Abstract
Our previous work has identified miR-125b as a negative regulator of melanogenesis. However, the specific melanogenesis-related genes targeted by this miRNA had not been identified. In this study, we established a screening strategy involving three consecutive analytical approaches—analysis of target genes of miR-125b, expression correlation analysis between each target gene and representative pigmentary genes, and functional analysis of candidate genes related to melanogenesis—to discover melanogenesis-related genes targeted by miR-125b. Through these analyses, we identified SRC homology 3 domain-binding protein 4 (SH3BP4) as a novel pigmentation gene. In addition, by combining bioinformatics analysis and experimental validation, we demonstrated that SH3BP4 is a direct target of miR-125b. Finally, we found that SH3BP4 is transcriptionally regulated by microphthalmia-associated transcription factor as its direct target. These findings provide important insights into the roles of miRNAs and their targets in melanogenesis.
Introduction
Melanin is synthesized within the melanosomes of melanocytes and transferred to surrounding epidermal cells, where it protects the skin from intrinsic and extrinsic stresses by decreasing reactive oxygen species and inhibiting ultraviolet radiation-induced DNA damage.1, 2 The amount and type of melanin determine the phenotypes of human skin and hair color, and many skin disorders involve abnormal melanin synthesis.3
MicroRNAs (miRNAs) are small noncoding RNAs of ~21–23 nucleotides in length that play a critical role in the regulation of mRNA expression. miRNAs primarily decrease the expression of targets by degradation of mRNA or inhibition of translation.4 Recently, we have identified miR-125b as a hypopigmentation gene involved in the regulation of steady-state melanogenesis.5 The tyrosinase (TYR) and dopachrome tautomerase (DCT) levels decrease when miR-125b is overexpressed; however, neither gene is a direct target of miR-125b. Therefore, we hypothesized that unknown pigmentation genes might be targeted by miR-125b in melanocytic cells. In addition, miR-125b is dysregulated in various cancers, including melanomas, and several direct targets of miR-125b have been identified in these cancers.6 However, no reported targets of miR-125b have been directly linked to pigmentation. Therefore, identification of novel targets of miR-125b that may be essential for melanocyte physiology, including pigmentary processes, is needed.
Accordingly, in this study, we developed a screening strategy for identifying novel pigmentation genes. By selecting genes that were experimentally regulated by miR-125b and theoretically predicted to be targets of miR-125b, we obtained a list of potential targets and further filtered the list by comparing gene expression levels of these targets with those of a representative pigmentary gene, TYR, by using a publically available data set of gene expression for melanocytic cells. From this analysis, combined with a melanin quantification assay, we identified SRC homology 3 domain-binding protein 4 (SH3BP4) as a melanogenesis-related regulatory target of miR-125b in melanocytic cells.
Materials and methods
Cell culture and transfection
WM266-4 human melanoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Eagle’s minimum essential medium (MEM) supplemented with 1% penicillin/streptomycin and 10% heat-inactivated fetal bovine serum (Gibco, Carlsbad, CA, USA). MNT1 human pigmented melanoma cells were kindly provided by Dr Ai-Young Lee at Dongguk University, who originally received these cells as a gift from Dr Vincent J. Hearing at the National Institutes of Health (Bethesda, MD, USA). These cells were maintained in MEM supplemented with Dulbecco’s modified Eagle’s medium, 1% penicillin/streptomycin, 20% FBS and 20 mM HEPES. Normal human melanocytes prepared from the foreskins of moderately pigmented donors were purchased from Cascade Biologics (Portland, OR, USA) and cultured in Medium 254 (Cascade Biologics) containing a human melanocyte growth supplement (Cascade Biologics). All cells were grown at 37 °C in an atmosphere containing 5% CO2.
Melanocytes, WM266-4 cells and MNT1 cells were transfected with miR-125b mimics (Ambion, Austin, TX, USA) or siRNAs against SH3BP4, sortilin 1 (SORT1), interferon regulatory factor 4 (IRF4) or microphthalmia-associated transcription factor (MITF; Bioneer, Daejeon, Korea) by using RNAiMAX Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The sequence of each siRNA is listed in Supplementary Table 1.
Microarray analysis
Total RNAs were isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The mRNA expression profiles were generated using an Illumina HumanHT-12 v4 Expression BeadChip (Illumina, San Diego, CA, USA).
Quantitative real-time PCR
Total RNA was reverse transcribed to generate cDNAs by using a Superscript Reverse Transcriptase II kit (Invitrogen). Quantitative mRNA measurements were performed for each sample with TaqMan Universal Master PCR mix and TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). The cDNA samples were analyzed for the following mRNAs with the following probes: SH3BP4, Hs00170950_m1; SORT1, Hs00361760_m1; IRF4, Hs01029057_m1; and MITF, Hs01117294_m1 (Applied Biosystems). Human glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 4333764F; Applied Biosystems) was also amplified and used to normalize variations in cDNA quantities. The quantitative real-time PCR (RT-qPCR) analyses were conducted using an ABI7500 FAST real-time PCR system (Applied Biosystems).
To determine whether miR-125b is incorporated into the RNA-induced silencing complex (RISC), we performed an immunoprecipitation analysis of MNT1 melanoma cells transfected with an miR-125b mimic or an N.C. mimic. Cells were lysed and subjected to AGO1/2/3 immunoprecipitation using a miRNA Target IP Kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. After elution, extracted RNAs were reverse-transcribed and subjected to RT-qPCR.
Melanin assay
The melanin content in melanocytes was determined as described previously.7 Briefly, cells were harvested using trypsin/EDTA and then centrifuged for 5 min at 1000 g. The cell pellets in tubes were imaged and then dissolved in 1 N NaOH. The relative melanin content was determined by measuring the absorbance at 450 nm using a spectrophotometer.
Western blotting
Total protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions, and the obtained proteins were subjected to SDS-PAGE. After protein transfer and membrane blocking, the membranes were incubated overnight at 4 °C with antibodies in PBS containing 0.1% Tween 20. The primary antibodies used for immunoblotting were as follows: anti-SH3BP4 antibodies (catalog number 17691-1-AP; Proteintech Group, Chicago, IL, USA) and anti-TYR antibodies (catalog number sc-20035; Santa Cruz Biotechnology, Dallas, TX, USA). All protein bands were detected using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Little Chalfont, UK).
Construction of vectors and luciferase assays
Partial segments of the 3′-untranslated regions (UTRs) of the TYR, DCT and SH3BP4 mRNAs were subjected to PCR amplification using the primer sets shown in Supplementary Table 2. The obtained PCR products were cloned into the pMIR-REPORT Firefly Luciferase reporter vector (Ambion). SH3BP4 promoter regions with the predicted MITF binding sites were amplified from human genomic DNA by PCR using the primer sets listed in Supplementary Table 2. PCR products were cloned into the pGL3-Promoter vector (Promega, Madison, WI, USA). Mutant constructs were generated using a QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA), according to the manufacturer’s instructions, with the primer sets listed in Supplementary Table 2. WM266-4 cells were cotransfected with 200 ng of each 3′-UTR reporter construct and 100 ng of a β-galactosidase expression vector (internal control) by using Lipofectamine 3000 (Invitrogen). Luciferase and β-galactosidase activities were measured using a Luciferase Assay Kit (Promega) and Enhanced β-Galactosidase Assay kit (Gelantis, San Diego, CA, USA) according to the manufacturers’ protocols.
In vitro TYR activity assay
Cellular TYR activity was determined as described previously.7 Melanocytes were extracted using extraction buffer (1% Nonidet P-40, 0.005% SDS, 0.1 M Tris-HCl, pH 7.2) containing a protease inhibitor cocktail purchased from Sigma-Aldrich Biotechnology (St Louis, MO, USA) for 1 h at 4 °C. The protein concentrations of the extracts were determined with a Bio-Rad protein assay kit (Bio-Rad). Equal amounts of protein (10 μg) from the cell extracts were incubated with 10 mM L-dihydroxyphenylalanine in 50 mM sodium phosphate buffer (pH 6.8) at 37 °C for 1 h. TYR activity was determined by measuring the absorbance at 490 nm using a spectrophotometer.
Bioinformatics analysis
Prediction of miR-125b targets was conducted using five algorithms (TargetScan, PicTar, RNA22, PITA, and miRanda). StarBase V2.0 (http://starbase.sysu.edu.cn/index.php), which provides an integrative analysis of RNA-Seq, miRNA-Seq and crosslinking immunoprecipitation (CLIP)-Seq, was used to analyze the relative expression levels of miR-125b and SH3BP4 and determine the binding sites of miR-125b on SH3BP4 mRNA.8, 9 The microarray data sets GSE7553, GSE32474 and GSE36879 were downloaded from NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/) and used for analysis of gene expression. The chromatin immunoprecipitation (ChIP)-Seq data set GSE50681 was downloaded from NCBI GEO, and the binding sites for MITF on the SH3BP4 gene were analyzed and visualized using an integrated genome viewer (https://www.broadinstitute.org/igv/).10 To explore the biologically enriched gene sets for high versus low SH3BP4-expressing melanocytic cells, gene set enrichment analysis (GSEA; http://software.broadinstitute.org/gsea/index.jsp) was performed.11
Results
Identification of SH3BP4 as a novel pigmentation gene
Previously, we have reported that miR-125b is an anti-pigmentation gene regulating steady-state melanogenesis.5 Although TYR and DCT expression levels are downregulated by miR-125b overexpression, computational analysis did not predict the presence of putative miR-125b-binding sites in the 3′-UTRs of TYR or DCT mRNA. Here we further tested whether TYR and DCT mRNAs are direct targets of miR-125b, by using luciferase vectors carrying the 3′-UTR of TYR or DCT mRNA (Supplementary Figure 1). In agreement with the prediction of miRNA target algorithms, luciferase activities were not decreased by miR-125b overexpression, thus indicating that TYR and DCT were not direct targets of miR-125b. These data suggested that unknown targets of miR-125b in melanocytic cells may be novel pigmentary genes.
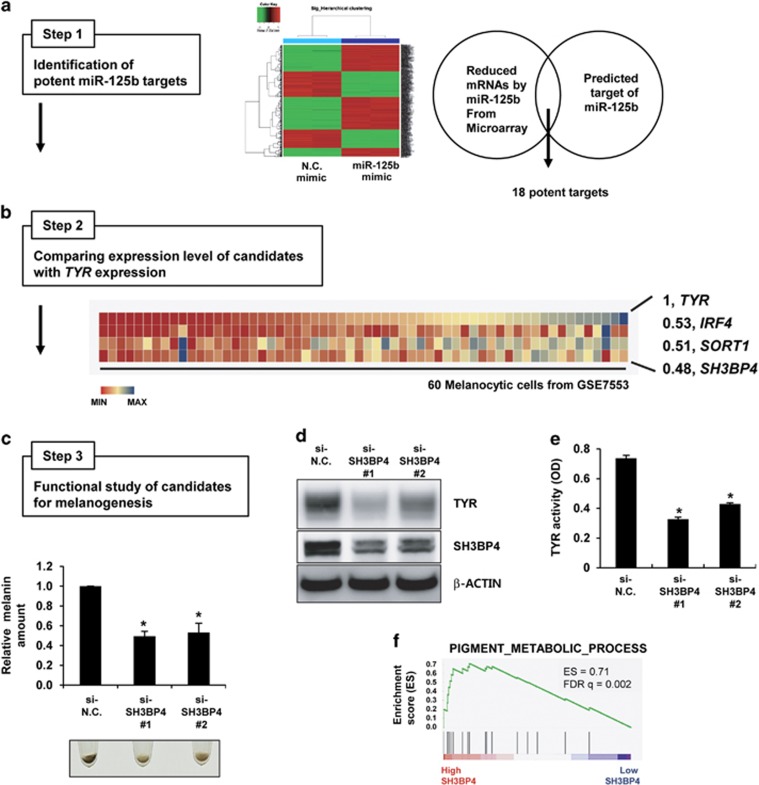
To identify melanogenesis-related targets regulated by miR-125b, we combined three analytical methods: (1) analysis of targets of miR-125b (Figure 1a, Step 1); (2) expression correlation analysis between each target gene and a representative pigmentation gene (Figure 1b, Step 2); and (3) functional analysis of candidates for melanogenesis (Figure 1c, Step 3). First, we identified targets of miR-125b by combining the genes downregulated by miR-125b overexpression in melanoma cells from microarray data (Supplementary Table 3) and the genes predicted as targets of miR-125b by more than three algorithms in five miRNA target prediction programs (TargetScan, PicTar, RNA22, PITA and miRanda). This analysis identified 18 genes as potential targets of miR-125b (Figure 1a and Supplementary Table 4). Next, we compared the mRNA expression profiles of candidates with that of TYR, a representative melanocytic lineage-specific gene, by analyzing a publicly available microarray data set of 60 melanocytic cell lines (Supplementary Figure S2). Among these 18 candidates, the mRNA levels of IRF4, SORT1 and SH3BP4 were correlated with that of TYR (correlation efficiency over 0.4; Figure 1b).
Figure 1.
Identification of SH3BP4 as a pigmentation gene. (a) WM266-4 melanoma cells were transfected with negative control (N.C.) or miR-125b mimics and subjected to microarray analysis. Genes that were downregulated by miR-125b overexpression were analyzed. Putative miR-125b targets were predicted using miRNA target prediction algorithms. From the intersection of both analyses, 18 candidate targets of miR-125b were selected (Step 1). (b) The expression of each gene was compared with that of TYR in the data set for 60 melanocytic cell lines (GSE7553; Step 2). Heat maps for the expression of IRF4, SORT1 and SH3BP4, which were highly correlated with TYR, and Pearson’s correlation coefficients (r) are shown. (c) Human primary melanocytes were transfected with negative control (si-N.C.) or SH3BP4 (si-SH3BP4) siRNAs for 7 days. Representative images of cell pellets (2 × 104 cells) are shown. The relative melanin content was determined. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-tests. (d) Melanocytes were transfected with the indicated siRNAs for 3 days. Protein expression was analyzed by western blotting using anti-TYR or anti-SH3PB4 antibodies. β-Actin was used as a loading control. (e) In vitro TYR activity was measured by incubating cell extracts as described in the Materials and Methods. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-tests. (f) GSEA plot for melanoma cells expressing high SH3BP4 versus low SH3BP4 levels. FDR, false-discovery rate; ES, enrichment score.
Finally, we performed functional melanin quantification assays for candidates after knocking down their expression by using specific siRNAs (Step 3). Two validated siRNAs for each gene (Supplementary Figure S3) were transfected into human primary melanocytes. IRF4 is a pigmentation gene12 that has recently been shown to be regulated by miR-125b.13 As expected, knockdown of IRF4 slightly decreased the melanin content (Supplementary Figure S4A). However, decreased SORT1 expression did not affect melanin levels (Supplementary Figure S4B). Compared with knockdown of these two targets, SH3BP4 knockdown significantly decreased melanin content, and resulted in downregulation of TYR expression and activity (Figures 1c–e). Next, we performed GSEA, which provides predetermined gene sets to identify the biological functions of samples.11 We clustered the melanoma samples of the microarray from GSE7553 into two groups: high SH3BP4-expressing and low SH3BP4-expressing melanocytic cells. Then, we analyzed the enriched gene sets in the high SH3BP4-expressing melanoma sample through GSEA (Supplementary Table 5). Among several gene sets analyzed, we found that high SH3BP4 expression was related to pigment metabolic/biosynthetic processes (Figure 1f), thus indicating that SH3BP4 expression in melanocytic cells is related to melanogenesis. Together, our results led us to conclude that the expression of SH3BP4 is related to the pigmentation process.
SH3BP4 mRNA is a direct target of miR-125b
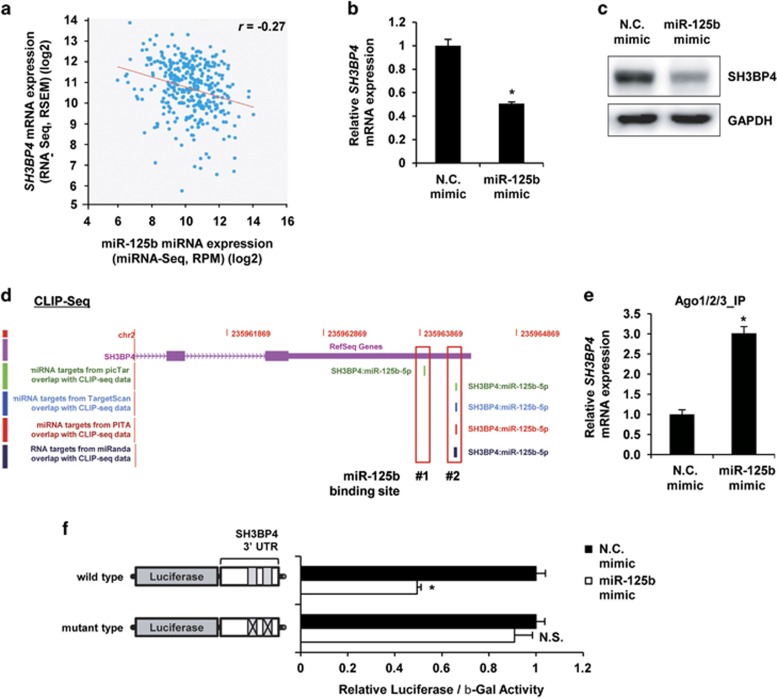
Using several analyses, we predicted that SH3BP4 is a potential target of miR-125b. Then, we confirmed these findings by combining our bioinformatics analyses and experimental validations. From the bioinformatics analysis using starBase V2.0 (http://starbase.sysu.edu.cn/index.php), which provides information on the systemic interactions between miRNAs and their targets through analysis of large-scale RNA-Seq, miRNA-Seq and CLIP-Seq data,8, 9 we found that SH3BP4 expression was inversely proportional to that of miR-125b in melanoma (Figure 2a). When WM266-4 melanoma cells were transfected with the miR-125b mimic, the expression of SH3BP4 mRNA was significantly decreased and was accompanied by decreased SH3BP4 protein levels (Figures 2b and c). In addition, miR-125b inhibition increased its expression (Supplementary Figure S5). On the basis of CLIP-Seq analysis, in which potential binding site(s) for specific miRNAs on target mRNAs were identified, we found that SH3BP4 has two putative binding sites for miR-125b in its 3′-UTR (Figure 2d). Moreover, we confirmed that the binding of SH3BP4 mRNA on RISC was increased by miR-125b overexpression (Figure 2e). Next, we confirmed that miR-125b targeted SH3BP4 mRNA, by using luciferase vectors carrying the 3′-UTR of SH3BP4 mRNA containing two putative miR-125b binding sites with or without mutations. The overexpression of miR-125b decreased the luciferase activity of the wild-type construct; however, this effect disappeared when mutations in the binding sites were introduced (Figure 2f), thus indicating that SH3BP4 is a direct target of miR-125b.
Figure 2.
SH3BP4 mRNA is a direct target of miR-125b. (a) Correlation scatter plot between miR-125b (x axis) and SH3BP4 (y axis). The regression line is shown in red (intercept=13.2, slope=−0.24). (b, c) MNT-1 cells were transfected with negative control or miR-125b mimics for 2 days. The mRNA (b) and protein (c) levels of SH3BP4 were analyzed using RT-qPCR and western blotting, respectively. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-test. (d) An integrative view of two putative binding sites for miR-125b on SH3BP4 mRNA from the starBase V2.0 analyses using CLIP-Seq data and miRNA-target prediction algorithms. (e) MNT1 melanoma cells transfected with miR-125b mimic or N.C. mimic were immunoprecipitated. RNAs were extracted from the immunoprecipitates, and SH3BP4 mRNA expression was analyzed. *P<0.05 versus N.C. mimic, unpaired Student's t-test. (f) Wild-type and mutant luciferase-SH3BP4 3′-UTR constructs were transfected into negative control or miR-125b mimic-expressing WM266-4 cells. At 1 day post transfection, the luciferase activity was measured and normalized to β-galactosidase activity. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-tests.
SH3BP4 is a target of MITF
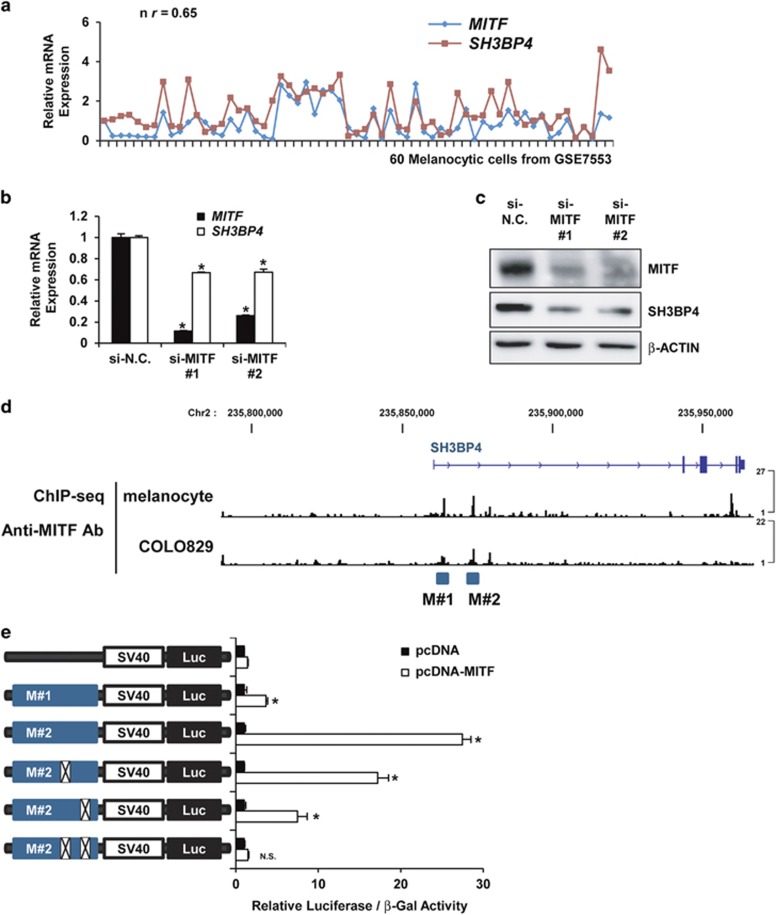
SH3BP4 was found to be expressed at relatively higher levels in melanoma than in other cancers when its expression was examined in NCI60 cancer cell lines (Supplementary Figure S6). Thus, we hypothesized that SH3BP4 expression is regulated by melanocytic lineage-specific traits. Accordingly, we examined whether SH3BP4 was transcriptionally regulated by MITF, which acts as a master regulator by inducing the expression of melanocytic lineage-specific genes.14 When the mRNA expression of SH3BP4 was compared with that of MITF in a microarray data set of 60 melanocytic samples, both genes were found to be highly correlated (r=0.65; Figure 3a). Moreover, loss-of-function studies indicated that MITF depletion decreased the mRNA (Figure 3b) and protein (Figure 3c) levels of SH3BP4. Through analysis of ChIP-Seq data using an anti-MITF antibody in human melanocytes and the melanoma cell line COLO829,10 we identified two potential MITF binding regions (M#1 and M#2) near the transcription start site of SH3BP4 (Figure 3d). Next, we constructed luciferase-reporter vectors carrying each of two DNA regions and measured the luciferase activity after cotransfecting cells with control or MITF plasmids. Compared with the vector carrying the M#1 region, the luciferase-reporter vector carrying the M#2 DNA region exhibited much stronger luciferase activity after MITF overexpression, and this effect was significantly decreased or completely abolished when two mutations were introduced into the M#2 region by site-directed mutagenesis (Figure 3e). Together, these results indicated that SH3BP4 is a target of MITF, thus potentially explaining why SH3BP4 is expressed at relatively higher levels in melanoma cells than in other cells.
Figure 3.
SH3BP4 is a target of MITF. (a) The relative expression of MITF and SH3BP4 mRNAs in 60 melanomas (microarray data set: GSE7553). (b, c) The mRNA (b) and protein (c) levels of MITF and SH3BP4 were analyzed using RT-qPCR and western blotting, respectively, after transfection of MNT-1 cells with negative control siRNA or siRNA against MITF. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-tests. (d) ChIP-Seq data showing binding of MITF to two chromosomal sites in the SH3BP4 gene (M#1 and M#2) in human melanocytes and COLO829 melanoma cells (GSE50681). (e) Mock or MITF plasmids were cotransfected with the luciferase vectors harboring the wild-type or mutant SH3BP4 promoter in WM266-4 cells. The two mutations in the M#2 region were introduced together or separately. At 24 h post transfection, luciferase activity was measured and normalized to β-galactosidase activity. The data are presented as the mean±s.d. of three independent experiments. *P<0.05, unpaired Student’s t-tests.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) has previously been identified as an upstream regulator of MITF in melanocytes.15 Thus, we hypothesized that PPARGC1A may regulate the expression of SH3BP4. To assess this hypothesis, we analyzed publicly available data and found that PPARGC1A expression was also highly correlated with that of SH3BP4 and that PPARGC1A depletion significantly decreased SH3BP4 mRNA levels (Supplementary Figures S7A and B), thus supporting our earlier conclusion that MITF regulates SH3BP4 transcription.
Discussion
To date, many genes have been shown to regulate melanogenesis directly or indirectly.16 With advancements in gene cloning and locus mapping, over 300 pigmentation genes have been identified (http://www.espcr.org/micemut/); these genes regulate melanogenesis through a variety of processes, such as development/differentiation into melanocytic lineages, melanosome formation/transport/transfer, melanin synthesis and protein trafficking, thereby indicating that melanogenesis is a complex process regulated by many genes and pathways. However, even with this long list of melanogenesis-related genes, no target genes of miR-125b have been identified to be involved in its hyperpigmentation effects. In this study, by combining systemic bioinformatics analyses and experimental assays, we demonstrated that SH3BP4 is a novel pigmentary gene regulated by miR-125b, which decreases SH3BP4 expression through direct binding to the 3′-UTR. Moreover, we verified that SH3BP4 is positively regulated by MITF, a master lineage regulator in melanocytic cells, as its direct target.
Although we identified SH3BP4 as a pigmentation gene, its function in melanogenesis was still unclear. Accordingly, we inferred its function from previous reports. Importantly, SH3BP4 may affect the trafficking of melanogenic enzymes. SH3BP4 has been reported to regulate the internalization of the transferrin receptor (TfR).17 SH3BP4 is localized to the plasma membrane, clathrin-coated pits, and clathrin-coated vesicles but not to endosomes in HeLa cells. Therefore, on the basis of its localization to clathrin-related structures and co-immunoprecipitation with clathrin, SH3BP4 may be tightly linked to clathrin or clathrin-mediated functions. Clathrin adaptor proteins are known to play critical roles in melanosome biogenesis in melanocytes.18, 19, 20 The interaction between clathrin adaptors and kinesin determines the localization of recycling endosomes and regulates the sorting of melanogenic enzymes and the recycling of TfR. From these studies, we hypothesized that SH3BP4 might be involved in the trafficking of melanogenic enzymes to melanosomes via clathrin-mediated sorting cargo.
Alternatively, SH3BP4 may negatively regulate mammalian target of rapamycin (mTOR) signaling, thus resulting in melanogenesis. Indeed, SH3BP4 has been shown to be a negative regulator of amino-acid-activated mTOR signaling.21 Amino acids stimulate mTOR signaling via Rag GTPases, such as GTP-bound RagA or RagB. However, SH3BP4 preferentially binds to inactive GDP-bound Rag GTPase and inhibits the formation of the active Rag GTPase complex, thus resulting in inactivation of mTOR signaling. Interestingly, mTOR signaling has been shown to be involved in melanogenesis.22, 23 WD repeat domain, phosphoinositide-interacting 1 (WIPI1) increases the expression of melanogenic enzymes by inhibiting mTOR signaling. Depletion of WIPI1 increases the activity of mTOR and reduces melanogenesis. In agreement with these results, inhibition of mTOR complex 1 (mTORC1) by using rapamycin increases the number of mature melanosomes in melanocytes, thereby indicating that the negative regulation of mTOR signaling promotes melanogenesis. Because Rag GTPases control the subcellular localization of mTORC1 in the lysosome, where mTORC1 signaling is integrated for biological functions,24 we hypothesized that SH3BP4 binding to GDP-bound inactive Rag GTPase inhibits mTOR signaling by preventing mTORC1 localization to the lysosomal surface membrane.
In summary, in this study, we found that SH3BP4 is a novel pigmentation gene directly regulated by two important melanogenesis-related molecules, miR-125b and MITF. Furthermore, we hypothesized that SH3BP4 may be involved in the clathrin-mediated sorting of melanogenic enzymes and the mTOR signal pathway, thus resulting in melanogenesis (Supplementary Figure S8).
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
The authors declare no conflict of interest.
Supplementary Material
References
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol 1998; 110: 806–810. [DOI] [PubMed] [Google Scholar]
- Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res 2006; 19: 572–594. [DOI] [PubMed] [Google Scholar]
- d'Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S et al. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res 2015; 28: 520–544. [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011; 12: 99–110. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bin BH, Kim J, Dong SE, Park PJ, Choi H et al. Novel inhibitory function of miR-125b in melanogenesis. Pigment Cell Melanoma Res 2014; 27: 140–144. [DOI] [PubMed] [Google Scholar]
- Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal 2014; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Ahn S, Lee BG, Chang I, Hwang JS. Inhibition of skin pigmentation by an extract of Lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment Cell Res 2005; 18: 439–446. [DOI] [PubMed] [Google Scholar]
- Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res 2011; 39(Database issue): D202–D209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 2014; 42(Database issue): D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DE, Barajas B, Bussat RT, Yan KJ, Neela PH, Flockhart RJ et al. Enhancer-targeted genome editing selectively blocks innate resistance to oncokinase inhibition. Genome Res 2014; 24: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius C, Grill C, Stacey SN, Metcalf AM, Gorkin DU, Robinson KC et al. A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell 2013; 155: 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So AY, Sookram R, Chaudhuri AA, Minisandram A, Cheng D, Xie C et al. Dual mechanisms by which miR-125b represses IRF4 to induce myeloid and B-cell leukemias. Blood 2014; 124: 1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Jackson IJ, Patton EE. The melanocyte lineage in development and disease. Development 2015; 142: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A et al. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell 2013; 49: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors 2009; 35: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni D, Puri C, Confalonieri S, Salcini AE, De Camilli P, Tacchetti C et al. TTP specifically regulates the internalization of the transferrin receptor. Cell 2005; 123: 875–888. [DOI] [PubMed] [Google Scholar]
- Chapuy B, Tikkanen R, Muhlhausen C, Wenzel D, von Figura K, Honing S. AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic 2008; 9: 1157–1172. [DOI] [PubMed] [Google Scholar]
- Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol 2009; 187: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J Biol Chem 2012; 287: 19550–19563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Stone M, Hwang TH, Kim YG, Dunlevy JR, Griffin TJ et al. SH3BP4 is a negative regulator of amino acid-Rag GTPase-mTORC1 signaling. Mol Cell 2012; 46: 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H, Kapadia R, Al-Tahan S, Ahmad S, Ganesan AK. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J Biol Chem 2011; 286: 12509–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah YS, Cho HY, Lim TY, Park DH, Kim HM, Yoon J et al. Induction of melanogenesis by rapamycin in human MNT-1 melanoma cells. Ann Dermatol 2012; 24: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol 2014; 36: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.