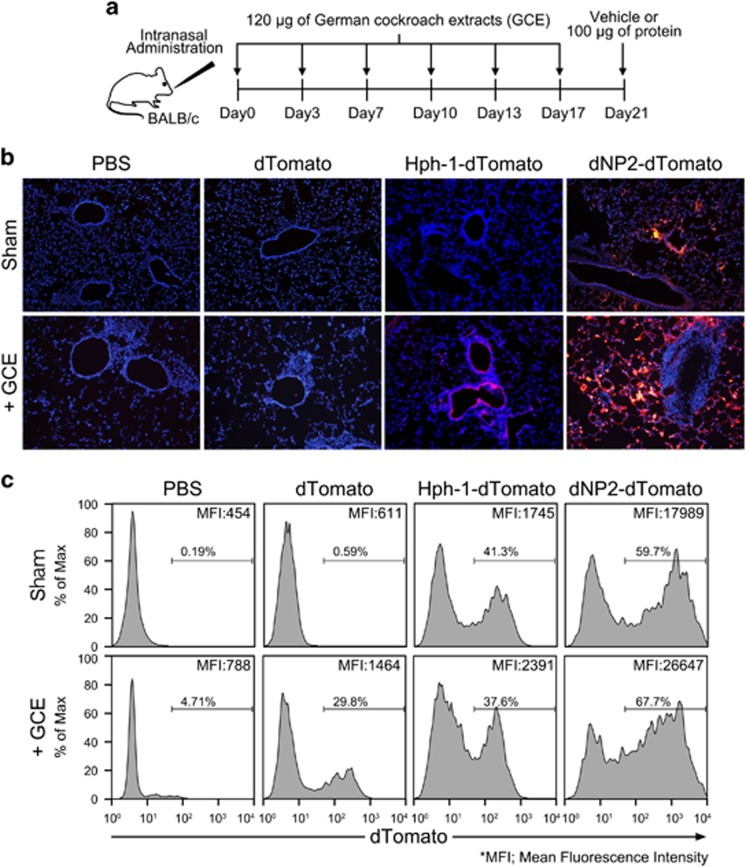
As dNP2 could more efficiently deliver a protein into activated or memory-like immune cells than it could into resting cells,23 we hypothesized that dNP2 could deliver a protein into the inflammatory lung resident cells more effectively than it could in sham mice. We intranasally administered 120 μg of GCE twice per week for 3 weeks into 8-week-old female BALB/c mice to induce chronic-like allergic inflammation in the lungs. On day 21, we intranasally administered 100 μg of dTomato, dNP2-dTomato or PBS (Figure 1a). After 15 min, the intracellular signals of dNP2-dTomato in the lung tissue were significantly increased in the airway epithelium and some resident cells; however, Hph-1-dTomato- and dTomato-treated lungs showed comparable levels with the PBS-treated group, suggesting superior protein delivery efficiency of dNP2 in sham mice (Figure 1b; upper panel). As expected, both dNP2-dTomato and Hph-1-dTomato were efficiently delivered into parenchymal cells in the inflammatory lungs, which had brighter signals, while there was no visible signal in dTomato- or PBS-treated mice (Figure 1b; bottom panel). In addition, we isolated BAL cells from the mice and analyzed them via flow cytometry. The mean fluorescence intensity (MFI) of the cells from the mice treated with dNP2-dTomato was 10 times higher than that of Hph-1, demonstrating the superior protein delivery efficiency of dNP2 compared to Hph-1 (Figure 1c). These results suggest that dNP2 efficiently delivers proteins to the lungs via intranasal administration and is much better than Hph-1.
Figure 1.
Efficiency of dNP2 protein delivery into lung cells by intranasal administration. (a–c) Eight-week-old BALB/c mice were intranasally administered 120 μg of German cockroach extract (GCE) or phosphate-buffered saline (PBS) twice per week for 3 weeks. On day 21, 100 μg of dTomato, Hph-1-dTomato, dNP2-dTomato, or PBS was intranasally administered. After 15 min, bronchoalveolar lavage (BAL) was performed twice for each mouse with 1 ml of PBS. The lungs were harvested and prepared as frozen blocks. (b) The tissues were sectioned to 8 μm thick and observed by fluorescence microscopy after nuclear staining using Hoechst. A representative image of three independent experiments is shown. (c) BAL fluid was collected, and intracellular fluorescence of the BAL cells was analyzed by flow cytometry. Histograms of red fluorescence and mean fluorescence intensities (MFIs) of the indicated populations are shown.
dNP2-ctCTLA-4 ameliorates airway hyper-responsiveness induced by GCE
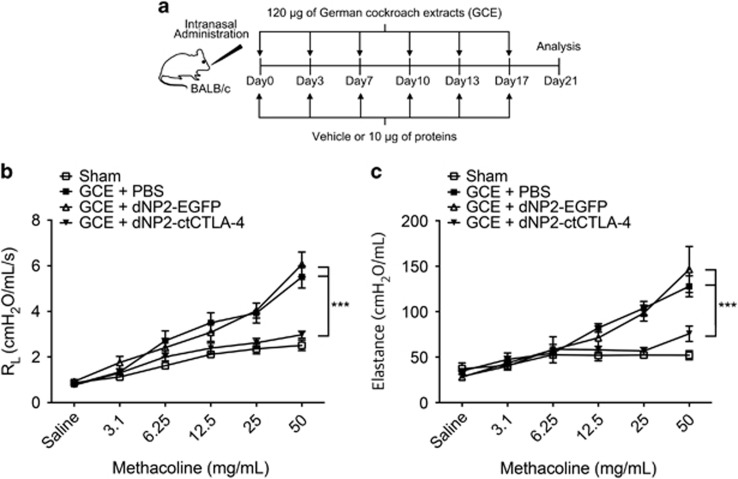
Next, to determine the inhibitory effects of dNP2-ctCTLA-4 in a chronic-like model of allergic asthma induced by GCE, we intranasally administered 120 μg of GCE as described above. In these experiments, 10 μg of dNP2-ctCTLA-4, dNP2-EGFP or vehicle (PBS) was intranasally administered on the same day of the allergen challenge (Figure 2a). On day 21, the mice were anesthetized, and airway resistance to methacholine (MeCh) was measured with a flexiVent system. PBS- or dNP2-EGFP-treated mice had increased airway resistance (Figure 2b) and airway elastance (Figure 2c), indicating significant airway hyper-responsiveness owing to the reduced airway space. We found that 10 μg of dNP2-ctCTLA-4 significantly reduced airway hyper-responsiveness, suggesting that intranasal treatment with dNP2-ctCTLA-4 ameliorates airway hyper-responsiveness induced by multiple exposures to GCE in the lungs.
Figure 2.
dNP2-ctCTLA-4 ameliorates airway hyper-responsiveness induced by German cockroach extract (GCE). (a) Eight-week-old BALB/c mice were intranasally administered 120 μg of GCE twice per week for 3 weeks and 10 μg of dNP2-ctCTLA-4, dNP2-EGFP or PBS was intranasally co-administered. (b, c) Airway hyper-responsiveness was analyzed by a flexiVent 5.1 small animal ventilator. (b) Airway resistance was measured with increasing doses of methacholine (MeCh). (c) Airway elastance was measured with increasing doses of MeCh. n=5 per group, and results are presented as the mean±s.e.m. ***P<0.001.
dNP2-ctCTLA-4 ameliorates airway inflammation and remodeling
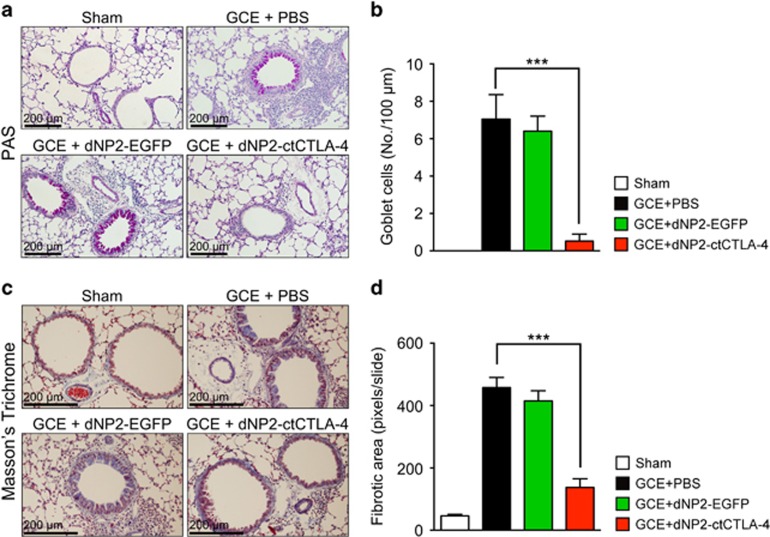
To evaluate the inhibitory effects of dNP2-ctCTLA-4 on the pathophysiology of asthmatic lungs induced by GCE, we analyzed lung tissue histology from the mice. Paraffin-embedded tissues were sectioned and stained with periodic acid-Schiff or Masson’s trichrome. GCE administration substantially increased infiltrated mononuclear cells around blood vessels and goblet cell metaplasia in the airway epithelium. However, intranasal dNP2-ctCTLA-4 treatment significantly reduced inflammatory cells in the lungs and goblet cell abundance (Figure 3a and b). In addition, we measured the fibrotic area based on Masson’s trichrome staining of the sectioned slide, which showed that GCE-treated mice exhibited a high level of peribronchial fibrosis in the lung, while dNP2-ctCTLA-4 treatment significantly reduced the fibrotic area around the bronchial regions (Figure 3c and d). These results collectively suggest that intranasal administration of dNP2-ctCTLA-4 ameliorates the pathophysiology of airway inflammation and remodeling.
Figure 3.
dNP2-ctCTLA-4 ameliorates the pathophysiology of airway inflammation and remodeling. (a) Paraffin-embedded lung tissues were prepared and sectioned. The tissue sections were stained with periodic acid-Schiff (PAS). The slides were observed using bright-field microscopy. Red regions indicate mucus-secreting goblet cells. (b) The number of goblet cells was analyzed using ImageJ 1.50i software. (c) The prepared slides were stained using Masson’s trichrome method and observed by bright-field microscopy. Blue regions indicate collagen deposition and fibrosis. (d) The fibrotic area was analyzed using ImageJ 1.50i software. A representative of each group is shown (n=5). Results are presented as the mean±s.e.m. ***P<0.001.
dNP2-ctCTLA-4 inhibits eosinophil infiltration and Th2 cytokine production
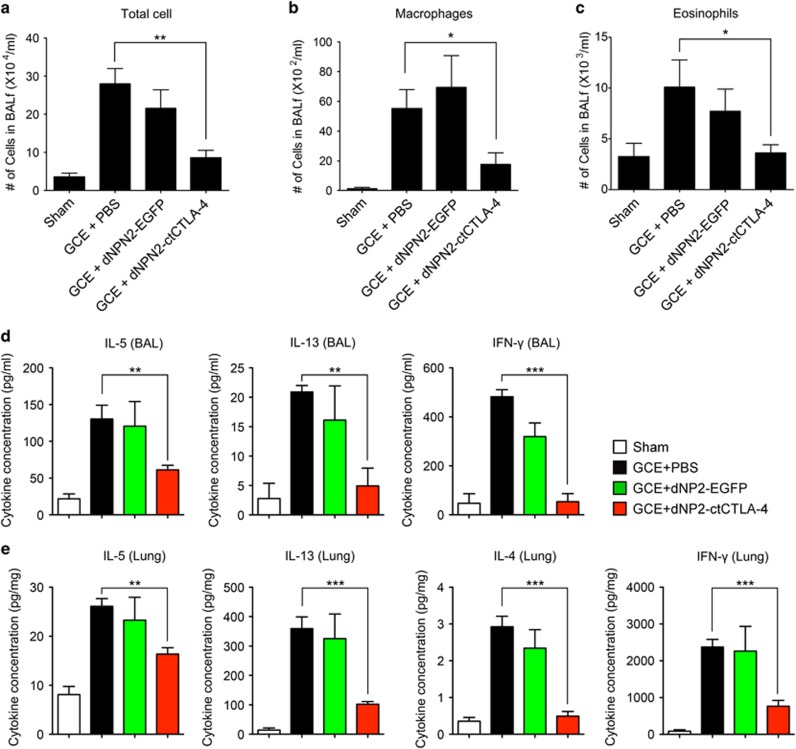
We further analyzed BAL cells, BAL fluid and lung lysate to examine the effects of dNP2-ctCTLA-4 on the recruitment of inflammatory cells and Th2 cytokine production. BAL cell quantification using a Hemacolor staining kit demonstrated that GCE administration increased the number of total cells (Figure 4a), macrophages (Figure 4b) and eosinophils (Figure 4c) in the BAL, while intranasal dNP2-ctCTLA-4 treatment significantly reduced the number of each cell compared to the control groups. Most cells in the GCE-induced asthmatic lung were eosinophils, and they were strongly reduced by dNP2-ctCTLA-4 treatment. Because IL-5 is critical for eosinophil activation,24 we examined the levels of IL-5 and other Th2 cytokines, including IL-13 and IL-4, in the BAL fluid (BALF) and the lung tissue homogenates. IL-5 and IL-13 levels in both BAL and lung lysate were significantly decreased by dNP2-ctCTLA-4, and IL-4 levels in the lung were also decreased (Figure 4d and e). In addition, the IFN-γ level in both BAL and the lungs was reduced by dNP2-ctCTLA-4 treatment, suggesting that intranasal administration of dNP2-ctCTLA-4 significantly reduces Th2 inflammation, including eosinophil recruitment and cytokine production.
Figure 4.
dNP2-ctCTLA-4 reduces Th2 responses, such as eosinophil infiltration and IL-5 production. Bronchoalveolar lavage fluids were collected, and the cells were stained with a Hemacolor staining kit after cytocentrifugation on slide glasses. The slides were observed on bright-field microscopy. (a) Total cell numbers were counted. (b) The number of macrophages were counted. (c) The numbers of eosinophils were counted. (d) The supernatants of bronchoalveolar lavage after centrifugation were collected, and the production of IL-5, IL-13 and IFN-γ was analyzed using an ELISA kit. (e) The lungs were harvested, and tissues were homogenized on ice. The concentration of IL-5, IL-13, IL-4 and IFN-γ was analyzed using an ELISA kit, and the values were normalized to the total protein concentration of the tissues, which was quantitated by Bradford assay. n=5 per group and results are presented as the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
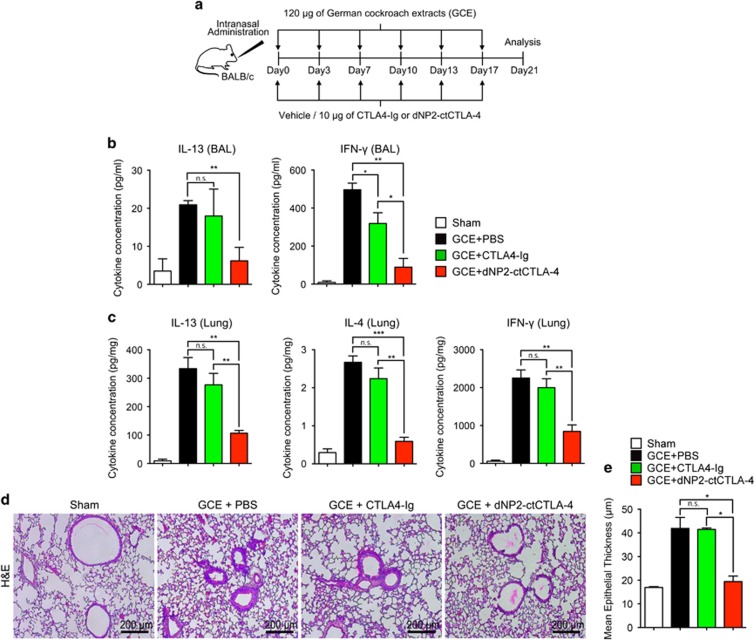
Intranasal administration of dNP2-ctCTLA-4, but not CTLA-Ig, inhibits airway inflammation
Abatacept, a commercial immune regulatory drug, is an immunoglobulin-like recombinant protein consisting of the extracellular domain of CTLA-4 and the Fc region of immunoglobulin (CTLA4-Ig). Systemic administration of CTLA4-Ig can inhibit T-cell activation and airway inflammation in experimental asthma models.18, 19, 20 However, we hypothesized that intranasal CTLA4-Ig cannot inhibit airway inflammation because of low tissue penetration efficiency, while dNP2-ctCTLA-4 can. We administered 10 μg of dNP2-ctCTLA-4, CTLA4-Ig or vehicle with 120 μg of GCE twice per week for 3 weeks (Figure 5a). Th2 cytokines and IFN-γ levels were measured in BAL fluid and lung tissue homogenates. dNP2-ctCTLA-4-treated mice showed significantly reduced IL-13 and IFN-γ in BAL fluid (Figure 5b) and reduced levels of IL-13, IL-4 and IFN-γ in lung tissues (Figure 5c). However, intranasal administration of CTLA4-Ig did not significantly inhibit cytokine production. In lung tissue, hematoxylin and eosin (H&E) staining also demonstrated that intranasal CTLA4-Ig treatment did not inhibit airway epithelium thickening and cell infiltration, while dNP2-ctCTLA-4-treated mice showed a dramatic reversal of these pathophysiologic features (Figure 5d and e). These results suggest that dNP2-ctCTLA-4 is more effective than CTLA4-Ig for intranasal treatment of GCE-induced asthma.
Figure 5.
Intranasal administration of CTLA4-Ig showed marginal inhibitory effects on allergic airway inflammation. (a) Eight-week-old BALB/c mice were intranasally administered 120 μg of German cockroach extract (GCE) twice per week for 3 weeks, and 10 μg of dNP2-ctCTLA-4, CTLA4-Ig or PBS was intranasally co-administered. (b) The supernatants of bronchoalveolar lavage after centrifugation were collected, and the production of IL-13 and IFN-γ was analyzed using an ELISA kit. (c) The lungs were harvested and tissues were homogenized on ice. The concentration of IL-4, IL-13 and IFN-γ was analyzed using an ELISA kit, and the values were normalized to the total protein concentration. (d) Paraffin-embedded lung tissues were prepared and sectioned. The tissue sections were stained with hematoxylin and eosin (H&E) and observed using bright-field microscopy. A representative of each group is shown (n=5). (e) The airway epithelium thickness was measured using ImageJ 1.50i Software. Results are presented as the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
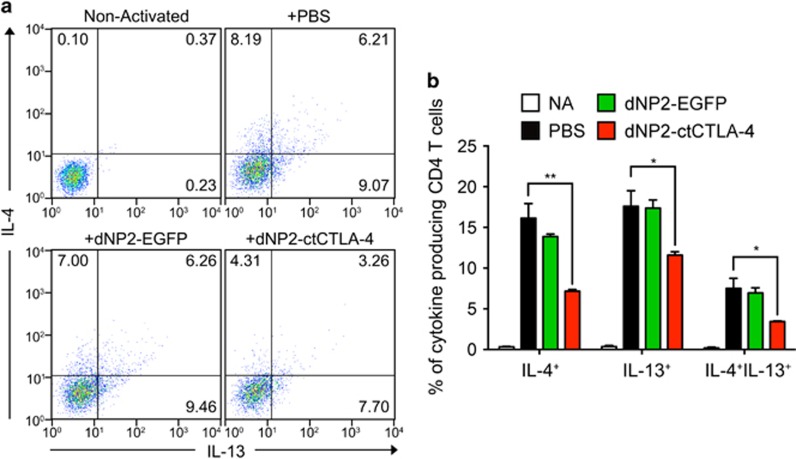
dNP2-ctCTLA-4 inhibits Th2 cell differentiation
Finally, to determine whether dNP2-ctCTLA-4 could directly inhibit Th2 cell differentiation, we utilized MACS-sorted CD4+CD62LhiCD44− naïve CD4 T cells from 8-week-old BALB/c mice and differentiated them into Th2 cells by addition of IL-4 during activation with anti-CD3/CD28 antibodies for 6 days. Th2 cells were analyzed as the IL-4 and IL-13-producing proportion by flow cytometry. Differentiated Th2 cells had a significant proportion of IL-4- and/or IL-13-producing CD4 T cells, while dNP2-ctCTLA-4 significantly reduced cytokine production, demonstrating that differentiation of Th2 cells is inhibited by dNP2-ctCTLA-4 (Figure 6a and b). These results suggest that dNP2-ctCTLA-4 directly inhibits Th2 differentiation, contributing to its inhibitory effect on GCE-induced Th2 inflammation in the lungs.
Figure 6.
dNP2-ctCTLA-4 inhibits Th2 cell differentiation in vitro. (a, b) CD4+CD62LhiCD44− naive CD4 T cells were isolated from 8-week-old BALB/c mice using magnetic activated cell sorting (MACS). The cells were incubated for 6 days in a 96-well plate with Th2 cell-skewing medium that contained coated anti-CD3/CD28 antibodies (0.1 μg ml−1), anti-IFN-γ neutralizing antibody (5 μg ml−1), IL-2 (50 U ml−1) and IL-4 (30 ng ml−1). Simultaneously, 1 μM of dNP2-EGFP, dNP2-ctCTLA-4 or PBS was added to the cell culture at day 0. On day 6, the cells were analyzed by flow cytometry after intracellular staining with anti-mouse IL-4-PE and anti-mouse IFN-γ-FITC FACS antibodies. (a) Dot plots are representative of three independent experiments. (b) The percentage of CD4+IL-4+ differentiated Th2 cells was analyzed. Results are presented as the mean±s.d. (n=3). Non-activated (NA): negative control group, which was not stimulated with anti-TcR antibodies nor cytokines. *P<0.05, **P<0.01.
Discussion
We examined the inhibitory effects of dNP2-ctCTLA-4 on cockroach allergen-induced airway inflammation in mice. dNP2 promoted substantial intranasal protein delivery into lung cells with higher efficiency in the asthmatic lung than in the sham group. dNP2-conjugated protein can penetrate the respiratory epithelium and efficiently localize into resident cells. Intranasal treatment with dNP2-ctCTLA-4 ameliorated airway hyper-responsiveness, Th2 inflammation, and airway remodeling, whereas CTLA-4-Ig could not. dNP2-ctCTLA-4 directly inhibited Th2 differentiation, suggesting it may have clinical applications for controlling allergic asthma.
Allergic asthma is a well characterized and common disorder that can be managed using glucocorticoids and β-2-agonist combination therapy.25 Although those therapeutics can relieve the physiological symptoms of asthma, they cannot ultimately cure the disease, and the symptoms of severe persistent asthmatic patients are not well controlled with those drugs.26 This is because inhibition of Th2 cells and their cytokine production, which have critical roles in asthma, is required to cure allergic asthma. Recently, clinical trials have assessed biological therapeutics that target Th2-related cytokines, including IL-4, IL-5, IL-13 and tumor necrosis factor-α, and thymic stromal lymphopoietin using monoclonal antibodies.27 Omalizumab, which targets IgE,28 and reslizumab or mepolizumab, which target IL-5,29 have been clinically approved for the treatment of asthma. Although they have long-lasting and significant regulatory effects, antibody-based drugs can only be applied through systemic routes and can cause systemic immunosuppression as a major side effect.11, 12 Thus, topical drugs with an appropriate drug delivery methodology are required for allergic asthma.
CTLA-4 negatively regulates T-cell activation, and there are CTLA-4-based drugs for immune modulation, which include the CTLA-4 extracellular domain and the Fc region of immunoglobulin G (CTLA4-Ig; Abatacept, ~92.3 kDa).30 CTLA4-Ig has been studied in clinical trials for allergen-induced airway inflammation as well as in vivo asthma models, which showed that CTLA4-Ig did not alter the inflammatory response in clinical asthma patients through intravenous injection.31 This suggests that systemic inhibition of T cell co-stimulation does not effectively modulate allergic asthma clinically, although it is effective in animal asthma models. In this study, we also demonstrated that CTLA4-Ig cannot be used for topical applications, presumably because of its large size and poor epithelial penetration. However, dNP2-ctCTLA-4 efficiently inhibited allergic airway inflammation in vivo through intranasal administration, which could have fewer adverse effects than systemic immune suppression.
In our previous study, we emphasized the role of the cytoplasmic domain of CTLA-4 (ctCTLA-4) in controlling T-cell activation and allergic autoimmune diseases; ctCTLA-4 could be an alternative drug based on CTLA-4.21, 32, 33 Based on cell-penetrating peptide-mediated protein delivery and its potent application in immune response regulation,34 we assessed a combination of Hph-1 with ctCTLA-4 in an ovalbumin (OVA)-induced allergic airway inflammation model, which showed significant regulation of airway inflammation and hyper-responsiveness.32 The mechanism underlying ctCTLA-4-mediated inhibition of T-cell receptor signaling involved a reduced phosphorylation of ZAP70, JNK, ERK, p38 and the T-cell receptor ζ-chain.33 This had comparable results to the study on liCTLA-4, which is a membrane-bound form of the cytoplasmic domain of CTLA-4.
We previously identified various human-derived CPPs, including LPIN35 and 2pIL-1αNLS.36 More recently, we identified dNP2, a novel CPP, and demonstrated that it could efficiently deliver proteins to various tissues from the bloodstream, even the brain and lymphocytes, while TAT or Hph-1 did not show a significant effect.21 We directly compared the intranasal protein delivery efficiency of dNP2 and Hph-1 and found that dNP2 has a much higher protein delivery efficiency than Hph-1. This enhanced drug delivery peptide could perhaps be used as a drug delivery system for other pulmonary diseases, such as lung cancer, chronic obstructive pulmonary disease and pulmonary fibrosis.
Based on the efficient tissue penetration of dNP2, we assessed the effects of ctCTLA-4 on a GCE-induced asthma model. Compared to a previous study using 25 μg Hph-1-ctCTLA-4 in an OVA-induced asthma model, we administered only 10 μg of protein, which is 500 μg kg−1, suggesting improved therapeutic efficacy. In addition, while a previous study focused on the acute allergic model with a model antigen, we examined dNP2-ctCTLA-4 in a chronic-like GCE allergen-induced asthma model. This could provide relevant clues for clinical trials of dNP2-ctCTLA-4 as an asthma drug candidate.
dNP2-ctCTLA-4 appears to control GCE-induced asthma through inhibition of Th2 inflammation, including eosinophilic inflammation, which is the most critical feature of allergic asthma.24 Immune responses against inhaled allergens in the airways induce differentiation of Th2 cells secreting IL-5, IL-4 and IL-13. IL-5 with eotaxin can stimulate the release of eosinophils from bone marrow,37 and multiple elements with IL-5 recruit eosinophils into the lung during allergic responses.38 Eosinophils are involved in airway hyper-responsiveness, remodeling, fibrosis and mucus secretion, which are key features of asthmatic pathophysiology.39, 40, 41 dNP2-ctCTLA-4 significantly inhibited eosinophil recruitment and Th2 cytokine production, with inhibitory effects on Th2 differentiation. dNP2-ctCTLA-4 also inhibited production of IFN-γ, which is regarded as a Th1 cytokine that contributes to airway inflammation and remodeling in chronic asthma.42 dNP2-ctCTLA-4 can directly regulate Th2-mediated allergic inflammation but can also regulate Th1-mediated chronic inflammation and airway remodeling.
Taken together, the findings of this study demonstrate that dNP2 is an efficient protein delivery peptide in the lung through intranasal administration. dNP2-ctCTLA-4 may be able to serve as a topically applicable biologic drug to control Th2 inflammation in allergic asthma.
Acknowledgments
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0234) to J-MC.
Footnotes
J-MC and SL are inventors who have patent applications describing the use of dNP2 and therapeutic use of dNP2-ctCTLA-4 in asthma. The remaining authors declare no conflict of interest.
References
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161: 1720–1745. [DOI] [PubMed] [Google Scholar]
- Calderon MA, Kleine-Tebbe J, Linneberg A, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC et al. House dust mite respiratory allergy: an overview of current therapeutic strategies. J Allergy Clin Immunol Pract 2015; 3: 843–855. [DOI] [PubMed] [Google Scholar]
- D'Amato G, Liccardi G, D'Amato M, Holgate S. Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy 2005; 35: 1113–1124. [DOI] [PubMed] [Google Scholar]
- Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol 2001; 107: 419–428. [DOI] [PubMed] [Google Scholar]
- Jeong KY, Son M, Lee JH, Hong CS, Park JW. Allergenic characterization of a novel allergen, homologous to chymotrypsin, from german cockroach. Allergy Asthma Immunol Res 2015; 7: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff C, Sidney J, Tripple V, Grey H, Wood R, Broide DH et al. Analysis of T cell responses to the major allergens from German cockroach: epitope specificity and relationship to IgE production. J Immunol 2012; 189: 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K et al. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol 2012; 5: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang M, Ma W, Jin S, Song W, He S. Cockroach allergen Bla g 7 promotes TIM4 expression in dendritic cells leading to Th2 polarization. Mediators Inflamm 2013; 2013: 983149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Iwamoto I, Tomoe S, Matsumura R, Tomioka H, Takatsu K et al. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis 1992; 146: 374–377. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999; 17: 255–281. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kaegi C, Akdis M, Bavbek S, Bossios A, Chatzipetrou A et al. EAACI IG Biologicals task force paper on the use of biologic agents in allergic disorders. Allergy 2015; 70: 727–754. [DOI] [PubMed] [Google Scholar]
- Cruz AA, Lima F, Sarinho E, Ayre G, Martin C, Fox H et al. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clin Exp Allergy 2007; 37: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc 2014; 11(Suppl 5): S244–S251. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W et al. Molecular basis of T cell inactivation by CTLA-4. Science 1998; 282: 2263–2266. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1: 405–413. [DOI] [PubMed] [Google Scholar]
- Ying L, Fu Z, Luo J, Zhou C, Chen Y, Wang L et al. Cytotoxic T lymphocyte antigen 4 immunoglobulin modified dendritic cells attenuate allergic airway inflammation and hyperresponsiveness by regulating the development of T helper type 1 (Th1)/Th2 and Th2/regulatory T cell subsets in a murine model of asthma. Clin Exp Immunol 2011; 165: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosterhout AJ, Hofstra CL, Shields R, Chan B, Van Ark I, Jardieu PM et al. Murine CTLA4-IgG treatment inhibits airway eosinophilia and hyperresponsiveness and attenuates IgE upregulation in a murine model of allergic asthma. Am J Respir Cell Mol Biol 1997; 17: 386–392. [DOI] [PubMed] [Google Scholar]
- Abdelshaeed R, Griffiths GB, Neighbour H. Use of abatacept in eosinophilic asthma. J Allergy Clin Immunol Pract 2014; 2: 220–221. [DOI] [PubMed] [Google Scholar]
- Lim S, Kim WJ, Kim YH, Lee S, Koo JH, Lee JA et al. dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat Commun 2015; 6: 8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol 2013; 161: 315–324. [DOI] [PubMed] [Google Scholar]
- Lim S, Lee JA, Koo JH, Kang TG, Ha SJ, Choi JM. Cell type preference of a novel human derived cell-permeable peptide dNP2 and TAT in murine splenic immune cells. PLoS ONE 2016; 11: e0155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possa SS, Leick EA, Prado CM, Martins MA, Tiberio IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol 2013; 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Current therapies for asthma. Promise and limitations. Chest 1997; 111(2 Suppl): 17S–26S. [DOI] [PubMed] [Google Scholar]
- Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med 2012; 18: 736–749. [DOI] [PubMed] [Google Scholar]
- Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep 2016; 16: 70. [DOI] [PubMed] [Google Scholar]
- Caminati M, Senna G, Guerriero M, Dama AR, Chieco-Bianchi F, Stefanizzi G et al. Omalizumab for severe allergic asthma in clinical trials and real-life studies: what we know and what we should address. Pulm Pharmacol Ther 2015; 31: 28–35. [DOI] [PubMed] [Google Scholar]
- Fainardi V, Pisi G, Chetta A. Mepolizumab in the treatment of severe eosinophilic asthma. Immunotherapy 2016; 8: 27–34. [DOI] [PubMed] [Google Scholar]
- von Kempis J, Dudler J, Hasler P, Kyburz D, Tyndall A, Zufferey P et al. Use of abatacept in rheumatoid arthritis. Swiss Med Wkly 2012; 142: w13581. [DOI] [PubMed] [Google Scholar]
- Parulekar AD, Boomer JS, Patterson BM, Yin-Declue H, Deppong CM, Wilson BS et al. A randomized controlled trial to evaluate inhibition of T-cell costimulation in allergen-induced airway inflammation. Am J Respir Crit Care Med 2013; 187: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med 2006; 12: 574–579. [DOI] [PubMed] [Google Scholar]
- Choi JM, Kim SH, Shin JH, Gibson T, Yoon BS, Lee DH et al. Transduction of the cytoplasmic domain of CTLA-4 inhibits TcR-specific activation signals and prevents collagen-induced arthritis. Proc Natl Acad Sci USA 2008; 105: 19875–19880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Koo JH, Choi JM. Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Netw 2016; 16: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Kim WJ, Kim YH, Choi JM. Identification of a novel cell-penetrating peptide from human phosphatidate phosphatase LPIN3. Mol Cells 2012; 34: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JH, Yoon H, Kim WJ, Lim S, Park HJ, Choi JM. Cell membrane penetrating function of the nuclear localization sequence in human cytokine IL-1alpha. Mol Biol Rep 2014; 41: 8117–8126. [DOI] [PubMed] [Google Scholar]
- Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood 1998; 91: 2240–2248. [PubMed] [Google Scholar]
- Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol 1993; 8: 349–355. [DOI] [PubMed] [Google Scholar]
- Obase Y, Shimoda T, Mitsuta K, Matsuo N, Matsuse H, Kohno S. Correlation between airway hyperresponsiveness and airway inflammation in a young adult population: eosinophil, ECP, and cytokine levels in induced sputum. Ann Allergy Asthma Immunol 2001; 86: 304–310. [DOI] [PubMed] [Google Scholar]
- Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 1997; 100: 2254–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraneveld AD, Folkerts G, Van Oosterhout AJ, Nijkamp FP. Airway hyperresponsiveness: first eosinophils and then neuropeptides. Int J Immunopharmacol 1997; 19: 517–527. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-gamma as a possible target in chronic asthma. Inflamm Allergy Drug Targets 2006; 5: 253–256. [DOI] [PubMed] [Google Scholar]